Silymarin and Cancer: A Dual Strategy in Both in Chemoprevention and Chemosensitivity
Abstract
1. Introduction
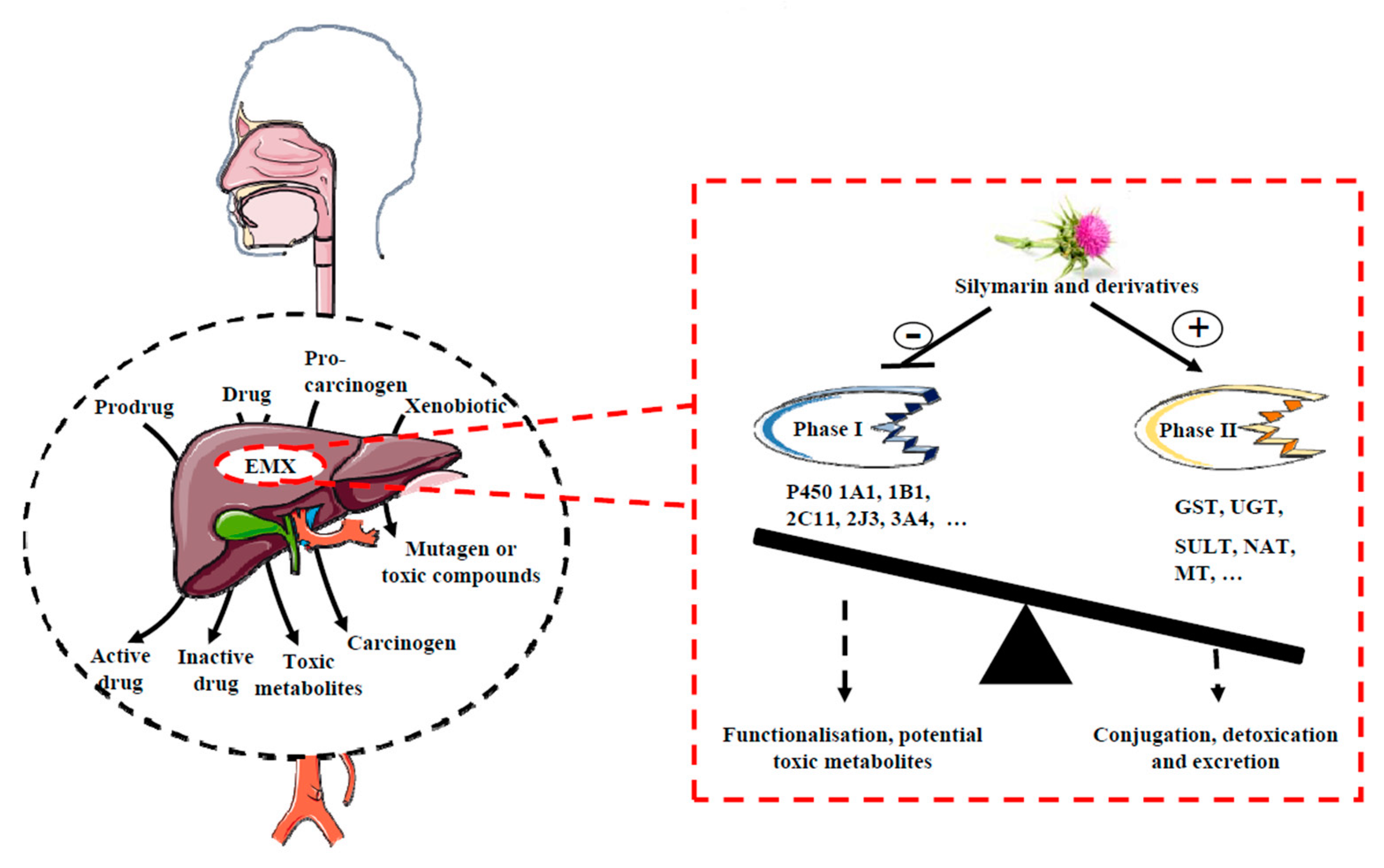
2. A Role for the Xenobiotics Metabolizing Enzymes (XME) Phase I and II in the Chemopreventive/Chemosensitivity Actions of Silymarin
2.1. Phase I Reactions
2.2. Phase II Reactions
2.3. Silymarin Exerts a Chemopreventive Action through an Inhibition of P450 Activity
2.4. Silymarin Exerts a Chemopreventive/Chemosensitivity Action through an Activation of Phase II Enzymes
2.5. Clinical Relevance of XME Modulation by Silymarin
3. A Role for Phase III Transporters in the Chemopreventive/Chemosensitivity Actions of Silymarin
3.1. Silymarin and OATP
3.2. Silymarin and ABC Transporters
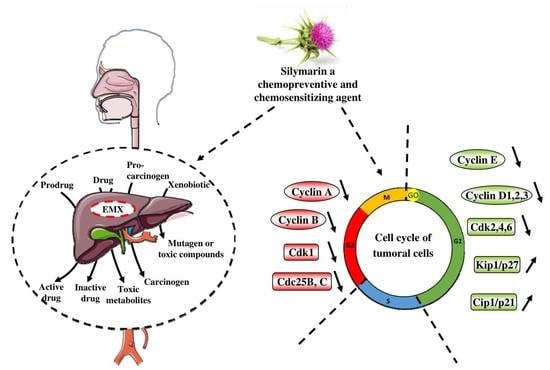
4. Cell Cycle and its Important Checkpoints for the Chemopreventive/Chemosensitivity Actions of Silymarin
4.1. Silymarin and G0/G1 Arrest
4.2. Silymarin and G2/M Arrest
4.3. Silymarin and Chemosensitization through a G0/G1 or a G2/M Arrest
5. Extrinsic and Intrinsic Cell Death Pathways for the Chemopreventive/Chemosensitivity Actions of Silymarin
5.1. Silymarin Modulates Mitochondrial and Death Receptors Pathways for its Chemopreventive Action
5.2. Silymarin Synergizes with Anticancer Drugs to Induce Apoptosis
6. Clinical Studies of Silymarin and Derivatives in a Cancer Context
7. Conclusions
Funding
Conflicts of Interest
References
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Smith, J.M. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed. Proc. 1976, 35, 1332–1338. [Google Scholar] [PubMed]
- Sporn, M.B.; Suh, N. Chemoprevention: An essential approach to controlling cancer. Nat. Rev. Cancer 2002, 2, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kannappan, R.; Reuter, S.; Kim, J.H.; Aggarwal, B.B. Chemosensitization of tumors by resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Lancon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef]
- Delmas, D.; Solary, E.; Latruffe, N. Resveratrol, a phytochemical inducer of multiple cell death pathways: Apoptosis, autophagy and mitotic catastrophe. Curr. Med. Chem. 2011, 18, 1100–1121. [Google Scholar] [CrossRef]
- Chambers, C.S.; Holeckova, V.; Petraskova, L.; Biedermann, D.; Valentova, K.; Buchta, M.; Kren, V. The silymarin composition and why does it matter? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 942. [Google Scholar] [CrossRef]
- Esmaeil, N.; Anaraki, S.B.; Gharagozloo, M.; Moayedi, B. Silymarin impacts on immune system as an immunomodulator: One key for many locks. Int. Immunopharmacol. 2017, 50, 194–201. [Google Scholar] [CrossRef]
- Mastron, J.K.; Siveen, K.S.; Sethi, G.; Bishayee, A. Silymarin and hepatocellular carcinoma: A systematic, comprehensive, and critical review. Anticancer Drugs 2015, 26, 475–486. [Google Scholar] [CrossRef]
- Polachi, N.; Bai, G.; Li, T.; Chu, Y.; Wang, X.; Li, S.; Gu, N.; Wu, J.; Li, W.; Zhang, Y.; et al. Modulatory effects of silibinin in various cell signaling pathways against liver disorders and cancer—A comprehensive review. Eur. J. Med. Chem. 2016, 123, 577–595. [Google Scholar] [CrossRef]
- Liakopoulou, C.; Kazazis, C.; Vallianou, N.G. Silimarin and Cancer. Anti-Cancer Agents Med. Chem. 2018, 18, 1970–1974. [Google Scholar] [CrossRef] [PubMed]
- Jahanafrooz, Z.; Motamed, N.; Rinner, B.; Mokhtarzadeh, A.; Baradaran, B. Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci. 2018, 213, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Barrera, J.; Menendez, J.A. Silibinin and STAT3: A natural way of targeting transcription factors for cancer therapy. Cancer Treat. Rev. 2015, 41, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [PubMed]
- Conney, A.H.; Chang, R.L.; Jerina, D.M.; Wei, S.J. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab. Rev. 1994, 26, 125–163. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, Z.; Vrzal, R.; Ulrichova, J. Silybin and dehydrosilybin inhibit cytochrome P450 1A1 catalytic activity: A study in human keratinocytes and human hepatoma cells. Cell Biol. Toxicol. 2006, 22, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zuber, R.; Modriansky, M.; Dvorak, Z.; Rohovsky, P.; Ulrichova, J.; Simanek, V.; Anzenbacher, P. Effect of silybin and its congeners on human liver microsomal cytochrome P450 activities. Phytother. Res. 2002, 16, 632–638. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Szaefer, H.; Krajka-Kuzniak, V. Inhibition of murine hepatic cytochrome P450 activities by natural and synthetic phenolic compounds. Xenobiotica 1998, 28, 735–743. [Google Scholar] [CrossRef]
- Kiruthiga, P.V.; Karthikeyan, K.; Archunan, G.; Pandian, S.K.; Devi, K.P. Silymarin prevents benzo(a)pyrene-induced toxicity in Wistar rats by modulating xenobiotic-metabolizing enzymes. Toxicol. Ind. Health 2015, 31, 523–541. [Google Scholar] [CrossRef]
- Tunca, R.; Sozmen, M.; Citil, M.; Karapehlivan, M.; Erginsoy, S.; Yapar, K. Pyridine induction of cytochrome P450 1A1, iNOS and metallothionein in Syrian hamsters and protective effects of silymarin. Exp. Toxicol. Pathol. 2009, 61, 243–255. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Anwar-Mohamed, A.; Aboutabl, M.E.; El-Kadi, A.O. Acute doxorubicin cardiotoxicity alters cardiac cytochrome P450 expression and arachidonic acid metabolism in rats. Toxicol. Appl. Pharm. 2010, 242, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Mooiman, K.D.; Maas-Bakker, R.F.; Moret, E.E.; Beijnen, J.H.; Schellens, J.H.; Meijerman, I. Milk thistle’s active components silybin and isosilybin: Novel inhibitors of PXR-mediated CYP3A4 induction. Drug Metab. Dispos. 2013, 41, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, G.; Kumar, A.; Singh, M.P. Effect of silymarin on pyrogallol- and rifampicin-induced hepatotoxicity in mouse. Eur. J. Pharm. 2007, 565, 190–201. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Fels, L.M.; Dunn, T.; Voigt, W.; Gaedeke, J.; Schmoll, H.J.; Stolte, H.; Lentzen, H. Silibinin protects against cisplatin-induced nephrotoxicity without compromising cisplatin or ifosfamide anti-tumour activity. Br. J. Cancer 1996, 74, 2036–2041. [Google Scholar] [CrossRef] [PubMed]
- Gaedeke, J.; Fels, L.M.; Bokemeyer, C.; Mengs, U.; Stolte, H.; Lentzen, H. Cisplatin nephrotoxicity and protection by silibinin. Nephrol. Dial. Transpl. 1996, 11, 55–62. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- Van Erp, N.P.; Baker, S.D.; Zhao, M.; Rudek, M.A.; Guchelaar, H.J.; Nortier, J.W.; Sparreboom, A.; Gelderblom, H. Effect of milk thistle (Silybum marianum) on the pharmacokinetics of irinotecan. Clin. Cancer Res. 2005, 11, 7800–7806. [Google Scholar] [CrossRef]
- Piscitelli, S.C.; Formentini, E.; Burstein, A.H.; Alfaro, R.; Jagannatha, S.; Falloon, J. Effect of milk thistle on the pharmacokinetics of indinavir in healthy volunteers. Pharmacotherapy 2002, 22, 551–556. [Google Scholar] [CrossRef]
- Mills, E.; Wilson, K.; Clarke, M.; Foster, B.; Walker, S.; Rachlis, B.; DeGroot, N.; Montori, V.M.; Gold, W.; Phillips, E.; et al. Milk thistle and indinavir: A randomized controlled pharmacokinetics study and meta-analysis. Eur. J. Clin. Pharm. 2005, 61, 1–7. [Google Scholar] [CrossRef]
- Wlcek, K.; Koller, F.; Ferenci, P.; Stieger, B. Hepatocellular organic anion-transporting polypeptides (OATPs) and multidrug resistance-associated protein 2 (MRP2) are inhibited by silibinin. Drug Metab. Dispos. 2013, 41, 1522–1528. [Google Scholar] [CrossRef]
- Kock, K.; Xie, Y.; Hawke, R.L.; Oberlies, N.H.; Brouwer, K.L. Interaction of silymarin flavonolignans with organic anion-transporting polypeptides. Drug Metab. Dispos. 2013, 41, 958–965. [Google Scholar] [CrossRef]
- Ferreira, A.; Rodrigues, M.; Fortuna, A.; Falcao, A.; Alves, G. Flavonoid compounds as reversing agents of the P-glycoprotein-mediated multidrug resistance: An in vitro evaluation with focus on antiepileptic drugs. Food Res. Int. 2018, 103, 110–120. [Google Scholar] [CrossRef]
- Ferreira, A.; Santos, A.O.; Falcao, A.; Alves, G. In vitro screening of dual flavonoid combinations for reversing P-glycoprotein-mediated multidrug resistance: Focus on antiepileptic drugs. Food Chem. Toxicol. 2018, 111, 84–93. [Google Scholar] [CrossRef]
- Noori-Daloii, M.R.; Saffari, M.; Raoofian, R.; Yekaninejad, M.; Dinehkabodi, O.S.; Noori-Daloii, A.R. The multidrug resistance pumps are inhibited by silibinin and apoptosis induced in K562 and KCL22 leukemia cell lines. Leuk. Res. 2014, 38, 575–580. [Google Scholar] [CrossRef]
- Lee, C.K.; Choi, J.S. Effects of silibinin, inhibitor of CYP3A4 and P-glycoprotein in vitro, on the pharmacokinetics of paclitaxel after oral and intravenous administration in rats. Pharmacology 2010, 85, 350–356. [Google Scholar] [CrossRef]
- Li, C.; Lee, M.Y.; Choi, J.S. Effects of silybinin, CYP3A4 and P-glycoprotein inhibitor in vitro, on the bioavailability of loratadine in rats. Pharmazie 2010, 65, 510–514. [Google Scholar]
- Nguyen, H.; Zhang, S.; Morris, M.E. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J. Pharm. Sci. 2003, 92, 250–257. [Google Scholar] [CrossRef]
- Mekhail, T.M.; Markman, M. Paclitaxel in cancer therapy. Expert Opin. Pharm. 2002, 3, 755–766. [Google Scholar] [CrossRef]
- Sparreboom, A.; van Asperen, J.; Mayer, U.; Schinkel, A.H.; Smit, J.W.; Meijer, D.K.; Borst, P.; Nooijen, W.J.; Beijnen, J.H.; van Tellingen, O. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. USA 1997, 94, 2031–2035. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.H.; Hur, H.J.; Woo, J.S.; Lee, H.J. Effects of silymarin and formulation on the oral bioavailability of paclitaxel in rats. Eur. J. Pharm. Sci. 2012, 45, 296–301. [Google Scholar] [CrossRef]
- Wang, Z.Y. Arsenic compounds as anticancer agents. Cancer Chemother. Pharm. 2001, 48, S72–S76. [Google Scholar] [CrossRef]
- Emadi, A.; Gore, S.D. Arsenic trioxide—An old drug rediscovered. Blood Rev. 2010, 24, 191–199. [Google Scholar] [CrossRef]
- Gulden, M.; Appel, D.; Syska, M.; Uecker, S.; Wages, F.; Seibert, H. Chrysin and silibinin sensitize human glioblastoma cells for arsenic trioxide. Food Chem. Toxicol. 2017, 105, 486–497. [Google Scholar] [CrossRef]
- Cooray, H.C.; Janvilisri, T.; Van Veen, H.W.; Hladky, S.B.; Barrand, M.A. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem. Biophys. Res. Commun. 2004, 317, 269–275. [Google Scholar] [CrossRef]
- Zhang, S.; Morris, M.E. Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J. Pharm. Exp. 2003, 304, 1258–1267. [Google Scholar] [CrossRef]
- Deep, G.; Singh, R.P.; Agarwal, C.; Kroll, D.J.; Agarwal, R. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: A comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene 2006, 25, 1053–1069. [Google Scholar] [CrossRef]
- Varghese, L.; Agarwal, C.; Tyagi, A.; Singh, R.P.; Agarwal, R. Silibinin efficacy against human hepatocellular carcinoma. Clin. Cancer Res. 2005, 11, 8441–8448. [Google Scholar] [CrossRef]
- Agarwal, C.; Singh, R.P.; Dhanalakshmi, S.; Tyagi, A.K.; Tecklenburg, M.; Sclafani, R.A.; Agarwal, R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene 2003, 22, 8271–8282. [Google Scholar] [CrossRef]
- Agarwal, R. Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem. Pharm. 2000, 60, 1051–1059. [Google Scholar] [CrossRef]
- Zi, X.; Feyes, D.K.; Agarwal, R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: Induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin. Cancer Res. 1998, 4, 1055–1064. [Google Scholar]
- Mateen, S.; Tyagi, A.; Agarwal, C.; Singh, R.P.; Agarwal, R. Silibinin inhibits human nonsmall cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol. Carcinog. 2010, 49, 247–258. [Google Scholar] [CrossRef]
- Deep, G.; Raina, K.; Singh, R.P.; Oberlies, N.H.; Kroll, D.J.; Agarwal, R. Isosilibinin inhibits advanced human prostate cancer growth in athymic nude mice: Comparison with silymarin and silibinin. Int. J. Cancer 2008, 123, 2750–2758. [Google Scholar] [CrossRef]
- Bhatia, N.; Agarwal, R. Detrimental effect of cancer preventive phytochemicals silymarin, genistein and epigallocatechin 3-gallate on epigenetic events in human prostate carcinoma DU145 cells. Prostate 2001, 46, 98–107. [Google Scholar] [CrossRef]
- Zi, X.; Agarwal, R. Modulation of mitogen-activated protein kinase activation and cell cycle regulators by the potent skin cancer preventive agent silymarin. Biochem. Biophys. Res. Commun. 1999, 263, 528–536. [Google Scholar] [CrossRef]
- Zi, X.; Grasso, A.W.; Kung, H.J.; Agarwal, R. A flavonoid antioxidant, silymarin, inhibits activation of erbB1 signaling and induces cyclin-dependent kinase inhibitors, G1 arrest, and anticarcinogenic effects in human prostate carcinoma DU145 cells. Cancer Res. 1998, 58, 1920–1929. [Google Scholar]
- Fan, L.; Ma, Y.; Liu, Y.; Zheng, D.; Huang, G. Silymarin induces cell cycle arrest and apoptosis in ovarian cancer cells. Eur. J. Pharm. 2014, 743, 79–88. [Google Scholar] [CrossRef]
- Vaid, M.; Singh, T.; Prasad, R.; Katiyar, S.K. Silymarin inhibits melanoma cell growth both in vitro and in vivo by targeting cell cycle regulators, angiogenic biomarkers and induction of apoptosis. Mol. Carcinog. 2015, 54, 1328–1339. [Google Scholar] [CrossRef]
- Karim, B.O.; Rhee, K.J.; Liu, G.; Zheng, D.; Huso, D.L. Chemoprevention utility of silibinin and Cdk4 pathway inhibition in Apc(-/+) mice. BMC Cancer 2013, 13, 157. [Google Scholar] [CrossRef]
- Kaur, M.; Velmurugan, B.; Tyagi, A.; Deep, G.; Katiyar, S.; Agarwal, C.; Agarwal, R. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol. Cancer 2009, 8, 2366–2374. [Google Scholar] [CrossRef]
- Singh, R.P.; Raina, K.; Deep, G.; Chan, D.; Agarwal, R. Silibinin suppresses growth of human prostate carcinoma PC-3 orthotopic xenograft via activation of extracellular signal-regulated kinase 1/2 and inhibition of signal transducers and activators of transcription signaling. Clin. Cancer Res. 2009, 15, 613–621. [Google Scholar] [CrossRef]
- Hogan, F.S.; Krishnegowda, N.K.; Mikhailova, M.; Kahlenberg, M.S. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J. Surg. Res. 2007, 143, 58–65. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, P.; Harper, J.W.; Elledge, S.J.; Leder, P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 1995, 82, 675–684. [Google Scholar] [CrossRef]
- Raina, K.; Blouin, M.J.; Singh, R.P.; Majeed, N.; Deep, G.; Varghese, L.; Glode, L.M.; Greenberg, N.M.; Hwang, D.; Cohen, P.; et al. Dietary feeding of silibinin inhibits prostate tumor growth and progression in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2007, 67, 11083–11091. [Google Scholar] [CrossRef]
- Tyagi, A.; Agarwal, C.; Agarwal, R. The cancer preventive flavonoid silibinin causes hypophosphorylation of Rb/p107 and Rb2/p130 via modulation of cell cycle regulators in human prostate carcinoma DU145 cells. Cell Cycle 2002, 1, 137–142. [Google Scholar] [CrossRef]
- Eo, H.J.; Park, G.H.; Song, H.M.; Lee, J.W.; Kim, M.K.; Lee, M.H.; Lee, J.R.; Koo, J.S.; Jeong, J.B. Silymarin induces cyclin D1 proteasomal degradation via its phosphorylation of threonine-286 in human colorectal cancer cells. Int. Immunopharmacol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.X.; Cai, H.; Jiang, G.; Zhou, T.B.; Wu, H. Silibinin inhibits proliferation, induces apoptosis and causes cell cycle arrest in human gastric cancer MGC803 cells via STAT3 pathway inhibition. Asian Pac. J. Cancer Prev. 2014, 15, 6791–6798. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.S.; Lee, S.K.; Kim, S.H.; Hur, S.M.; Kim, J.S.; Kim, J.H.; Choe, J.H.; Shin, I.; Yang, J.H.; et al. 12-O-Tetradecanoyl phorbol-13-acetate (TPA)-induced growth arrest is increased by silibinin by the down-regulation of cyclin B1 and cdc2 and the up-regulation of p21 expression in MDA-MB231 human breast cancer cells. Phytomedicine 2010, 17, 1127–1132. [Google Scholar] [CrossRef]
- Prajapati, V.; Kale, R.K.; Singh, R.P. Silibinin combination with arsenic strongly inhibits survival and invasiveness of human prostate carcinoma cells. Nutr. Cancer 2015, 67, 647–658. [Google Scholar] [CrossRef]
- Chen, C.H.; Huang, T.S.; Wong, C.H.; Hong, C.L.; Tsai, Y.H.; Liang, C.C.; Lu, F.J.; Chang, W.H. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem. Toxicol. 2009, 47, 638–644. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Agarwal, P.; Glode, L.M.; Agarwal, R. Silibinin sensitizes human prostate carcinoma DU145 cells to cisplatin- and carboplatin-induced growth inhibition and apoptotic death. Int. J. Cancer 2003, 106, 699–705. [Google Scholar] [CrossRef]
- Singh, R.P.; Agarwal, R. Prostate cancer prevention by silibinin. Curr. Cancer Drug Targets 2004, 4, 1–11. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Singh, R.P.; Agarwal, C.; Chan, D.C.; Agarwal, R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin. Cancer Res. 2002, 8, 3512–3519. [Google Scholar]
- Tyagi, A.K.; Agarwal, C.; Chan, D.C.; Agarwal, R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncol. Rep. 2004, 11, 493–499. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharm. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Raskovic, A.; Stilinovic, N.; Kolarovic, J.; Vasovic, V.; Vukmirovic, S.; Mikov, M. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules 2011, 16, 8601–8613. [Google Scholar] [CrossRef]
- Chlopcikova, S.; Psotova, J.; Miketova, P.; Simanek, V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part I. Silymarin and its flavonolignans. Phytother. Res. 2004, 18, 107–110. [Google Scholar] [CrossRef]
- Li, W.G.; Wang, H.Q. Inhibitory effects of Silibinin combined with doxorubicin in hepatocellular carcinoma; an in vivo study. J. BUON 2016, 21, 917–924. [Google Scholar]
- Pashaei-Asl, F.; Pashaei-Asl, R.; Khodadadi, K.; Akbarzadeh, A.; Ebrahimie, E.; Pashaiasl, M. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1483–1487. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, P.; Chen, B.; Wang, Y.; Wang, X.; Chiriva Internati, M.; Wachtel, M.S.; Frezza, E.E. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Res. 2008, 28, 1119–1127. [Google Scholar]
- Zhang, Y.; Ge, Y.; Ping, X.; Yu, M.; Lou, D.; Shi, W. Synergistic apoptotic effects of silibinin in enhancing paclitaxel toxicity in human gastric cancer cell lines. Mol. Med. Rep. 2018, 18, 1835–1841. [Google Scholar] [CrossRef]
- Dogan Sigva, Z.O.; Balci Okcanoglu, T.; Biray Avci, C.; Yilmaz Susluer, S.; Kayabasi, C.; Turna, B.; Dodurga, Y.; Nazli, O.; Gunduz, C. Investigation of the synergistic effects of paclitaxel and herbal substances and endemic plant extracts on cell cycle and apoptosis signal pathways in prostate cancer cell lines. Gene 2019, 687, 261–271. [Google Scholar] [CrossRef]
- Delmas, D.; Xiao, J. Natural Polyphenols Properties: Chemopreventive and Chemosensitizing Activities. Anticancer Agents Med. Chem. 2012, 12, 835. [Google Scholar] [CrossRef]
- Jahanafrooz, Z.; Motameh, N.; Bakhshandeh, B. Comparative Evaluation of Silibinin Effects on Cell Cycling and Apoptosis in Human Breast Cancer MCF-7 and T47D Cell Lines. Asian Pac. J. Cancer Prev. 2016, 17, 2661–2665. [Google Scholar]
- Zhang, M.; Liu, Y.; Gao, Y.; Li, S. Silibinin-induced glioma cell apoptosis by PI3K-mediated but Akt-independent downregulation of FoxM1 expression. Eur. J. Pharm. 2015, 765, 346–354. [Google Scholar] [CrossRef]
- Su, C.H.; Chen, L.J.; Liao, J.F.; Cheng, J.T. Increase of phosphatase and tensin homolog by silymarin to inhibit human pharynx squamous cancer. J. Med. Food 2013, 16, 778–784. [Google Scholar] [CrossRef]
- Kauntz, H.; Bousserouel, S.; Gosse, F.; Marescaux, J.; Raul, F. Silibinin, a natural flavonoid, modulates the early expression of chemoprevention biomarkers in a preclinical model of colon carcinogenesis. Int. J. Oncol. 2012, 41, 849–854. [Google Scholar] [CrossRef]
- Yu, H.C.; Chen, L.J.; Cheng, K.C.; Li, Y.X.; Yeh, C.H.; Cheng, J.T. Silymarin inhibits cervical cancer cell through an increase of phosphatase and tensin homolog. Phytother. Res 2012, 26, 709–715. [Google Scholar] [CrossRef]
- Deep, G.; Oberlies, N.H.; Kroll, D.J.; Agarwal, R. Identifying the differential effects of silymarin constituents on cell growth and cell cycle regulatory molecules in human prostate cancer cells. Int. J. Cancer 2008, 123, 41–50. [Google Scholar] [CrossRef]
- Bhatia, N.; Agarwal, C.; Agarwal, R. Differential responses of skin cancer-chemopreventive agents silibinin, quercetin, and epigallocatechin 3-gallate on mitogenic signaling and cell cycle regulators in human epidermoid carcinoma A431 cells. Nutr. Cancer 2001, 39, 292–299. [Google Scholar] [CrossRef]
- Zi, X.; Agarwal, R. Silibinin decreases prostate-specific antigen with cell growth inhibition via G1 arrest, leading to differentiation of prostate carcinoma cells: Implications for prostate cancer intervention. Proc. Natl. Acad. Sci. USA 1999, 96, 7490–7495. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Agarwal, R. Combinatorial strategies for cancer eradication by silibinin and cytotoxic agents: Efficacy and mechanisms. Acta Pharmacol. Sin. 2007, 28, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Bhatia, N.; Condon, M.S.; Bosland, M.C.; Agarwal, C.; Agarwal, R. Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells. Prostate 2002, 53, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kauntz, H.; Bousserouel, S.; Gosse, F.; Raul, F. The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells. Apoptosis Int. J. Program. Cell Death 2012, 17, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fan, S.M.; Yuan, S.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Nitric oxide (*NO) generation but not ROS plays a major role in silibinin-induced autophagic and apoptotic death in human epidermoid carcinoma A431 cells. Free Radic. Res. 2012, 46, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yu, Y.; Qi, M.; Sun, Z.; Li, L.; Yao, G.; Tashiro, S.; Onodera, S.; Ikejima, T. P53-mediated GSH depletion enhanced the cytotoxicity of NO in silibinin-treated human cervical carcinoma HeLa cells. Free Radic. Res. 2012, 46, 1082–1092. [Google Scholar] [CrossRef]
- Tyagi, A.; Agarwal, C.; Agarwal, R. Inhibition of retinoblastoma protein (Rb) phosphorylation at serine sites and an increase in Rb-E2F complex formation by silibinin in androgen-dependent human prostate carcinoma LNCaP cells: Role in prostate cancer prevention. Mol. Cancer 2002, 1, 525–532. [Google Scholar]
- Bousserouel, S.; Bour, G.; Kauntz, H.; Gosse, F.; Marescaux, J.; Raul, F. Silibinin inhibits tumor growth in a murine orthotopic hepatocarcinoma model and activates the TRAIL apoptotic signaling pathway. Anticancer Res. 2012, 32, 2455–2462. [Google Scholar]
- Kauntz, H.; Bousserouel, S.; Gosse, F.; Raul, F. Silibinin triggers apoptotic signaling pathways and autophagic survival response in human colon adenocarcinoma cells and their derived metastatic cells. Apoptosis Int. J. Program. Cell Death 2011, 16, 1042–1053. [Google Scholar] [CrossRef]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Dizaji, M.Z.; Malehmir, M.; Ghavamzadeh, A.; Alimoghaddam, K.; Ghaffari, S.H. Synergistic effects of arsenic trioxide and silibinin on apoptosis and invasion in human glioblastoma U87MG cell line. Neurochem. Res. 2012, 37, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Leon, I.E.; Cadavid-Vargas, J.F.; Tiscornia, I.; Porro, V.; Castelli, S.; Katkar, P.; Desideri, A.; Bollati-Fogolin, M.; Etcheverry, S.B. Oxidovanadium(IV) complexes with chrysin and silibinin: Anticancer activity and mechanisms of action in a human colon adenocarcinoma model. J. Biol. Inorg. Chem. JBIC 2015, 20, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, D.; Wang, Y.; Li, Z.; Zhu, C. Co-delivery doxorubicin and silybin for anti-hepatoma via enhanced oral hepatic-targeted efficiency. Int. J. Nanomed. 2019, 14, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Gioti, K.; Papachristodoulou, A.; Benaki, D.; Havaki, S.; Beloukas, A.; Vontzalidou, A.; Aligiannis, N.; Skaltsounis, A.L.; Mikros, E.; Tenta, R. Silymarin Enriched Extract (Silybum marianum) Additive Effect on Doxorubicin-Mediated Cytotoxicity in PC-3 Prostate Cancer Cells. Planta Med. 2019, 85, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Molavi, O.; Narimani, F.; Asiaee, F.; Sharifi, S.; Tarhriz, V.; Shayanfar, A.; Hejazi, M.; Lai, R. Silibinin sensitizes chemo-resistant breast cancer cells to chemotherapy. Pharm. Biol. 2017, 55, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, H.; Ahmadi Ashtiani, H.; Anjarani, S.; Bokaee, S.; Khaki, A.; Javadi, L. The role of milk thistle extract in breast carcinoma cell line (MCF-7) apoptosis with doxorubicin. Acta Med. Iran. 2013, 51, 591–598. [Google Scholar]
- Singh, R.P.; Mallikarjuna, G.U.; Sharma, G.; Dhanalakshmi, S.; Tyagi, A.K.; Chan, D.C.; Agarwal, C.; Agarwal, R. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin. Cancer Res. 2004, 10, 8641–8647. [Google Scholar] [CrossRef]
- Son, Y.G.; Kim, E.H.; Kim, J.Y.; Kim, S.U.; Kwon, T.K.; Yoon, A.R.; Yun, C.O.; Choi, K.S. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res. 2007, 67, 8274–8284. [Google Scholar] [CrossRef]
- Manouchehri, J.M.; Kalafatis, M. Sensitization of rhTRAIL-resistant Triple-negative Breast Carcinoma Through Silibinin Co-Treatment. Anticancer Res. 2017, 37, 6593–6599. [Google Scholar] [CrossRef]
- Li, L.H.; Wu, L.J.; Jiang, Y.Y.; Tashiro, S.; Onodera, S.; Uchiumi, F.; Ikejima, T. Silymarin enhanced cytotoxic effect of anti-Fas agonistic antibody CH11 on A375-S2 cells. J. Asian Nat. Prod. Res. 2007, 9, 593–602. [Google Scholar] [CrossRef]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, R.; Bernuzzi, S.; Ciani, D.; Ascari, E. Silymarine during maintenance therapy of acute promyelocytic leukemia. Haematologica 1993, 78, 340–341. [Google Scholar] [PubMed]
- Schroder, F.H.; Roobol, M.J.; Boeve, E.R.; de Mutsert, R.; Zuijdgeest-van Leeuwen, S.D.; Kersten, I.; Wildhagen, M.F.; van Helvoort, A. Randomized, double-blind, placebo-controlled crossover study in men with prostate cancer and rising PSA: Effectiveness of a dietary supplement. Eur. Urol. 2005, 48, 922–930, discussion 930-921. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, M.; Guerrieri-Gonzaga, A.; Gandini, S.; Johansson, H.; Serrano, D.; Cazzaniga, M.; Aristarco, V.; Puccio, A.; Mora, S.; Caldarella, P.; et al. A Presurgical Study of Oral Silybin-Phosphatidylcholine in Patients with Early Breast Cancer. Cancer Prev. Res. 2016, 9, 89–95. [Google Scholar] [CrossRef]
- Siegel, A.B.; Narayan, R.; Rodriguez, R.; Goyal, A.; Jacobson, J.S.; Kelly, K.; Ladas, E.; Lunghofer, P.J.; Hansen, R.J.; Gustafson, D.L.; et al. A phase I dose-finding study of silybin phosphatidylcholine (milk thistle) in patients with advanced hepatocellular carcinoma. Integr. Cancer Ther. 2014, 13, 46–53. [Google Scholar] [CrossRef]
- Vidlar, A.; Vostalova, J.; Ulrichova, J.; Student, V.; Krajicek, M.; Vrbkova, J.; Simanek, V. The safety and efficacy of a silymarin and selenium combination in men after radical prostatectomy—A six month placebo-controlled double-blind clinical trial. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2010, 154, 239–244. [Google Scholar] [CrossRef]
- Hoh, C.; Boocock, D.; Marczylo, T.; Singh, R.; Berry, D.P.; Dennison, A.R.; Hemingway, D.; Miller, A.; West, K.; Euden, S.; et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: Silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin. Cancer Res. 2006, 12, 2944–2950. [Google Scholar] [CrossRef]
- Flaig, T.W.; Gustafson, D.L.; Su, L.J.; Zirrolli, J.A.; Crighton, F.; Harrison, G.S.; Pierson, A.S.; Agarwal, R.; Glode, L.M. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investig. New Drugs 2007, 25, 139–146. [Google Scholar] [CrossRef]
- Mateen, S.; Raina, K.; Agarwal, C.; Chan, D.; Agarwal, R. Silibinin synergizes with histone deacetylase and DNA methyltransferase inhibitors in upregulating E-cadherin expression together with inhibition of migration and invasion of human non-small cell lung cancer cells. J. Pharm. Exp. 2013, 345, 206–214. [Google Scholar] [CrossRef]
- El-Awady, E.S.E.; Moustafa, Y.M.; Abo-Elmatty, D.M.; Radwan, A. Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur. J. Pharm. 2011, 650, 335–341. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; El-Haggar, S.; El-desoky, K. Silymarin prevents adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food Chem. Toxicol. 2008, 46, 2422–2428. [Google Scholar] [CrossRef] [PubMed]


| Compound | Target | Cellular or Animal Model | Reference | ||
|---|---|---|---|---|---|
| Inhibition | Silybin, dehydrosilybin | Phase I enzymes | Ethoxyresorufin O-dealkylase (EROD) (P450 1A1) | human keratinocytes (HaCaT), human hepatoma cells (HepG2) | [16] |
| Silybin, silibinin | P450 1A1, methoxyresorufin O-dealkylase (MROD) (P450 1A2), pentoxy-O-dealkylase (PROD) (P450 2B) | mouse liver microsomes | [18] | ||
| Silymarin | P450 1A1 | Wistar rats, Syrian hamsters | [19,20] | ||
| Silymarin | CYP3A4 | LS180 colon adenocarcinoma cells | [22] | ||
| Silibinin | Phase III transporters | Organic Anion Transporters (OAT)P1B1, OATP1B3, OATP2B1, MRP2 | Chinese hamster ovary cells | [31] | |
| Silymarin and silibinin | OATP1B1, OATP1B3 and OATP2B1 | human hepatocytes | [32] | ||
| Silymarin | P-glycoprotein (P-gP) | Madin–Darby canine kidney II cells, MCF-7 | [33,34] | ||
| Silibinin | MDR1, MRP3, MRP2, MRP1, MRP5, MRP4, ABCG2, ABCB11, MRP6 and MRP7 | K562 and KCL22 cell lines | [35] | ||
| Silibinin | P-gP | Rats | [36,37] | ||
| Silymarin | MRP1 | human pancreatic adenocarcinoma cell Panc-1 | [38] | ||
| Silibinin | Phase I enzymes | cytochrome P4503A4 | rats | [37] | |
| Activation | Silymarin | Phase II enzymes | glutathione S-transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPO) | mouse liver | [23] |
| Silymarin | GST, UDP-glucuronosyltransferases (UGT), epoxide transferase, sulfotransferase | Wistar rats | [19] | ||
| Compound | Target | Cellular or Animal Model | Reference | ||
|---|---|---|---|---|---|
| Inhibition | Silibinin | G1 phase | cyclins D1, D3, E/cyclin-dependent kinases, Cdk 2, 4, 6 | Human prostate, hepatoma, colon, non-small cell lung cancer, epidermoid carcinoma, ovarian cancer, melanoma cells | [47,48,49,51,52,57,58,59,60,62,89,90,91] |
| cyclins A, B1 and E and their respective Cdks | Transgenic adenocarcinoma of the mouse prostate | [92] | |||
| pRb | Human non-small cell lung cancer cell, apc (−/+) mice, human prostate carcinoma DU145 cells, human hepatoma HepG2 cells | [52,59,70,93] | |||
| Silymarin and silibinin | G2/M phase | cyclins B1/A; Cdk1; Cdc25B/Cdc25C phosphatases | Human prostate cancer, LoVo cells, human colon cancer cells, human gastric cancer MGC803, MDA-MB231 human breast cancer cells | [47,58,60,62,67,68] | |
| Silibinin | Intrinsic cell death pathway | Bcl-2, PI3K pathway | Human breast cancer call (MCF-7, T47D), glioma cells, ovarian cancer cells, in melanoma cells, in pharynx squamous cell carcinoma, in colon cancer cells and in cervical cancer cells. | [57,58,84,85,94,95,96] | |
| Activation | Silibinin | G1 phase | Cdk inhibitors: Kip1/p27, Cip1/p21 and p18/INK4C | Breast cancer cells MDA-MB 468, human prostate cancer PC3 cells, human hepatocellular carcinoma, human colon carcinoma HT-29 cells, human non-small cell lung cancer cell, human prostate carcinoma DU145 cells, ovarian cancer cells, mouse prostate model, HepG2 cells | [47,48,49,51,52,56,57,64,65,69,70,91,97] |
| Rb | Human non-small cell lung cancer cell, Apc (−/+) mice, human prostate carcinoma DU145 cells, HepG2 cells | [52,59,65,70,85] | |||
| Intrinsic cell death pathway | Bax protein, capsase-3 | Glioma cells | [85] | ||
| Extrinsic cell death pathway | TRAIL/TRAIL Death receptor 5 (DR5), DR4, caspase-3, -8, -10 | Hepatocarcinoma cells, colon cancer SW480 cells | [98,99] | ||
| Compounds | Silymarin ± FOLFIRI | Identifier | NCT03130634 | Ref |
| Cancer type | Metastatic colorectal cancer and received chemotherapy with FOLFIRI regimen | N/A | ||
| Study design/type | Interventional | |||
| Sample size and phase | 70 patients (between 20 and 80 years old)/Phase 4 study | |||
| Dose/administration route | Experimental arm: during six cycles of FOLFIRI chemotherapy, silymarin (150 mg) three times daily from day 1 to day 7 during one cycle of treatment Control arm: during six cycles of FOLFIRI chemotherapy, patients did not received silymarin during chemotherapy | |||
| Outcome measures | Silymarin to improve the intestinal side effect of the patients undergoing FOLFIRI chemotherapy | |||
| Results | Not yet available | |||
| Oral Green Tea Extract and Milk Thistle Extract | Identifier | NCT01239095 | N/A | |
| Cancer type | Colorectal cancer patients undergoing resection | |||
| Study design/type | Interventional, single group assignment | |||
| Sample size and phase | 30 patients (between 18 and 85 years old) Phase 1 study | |||
| Dose/administration route | Experimental arm: Green tea extract (3200 mg per day) plus Milk thistle extract with phosphatidylcholine (2700 mg per day) For one week prior to surgery and for 30 days after surgery | |||
| Outcome measures | Number of patients with adverse events or complications (time frame 60 days) | |||
| Results | Not yet available | |||
| Silybin formulated with phosphatidylcholine (Siliphos; improves its systemic availability compared with silymarin) | Identifier | R621-IEO661/511 | [114] | |
| Cancer type | Breast cancer patients with newly diagnosed breast cancer not eligible for neoadjuvant treatment and candidate for surgical lumpectomy or mastectomy | |||
| Study design/type | Pilot presurgical study | |||
| Sample size and phase | 12 consecutive patients (women of 18 years old or older), Phase 1study | |||
| Dose/administration route | Silybin formulated in granules to be suspended in drinkable water. Each sachet contained 2.8 g of Siliphos (containing between 29.7 and 36.3% of silybin). A single sachet once daily for 4 weeks until surgery, in an empty stomach (30 min before eating, at least 2 h after the previous meal) | |||
| Outcome measures | Silybin pharmacokinetic profile and pharmacodynamic effects on malignant as well as surrounding normal tissue | |||
| Results | Silybin levels were measured before (SIL) and after (TOT-SIL) enzymatic hydrolysis by HPLC-MS/MS in biologic samples (plasma, urine, breast cancer and surrounding normal tissue). Despite a high between-subject variability, repeated administration of Siliphos achieved levels of TOT-SIL of 31,121 to 7654 ng/mL in the plasma and up to 1375 ng/g in breast cancer tissue. SIL concentrations ranged from 10,861 to 1818 ng/mL in plasma and up to 177 ng/g in breast cancer tissue. Median TOT-SIL concentration was higher in the tumor as compared with the adjacent normal tissue (P = 0.018). No significant change in either blood levels of IGF-I and nitric oxide or Ki-67 in tumors was noted. | |||
| Silybin formulated with phosphatidylcholine (Siliphos) | Identifier | FDA approval #107662 | [115] | |
| Cancer type | Advanced Hepatocellular Carcinoma patients | |||
| Study design/type | ||||
| Sample size and phase | 30 patients were supposed to be enrolled in the study but only 3 patients could be included (Male aged of 47, 54 and 60 years old)/Phase 1study | |||
| Dose/administration route | Siliphos powder (1:1 ratio of silybin to phosphatidylcholine, which increases drug absorption). All patients orally received 2g of Siliphos per day over 12 weeks | |||
| Outcome measures | Primary endpoint was to determine maximal tolerated dose (MTD) of Siliphos. The secondary endpoints were to (a) mean intrapatient percentage change in AST, ALT and total serum bilirubin levels; (b) quality of life as measured by the FACT (Functional Assessment of Cancer Therapy)–Hepatobiliary questionnaire; (c) plasma concentrations of silibinin and silibinin glucuronide; (d) mean intrapatient percentage change in serum inflammatory biomarkers; and (e) tumor response as measured by RECIST criteria and α-fetoprotein (AFP) concentrations. Exploratory aims were to evaluate a) tumor response as measured by RECIST criteria and AFP concentrations and (b) survival at 12 months | |||
| Results | Increased plasma concentrations of silybinin and silibinin glucuronide within 1 to 3 weeks were observed. Only one patient out of 3 showed some improvements in liver function abnormalities and inflammatory biomarkers but after 56 days of intervention. All patients died within 23 to 69 days of enrolling into the trial. No MTD could be determined | |||
| Silymarin and selenium combination (SM-Se formulation) | Identifier | N/A | [116] | |
| Cancer type | Prostate cancer patients | |||
| Study design/type | 6 months randomized controlled double-blind trial | |||
| Sample size and phase | 37 patients (men) 2 to 3 months after radical prostatectomy and aged between 51 to 72 years’ old, Phase 1 study | |||
| Dose/administration route | Experimental arm: SM-Se tablet containing 190 mg silymarin of the following composition (%; w/w): taxifolin 4.13, silychristin 17.00, silydianin 7.70, silibinin A 23.66, silibinin B 29.01, isosilibinin A+B 11.38 and undefined components 7.11; 80 μg selenium as selenomethionine Control arm: Placebo tablet contained microcrystalline cellulose (250 mg), isomalt (250 mg) and hydroxypropyl cellulose (10 mg). Patients received either SM-Se or placebo tablets for 6 months (3 tablets/day) | |||
| Outcome measures | Evaluation of the safety and tolerability of a 6 months’ daily consumption of 570 mg silymarin and 240 µg selenium and evaluation of the efficacy to reduce prostate cancer progression markers | |||
| Results | Physical examination, quality of life score (QoL), hematology, basic clinical chemistry and oxidative stress markers, selenium and testosterone levels, antioxidant status were evaluated at baseline, at 3 and 6 months. Data showed that the combination of silymarin and selenium improved the quality of life (QoL) score, decreased the low-density lipoproteins (LDL) and total cholesterol (markers of prostate cancer progression) and, increased serum selenium levels. The formulation did not show any adverse effects in patients. No improvements were observed in the placebo group. | |||
| Silibinin | Identifier | N/A | [117] | |
| Cancer type | Colorectal cancer patients | |||
| Study design/type | Interventional, single group assignment | |||
| Sample size and phase | 12 patients (1 female and 11 male) aged between 55 and 78 years’ old with confirmed colorectal carcinoma of stages Dukes A (2 patients), B (5) or C (5), who were to undergo colorectal resection and 12 patients (7 females and 5 males, aged between 49 and 78 years’ old, all Dukes D with hepatic metastatic disease originating from primary colorectal carcinoma, who were to undergo hepatic surgery. One patient who underwent colectomy had preoperative radiotherapy and none preoperative chemotherapy. All, except two patients who underwent hepatic surgery, had received 5-fluorouracil with folinic acid, oxaliplatin and/or irinotecan before recruitment/Phase 1 study | |||
| Dose/administration route | Silibinin was formulated in capsules as silipide (IdB 1016), a phytosome product marketed by Indena SpA. The capsules contained 120 mg of silibinin and soy phosphatidylcholine at a molar ratio of 1:1, constituting in terms of percentage weight ∼40% silibinin and 60% phosphatidylcholine. Patients received silipide at dosages of either 360, 720 or 1440 mg silibinin daily for 7 days before surgery; each daily dose was divided in three equal portions taken in the morning, at noon and in the evening. There were eight individuals per dose level (four patients who underwent colectomy and four who had liver resection). The first and second portions of the first dose were taken at noon and in the evening, respectively, of day 1; the last dose portion was ingested in the morning of day 8 before surgery so that, in total, the seven daily doses were distributed >8 days. | |||
| Outcome measures | Evaluation of silibinin pharmacokinetics and pharmacodynamic parameters. Blood and biopsy samples of normal and malignant colorectum or liver were obtained before dosing, and blood and colorectal or hepatic tissues were collected at resection surgery after the final silipide dose. Levels of silibinin were quantified by high-pressure liquid chromatography-UV, and plasma metabolites were identified by LC-MS. Blood levels of IGFBP-3, IGF-I and the oxidative DNA damage pyrimidopurinone adduct of deoxyguanosine (M1dG) were determined. | |||
| Results | Patients silipide supplementation for 7 days, was safe. Plasma levels of silibinin reached 0.3 to 4 μmol/L, with silibinin monoglucuronide, silibinin diglucuronide, silibinin monosulfate and silibinin glucuronide as major metabolites. Silibin levels in liver and colorectal tissues reached 0.3 to 2.5 nmol/g and 20 to 141 nmol/g, respectively. No significant modifications in plasma levels of IGFBP-3, IGF-1 and M1dG were observed at the end of the intervention. | |||
| Silybin-phytosome formulation | Identifier | N/A | [118] | |
| Cancer type | Prostate cancer patients | |||
| Study design/type | Interventional, single group assignment | |||
| Sample size and phase | 13 patients (18 years old or older), with histologically confirmed prostate cancer, with progressive disease defined by a rising Prostate-Specific Antigen (PSA) or measurable disease by radiological assessment/Phase 1 study | |||
| Dose/administration route | Silybin-phytosome (Siliphos®) formulation obtained from Indena Corporation (Seattle, WA). It is a silibinin and phosphatidylcholine powder containing approximately 30% silibinin by weight, which is mixed with applesauce at the ratio of 1/4 teaspoon of silybin-phytosome to 1 Tablespoon of applesauce. Patients received 3 times a day for 4 weeks the silybin-phytosome formulation. The first daily dose-level was 2.5 g, then 5 g and then increased by increments of 5 g (i.e., 10, 15, 20 g daily); due to the toxicity observed with chronic administration of 15 and 20 g daily, the dose level was reduced to 13 g daily. | |||
| Outcome measures | Evaluation of a high-dose Silybin-phytosome pharmacokinetics in blood and urine samples. Evaluation of the safety and tolerability of the formulation | |||
| Results | For a high dose of the formulation (13 g/day in 3-divided doses), the most notable toxicity observed was gastrointestinal, with grade 1 or 2 unconjugated hyperbilirubinemia observed commonly. The only grade 3 or 4 toxicity noted was one patient with transient grade 3 elevation of Alanine-transaminase (ALT). Silibinin plasma half-life was ranging from 1.79–4.99 h. Interpatient great variability was found notably in urine samples. Silibinin level in urine ranged from undetectable to 28.2 µM. Its mean urine level was found to be 6.4 µM. The mean silibinin-glucuronide level was 253.4 (range of 1.5–982 μM). Maximum tolerated dose (MTD) could not be accurately defined. Finally, no objective PSA responses were found with the formulation. | |||
| Silibinin-phytosome formulation (Siliphos) +/- Erlotinib (Tarceva) | Identifier | NCT0214611 | N/A | |
| Cancer type | EGFR mutant lung adenocarcinoma patients | |||
| Study design/type | - | |||
| Sample size and phase | 42 patients with stage IV lung adenocarcinoma and confirmed EGFR (Epidermal Growth Factor Receptor) mutation, aged between 30 and 80 years’ old, who have not received chemotherapy before or who have received postoperative adjuvant chemotherapy more than 6 months before enrollment/Phase 2 study | |||
| Dose/administration route | Patients group receiving 150 mg/day for 4 weeks of Erlotinib (Tarceva) patients group receiving for 4 weeks 1g/day of Silybin-phytosome. No additional information available | |||
| Outcome measures | The primary endpoint of the study is to evaluate the tumor response rate in patients and secondary endpoint is to evaluate progression-free survival, overall survival and safety of Siliphos | |||
| Results | Not yet available |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delmas, D.; Xiao, J.; Vejux, A.; Aires, V. Silymarin and Cancer: A Dual Strategy in Both in Chemoprevention and Chemosensitivity. Molecules 2020, 25, 2009. https://doi.org/10.3390/molecules25092009
Delmas D, Xiao J, Vejux A, Aires V. Silymarin and Cancer: A Dual Strategy in Both in Chemoprevention and Chemosensitivity. Molecules. 2020; 25(9):2009. https://doi.org/10.3390/molecules25092009
Chicago/Turabian StyleDelmas, Dominique, Jianbo Xiao, Anne Vejux, and Virginie Aires. 2020. "Silymarin and Cancer: A Dual Strategy in Both in Chemoprevention and Chemosensitivity" Molecules 25, no. 9: 2009. https://doi.org/10.3390/molecules25092009
APA StyleDelmas, D., Xiao, J., Vejux, A., & Aires, V. (2020). Silymarin and Cancer: A Dual Strategy in Both in Chemoprevention and Chemosensitivity. Molecules, 25(9), 2009. https://doi.org/10.3390/molecules25092009