Effects of Exercise Training on Renal Carnitine Biosynthesis and Uptake in the High-Fat and High-Sugar-Fed Mouse
Abstract
1. Introduction
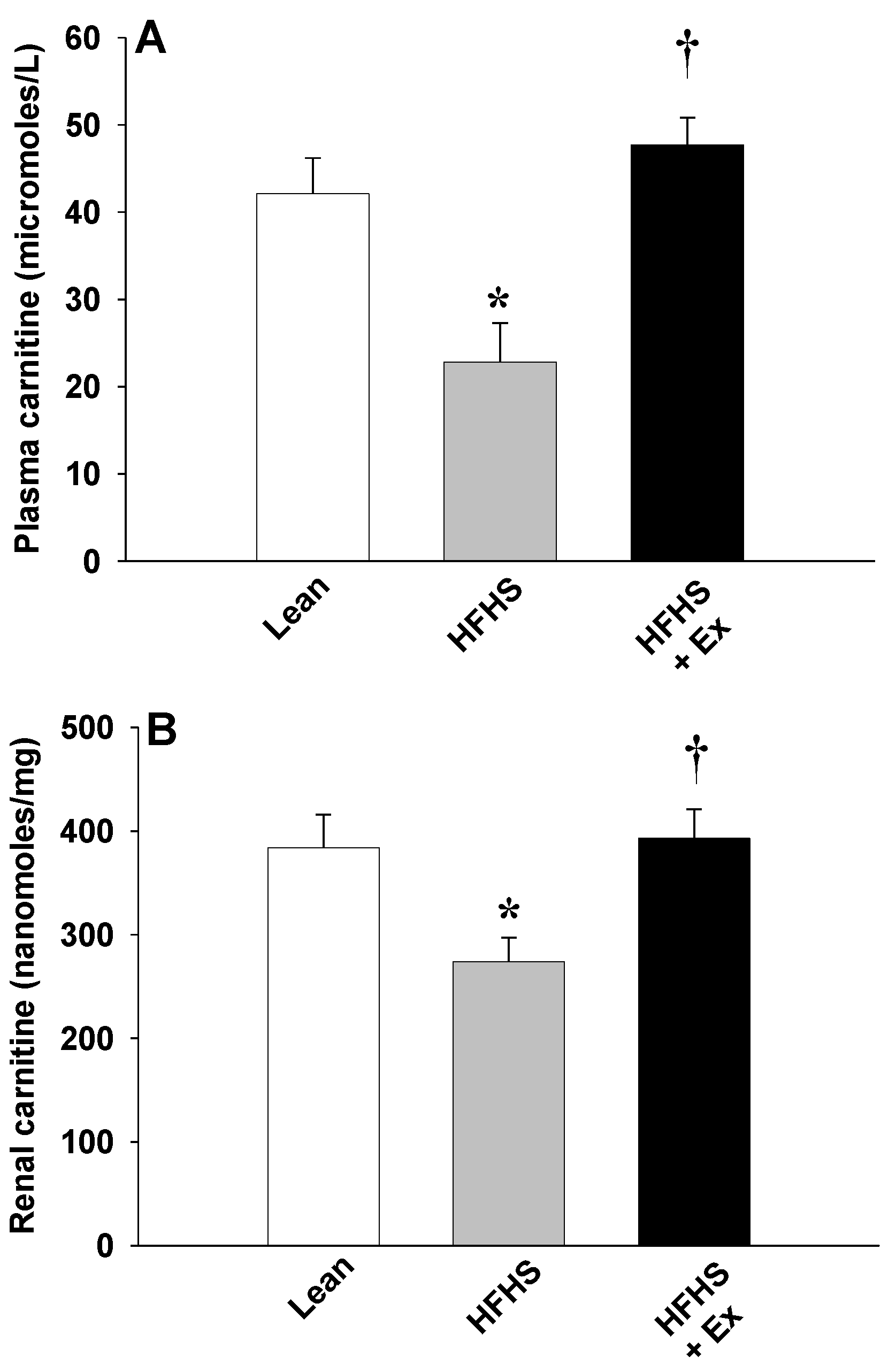
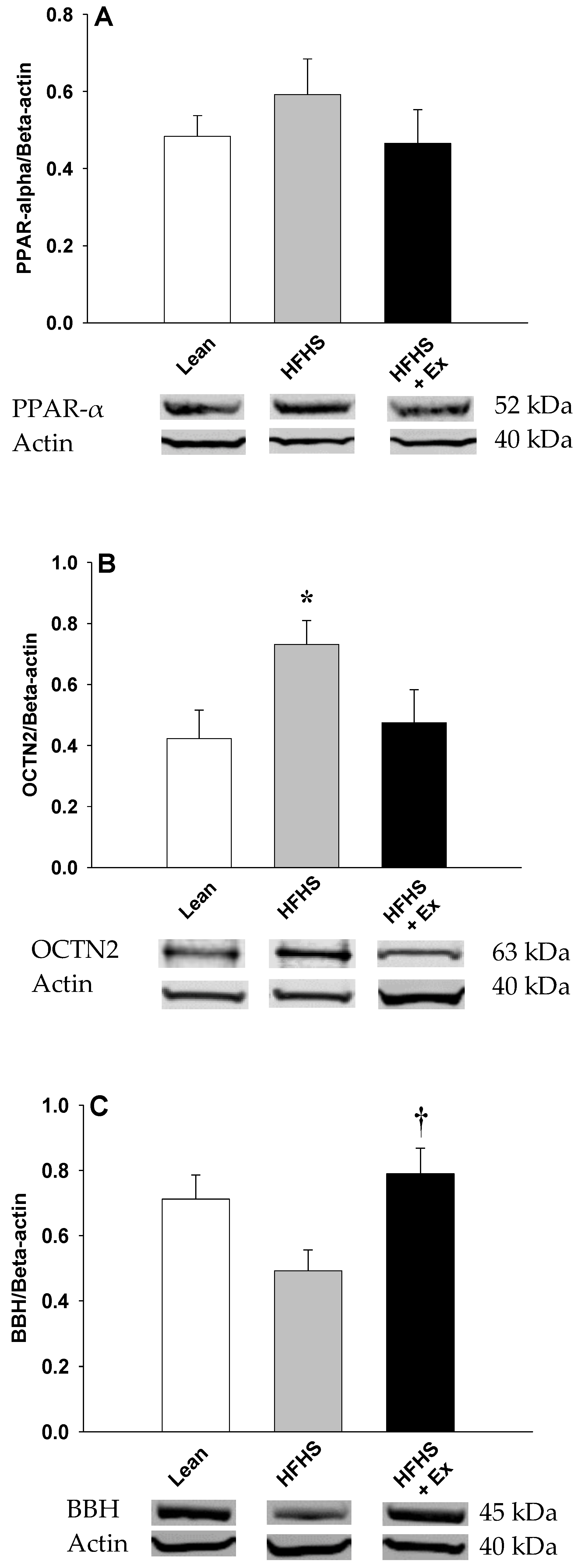
2. Results
3. Discussion
4. Material and Methods
4.1. Mouse Model and Treatment Protocols
4.2. Tissue and Plasma Collection
4.3. Western Blot Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bremer, J. Carnitine metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef]
- Vaz, F.M.; Wanders, R.J.A. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef]
- Strijbis, K.; Vaz, F.M.; Distel, B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life 2010, 62, 357–362. [Google Scholar] [CrossRef]
- Tamai, I.; Ohashi, R.; Nezu, J.I.; Sai, Y.; Kobayashi, D.; Oku, A.; Shimane, M.; Tsuji, A. Molecular and functional characterization of organic cation/carnitine transporter family in mice. J. Biol. Chem. 2000, 275, 40064–40072. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Bosch, E.P.; Chenard, C.A.; Schabold, K.J.; Nelson, S.E. Utilization of dietary precursors for carnitine synthesis in human adults. J. Nutr. 1989, 119, 1907–1913. [Google Scholar] [CrossRef]
- Long, C.S.; Haller, R.J.; Foster, D.W.; McGarry, J.D. Kinetics of carnitine-dependent fatty acid oxidation: implications for human carnitine deficiency. Neurology 1982, 32, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochem. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Luci, S.; Hirche, F.; Eder, K. Fasting and caloric restriction increase mRNA concentrations of novel organic cation transporter-2 and carnitine concentrations in rat tissues. Ann. Nutri. Metabol. 2008, 52, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Wege, N.; Wen, G.; Rauer, C.; Hirche, F.; Kluge, H.; Eder, K. Carnitine synthesis and uptake into cells are stimulated by fasting in pigs as a model of nonproliferating species. J. Nutr. Biochem. 2009, 20, 840–847. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauҫa, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef]
- van Vlies, N.; Ferdinandusse, S.; Turkenburg, M.; Wanders, R.J.; Vaz, F.M. PPARα-activation results in enhanced carnitine biosynthesis and OCTN2-medicated hepatic carnitine accumulation. Biochem. Biophys. Acta 2007, 1767, 1134–1142. [Google Scholar]
- Maeda, T.; Wakasawa, T.; Funabashi, M.; Fukushi, A.; Fujita, M.; Motojima, K.; Tamai, I. Regulation of Octn2 transporter (SLC22A5) by peroxisome proliferator activated receptor alpha. Biol. Pharm. Bull. 2008, 31, 1230–1236. [Google Scholar] [CrossRef]
- Broderick, T.L.; El Midaoui, A.; Chiasson, J.-L.; Wang, D.; Jankowski, M.; Gutkowska, J. The effects of exercise training on γ-butyrobetaine hydroxylase and novel organic cation transporter-2 gene expression in the rat. Appl. Physiol. Nutr. Metab. 2011, 36, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Broderick, T.L.; Cusimano, F.A.; Carlson, C.; Tamura, L.M. Acute exercise stimulates carnitine biosynthesis and OCTN2 expression in mouse kidney. Kidney Blood Press. Res. 2017, 42, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Broderick, T.L.; Cusimano, F.A.; Carlson, C.; Babu, J.R. Biosynthesis of the essential fatty acid oxidation cofactor carnitine is stimulated in heart and liver after a single bout of exercise in mice. J. Nutr. Metab. 2018, 2018, 2785090. [Google Scholar] [CrossRef] [PubMed]
- Ringeis, R.; Mooren, F.-C.; Keller, J.; Couturier, A.; Wen, G.; Hirche, F.; Stangle, G.I.; Eder, K.; Krüger, K. Regular endurance exercise improves the diminished hepatic carnitine status in mice fed a high-fat diet. Mol. Nutr. Food Res. 2011, 55, S193–S202. [Google Scholar] [CrossRef] [PubMed]
- Noland, R.C.; Koves, T.R.; Seiler, S.E.; Lum, H.; Lust, R.M.; Ilkayeva, O.; Stevens, R.D.; Hegardt, F.G.; Muoio, D.M. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009, 284, 22840–22852. [Google Scholar] [CrossRef]
- Ciman, M.; Rizzoli, V.; Siliprandi, N. Effect of physical training on carnitine concentration in liver, heart and gastrocnemius muscle of rat. Inter. J. Vitam. Nutr. Res. 1980, 50, 40–43. [Google Scholar]
- Makowski, L.; Noland, R.C.; Koves, T.R.; Xing, W.; Ilkayeva, O.R.; Muehlbauer, M.J.; Stevens, R.D.; Muoio, D.M. Metabolic profiling of PPARalpha-/- mice reveals defects in carnitine and amino acid homeostasis that are partially reversed by oral carnitine supplementation. FASEB J. 2009, 23, 586–604. [Google Scholar] [CrossRef]
- Hoydal, M.A.; Wislofff, U.; Kemi, O.J.; Ellingsen, O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.M.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: practice Guidelines by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Lean | HFHS | HFHS + Exercise | |
|---|---|---|---|
| Body weight (g) | |||
| Initial body weight | 23.5 ± 0.4 | 22.5 ± 0.9 | 22.2 ± 0.8 |
| Final body weight | 37.6 ± 0.9 | 47.9 ± 2.3 * | 45.7 ± 2.0 * |
| Organ weight (g) | |||
| Liver | 1.39 ± 0.08 | 2.34 ± 0.43 ‡ | 1.89 ± 0.16 |
| Kidney | 0.41 ± 0.02 | 0.40 ± 0.02 | 0.44 ± 0.03 |
| Total fat | 2.21 ± 0.41 | 4.57 ± 0.33 * | 4.33 ± 0.40 * |
| Plasma | |||
| Glucose (mg/dL) | 149 ± 12 | 236 ± 26 | 216 ± 34 |
| Insulin (ng/mL) | 0.46 ± 0.06 | 1.07 ± 0.09 * | 0.62 ± 0.06 † |
| Fructosamine (μmol/L) | 114 ± 14 | 279 ± 22 * | 188 ± 18 *,† |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, A.; Al-Nakkash, L.; Broderick, T.L. Effects of Exercise Training on Renal Carnitine Biosynthesis and Uptake in the High-Fat and High-Sugar-Fed Mouse. Molecules 2020, 25, 2100. https://doi.org/10.3390/molecules25092100
Upadhyay A, Al-Nakkash L, Broderick TL. Effects of Exercise Training on Renal Carnitine Biosynthesis and Uptake in the High-Fat and High-Sugar-Fed Mouse. Molecules. 2020; 25(9):2100. https://doi.org/10.3390/molecules25092100
Chicago/Turabian StyleUpadhyay, Aman, Layla Al-Nakkash, and Tom L. Broderick. 2020. "Effects of Exercise Training on Renal Carnitine Biosynthesis and Uptake in the High-Fat and High-Sugar-Fed Mouse" Molecules 25, no. 9: 2100. https://doi.org/10.3390/molecules25092100
APA StyleUpadhyay, A., Al-Nakkash, L., & Broderick, T. L. (2020). Effects of Exercise Training on Renal Carnitine Biosynthesis and Uptake in the High-Fat and High-Sugar-Fed Mouse. Molecules, 25(9), 2100. https://doi.org/10.3390/molecules25092100







