Energetic Performance of Pure Silica Zeolites under High-Pressure Intrusion of LiCl Aqueous Solutions: An Overview
Abstract
:1. Introduction
1.1. Heterogeneous Lyophobic Systems
1.2. Potential Applications of Heterogeneous Lyophobic Systems
1.3. Heterogeneous Lyophobic Systems Based on Hydrophobic Zeolites
2. Water Intrusion in Zeosils
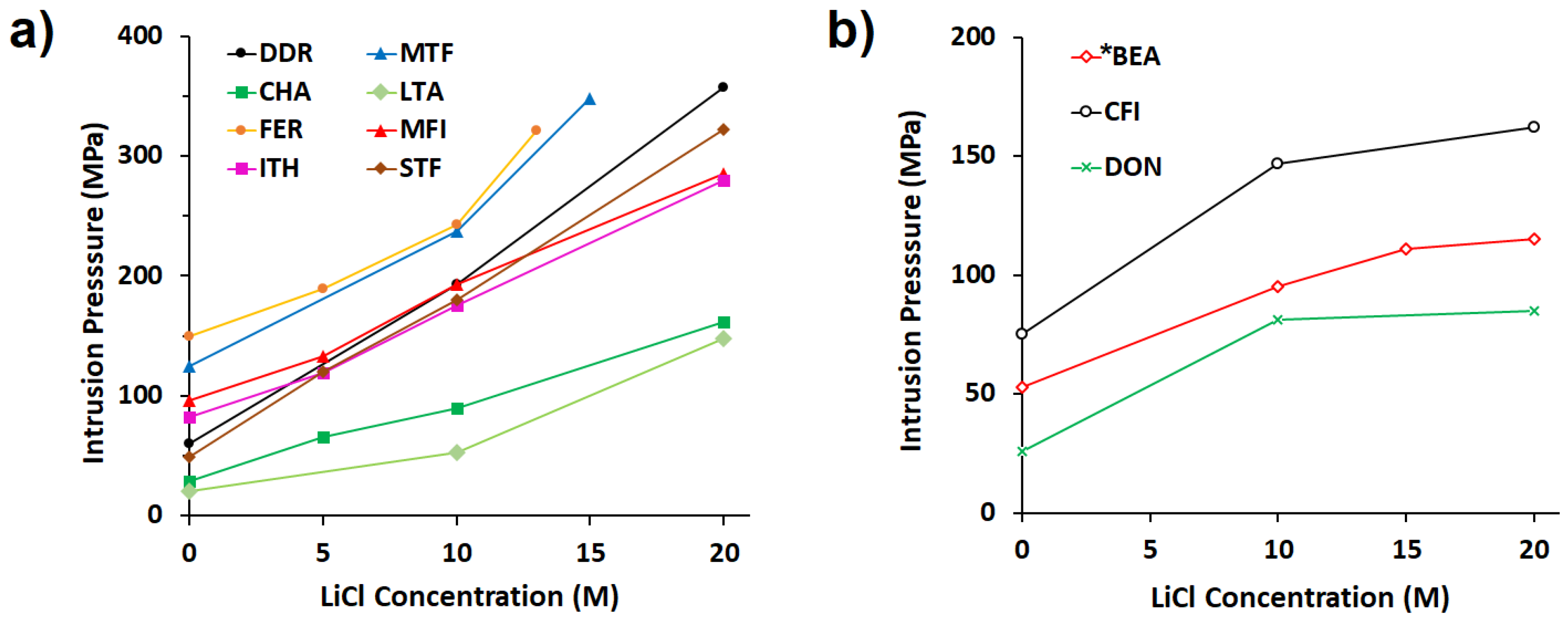
3. Influence of LiCl Aqueous Solutions on Intrusion Pressure
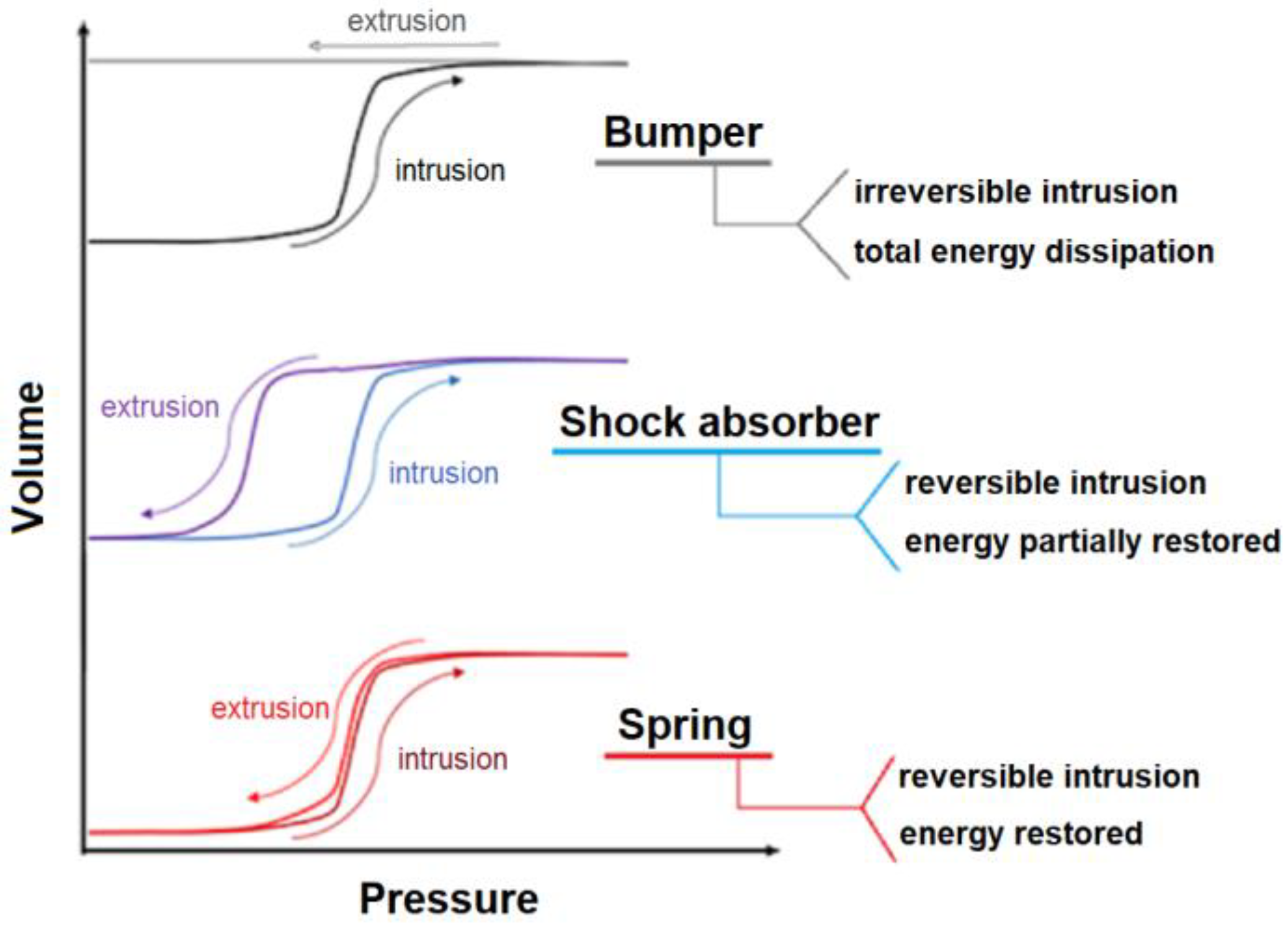
4. Influence of LiCl Aqueous Solutions on Intrusion–Extrusion Behavior
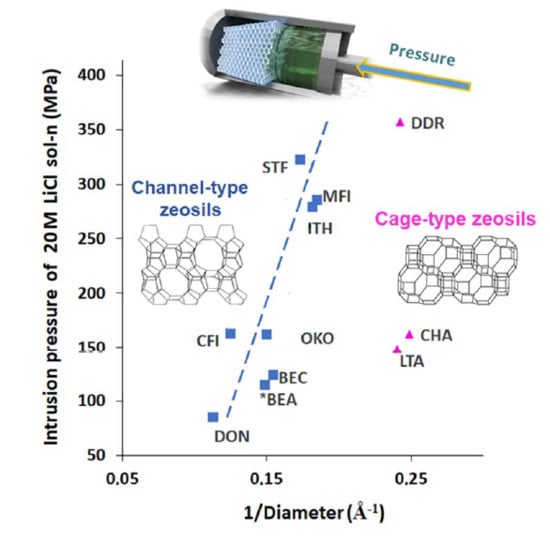
5. Influence of Particle Size and Morphology on Intrusion of LiCl Aqueous Solutions
6. Energetic Performance of “Zeosil–LiCl Aqueous Solution” Systems
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eroshenko, V.A. Dimensionnalité de l’espace comme potentiel thermodynamique d’un système. Entropie 1997, 33, 110–114. [Google Scholar]
- Eroshenko, V.A. Heterogeneous Structure for Accumulation or Dissipation of Energy, Process to Use it and Associated Devices. Int. Pat. WO 96/18040, 13 June 1996. [Google Scholar]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 374–375. [Google Scholar] [CrossRef]
- Eroshenko, V.A.; Piatiletov, I.; Coiffard, L.; Stoudenets, V.P. A new paradigm of mechanical energy dissipation: Experimental investigation and effectiveness of a novel car damper. Proc. Mech. Eng. D 2007, 221, 301–312. [Google Scholar] [CrossRef]
- Eroshenko, V.A. A new paradigm of mechanical energy dissipation: Theoretical aspects and practical solutions. Proc. Mech. Eng. D 2007, 221, 285–300. [Google Scholar] [CrossRef]
- Suciu, C.V.; Iwatsubo, T.; Yaguchi, K.; Ikenaga, M. Novel and global approach of the complex and interconnected phenomena related to the contact line movement past a solid surface from hydrophobized silica gel. J. Coll. Inter. Sci. 2005, 283, 196–214. [Google Scholar] [CrossRef]
- Suciu, C.V.; Yaguchi, K. Endurance tests on a colloidal damper destined to vehicle suspension. Exp. Mech. 2009, 49, 383–393. [Google Scholar] [CrossRef]
- Eroshenko, V.A. Interfacial energy in the lyophobic systems and challenge to all physico-chemists. In The 8th International Conference on Material Technologies and Modeling; MMT-2014 Organizing Committee: Ariel, Israel, 2014; pp. 4–212. [Google Scholar]
- Eroshenko, V.A. Repulsive clathrates. A new operational material for efficient seismic isolation. In Proc. of Int. Post–SMITR Conf. Seismic Isolation, Passive Energy Dissipation and Active Control of Seismic Vibrations of Structure; IAEA: Wien, Austria, 1997; p. 783. [Google Scholar]
- Eroshenko, V.A. Damper with High Dissipating Power. Int. Pat. WO 01/55616 A1, 2 August 2001. [Google Scholar]
- Grujicic, M.; Yavari, R.; Snipes, J.S.; Ramaswami, S. A zeolite absorbent/nano-fluidics protection-based blast and ballistic-impact-mitigation system. J. Mater Sci. 2015, 50, 2019–2037. [Google Scholar] [CrossRef]
- Xu, B.; Qiao, Y.; Chen, X. Mitigating impact/blast energy via a novel nanofluidic energy capture mechanism. J. Mech. Phys. Solid. 2014, 62, 194–208. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, M.C.; Gao, X. Study of the mechanical properties and vibration isolation performance of a molecular spring isolator. Shock Vib. 2016. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Surani, F.B.; Kong, X.; Punyamurtula, V.K.; Qiao, Y. Energy absorption performance of steel tubes enhanced by a nanoporous material functionalized liquid. Appl. Phys. Lett. 2006, 89, 241819. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Punyamurtula, V.K.; Han, A. Temperature dependence of working pressure of a nanoporous liquid spring. Appl. Phys. Lett. 2006, 89, 251905. [Google Scholar] [CrossRef]
- Eroshenko, V.A.; Popyk, A. Current status and perspectives of thermomolecular engine developments. Int. J. Thermodyn. 2014, 17, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Coiffard, L.; Eroshenko, V.; Grolier, J.-P.E. Thermomechanics of the variation of interfaces in heterogeneous lyophobic systems. Aiche J. 2005, 51, 1246–1257. [Google Scholar] [CrossRef]
- Laouir, A.; Luo, L.; Tondeur, D.; Cachot, T.; Le Goff, P. Thermal machines based on surface energy of wetting. Aiche J. 2003, 49, 764–781. [Google Scholar] [CrossRef]
- Eroshenko, V.A.; Grosu, Y. Importance of synthesis of lyophobic porous materials and corresponding liquids for development of thermomolecular energy devices of new generation. In The 8th International Conference on Material Technologies and Modeling; MMT-2014 Organizing Committee: Ariel, Israel, 2014; pp. 4–55. [Google Scholar]
- Han, A.; Qiao, Y. A volume-memory liquid. Appl. Phys. Lett. 2007, 91, 173123. [Google Scholar] [CrossRef] [Green Version]
- Eroshenko, V.; Grosu, Y.; Tsyrin, N.; Nedelec, J.-M.; Grolier, J.P.E. Exceptionally large and controlled effect of negative thermal expansion in porous heterogeneous lyophobic systems. J. Phys. Chem. C 2015, 119, 10266–10272. [Google Scholar] [CrossRef]
- Eroshenko, V.A. Eroshenko hydrocapillary accumulator. URSS Patent 1333870, 24 May 1985. [Google Scholar]
- Eroshenko, V.A.; Fadeev, A.Y. Intrusion and extrusion of water in hydrophobized porous silica. Kolloidn. Zhur. (Rus) 1995, 57, 446–449. [Google Scholar]
- Fadeev, A.Y.; Eroshenko, V.A. Study of penetration of water into hydrophobized porous silicas. J. Coll. Inter. Sci. 1997, 187, 275–282. [Google Scholar] [CrossRef]
- Martin, T.; Lefevre, B.; Brunel, D.; Galarneau, A.; Di Renzo, F.; Fajula, F.; Gobin, P.F.; Quinson, J.; Vigier, G. Dissipative water intrusion in hydrophobic MCM-41 type materials. Chem. Comm. 2002, 1, 24–25. [Google Scholar] [CrossRef]
- Eroshenko, V.; Regis, R.C.; Soulard, M.; Patarin, J. Energetics: A new field of applications for hydrophobic zeolites. J. Am. Chem. Soc. 2001, 123, 8129–8130. [Google Scholar] [CrossRef]
- Čejka, J.; Van Bekkum, H.; Corma, A.; Schüth, F. Introduction to Zeolite Science and Practice, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 1–1068. [Google Scholar] [CrossRef]
- Tzanis, L.; Trzpit, M.; Soulard, M.; Patarin, J. Energetic performances of channel and cage-type zeosils. J. Phys. Chem. C 2012, 116, 20389–20395. [Google Scholar] [CrossRef]
- Ronchi, L. Synthesis of Hydrophobic Zeolites for Energetic Applications. Ph.D. Thesis, University of Haute-Alsace, Mulhouse, France, 17 October 2017. [Google Scholar]
- Tzanis, L.; Trzpit, M.; Soulard, M.; Patarin, J. High pressure water intrusion investigation of pure silica 1D channel AFI, MTW and TON-type zeolites. Micropor. Mesopor. Mater. 2011, 146, 119–126. [Google Scholar] [CrossRef]
- Khay, I.; Tsanis, L.; Daou, T.J.; Nouali, H.; Ryzhikov, A.; Patarin, J. Energetic behavior of the pure silica ITQ-12 (ITW) zeolite under high pressure water intrusion. Phys. Chem. Chem. Phys. 2013, 15, 20320–20325. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikov, A.; Khay, I.; Nouali, H.; Daou, T.J.; Patarin, J. Energetic performances of pure silica STF and MTT-type zeolites under high pressure water intrusion. RSC Adv. 2014, 4, 37655–37661. [Google Scholar] [CrossRef]
- Saada, M.A.; Rigolet, S.; Paillaud, J.-L.; Bats, N.; Soulard, M.; Patarin, J. Investigation of the energetic performance of pure silica ITQ-4 (IFR) zeolite under high pressure water intrusion. J. Phys. Chem. C 2010, 114, 11650–11658. [Google Scholar] [CrossRef]
- Saada, M.A.; Soulard, M.; Marler, B.; Gies, H.; Patarin, J. High-pressure water intrusion investigation of pure silica RUB-41 and S-SOD zeolite materials. J. Phys. Chem. C 2011, 115, 425–430. [Google Scholar] [CrossRef]
- Fraux, G.; Coudert, F.-X.; Boutin, A.; Fuchs, A.H. Forced intrusion of water and aqueous solutions in microporous materials: From fundamental thermodynamics to energy storage devices. Chem. Soc. Rev. 2017, 46, 7421–7437. [Google Scholar] [CrossRef] [Green Version]
- Tzanis, L.; Marler, B.; Gies, H.; Patarin, J. High-pressure water intrusion investigation of pure silica ITQ-7 zeolite. J. Phys. Chem. C 2013, 117, 4098–4103. [Google Scholar] [CrossRef]
- Ievtushenko, O.V.; Eroshenko, V.A.; Grosu, Y.G.; Nedelec, J.M.; Grolier, J.P.E. Evolution of the energetic characteristics of silicalite-1 + water repulsive clathrates in a wide temperature range. Phys. Chem. Chem. Phys. 2013, 15, 4451–4457. [Google Scholar] [CrossRef]
- Humplik, T.; Shalabh, R.; Maroo, C.; Laoui, T.; Wang, E.N. Framework water capacity and infiltration pressure of MFI zeolites. Micropor. Mesopor. Mater. 2014, 190, 84–91. [Google Scholar] [CrossRef]
- Fasano, M.; Humplik, T.; Bevilacqua, A.; Tsapatsis, M.; Chiavazzo, E.; Wang, E.N.; Asinari, P. Interplay between hydrophilicity and surface barriers on water transport in zeolite membranes. Nat. Commun. 2016, 7, 12762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Liu, L.; Chen, X. Pressurized liquid in nanopores: A modified Laplace-Young equation. Nano Lett. 2009, 9, 984–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzanis, L.; Nouali, H.; Daou, T.J.; Soulard, M.; Patarin, J. Influence of the aqueous medium on the energetic performances of silicalite-1. Mater. Lett. 2014, 115, 229–232. [Google Scholar] [CrossRef]
- Soulard, M.; Patarin, J. Process for high-pressure energy storage by solvation/desolvation and associated storage device. Patent FR2976030, 30 May 2011. [Google Scholar]
- Qiao, Y.; Han, K. Infiltration pressure of a nanoporous liquid spring modified by an electrolyte. J. Mater. Res. 2007, 22, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Ryzhikov, A.; Khay, I.; Nouali, H.; Daou, T.J.; Patarin, J. Drastic change of the intrusion-extrusion behavior of electrolyte solutions in pure silica *BEA-type zeolite. Phys. Chem. Chem. Phys. 2014, 16, 17893–17899. [Google Scholar] [CrossRef] [Green Version]
- Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Drastic influence of the anion nature and concentration on high pressure intrusion-extrusion of electrolyte solutions in silicalite-1. Phys. Chem. Chem. Phys. 2018, 20, 6462–6468. [Google Scholar] [CrossRef]
- Ryzhikov, A.; Ronchi, L.; Nouali, H.; Daou, T.J.; Paillaud, J.-L.; Patarin, J. High-pressure intrusion–extrusion of water and electrolyte solutions in pure-silica LTA zeolite. J. Phys. Chem. C 2015, 119, 28319–28325. [Google Scholar] [CrossRef]
- Khay, I.; Daou, T.J.; Nouali, H.; Ryzhikov, A.; Rigolet, S.; Patarin, J. High pressure intrusion-extrusion of LiCl aqueous solutions in silicalite-1 zeolite: Influence on energetic performances. J. Phys. Chem. C 2014, 118, 3935–3941. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Energetic performances of FER-type zeolite in the presence of electrolyte solutions under high pressure. Energy 2017, 130, 29–37. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Influence of LiCl aqueous solution concentration on the energetic performances of pure silica chabazite. New J. Chem. 2017, 47, 2586–2592. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Extra-large pore opening CFI and DON-type zeosils for mechanical energy storage. Micropor. Mesopor. Mater. 2018, 255, 211–219. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Albrecht, S.; Patarin, J. Investigation of the energetic performance of pure silica BEC-type zeolite under high pressure water and 20 M LiCl intrusion-extrusion experiments. Micropor. Mesopor. Mater. 2017, 254, 153–159. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Heterogeneous lyophobic systems based on pure silica ITH-type zeolites: High pressure intrusion of water and electrolyte solutions. New J. Chem. 2017, 41, 15087–15093. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Energetic performances of pure-silica DDR zeolite by high-pressure intrusion–extrusion of electrolyte aqueous solutions: A shock-absorber with huge absorbed energy. J. Phys. Chem. C 2018, 122, 2726–2733. [Google Scholar] [CrossRef]
- Isaac, C.; Confalonieri, G.; Nouali, H.; Paillaud, J.-L.; Arletti, R.; Daou, T.J.; Ryzhikov, A. Unusual high-pressure intrusion-extrusion behavior of electrolyte solutions in Mu-26, a pure silica zeolite of topology STF. Micropor. Mesopor. Mater. 2020, 298, 110047. [Google Scholar] [CrossRef]
- Kirschhock, C.E.; De Prins, M.; Verheijen, E.; Ryzhikov, A.; Daou, T.J.; Nouali, H.; Taulelle, F.; . Martens, J.A.; Patarin, J. Intrusion–extrusion spring performance of–COK-14 zeolite enhanced by structural changes. Phys. Chem. Chem. Phys. 2016, 18, 18795–18801. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org (accessed on 15 January 2020).
- Bushuev, Y.G.; Sastre, G.; De Julian-Ortiz, J.V.; Galvez, J. Water−hydrophobic zeolite systems. J. Phys. Chem. C 2012, 116, 24916–24929. [Google Scholar] [CrossRef]
- Desbiens, N.; Demachy, I.; Fuchs, A.H.; Kirsch-Rodeschini, H.; Soulard, M.; Patarin, J. Water condensation in hydrophobic nanopores. Angew. Chem. Int. Ed. 2005, 44, 5310–5313. [Google Scholar] [CrossRef]
- Trzpit, M.; Soulard, M.; Patarin, J.; Desbiens, N.; Cailliez, F.; Boutin, A.; Demachy, I.; Fuchs, A.H. The effect of local defects on water adsorption in silicalite-1 zeolite: A joint experimental and molecular simulation study. Langmuir 2007, 23, 10131–10139. [Google Scholar] [CrossRef]
- Michelin-Jamois, M.; Picard, C.; Vigier, G.; Charlaix, E. Giant osmotic pressure in the forced wetting of hydrophobic nanopores. Phys. Rev. Lett. 2015, 115, 036101. [Google Scholar] [CrossRef] [Green Version]
- Han, A.; Punyamurtula, V.K.; Qiao, Y. Influence of anions on liquid infiltration and defiltration in a zeolite Y. Phys. Rev. E 2008, 78, 031408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Anderko, A.; Young, R.D. Modeling surface tension of concentrated and mixed-solvent electrolyte systems. Ind. Eng. Chem. Res. 2011, 50, 4086–4098. [Google Scholar] [CrossRef]
- Arletti, R.; Ronchi, L.; Quartieri, S.; Vezzalini, G.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Intrusion-extrusion experiments of MgCl2 aqueous solution in pure silica ferrierite: Evidence of the nature of intruded liquid by in situ high pressure synchrotron X-ray powder diffraction. Micropor. Mesopor. Mater. 2016, 235, 253–260. [Google Scholar] [CrossRef]
- Confalonieri, G.; Ryzhikov, A.; Arletti, R.; Nouali, H.; Quartieri, S.; Daou, T.J.; Patarin, J. Intrusion–extrusion of electrolyte aqueous solutions in pure silica chabazite by in situ high pressure synchrotron x-ray powder diffraction. J. Phys. Chem. C 2018, 122, 28001–28012. [Google Scholar] [CrossRef]
- Confalonieri, G.; Ryzhikov, A.; Arletti, R.; Quartieri, S.; Vezzalini, G.; Isaac, C.; Paillaud, J.-L.; Nouali, H.; Daou, T.J. Structural interpretation of the energetic performances of a pure silica LTA-type zeolite. Phys. Chem. Chem. Phys. 2020, 22, 5178–5187. [Google Scholar] [CrossRef]
- Ryzhikov, A.; Khay, I.; Nouali, H.; Daou, T.J.; Patarin, J. High pressure intrusion–extrusion of electrolyte solutions in aluminosilicate FAU and *BEA-type zeolites. Micropor. Mesopor. Mater. 2016, 221, 1–7. [Google Scholar] [CrossRef]
- Kabalan, I.; Khay, I.; Nouali, H.; Ryzhikov, A.; Lebeau, B.; Albrecht, S.; Rigolet, S.; Fadlallah, M.-B.; Toufaily, J.; Hamieh, T.; et al. Influence of the particle sizes on the energetic performances of MFI-type zeolites. J. Phys. Chem. C 2015, 119, 18074–18083. [Google Scholar] [CrossRef]
- Huve, J.; Daou, T.J.; Nouali, H.; Patarin, J.; Ryzhikov, A. The effect of nanostructures on high pressure intrusion–extrusion of water and electrolyte solutions in hierarchical nanoboxes of silicalite-1. New J. Chem. 2020, 44, 273–281. [Google Scholar] [CrossRef]


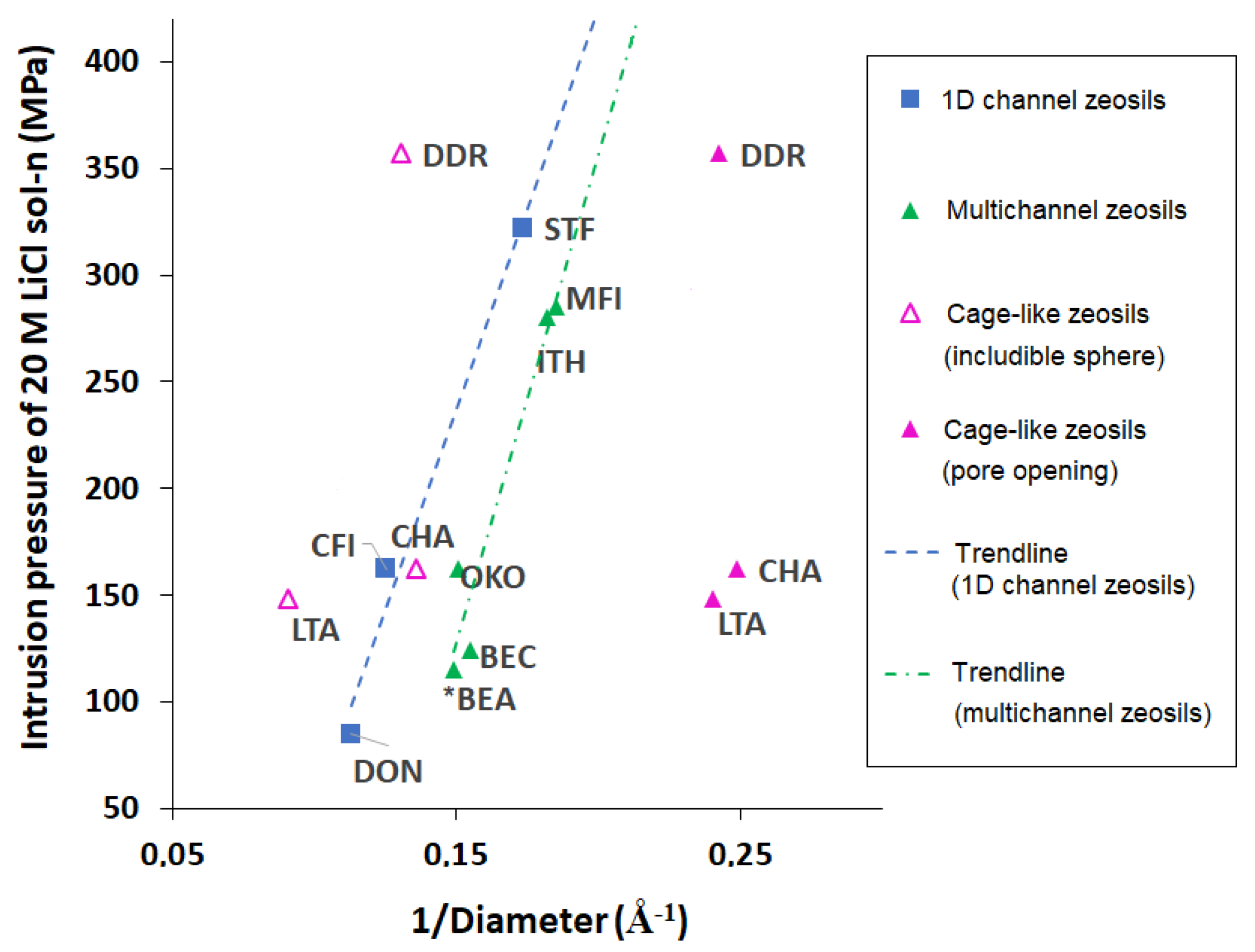
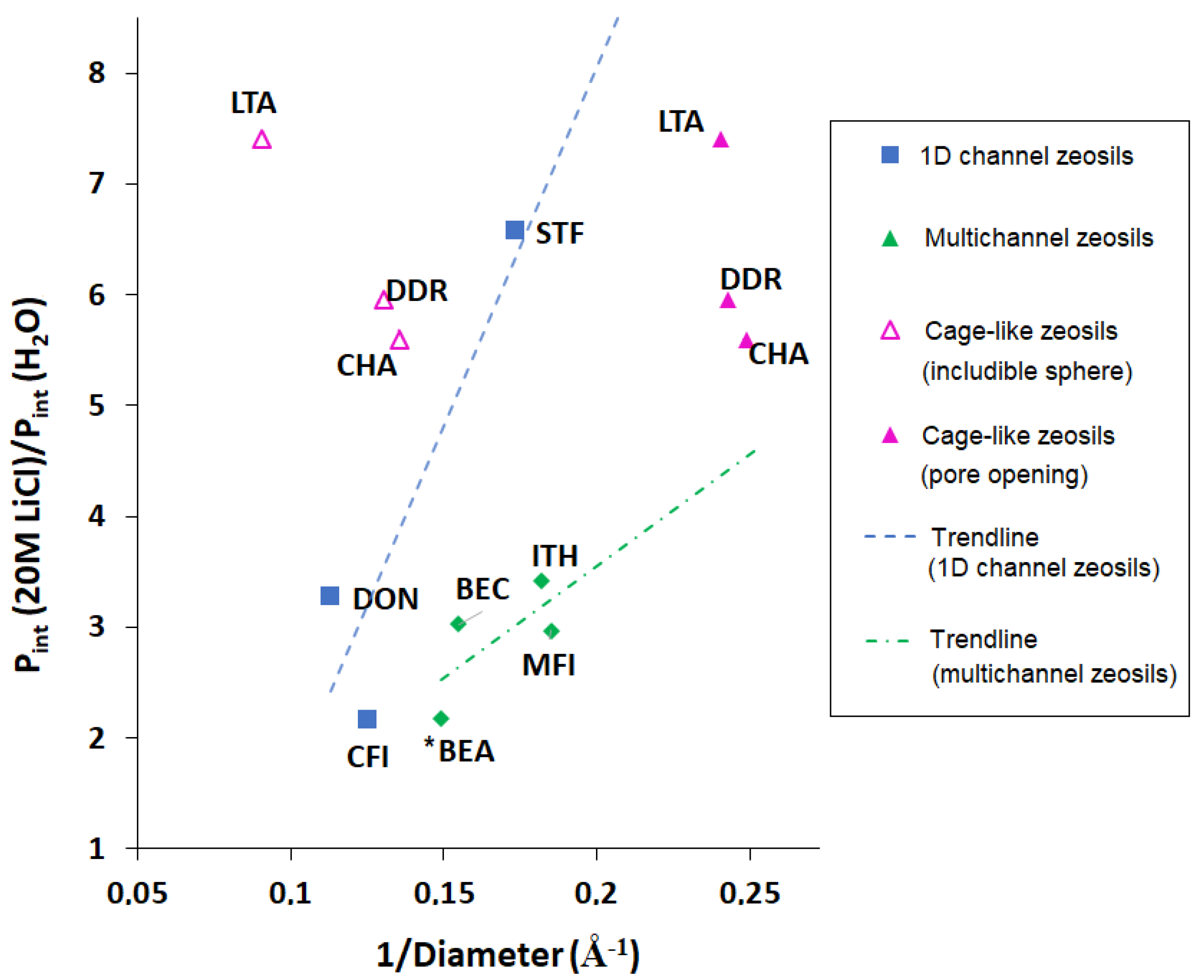
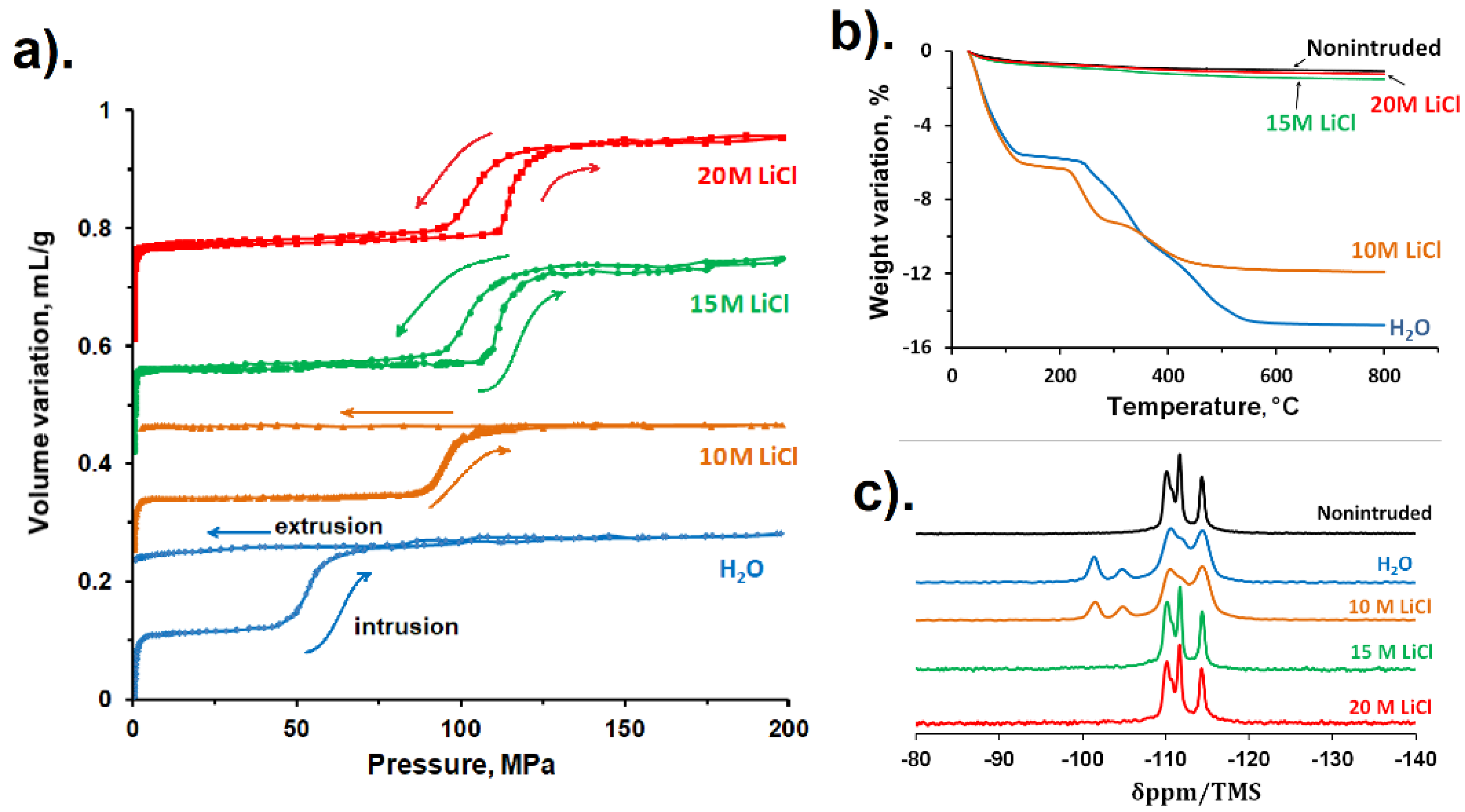
| Framework Type | Pore System | Ring Size (T atoms) | Average Free Diameter (Å) | Max. Diam. Includible Sphere (Å) |
|---|---|---|---|---|
| CDO | Multichannel (2D) | 8 | 3.971 | 5.78 |
| CHA | Cages | 8 | 4.021 | 7.37 |
| MTF | 1D Channels with side pockets | 8 | 4.113 | 6.25 |
| DDR | Cages | 8 | 4.121 | 7.66 |
| LTA | Cages | 8 | 4.157 | 11.05 |
| FER | Multichannel (2D) | 10 and 8 | 5.242 | 6.31 |
| MFI | Multichannel (3D) | 10 | 5.405 | 6.36 |
| ITH | Multichannel (3D) | 10 and 9 | 5.502 | 6.72 |
| STF | 1D Channels with side pockets | 10 | 5.762 | 7.63 |
| BEC | Multichannel (3D) | 12 | 6.462 | 6.95 |
| OKO | Multichannel (2D) | 12 and 10 | 6.638 | 6.70 |
| *BEA | Multichannel (3D) | 12 | 6.709 | 6.68 |
| CFI | 1D Channels | 14 | 7.976 | 7.47 |
| DON | 1D Channels | 14 | 8.856 | 8.79 |
| R | C (M) | Pint (MPa) | Vint (mLg−1) | Pext (MPa) | Vext (mLg−1) | Eint (Jg−1) | Eext (Jg−1) | E.Y. (%) | Beh. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1D Channels | CFI [50] | 14 MR | 0 | 75 | 0.08 | 75 | 0.08 | 6.0 | 6.0 | 100 | S | |
| 10 | 147 | 0.09 | 143 | 0.09 | 13.2 | 12.9 | 97 | S | ||||
| 20 | 162 | 0.09 | 158 | 0.09 | 14.6 | 14.2 | 97 | S | ||||
| DON [50] | 14 MR | 0 | 26 | 0.04 | 21 | 0.04 | 1.0 | 0.8 | 81 | S | ||
| 10 | 81 | 0.06 | 70 | 0.06 | 4.9 | 4.2 | 86 | S | ||||
| 20 | 85 | 0.08 | 75 | 0.08 | 6.8 | 6.0 | 88 | S | ||||
| MTF [29] | 8 MR | 0 | 125 | 0.008 | 125 | 0.008 | 1.0 | 1.0 | 100 | S | ||
| 10 | 237 | 0.009 | 237 | 0.009 | 2.1 | 2.1 | 100 | S | ||||
| 15 | 348 | 0.012 | 348 I/32 II | 0.007 I/0.005 II | 4.2 | 2.6 | 62 | S + SA | ||||
| STF [54] | 10 MR | 0 | 49 */26 ** | 0.055 */0.025 ** | 24 | 0.025 | 2.7 */0.7 ** | 0.6 | 22 */86 ** | B + SA */S ** | ||
| 5 | 120 */66 ** | 0.07 */0.02 ** | 48 | 0.02 | 8.4 */1.3 ** | 1 | 11 */72 ** | B + SA */SA ** | ||||
| 10 | 180 */133 ** | 0.08 */0.04 ** | 109 */95 ** | 0.04 | 14.4 */5.3 ** | 4.4 */3.8 ** | 30 */72 ** | B + SA */SA ** | ||||
| 20 | 322 */225–252 ** | 0.125 */0.08 ** | 115 | 0.08 | 40.2 */19.2 ** | 9.2 | 23 */48 ** | B+ SA */SA ** | ||||
| Multichannels | 2D | FER [48] | 10 and 8 MR | 0 | 150 | 0.056 | 143 | 0.056 | 8.4 | 8.2 | 97 | S |
| 5 | 189 | 0.052 | 184 | 0.052 | 9.8 | 9.6 | 98 | S | ||||
| 10 | 243 | 0.052 | 231 | 0.052 | 12.6 | 12.0 | 91 | S | ||||
| 13 | 321 | 0.055 | 300 | 0.055 | 17.7 | 16.5 | 93 | S | ||||
| OKO [55] | 12 and 10 MR | 0 | / | / | / | / | / | / | / | SI | ||
| 20 | 162 */143 ** | 0.12 */0.105 ** | 131 | 0.105 | 19.4 */15.0 ** | 13.7 | 70 */98 ** | B + SA */ S ** | ||||
| CDO [29] | 8 MR | 0 | 210 | 0.03 | 180 | 0.03 | 6.3 | 5.4 | 84 | S | ||
| 5 | 294 | 0.035 | 251 | 0.035 | 10.3 | 8.8 | 85 | S | ||||
| 3D | ITH [52] | 10 and 9 MR | 0 | 82 | 0.08 | / | / | 6.6 | / | / | B | |
| 5 | 119 | 0.08 | / | / | 9.5 | / | / | B | ||||
| 10 | 175 | 0.08 | / | / | 14 | / | / | B | ||||
| 20 | 280 */138 ** | 0.11 */0.06 ** | 117 | 0.06 | 30.8 */8.3 ** | 7.0 | 22 */84 ** | B +SA */ S ** | ||||
| MFI [47] | 10 MR | 0 | 96 | 0.1 | 95 | 0.1 | 9.6 | 9.5 | 99 | S | ||
| 5 | 133 | 0.10 | 128 | 0.10 | 13.3 | 12.8 | 96 | S | ||||
| 10 | 193 | 0.10 | 179 | 0.10 | 19.3 | 17.9 | 93 | S | ||||
| 20 | 285 | 0.11 | 273 | 0.10 | 31.3 | 27.3 | 87 | S | ||||
| *BEA [44] | 12 MR | 0 | 53 | 0.14 | / | / | 8.3 | / | / | B | ||
| 10 | 95 | 0.12 | / | / | 11.4 | / | / | B | ||||
| 15 | 111 | 0.16 | 102 | 0.16 | 17.8 | 16.3 | 91 | S | ||||
| 20 | 115 | 0.16 | 103 | 0.16 | 18.4 | 16.5 | 90 | S | ||||
| BEC [51] | 12 MR | 0 | 41 | 0.08 | / | / | 3.3 | / | / | B | ||
| 20 | 124 */119 ** | 0.11 | 82 | 0.11 | 13.6 */13.1 ** | 9.02 | 66 */69 ** | SA | ||||
| Cages | DDR [53] | 8 MR | 0 | 60 | 0.112 | 51 | 0.112 | 6.7 | 5.7 | 85 | S | |
| 10 | 193 */166 ** | 0.08 */0.07 ** | 166 | 0.07 | 15.4 */11.6 ** | 11.6 | 75 */100 ** | B + SA */S ** | ||||
| 20 | 357 */253 ** | 0.26 */0.24 ** | 130 | 0.24 | 92.8 */60.7 ** | 31 | 33 */51 ** | B + SA */SA ** | ||||
| CHA [49] | 8 MR | 0 | 29 */22 ** | 0.15 */0.13 ** | 22 */20 ** | 0.13 | 4.4 */2.9 ** | 2.9 */2.6 ** | 65 */90 ** | B + SA */S ** | ||
| 5 | 66 */63 ** | 0.15 | 54 | 0.15 | 9.9 */9.4 ** | 8.1 | 82 */86 ** | S | ||||
| 10 | 90 */86 ** | 0.15 | 79 | 0.15 | 13.5 */12.9 ** | 11.8 */11.8 ** | 88 */92 ** | S | ||||
| 20 | 162 */153 ** | 0.15 | 137 | 0.15 | 24.3 */22.9 ** | 20.5 | 85 */89 ** | S | ||||
| LTA [46] | 8 MR | 0 | 20 | 0.17 | / | / | 3.4 | / | / | B | ||
| 10 | 53 */46 ** | 0.20 */0.12 ** | 39 | 0.12 | 10.6 */5.5 ** | 4.7 | 42 */85 ** | B + SA */S ** | ||||
| 20 | 148 */133 ** | 0.22 */0.12 ** | 98 | 0.12 | 32.6 */16.0 ** | 11.8 | 36 */74 ** | B + SA */SA ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Confalonieri, G.; Daou, T.J.; Nouali, H.; Arletti, R.; Ryzhikov, A. Energetic Performance of Pure Silica Zeolites under High-Pressure Intrusion of LiCl Aqueous Solutions: An Overview. Molecules 2020, 25, 2145. https://doi.org/10.3390/molecules25092145
Confalonieri G, Daou TJ, Nouali H, Arletti R, Ryzhikov A. Energetic Performance of Pure Silica Zeolites under High-Pressure Intrusion of LiCl Aqueous Solutions: An Overview. Molecules. 2020; 25(9):2145. https://doi.org/10.3390/molecules25092145
Chicago/Turabian StyleConfalonieri, Giorgia, T. Jean Daou, Habiba Nouali, Rossella Arletti, and Andrey Ryzhikov. 2020. "Energetic Performance of Pure Silica Zeolites under High-Pressure Intrusion of LiCl Aqueous Solutions: An Overview" Molecules 25, no. 9: 2145. https://doi.org/10.3390/molecules25092145
APA StyleConfalonieri, G., Daou, T. J., Nouali, H., Arletti, R., & Ryzhikov, A. (2020). Energetic Performance of Pure Silica Zeolites under High-Pressure Intrusion of LiCl Aqueous Solutions: An Overview. Molecules, 25(9), 2145. https://doi.org/10.3390/molecules25092145