Iron Absorption in Celiac Disease and Nutraceutical Effect of 7-Hydroxymatairesinol. Mini-Review
Abstract
1. Introduction
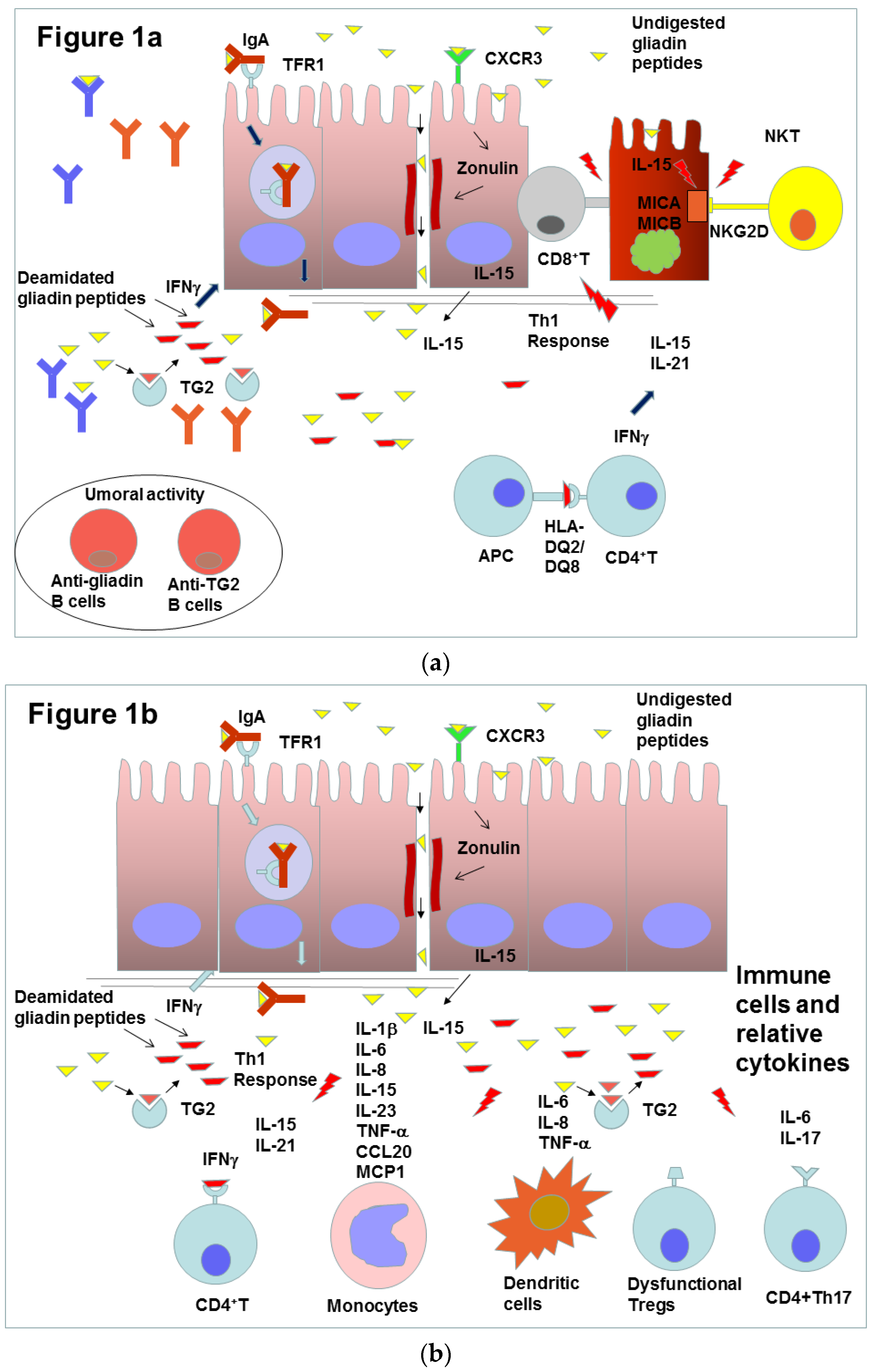
2. Celiac Disease and Inflammation
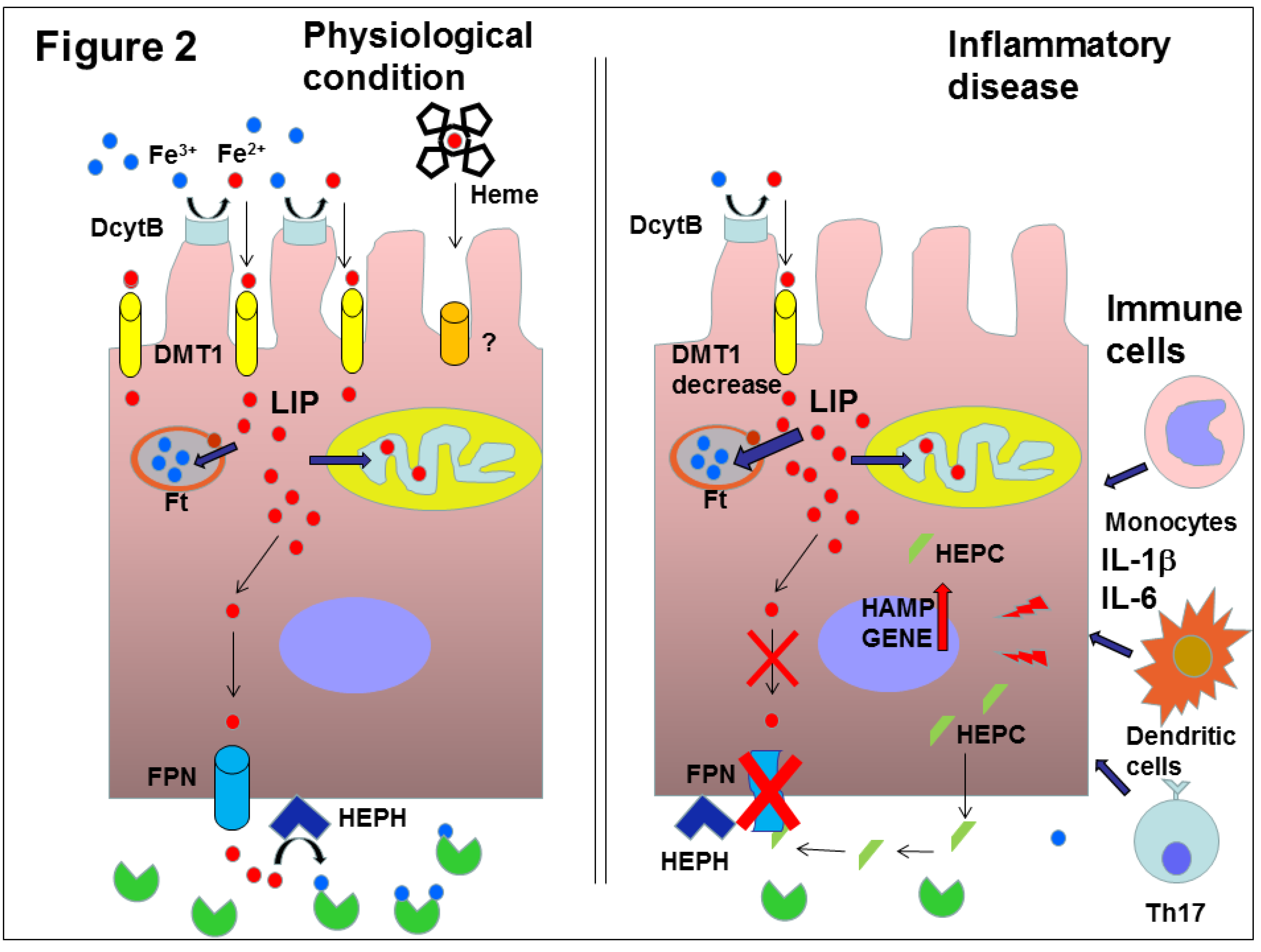
3. Iron Homeostasis and Hepcidin
4. 7-Hydroxymatairesinol
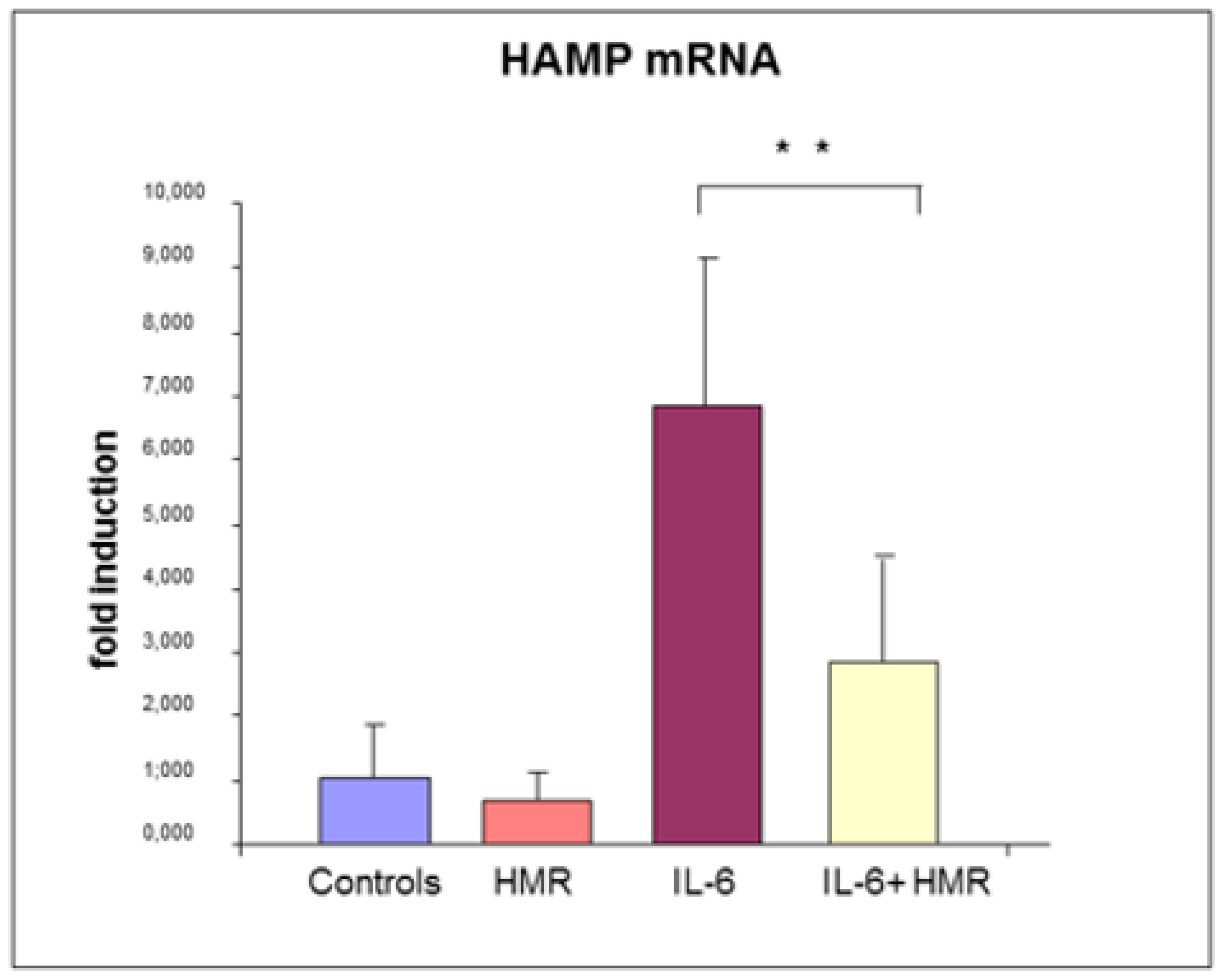
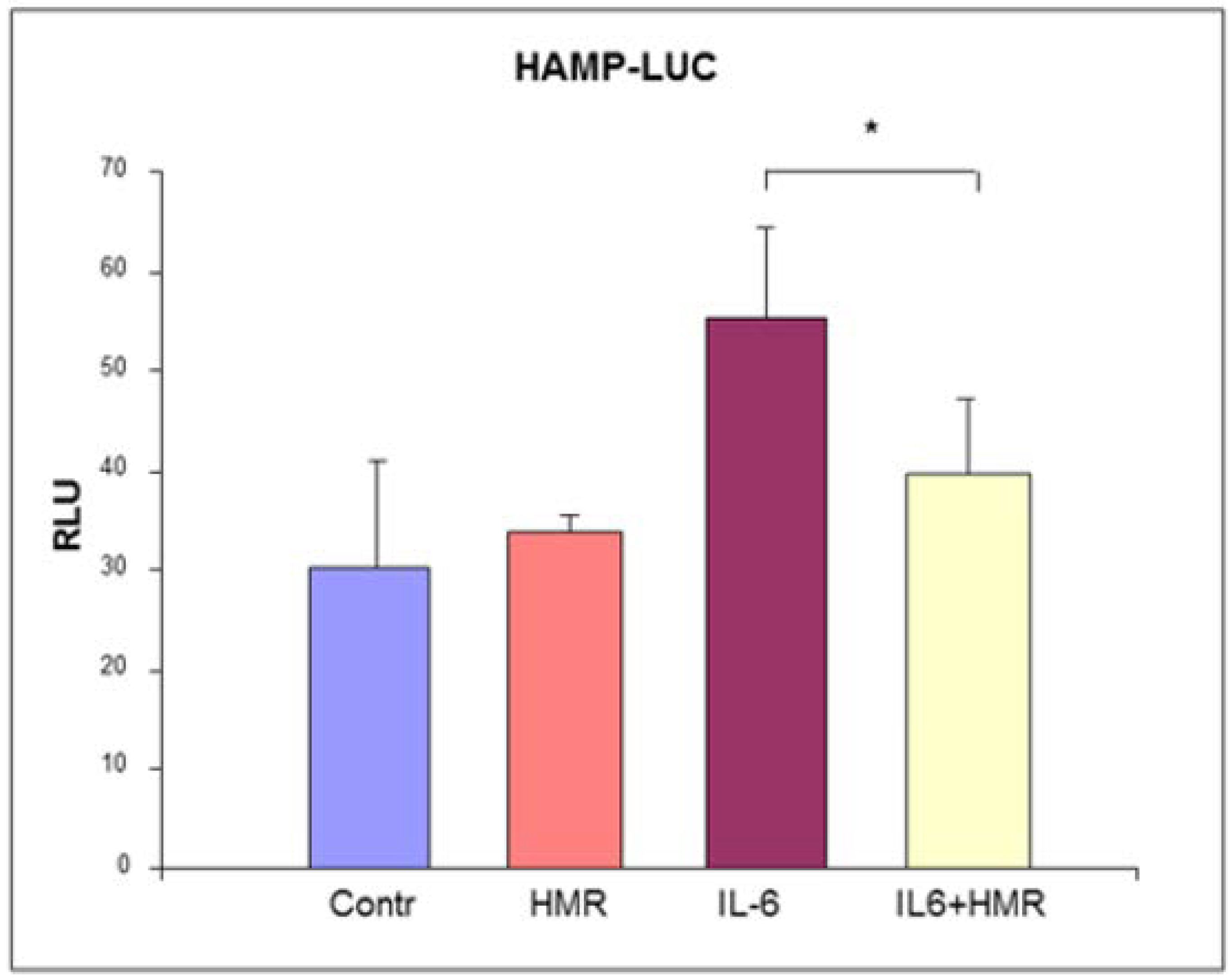
5. Experimental Results and Discussion
5.1. Anti-Inflammatory Effect of 7-HMR
5.2. Effect on Hepcidin Promoter
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacDonald, T.T. The gut is still the biggest lymphoid organ in the body. Mucosal Immunol. 2008, 1, 246–247. [Google Scholar]
- Bergamaschi, G.; Di Sabatino, A.; Corazza, G.R. Pathogenesis, diagnosis and treatment of anaemia in immune-mediated gastrointestinal disorders. Br. J. Haematol. 2018, 182, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Martín-Masot, R.; Nestares, M.T.; Diaz-Castro, J.; López-Aliaga, I.; Alférez, M.J.M.; Moreno-Fernandez, J.; Maldonado, J. Multifactorial Etiology of Anemia in Celiac Disease and Effect of Gluten-Free Diet: A Comprehensive Review. Nutrients 2019, 11, 2557. [Google Scholar] [CrossRef]
- Sollid, L.M.; Lie, B.A. Celiac disease genetics: Current concepts and practical applications. Clin Gastroenterol. Hepatol. 2005, 3, 843–851. [Google Scholar] [CrossRef]
- Dubois, P.C.; Trynka, G.; Franke, L.; Hunt, K.A.; Romanos, J.; Curtotti, A.; Zhernakova, A.; Heap, G.A.; Adány, R.; Aromaa, A.; et al. Multiple common variants for celiac disease influencing immune gene expression. Nat. Genet. 2010, 42, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Jabri, B.; Sollid, L.M. Tissue-mediated control of immunopathology in celiac disease. Nat. Rev. Immunol. 2009, 9, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of celiac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Kruppa, K. Endomysial antibodies predict celiac disease irrespective of the titers or clinical presentation. World J. Gastroenterol. 2012, 18, 2511–2516. [Google Scholar] [CrossRef]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008, 135, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Valitutti, F.; Fasano, A. Breaking Down Barriers: How Understanding Celiac Disease Pathogenesis Informed the Development of Novel Treatments. Dig. Dis. Sci. 2019, 64, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Matysiak-Budnik, T.; Moura, I.C.; Arcos-Fajardo, M.; Lebreton, C.; Ménard, S.; Candalh, C.; Ben-Khalifa, K.; Dugave, C.; Tamouza, H.; van Niel, G.; et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J. Exp. Med. 2008, 205, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, S.S.; Jabri, B. Pathophysiology of celiac disease. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.Y.; Tye-Din, J.A. T cells in coeliac disease: A rational target for diagnosis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 583–584. [Google Scholar] [CrossRef]
- De Re, V.; Magris, R.; Cannizzaro, R. New Insights into the Pathogenesis of Celiac Disease. Front. Med. (Lausanne). 2017, 4, 137. [Google Scholar] [CrossRef]
- Troncone, R.; Jabri, B. Coeliac disease and gluten sensitivity. J. Intern. Med. 2011, 269, 582–590. [Google Scholar] [CrossRef]
- Meresse, B.; Chen, Z.; Ciszewski, C.; Tretiakova, M.; Bhagat, G.; Krausz, T.N.; Raulet, D.H.; Lanier, L.L.; Groh, V.; Spies, T.; et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 2004, 21, 357–366. [Google Scholar] [CrossRef]
- Stamnaes, J.; Sollid, L.M. Celiac disease: Autoimmunity in response to food antigen. Semin. Immunol. 2015, 27, 343–352. [Google Scholar] [CrossRef]
- Iversen, R.; Roy, B.; Stamnaes, J.; Høydahl, L.S.; Hnida, K.; Neumann, R.S.; Korponay-Szabó, I.R.; Lundin, K.E.A.; Sollid, L.M. Efficient T cell-B cell collaboration guides autoantibody epitope bias and onset of celiac disease. Proc. Natl. Acad. Sci. USA 2019, 116, 15134–15139. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Fantini, M.C.; Fina, D.; Caruso, R.; Boirivant, M.; MacDonald, T.T.; Pallone, F.; Monteleone, G. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J. Immunol. 2007, 178, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Bodd, M.; Ráki, M.; Tollefsen, S.; Fallang, L.E.; Bergseng, E.; Lundin, K.E.; Sollid, L.M. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol. 2010, 3, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Cellier, C.; Patey, N.; Mauvieux, L.; Jabri, B.; Delabesse, E.; Cervoni, J.P.; Burtin, M.L.; Guy-Grand, D.; Bouhnik, Y.; Modigliani, R.; et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology 1998, 114, 471–481. [Google Scholar] [CrossRef]
- Lahdenperä, A.I.; Hölttä, V.; Ruohtula, T.; Salo, H.M.; Orivuori, L.; Westerholm-Ormio, M.; Savilahti, E.; Fälth-Magnusson, K.; Högberg, L.; Ludvigsson, J.; et al. Up-regulation of small intestinal interleukin-17 immunity in untreated coeliac disease but not in potential coeliac disease or in type 1 diabetes. Clin. Exp. Immunol. 2012, 167, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Palová-Jelínková, L.; Rozková, D.; Pecharová, B.; Bártová, J.; Sedivá, A.; Tlaskalová-Hogenová, H.; Spísek, R.; Tucková, L. Gliadin fragments induce phenotypic and functional maturation of human dendritic cells. J. Immunol. 2005, 175, 7038–7045. [Google Scholar] [CrossRef]
- Harris, K.M.; Fasano, A.; Mann, D.L. Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: Implications for celiac disease. Clin. Immunol. 2010, 135, 430–439. [Google Scholar] [CrossRef]
- Lissner, D.; Schumann, M.; Batra, A.; Kredel, L.I.; Kuhl, A.A.; Erben, U.; May, C.; Schulzke, J.D.; Siegmund, B. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm. Bowel. Dis. 2015, 21, 1297–1305. [Google Scholar] [CrossRef]
- Managlia, E.; Liu, S.X.L.; Yan, X.; Tan, X.D.; Chou, P.M.; Barrett, T.A.; De Plaen, I.G. Blocking NF-kappaB Activation in Ly6c(+) Monocytes Attenuates Necrotizing Enterocolitis. Am. J. Pathol. 2019, 189, 604–618. [Google Scholar] [CrossRef]
- Morhardt, T.L.; Hayashi, A.; Ochi, T.; Quiros, M.; Kitamoto, S.; Nagao-Kitamoto, H.; Ku_a, P.; Atarashi, K.; Honda, K.; Kao, J.Y.; et al. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019, 9, 1223. [Google Scholar] [CrossRef]
- Bain, C.C.; Schridde, A. Origin, Diferentiation, and Function of Intestinal Macrophages. Front. Immunol. 2018, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Cinova, J.; Palova-Jelinkova, L.; Smythies, L.E.; Cerna, M.; Pecharova, B.; Dvorak, M.; Fruhauf, P.; Tlaskalova-Hogenova, H.; Smith, P.D.; Tuckova, L. Gliadin peptides activate blood monocytes from patients with celiac disease. J. Clin. Immunol. 2007, 27, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Delbue, D.; Cardoso-Silva, D.; Branchi, F.; Itzlinger, A.; Letizia, M.; Siegmund, B.; Schumann, M. Celiac Disease Monocytes Induce a Barrier Defect in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2019, 20, 5597. [Google Scholar] [CrossRef] [PubMed]
- Zanzi, D.; Stefanile, R.; Santagata, S.; Iaffaldano, L.; Iaquinto, G.; Giardullo, N.; Lania, G.; Vigliano, I.; Vera, A.R.; Ferrara, K.; et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am. J. Gastroenterol. 2011, 106, 1308–1317. [Google Scholar] [CrossRef]
- Barone, M.V.; Zanzi, D.; Maglio, M.; Nanayakkara, M.; Santagata, S.; Lania, G.; Miele, E.; Ribecco, M.T.; Maurano, F.; Auricchio, R.; et al. Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking. PLoS ONE 2011, 25, e17039. [Google Scholar] [CrossRef]
- Capozzi, A.; Vincentini, O.; Gizzi, P.; Porzia, A.; Longo, A.; Felli, C.; Mattei, V.; Mainiero, F.; Silano, M.; Sorice, M.; et al. Modulatory Effect of Gliadin Peptide 10-mer on Epithelial Intestinal CACO-2 Cell Inflammatory Response. PLoS ONE 2013, 8, e66561. [Google Scholar] [CrossRef]
- Mamone, G.; Ferranti, P.; Rossi, M.; Roepstorff, P.; Fierro, O.; Malorni, A.; Addeo, F. Identification of a peptide from alpha-gliadin resistant to digestive enzymes: Implications for celiac disease. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 855, 236–241. [Google Scholar] [CrossRef]
- Iacomino, G.; Fierro, O.; D’Auria, S.; Picariello, G.; Ferranti, P.; Liguori, C.; Addeo, F.; Mamone, G. Structural analysis and Caco-2 cell permeability of the celiac-toxic A-gliadin peptide 31-55. J. Agric. Food Chem. 2013, 61, 1088–1096. [Google Scholar] [CrossRef]
- Nanayakkara, M.; Lania, G.; Maglio, M.; Auricchio, R.; De Musis, C.; Discepolo, V.; Miele, E.; Jabri, B.; Troncone, R.; Auricchio, S.; et al. P31-43, an undigested gliadin peptide, mimics and enhances the innate immune response to viruses and interferes with endocytic trafficking: A role in celiac disease. Sci. Rep. 2018, 8, 10821. [Google Scholar] [CrossRef]
- Drakesmith, H.; Prentice, A.M. Hepcidin and the iron-infection axis. Science. 2012, 338, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Ohgami, R.S.; Campagna, D.R.; Greer, E.L.; Antiochos, B.; McDonald, A.; Chen, J.; Sharp, J.J.; Fujiwara, Y.; Barker, J.E.; Fleming, M.D. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 2005, 37, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, A.; Roshan, T.M.; Kahawita, T.M.; Mason, A.B.; Sheftel, A.D.; Ponka, P. Erythroid cell mitochondria receive endosomal iron by a “kiss-and-run” mechanism. Biochim. Biophys. Acta 2016, 1863, 2859–2867. [Google Scholar] [CrossRef]
- Bradley, J.M.; Le Brun, N.E.; Moore, G.R. Ferritins: Furnishing proteins with iron. J. Biol. Inorg. Chem. 2016, 21, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Jordan, J.B.; Poppe, L.; Haniu, M.; Arvedson, T.; Syed, R.; Li, V.; Kohno, H.; Kim, H.; Schnier, P.D.; Harvey, T.S.; et al. Hepcidin revisited, disulfide connectivity, dynamics, and structure. J. Biol. Chem. 2009, 284, 24155–24167. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Chaston, T.; Chung, B.; Mascarenhas, M.; Marks, J.; Patel, B.; Srai, S.K.; Sharp, P. Evidence for differential effects of hepcidin in macrophages and intestinal epithelial cells. Gut 2008, 57, 374–382. [Google Scholar] [CrossRef]
- Chung, B.; Chaston, T.; Marks, J.; Srai, S.K.; Sharp, P.A. Hepcidin decreases iron transporter expression in vivo in mouse duodenum and spleen and in vitro in THP-1 macrophages and intestinal Caco-2 cells. J. Nutr. 2009, 139, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Brasse-Lagnel, C.; Karim, Z.; Letteron, P.; Bekri, S.; Bado, A.; Beaumont, C. Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology 2011, 140, 1261–1271.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Louis, S.; Cuvellier, S.; Shambat, S.M.; Hua, C.; Gomart, C.; Fouet, A.; Ortonne, N.; Decousser, J.W.; Zinkernagel, A.S.; et al. Epidermal hepcidin is required for neutrophil response to bacterial infection. J. Clin. Investig. 2020, 130, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Porrini, V.; Derosas, M.; Nardi, V.; Biasiotto, G.; Maccarinelli, F.; Zanella, I. Protective effect of mitochondrial ferritin on cytosolic iron dysregulation induced by doxorubicin in HeLa cells. Mol. Biol. Rep. 2013, 40, 6757–6764. [Google Scholar] [CrossRef] [PubMed]
- Daher, R.; Lefebvre, T.; Puy, H.; Karim, Z. Extrahepatic hepcidin production: The intriguing outcomes of recent years. World J. Clin. Cases. 2019, 7, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, J.; Diaz-Castro, J.; Pulido-Moran, M.; Alferez, M.J.; Boesch, C.; Sanchez-Alcover, A.; López-Aliaga, I. Fermented Goat’s Milk Consumption Improves Duodenal Expression of Iron Homeostasis Genes during Anemia Recovery. J. Agric. Food Chem. 2016, 64, 2560–2568. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, D.; Zhang, W.; Quan, X.; Dong, W.; Xu, Y.; Zhang, L. Cardioprotection by Hepc1 in cTnT(R141W) transgenic mice. Transgenic. Res. 2012, 21, 867–878. [Google Scholar] [CrossRef]
- Yang, Q.; Jian, J.; Katz, S.; Abramson, S.B.; Huang, X. 17β-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 2012, 153, 3170–3178. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, S.; Wang, L.; Li, J.; Qu, G.; He, J.; Rong, H.; Ji, H.; Liu, S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 2012, 511, 398–403. [Google Scholar] [CrossRef]
- Bajbouj, K.; Shafarin, J.; Abdalla, M.Y.; Ahmad, I.M.; Hamad, M. Estrogen-induced disruption of intracellular iron metabolism leads to oxidative stress, membrane damage, and cell cycle arrest in MCF-7 cells. Tumour Biol. 2017, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lehtihet, M.; Bonde, Y.; Beckman, L.; Berinder, K.; Hoybye, C.; Rudling, M.; Sloan, J.H.; Konrad, R.J.; Angelin, B. Circulating Hepcidin-25 Is Reduced by Endogenous Estrogen in Humans. PLoS ONE 2016, 11, e0148802. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.; Bajbouj, K.; Taneera, J. The Case for an Estrogen-iron Axis in Health and Disease. Exp. Clin. Endocrinol. Diabetes. 2019, 12. [Google Scholar] [CrossRef]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Anemia of Inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Theurl, I.; Aigner, E.; Theurl, M.; Nairz, M.; Seifert, M.; Schroll, A.; Sonnweber, T.; Eberwein, L.; Witcher, D.R.; Murphy, A.T.; et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: Diagnostic and therapeutic implications. Blood 2009, 113, 5277–5286. [Google Scholar] [CrossRef]
- Feder, J.N.; Gnirke, A.; Thomas, W.; Tsuchihashi, Z.; Ruddy, D.A.; Basava, A.; Dormishian, F.; Domingo, R., Jr.; Ellis, M.C.; Fullan, A.; et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat. Genet 1996, 13, 399–408. [Google Scholar] [CrossRef]
- Finberg, K.E.; Heeney, M.M.; Campagna, D.R.; Aydinok, Y.; Pearson, H.A.; Hartman, K.R.; Mayo, M.M.; Samuel, S.M.; Strouse, J.J.; Markianos, K.; et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat. Genet. 2008, 40, 569–571. [Google Scholar] [CrossRef]
- Barisani, D.; Ceroni, S.; Del Bianco, S.; Meneveri, R.; Bardella, M.T. Hemochromatosis gene mutations and iron metabolism in celiac disease. Haematologica 2004, 89, 1299–1305. [Google Scholar]
- Elli, L.; Poggiali, E.; Tomba, C.; Andreozzi, F.; Nava, I.; Bardella, M.T.; Campostrini, N.; Girelli, D.; Conte, D.; Cappellini, M.D. Does TMPRSS6 RS855791 polymorphism contribute to iron deficiency in treated celiac disease? Am. J. Gastroenterol. 2015, 110, 200–202. [Google Scholar] [CrossRef]
- De Falco, L.; Tortora, R.; Imperatore, N.; Bruno, M.; Capasso, M.; Girelli, D.; Castagna, A.; Caporaso, N.; Iolascon, A.; Rispo, A. The role of TMPRSS6 and HFE variants in iron deficiency anemia in celiac disease. Am. J. Hematol. 2018, 93, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Zanella, I.; Caimi, L.; Biasiotto, G. About TMPRSS6 rs855791 polymorphism, iron metabolism and celiac disease. Am. J. Gastroenterol. 2015, 110, 1240. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Jia, R.; Fang, F.; Liu, Y.; Cui, W. Computational and biological investigation of the soybean lecithin-gallic acid complex for ameliorating alcoholic liver disease in mice with iron overload. Food Funct. 2019, 10, 5203–5214. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Qian, X.H.; Qian, X.L. Astragalus polysaccharide upregulates hepcidin and reduces iron overload in mice via activation of p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 2016, 472, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Manoj, P.; Shetty, N.P.; Srinivasan, K.; Giridhar, P. Dietary iron supplements and Moringa oleifera leaves influence the liver hepcidin messenger RNA expression and biochemical indices of iron status in rats. Nutr. Res. 2014, 34, 630–638. [Google Scholar] [CrossRef]
- Mu, M.; Wu, A.; An, P.; Du, X.; Wu, Q.; Shen, X.; Wang, F. Black soyabean seed coat extract regulates iron metabolism by inhibiting the expression of hepcidin. Br. J. Nutr. 2014, 111, 1181–1189. [Google Scholar] [CrossRef]
- Guan, Y.; An, P.; Zhang, Z.; Zhang, F.; Yu, Y.; Wu, Q.; Shi, Y.; Guo, X.; Tao, Y.; Wang, F. Screening identifies the Chinese medicinal plant Caulis Spatholobi as an effective HAMP expression inhibitor. J. Nutr. 2013, 143, 1061–1066. [Google Scholar] [CrossRef]
- Wang, K.P.; Zeng, F.; Liu, J.Y.; Guo, D.; Zhang, Y. Inhibitory effect of polysaccharides isolated from Angelica sinensis on hepcidin expression. J. Ethnopharmacol. 2011, 134, 944–948. [Google Scholar] [CrossRef]
- Milder, I.E.; Feskens, E.J.; Arts, I.C.; Bueno de Mesquita, H.B.; Hollman, P.C.; Kromhout, D. Intake of the plant lignans secoisolariciresinol, matairesinol, lariciresinol, and pinoresinol in Dutch men and women. J. Nutr. 2005, 135, 1202–1207. [Google Scholar] [CrossRef]
- Zanella, I.; Biasiotto, G.; Holm, F.; Di Lorenzo, D. Cereal Lignans, Natural Compounds of Interest for Human Health. Nat. Prod. Commun. 2017, 12, 139–146. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Sok, D.E.; Cui, H.S.; Kim, M.R. Isolation and bioactivities of furfuran type lignan compounds from edible plants. Recent. Pat. Food. Nutr. Agric. 2009, 1, 87–95. [Google Scholar] [CrossRef]
- Biasiotto, G.; Penza, M.; Zanella, I.; Cadei, M.; Caimi, L.; Rossini, C.; Smeds, A.I.; Di Lorenzo, D. Oilseeds ameliorate metabolic parameters in male mice, while contained lignans inhibit 3T3-L1 adipocyte differentiation in vitro. Eur. J. Nutr. 2014, 53, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, H.B.; Metzler, M. Differences in the antioxidant activity of plant and mammalian lignans. J. Food Eng. 2003, 56, 255–256. [Google Scholar] [CrossRef]
- Yang, D.; Xiao, C.X.; Su, Z.H.; Huang, M.W.; Qin, M.; Wu, W.J.; Jia, W.W.; Zhu, Y.Z.; Hu, J.F.; Liu, X.H. (-)-7(S)-hydroxymatairesinol protects against tumor necrosis factor-α-mediated inflammation response in endothelial cells by blocking the MAPK/NF-κB and activating Nrf2/HO-1. Phytomedicine 2017, 32, 15–23. [Google Scholar] [CrossRef]
- Hovelstad, H.; Leirset, I.; Oyaas, K.; Fiksdahl, A. Screening analyses of pinosylvin stilbenes, resin acids and lignans in Norwegian conifers. Molecules 2006, 11, 103–114. [Google Scholar] [CrossRef]
- Smeds, A.I.; Jauhiainen, L.; Tuomola, E.; Peltonen-Sainio, P. Characterization of variation in the lignan content and composition of winter rye, spring wheat, and spring oat. J. Agric. Food Chem. 2009, 57, 5837–5842. [Google Scholar] [CrossRef]
- Eklund, P.; Raitanen, J.E. 9-Norlignans: Occurrence, Properties and Their Semisynthetic Preparation from Hydroxymatairesinol. Molecules 2019, 24, 220. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Wärri, A.; Mäkelä, S.I.; Eckerman, C.; Reunanen, M.; Ahotupa, M.; Salmi, S.M.; Franke, A.A.; Kangas, L.; Santti, R. Hydroxymatairesinol, a novel enterolactone precursor with antitumor properties from coniferous tree (Picea abies). Nutr. Cancer. 2000, 36, 207–216. [Google Scholar] [CrossRef]
- Katsuda, S.; Yoshida, M.; Saarinen, N.; Smeds, A.; Nakae, D.; Santti, R.; Maekawa, A. Chemopreventive effects of hydroxymatairesinol on uterine carcinogenesis in Donryu rats. Exp. Biol. Med. (Maywood) 2004, 229, 417–424. [Google Scholar] [CrossRef]
- Oikarinen, S.I.; Pajari, A.; Mutanen, M. Chemopreventive activity of crude hydroxymatairesinol (HMR) extract in Apc(Min) mice. Cancer Lett. 2000, 161, 253–258. [Google Scholar] [CrossRef]
- Bylund, A.; Saarinen, N.; Zhang, J.X.; Bergh, A.; Widmark, A.; Johansson, A.; Lundin, E.; Adlercreutz, H.; Hallmans, G.; Stattin, P.; et al. Anticancer effects of a plant lignan 7-hydroxymatairesinol on a prostate cancer model in vivo. Exp. Biol. Med. (Maywood) 2005, 230, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hedelin, M.; Klint, A.; Chang, E.T.; Bellocco, R.; Johansson, J.E.; Andersson, S.O.; Heinonen, S.M.; Adlercreutz, H.; Adami, H.O.; Grönberg, H.; et al. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: The cancer prostate Sweden study (Sweden). Cancer Causes Control. 2006, 17, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Spilioti, E.; Holmbom, B.; Papavassiliou, A.G.; Moutsatsou, P. Lignans 7-hydroxymatairesinol and 7-hydroxymatairesinol 2 exhibit anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2014, 58, 749–759. [Google Scholar] [CrossRef]
- Lina, B.; Korte, H.; Nyman, L.; Unkila, M. A thirteen week dietary toxicity study with 7-hydroxymatairesinol potassium acetate (HMRlignan) in rats. Regul. Toxicol. Pharmacol. 2005, 41, 28–38. [Google Scholar] [CrossRef]
- Wolterbeek, A.P.; Roberts, A.; Korte, H.; Unkila, M.; Waalkens-Berendsen, D.H. Prenatal developmental toxicity study with 7-hydroxymatairesinol potassium acetate (HMRlignan) in rats. Regul. Toxicol. Pharmacol. 2004, 40, 1–8. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Huovinen, R.; Wärri, A.; Mäkelä, S.I.; Valentín-Blasini, L.; Needham, L.; Eckerman, C.; Collan, Y.U.; Santti, R. Uptake and metabolism of hydroxymatairesinol in relation to its anticarcinogenicity in DMBA-induced rat mammary carcinoma model. Nutr. Cancer. 2001, 41, 82–90. [Google Scholar] [CrossRef]
- Udani, J.K.; Brown, D.J.; Tan, M.O.; Hardy, M. Pharmacokinetics and bioavailability of plant lignan 7-hydroxymatairesinol and effects on serum enterolactone and clinical symptoms in postmenopausal women: A single-blinded, parallel, dose-comparison study. J. Am. Coll. Nutr. 2013, 32, 428–435. [Google Scholar] [CrossRef]
- Biasiotto, G.; Zanella, I.; Predolini, F.; Archetti, I.; Cadei, M.; Monti, E.; Luzzani, M.; Pacchetti, B.; Mozzoni, P.; Andreoli, R.; et al. 7-Hydroxymatairesinol improves body weight, fat and sugar metabolism in C57BJ/6 mice on a high-fat diet. Br. J. Nutr. 2018, 120, 751–762. [Google Scholar] [CrossRef]
- Giuliano, C.; Siani, F.; Mus, L.; Ghezzi, C.; Cerri, S.; Pacchetti, B.; Bigogno, C.; Blandini, F. Neuroprotective effects of lignan 7-hydroxymatairesinol (HMR/lignan) in a rodent model of Parkinson’s disease. Nutrition 2020, 69, 110494. [Google Scholar] [CrossRef]
- Cosentino, M.; Marino, F.; Ferrari, M.; Rasini, E.; Bombelli, R.; Luini, A.; Legnaro, M.; Delle Canne, M.G.; Luzzani, M.; Crema, F.; et al. Estrogenic activity of 7-hydroxymatairesinol potassium acetate (HMR/lignan) from Norway spruce (Picea abies) knots and of its active metabolite enterolactone in MCF-7 cells. Pharmacol. Res. 2007, 56, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zanella, I.; Derosas, M.; Corrado, M.; Cocco, E.; Cavadini, P.; Biasiotto, G.; Poli, M.; Verardi, R.; Arosio, P. The effects of frataxin silencing in HeLa cells are rescued by the expression of human mitochondrial ferritin. Biochim. Biophys. Acta 2008, 1782, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Angmo, S.; Rana, S.; Yadav, K.; Sandhir, R.; Singhal, N.K. Novel Liposome Eencapsulated Guanosine Di Phosphate based Therapeutic Target against Anemia of Inflammation. Sci. Rep. 2018, 8, 17684. [Google Scholar] [CrossRef] [PubMed]
- Jacolot, S.; Férec, C.; Mura, C. Iron responses in hepatic, intestinal and macrophage/monocyte cell lines under different culture conditions. Blood Cells Mol. Dis. 2008, 41, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Silvestri, L.; Nai, A.; Camaschella, C. Hemojuvelin N-terminal mutants reach the plasma membrane but do not activate the hepcidin response. Haematologica 2008, 93, 1466–1472. [Google Scholar] [CrossRef]
- Verga Falzacappa, M.V.; Vujic Spasic, M.; Kessler, R.; Stolte, J.; Hentze, M.W.; Muckenthaler, M.U. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 2007, 109, 353–358. [Google Scholar] [CrossRef]
| Nutrient Sources | 7-Hydroxymatairesinol (μg/100 g) |
|---|---|
| Amaranth | 519 |
| Barley bran | 541 |
| Buckwheat | 142 |
| Corn | 407 |
| Flaxseed | 35 |
| Millet | 160 |
| Oat bran | 712 |
| Quinoa | 163 |
| Rye Bran | 1017 |
| Sesame | 7209 |
| Wheat Bran | 2787 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanella, I.; Paiardi, G.; Di Lorenzo, D.; Biasiotto, G. Iron Absorption in Celiac Disease and Nutraceutical Effect of 7-Hydroxymatairesinol. Mini-Review. Molecules 2020, 25, 2041. https://doi.org/10.3390/molecules25092041
Zanella I, Paiardi G, Di Lorenzo D, Biasiotto G. Iron Absorption in Celiac Disease and Nutraceutical Effect of 7-Hydroxymatairesinol. Mini-Review. Molecules. 2020; 25(9):2041. https://doi.org/10.3390/molecules25092041
Chicago/Turabian StyleZanella, Isabella, Giulia Paiardi, Diego Di Lorenzo, and Giorgio Biasiotto. 2020. "Iron Absorption in Celiac Disease and Nutraceutical Effect of 7-Hydroxymatairesinol. Mini-Review" Molecules 25, no. 9: 2041. https://doi.org/10.3390/molecules25092041
APA StyleZanella, I., Paiardi, G., Di Lorenzo, D., & Biasiotto, G. (2020). Iron Absorption in Celiac Disease and Nutraceutical Effect of 7-Hydroxymatairesinol. Mini-Review. Molecules, 25(9), 2041. https://doi.org/10.3390/molecules25092041






