Subcritical Water Extraction of Valuable Metals from Spent Lithium-Ion Batteries
Abstract
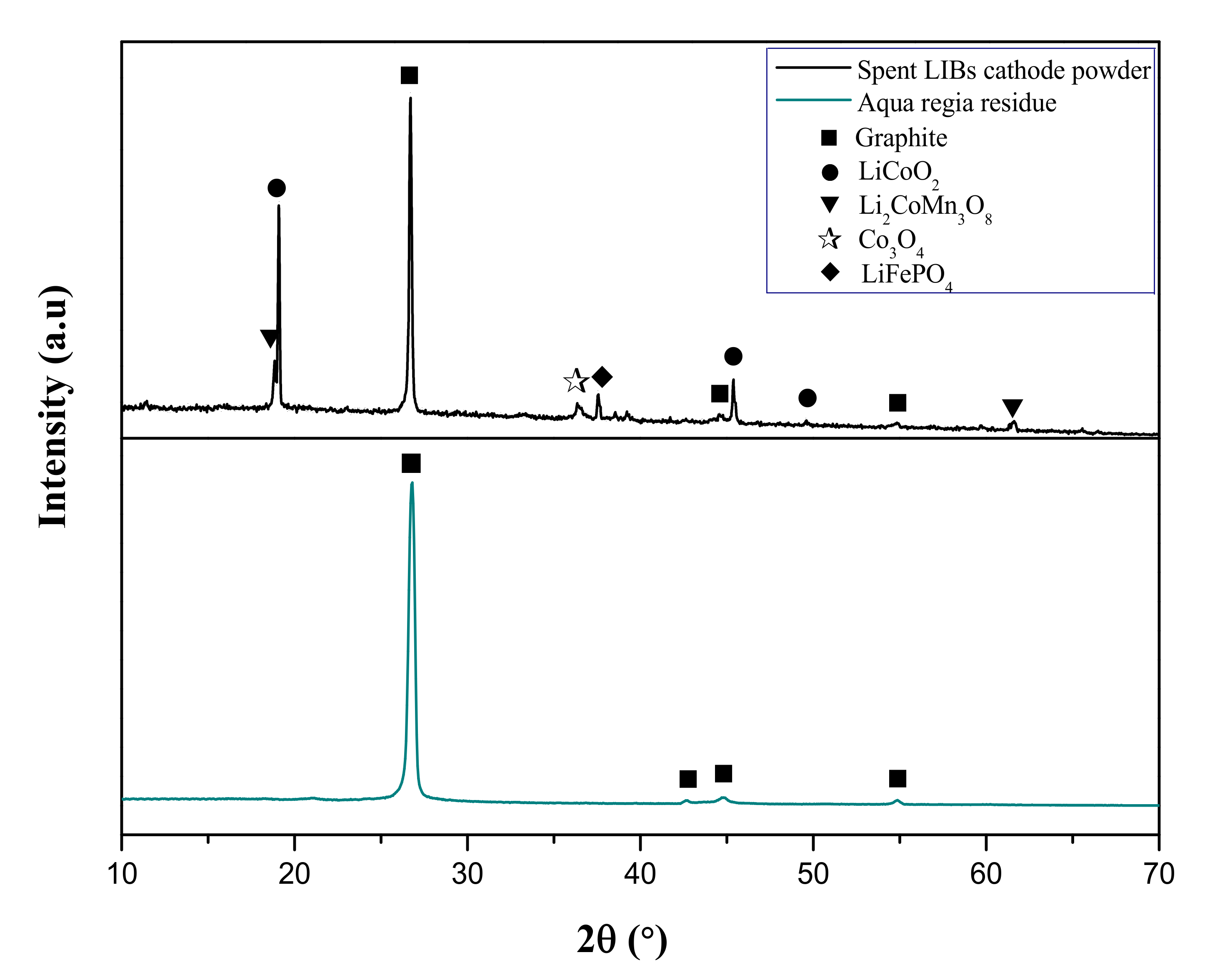
1. Introduction
2. Results
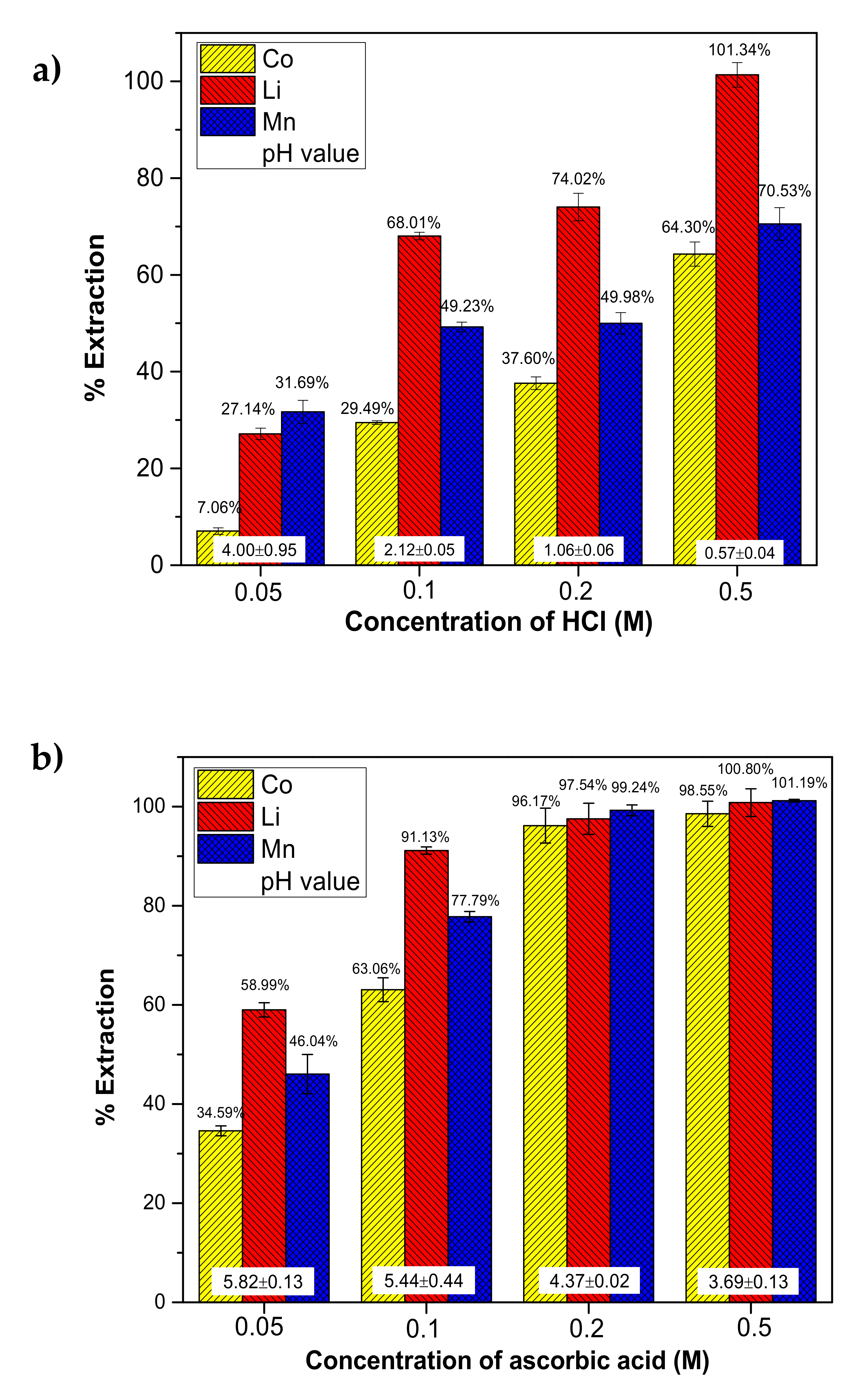
2.1. Effect of Acid Concentration
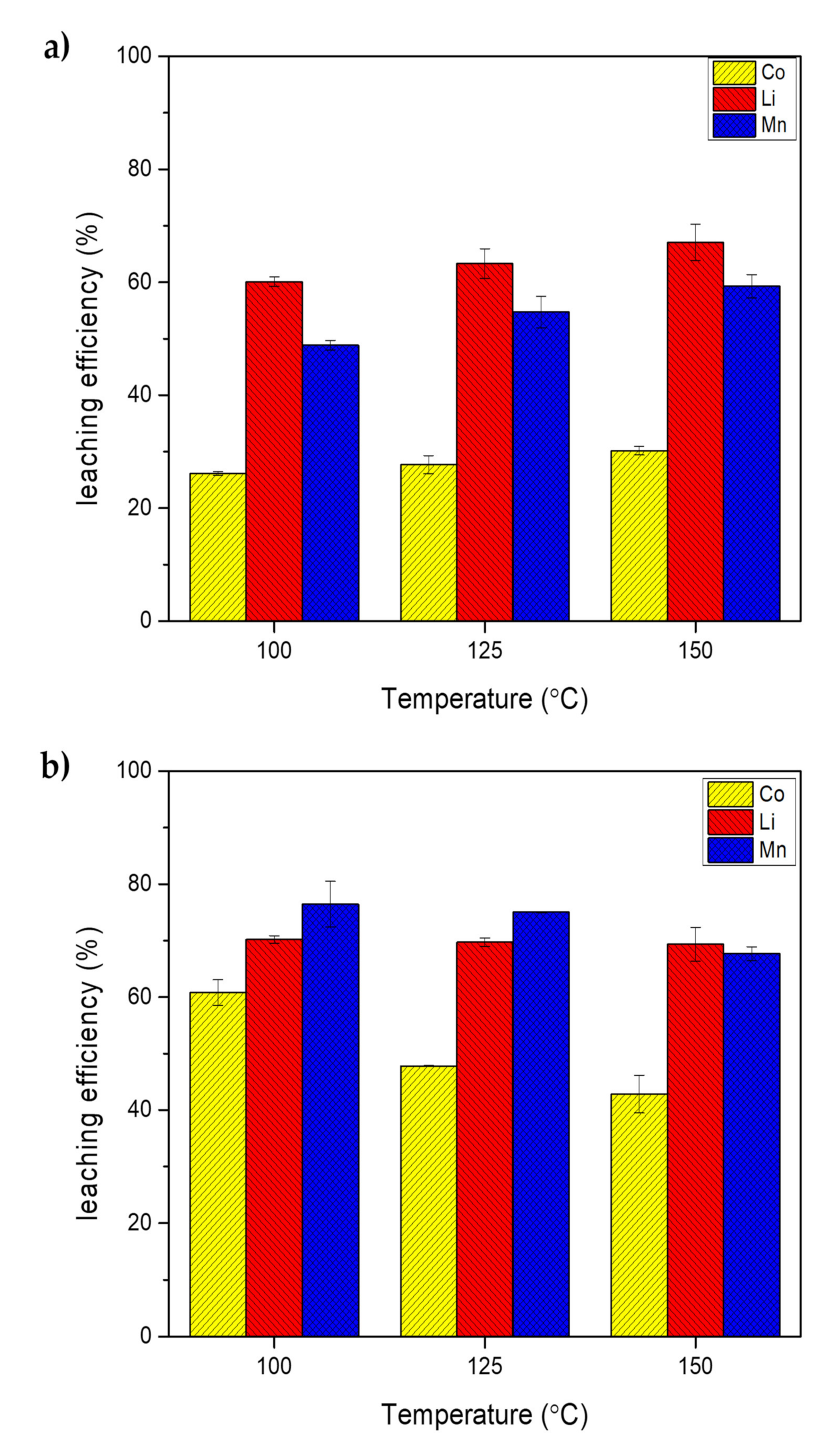
2.2. Effect of Temperature
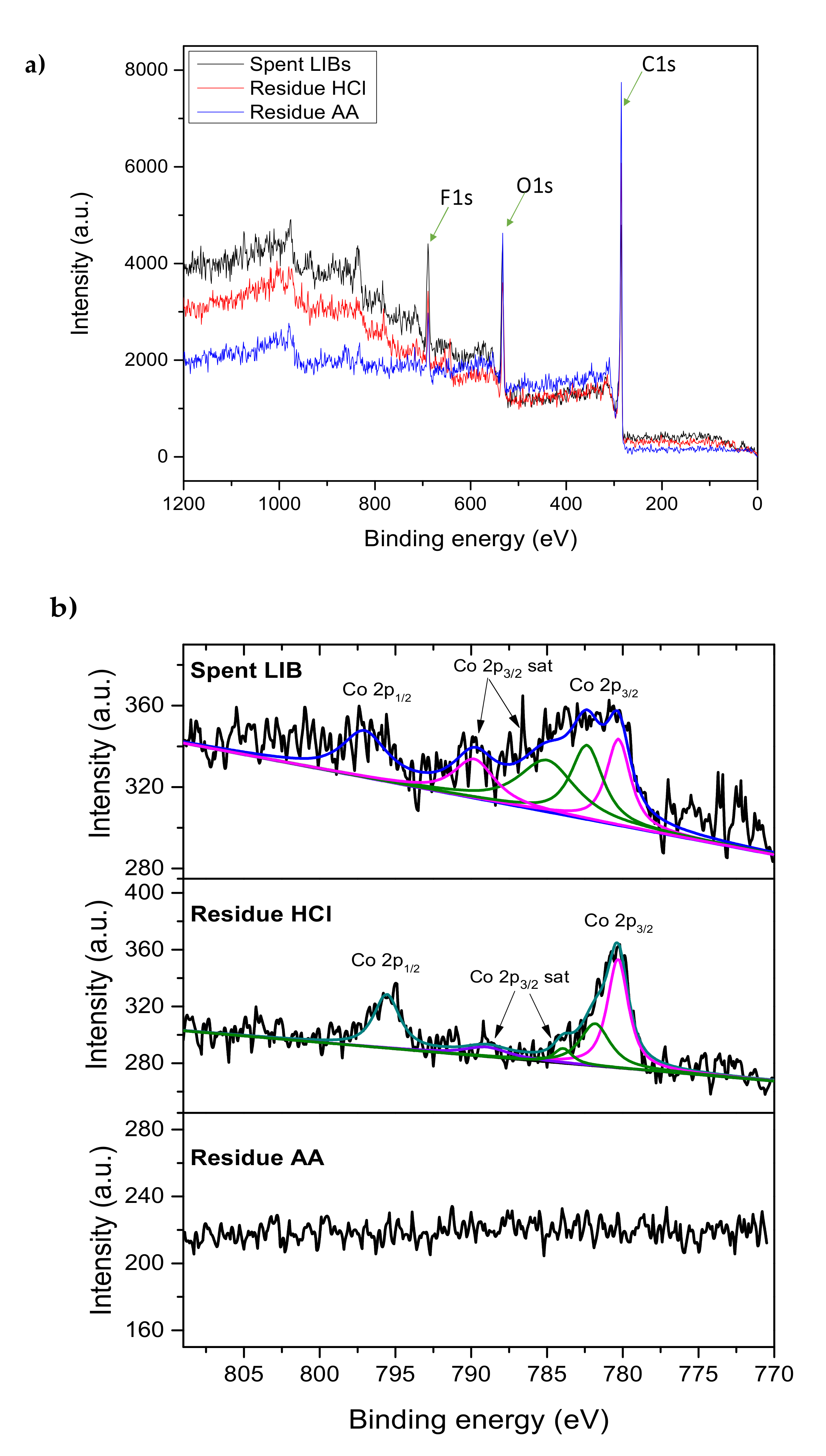
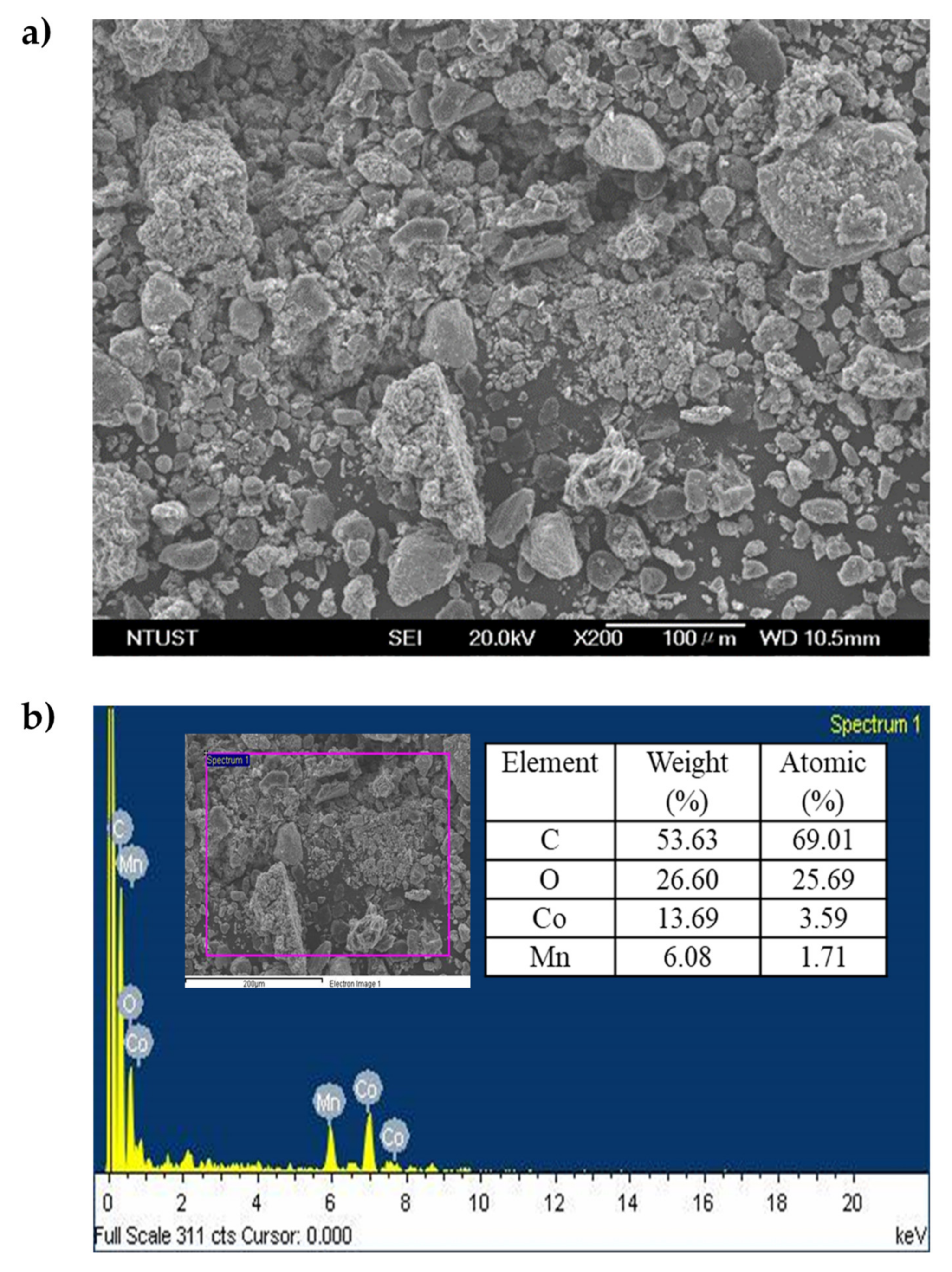
2.3. Dissolution of Cobalt from Spent LIBs
2.4. Conventional Leaching
3. Material and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Song, X.F.; Yu, J.G. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2cathode of lithium-ion batteries. Waste Manag. 2017, 64, 171–181. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical processes for recycling spent lithium-Ion batteries: A critical review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective recovery of valuable metals from spent lithium-ion batteries—Process development and kinetics evaluation. J. Clean. Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- Barik, S.P.; Prabaharan, G.; Kumar, B. An innovative approach to recover the metal values from spent lithium-ion batteries. Waste Manag. 2016, 51, 222–226. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Meshram, P.; Mishra, A.; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Lee, J.C.; Kim, M.S.; Jeong, J.; Kim, B.S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of spent lithium-ion battery: A critical review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Lin, E.Y.; Rahmawati, A.; Ko, J.H.; Liu, J.C. Extraction of yttrium and europium from waste cathode-ray tube (CRT) phosphor by subcritical water. Sep. Purif. Technol. 2018, 192, 166–175. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, F.S. Innovative leaching of cobalt and lithium from spent lithium-ion batteries and simultaneous dechlorination of polyvinyl chloride in subcritical water. J. Hazard. Mater. 2016, 316, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Nshizirungu, T.; Agarwal, A.; Jo, Y.T.; Rana, M.; Shin, D.; Park, J.H. Chlorinated polyvinyl chloride (CPVC) assisted leaching of lithium and cobalt from spent lithium-ion battery in subcritical water. J. Hazard. Mater. 2020, 393, 122367. [Google Scholar] [CrossRef] [PubMed]
- Nshizirungu, T.; Rana, M.; Jo, Y.-T.; Park, J.-H. Rapid leaching and recovery of valuable metals from spent lithium ion batteries (LIBs) via environmentally benign subcritical nickel-containing water over chlorinated polyvinyl chloride. J. Hazard. Mater. 2020, 122667. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Kitagawa, Y.; Okumura, M.; Shigeta, Y. Accurate standard hydrogen electrode potential and applications to the redox potentials of vitamin C and NAD/NADH. J. Phys. Chem. A 2015, 119, 369–376. [Google Scholar] [CrossRef]
- Shih, Y.J.; Chien, S.K.; Jhang, S.R.; Lin, Y.C. Chemical leaching, precipitation and solvent extraction for sequential separation of valuable metals in cathode material of spent lithium ion batteries. J. Taiwan Inst. Chem. Eng. 2019, 100, 151–159. [Google Scholar] [CrossRef]
- Gao, W.; Liu, C.; Cao, H.; Zheng, X.; Lin, X.; Wang, H.; Zhang, Y.; Sun, Z. Comprehensive evaluation on effective leaching of critical metals from spent lithium-ion batteries. Waste Manag. 2018, 75, 477–485. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process for recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Chen, G.; Yang, H.; Li, H.; Tong, L. Recovery of cobalt as cobalt oxalate from cobalt tailings using moderately thermophilic bioleaching technology and selective sequential extraction. Minerals 2016, 6, 67. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Harris, D., Swain, E., Robey, C., Aiello, E., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2012; Volume 2, ISBN 0-471-04372-9. [Google Scholar]
- Tur’yan, Y.I.; Kohen, R. Formal redox potentials of the dehydro-l-ascorbic acid/l-ascorbic acid system. J. Electroanal. Chem. 1995, 380, 273–277. [Google Scholar] [CrossRef]
- Karpushkin, E.A.; Kharochkina, E.S.; Iarchuk, A.R.; Gallyamov, M.O.; Sergeyev, V.G. Hydrothermal transformations of ascorbic acid. Russ. J. Gen. Chem. 2017, 87, 2858–2864. [Google Scholar] [CrossRef]
- Mogol, B.A.; Gökmen, V. Kinetics of furan formation from ascorbic acid during heating under reducing and oxidizing conditions. J. Agric. Food Chem. 2013, 61, 10191–10196. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Influence of the PVdF binder on the stability of LiCoO2 electrodes. Electrochem. commun. 2005, 7, 1298–1304. [Google Scholar] [CrossRef]
- Verdier, S.; El Ouatani, L.; Dedryvère, R.; Bonhomme, F.; Biensan, P.; Gonbeau, D. XPS study on Al2O3- and AlPO4-coated LiCoO2 cathode material for high-capacity Li ion batteries. J. Electrochem. Soc. 2007, 154, A1088. [Google Scholar] [CrossRef]
- Peng, C.; Hamuyuni, J.; Wilson, B.P.; Lundström, M. Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system. Waste Manag. 2018, 76, 582–590. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, B.; Lee, J. Effect of mechanical activation on the kinetics of copper leaching from copper sulfide (CuS). Metals (Basel) 2018, 8, 150. [Google Scholar] [CrossRef]
- Baba, A.A.; Ayinla, K.I.; Adekola, F.A.; Bale, R.B.; Ghosh, M.K.; Alabi, A.G.F.; Sheik, A.R.; Folorunso, I.O. Hydrometallurgical application for treating a Nigerian chalcopyrite ore in chloride medium: Part I. Dissolution kinetics assessment. Int. J. Miner. Metall. Mater. 2013, 20, 1021–1028. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Leaching by HCl | Leaching by Ascorbic Acid | ||
|---|---|---|---|
| Reaction | E°/V * | Reaction | E°/V * |
| Co3+ + e− → Co2+ | 1.92 a | Co3+ + e− → Co2+ | 1.92 a |
| Cl2(g) + 2 e− → 2 Cl− | 1.36 a | DAsc + 2e− + H+ → HAsc− | 0.28 b |
| DAsc + 2e− + 2H+ → H2Asc | 0.39 b | ||
| 2 Cl− + 2 Co3+ → 2 Co2+ + Cl2(g) | 1.24 | HAsc− + 2 Co3+ → DAsc + 2Co2+ + H+ | 1.78 |
| H2Asc + 2 Co3+ → DAsc + 2Co2+ + 2H+ | 1.73 | ||
| Peak | Spent LIBs | Leaching Residue HCl | Leaching Residue AA | Assignment | |||
|---|---|---|---|---|---|---|---|
| BE (eV) | Ratio | BE (eV) | Ratio | BE (eV) | Ratio | ||
| Co 2p | 780.28 | 1.000 | 780.32 | 1.000 | n.d. | n.d. | Co 2p3/2: Co(III) |
| 782.36 | 1.141 | 781.82 | 0.550 | n.d. | n.d. | Co 2p3/2: Co (II) | |
| Component | Content (mg/g) | Mass Fraction (%) * |
|---|---|---|
| Co | 188.93 ± 3.01 | 51.34 |
| Mn | 103.46 ± 2.09 | 28.12 |
| Li | 28.31 ± 0.36 | 7.69 |
| Ni | 17.79 ± 0.11 | 4.83 |
| Al | 12.07 ± 0.07 | 3.28 |
| Fe | 9.27 ± 0.25 | 2.52 |
| Cu | 8.14 ± 0.06 | 2.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lie, J.; Tanda, S.; Liu, J.-C. Subcritical Water Extraction of Valuable Metals from Spent Lithium-Ion Batteries. Molecules 2020, 25, 2166. https://doi.org/10.3390/molecules25092166
Lie J, Tanda S, Liu J-C. Subcritical Water Extraction of Valuable Metals from Spent Lithium-Ion Batteries. Molecules. 2020; 25(9):2166. https://doi.org/10.3390/molecules25092166
Chicago/Turabian StyleLie, Jenni, Stefani Tanda, and Jhy-Chern Liu. 2020. "Subcritical Water Extraction of Valuable Metals from Spent Lithium-Ion Batteries" Molecules 25, no. 9: 2166. https://doi.org/10.3390/molecules25092166
APA StyleLie, J., Tanda, S., & Liu, J.-C. (2020). Subcritical Water Extraction of Valuable Metals from Spent Lithium-Ion Batteries. Molecules, 25(9), 2166. https://doi.org/10.3390/molecules25092166





