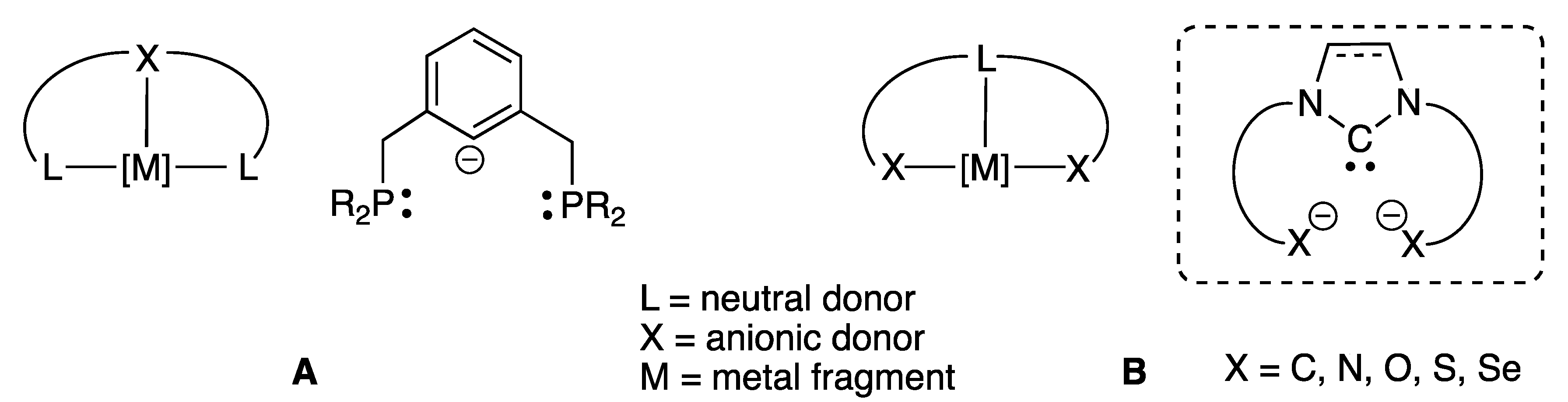
NHC Core Pincer Ligands Exhibiting Two Anionic Coordinating Extremities
Abstract
1. Introduction
2. NHC Core Pincer Ligands of LX2-Type
2.1. Based on Two Identical Side Arms
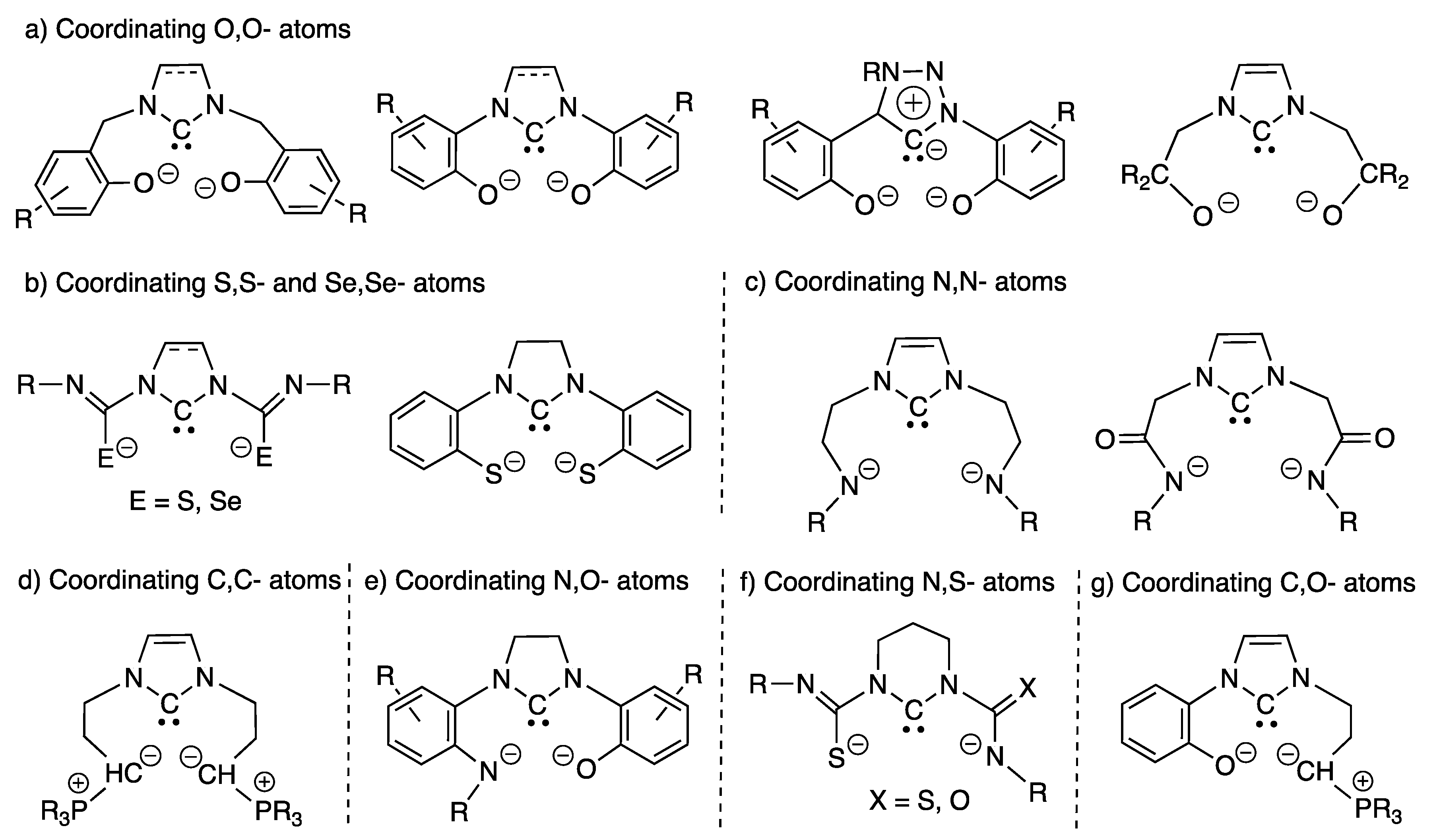
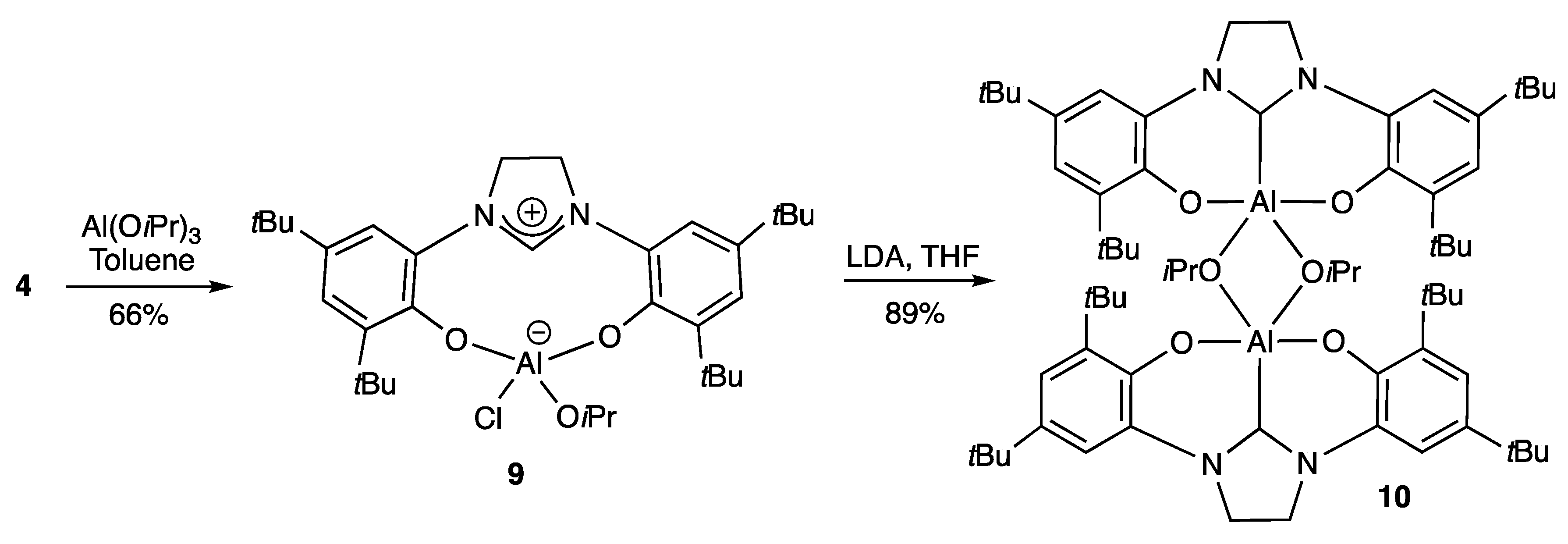
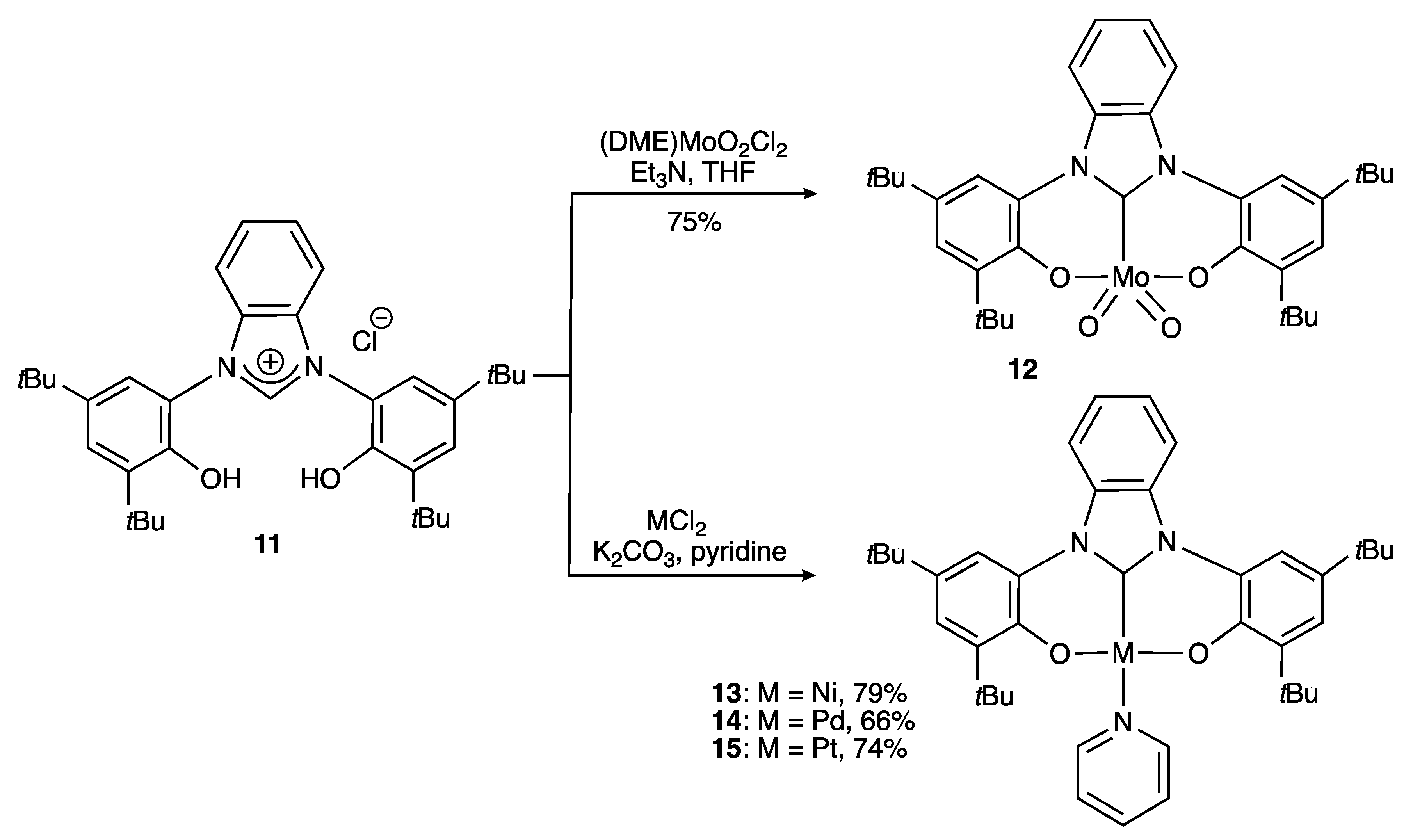
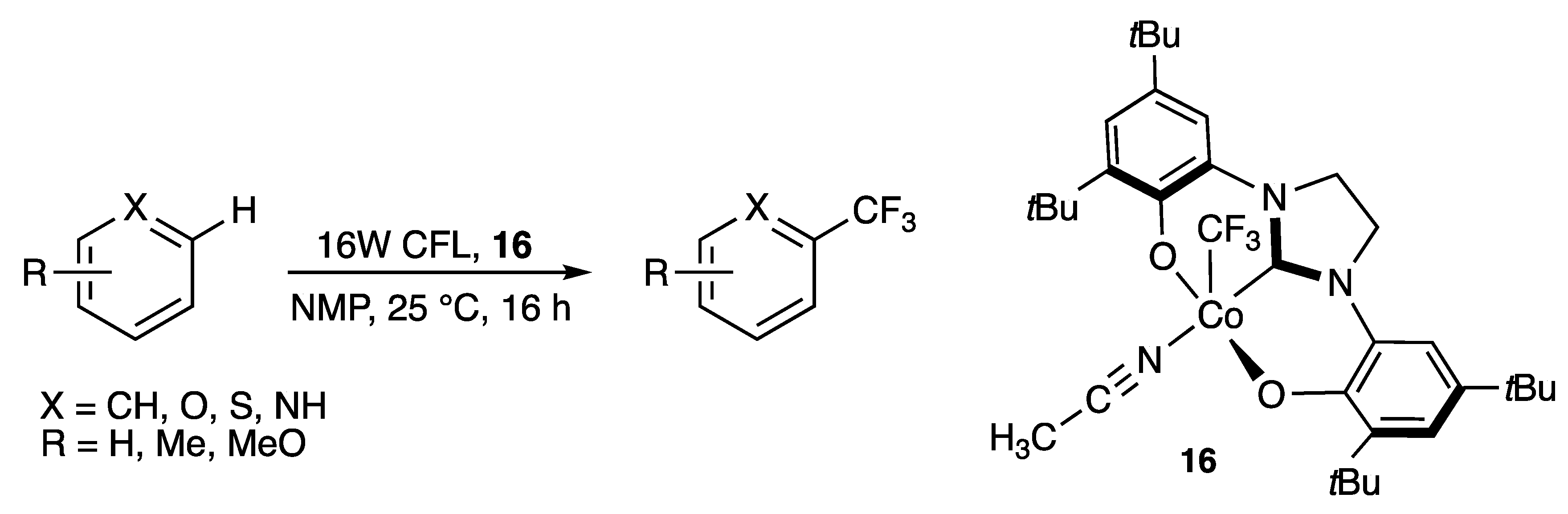
2.1.1. With Two Coordinating O- Atoms
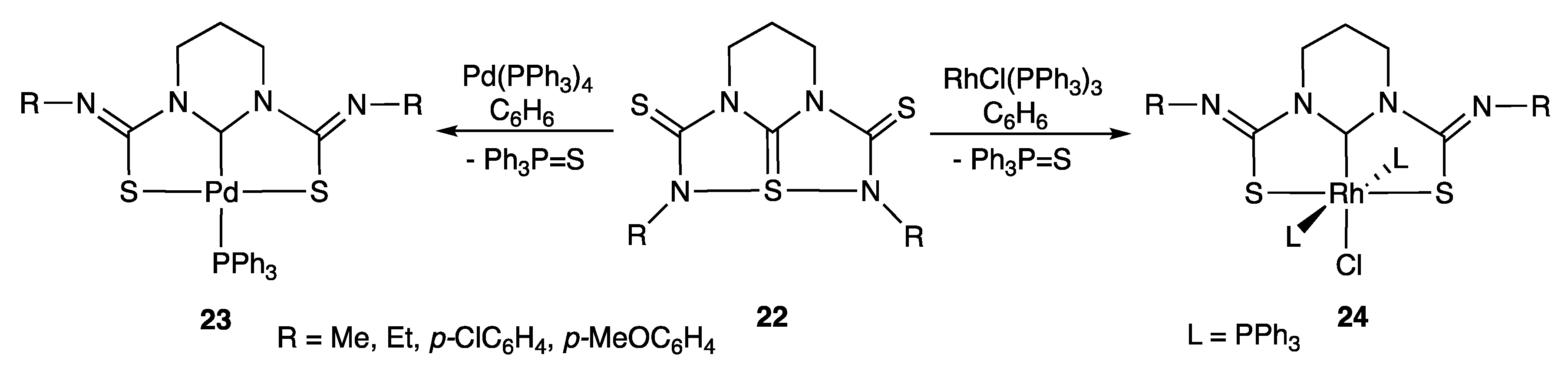
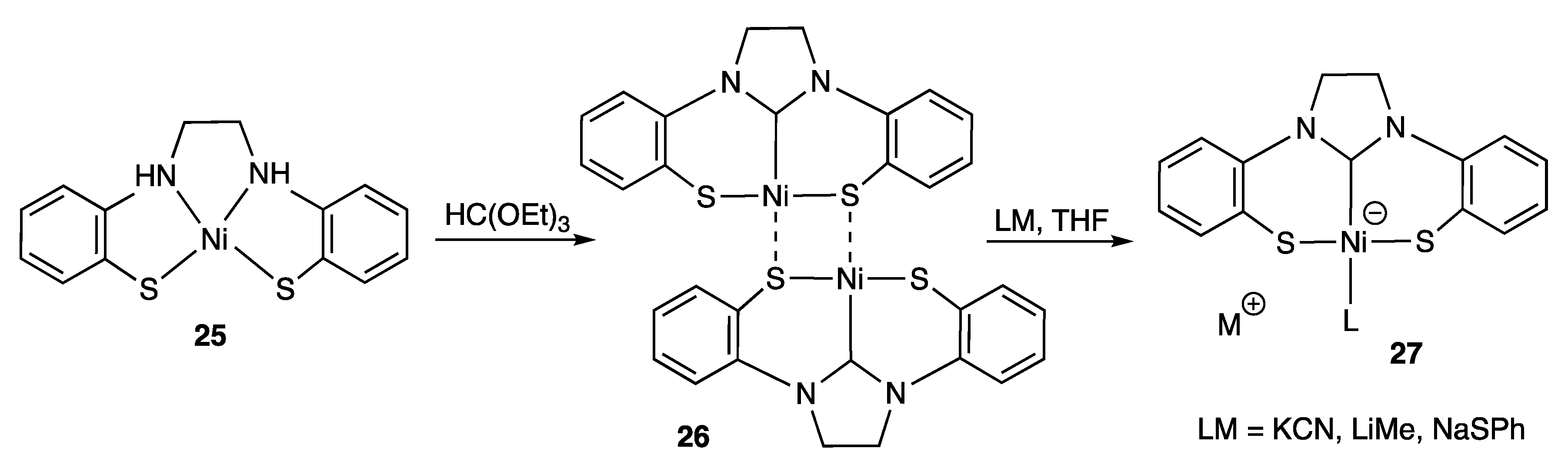
2.1.2. With Two Coordinating S- (or Se) Atoms
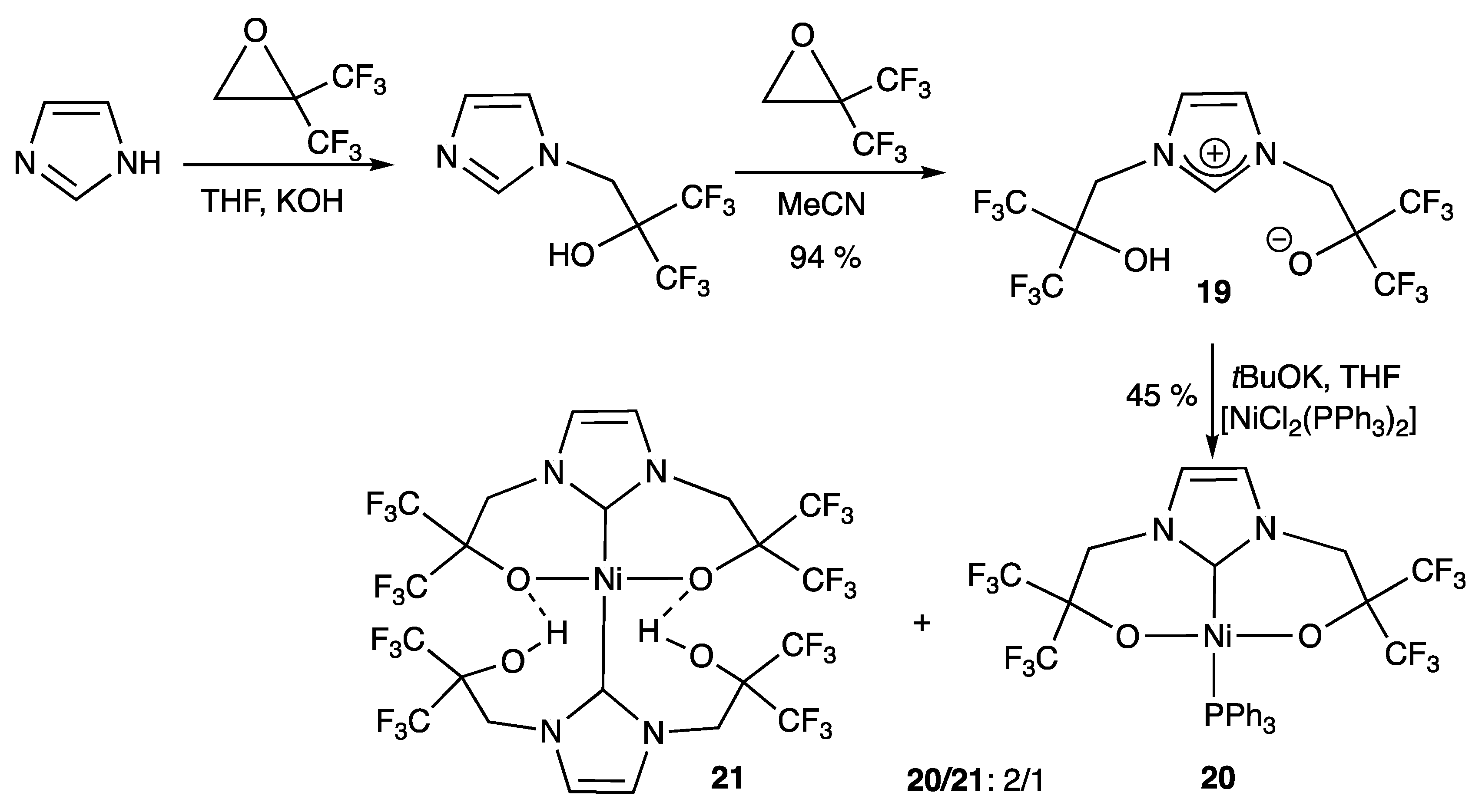
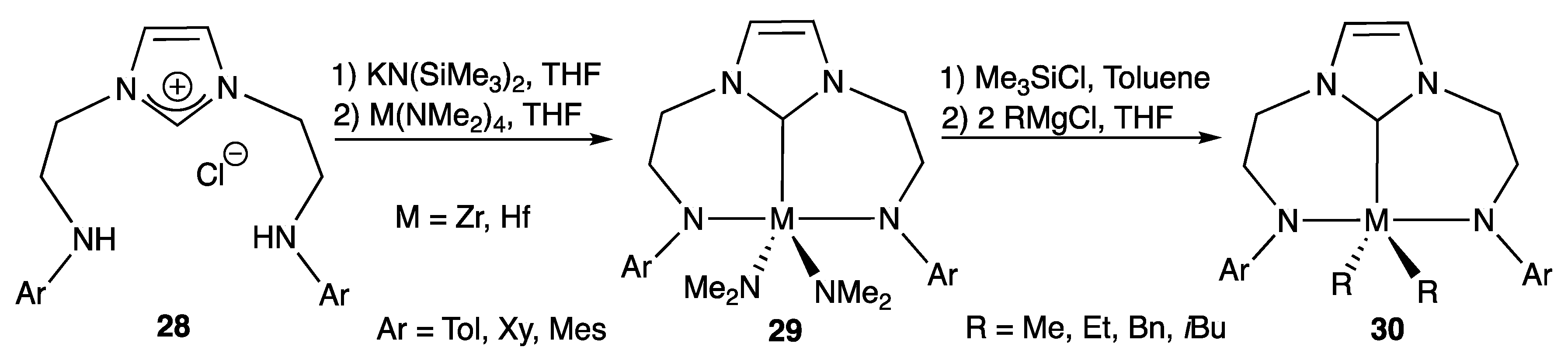
2.1.3. With Two Coordinating N- Atoms
2.1.4. With Two Coordinating C- Atoms
2.2. Based on Two Different Side Arms
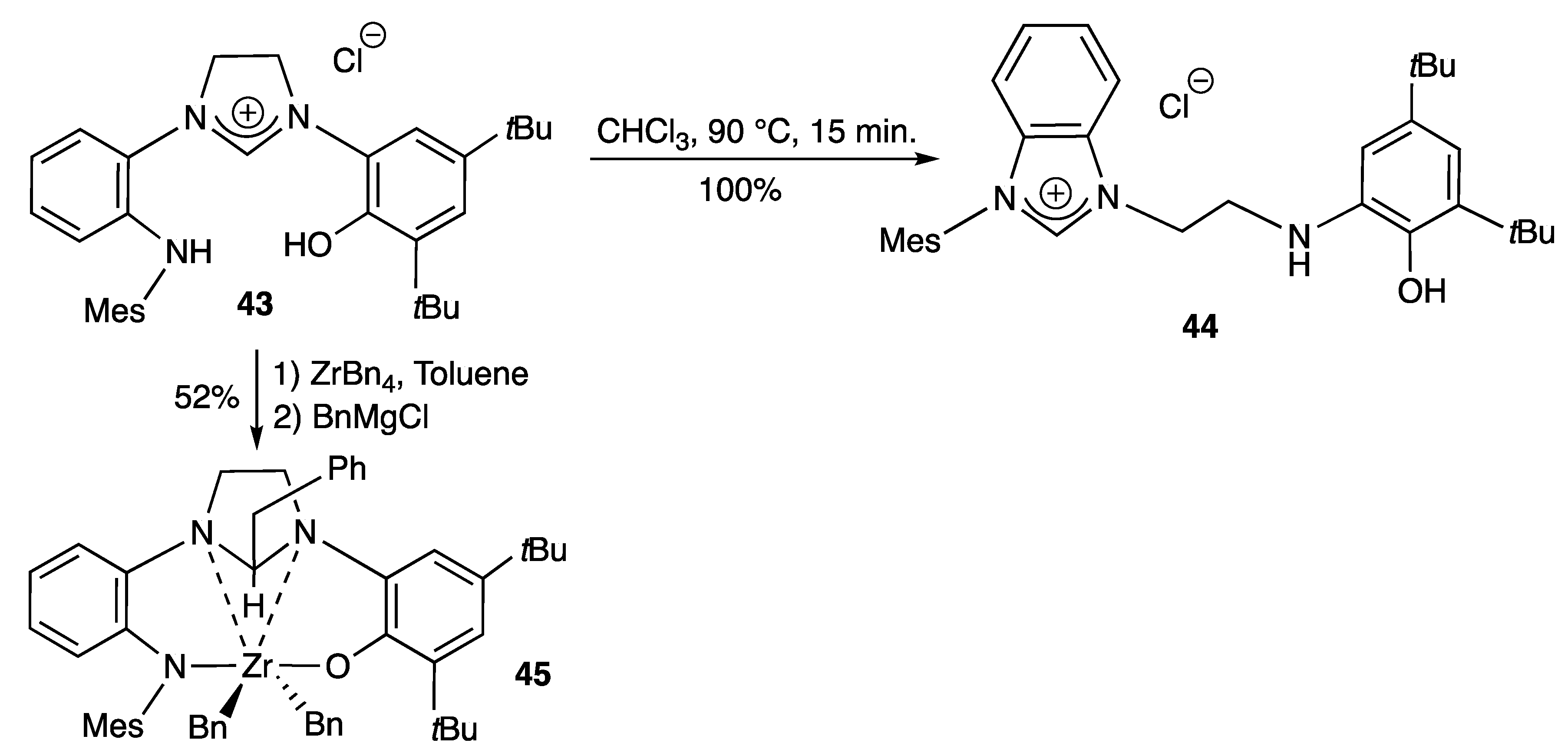
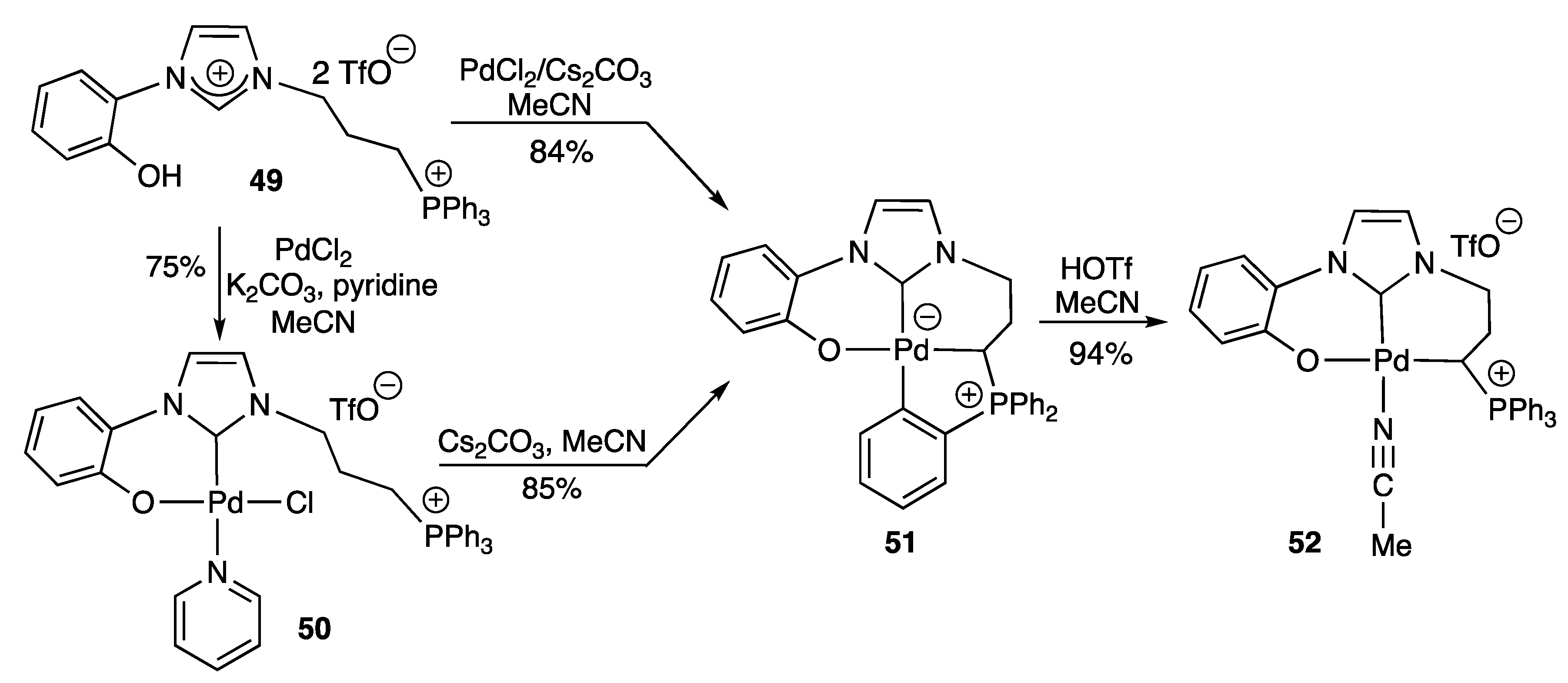
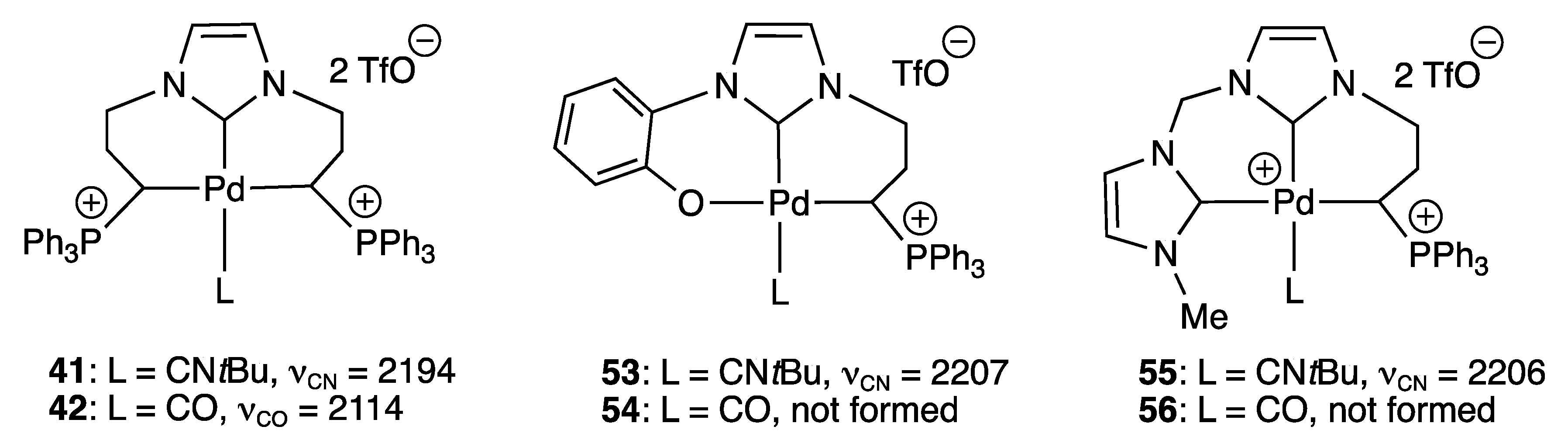
2.2.1. With N,O- Coordinating Atoms
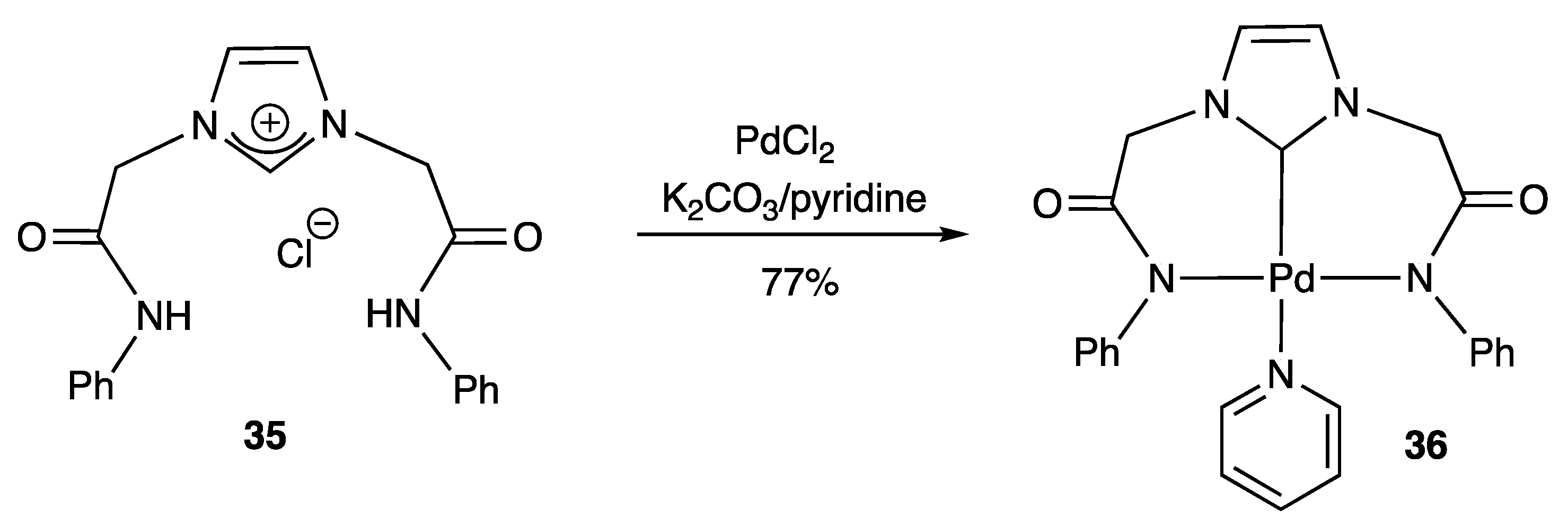
2.2.2. With N,S- Coordinating Atoms
2.2.3. With C,O- Coordinating Atoms
3. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Koten, G. Tuning the reactivity of metals held in a rigid ligand environment. Pure Appl. Chem. 1989, 61, 1681–1694. [Google Scholar] [CrossRef]
- Morales-Morales, D.; Jensen, C.G.M. The Chemistry of Pincer Compounds, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Van Koten, G.; Milstein, D. Organometallic Pincer Chemistry; Van Koten, G., Milstein, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 40, pp. 1–356. [Google Scholar]
- Adams, G.M.; Weller, A.S. POP-type ligands: Variable coordination and hemilabile behaviour. Coord. Chem. Rev. 2018, 355, 150–172. [Google Scholar] [CrossRef]
- Benito-Garagorri, D.; Kirchner, K. Modularly designed transition metal PNP and PCP pincer complexes based on aminophosphines: Synthesis and catalytic applications. Acc. Chem. Res. 2008, 41, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Roddick, D.M.; Zargarian, D. Pentacoordination for pincer and related tertendate coordination compounds: Revisiting structural properties and trends for d8 transition metal systems. Inorg. Chim. Acta 2014, 422, 251–264. [Google Scholar] [CrossRef]
- Szabó, K.J.; Wendt, O.F. (Eds.) Pincer and Pincer-Type Complexes: Applications in Organic Synthesis and Catalysis; Willey-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Gunanathan, G.; Milstein, D. Bond activation and catalysis by ruthenium pincer complexes. Chem. Rev. 2014, 114, 12024–12087. [Google Scholar] [CrossRef]
- Maser, L.; Vondung, L.; Langer, R. The ABC in pincer chemistry–from amine to borylene and carbon-based pincer ligands. Polyhedron 2018, 143, 28–42. [Google Scholar] [CrossRef]
- Moulton, C.J.; Shaw, B.L. Transition metal-carbon bonds. Part XLII. Complexes of nickel, palladium, platinum, rhodium and iridium with the tridentate ligand 2,6-bis[(di-t-butylphosphino)methyl]phenyl. J. Chem. Soc. Dalton Trans. 1976, 11, 1020–1024. [Google Scholar] [CrossRef]
- Van Koten, G.; Timmer, K.; Noltes, J.G.; Spek, A.L. A novel type of Pt–C interaction and a model for the final stage in reductive elimination processes involving C–C coupling at Pt; synthesis and molecular geometry of [1,N,N′-η-2,6-bis{(dimethylamino)methyl}toluene]iodoplatinum(II) tetrafluoroborate. J. Chem. Soc. Chem. Commun. 1978, 6, 250–252. [Google Scholar] [CrossRef]
- Parkin, G. The bioinorganic chemistry of zinc: Synthetic analogues of zinc enzymes that feature tripodal ligands. Chem. Commun. 2000, 20, 1971–1985. [Google Scholar] [CrossRef]
- Blackman, A.G. Tripodal tetraamine ligands containing three pyridine units: The other polypyridyl ligands. Eur. J. Inorg. Chem. 2008, 2008, 2633–2647. [Google Scholar] [CrossRef]
- Gamble, A.J.; Lynam, J.M.; Thatcher, R.J.; Walton, P.H.; Whitwood, A.C. Cis-1,3,5-triaminocyclohexane as a facially cappind ligand for ruthenium(II). Inorg. Chem. 2013, 52, 4517–4527. [Google Scholar] [CrossRef] [PubMed]
- Green, M.L.H. A new approach for the formal classification of covalents compounds of the elements. J. Organomet. Chem. 1995, 500, 127–148. [Google Scholar] [CrossRef]
- Pugh, D.; Danapoulos, A.A. Metal complexes with ‘pincer’-type ligands incorporating N-heterocyclic carbene functionalities. Coord. Chem. Rev. 2007, 251, 610–641. [Google Scholar] [CrossRef]
- Niu, J.L.; Hao, X.Q.; Gong, J.F.; Song, M.P. Symmetrical and unsymmetrical pincer complexes with group 10 metals: Synthesis via aryl C–H activation and some catalytic applications. Dalton Trans. 2011, 40, 5135–5150. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.E.; Veige, A.S. Trianionic pincer and pincer-type metal complexes and catalysts. Chem. Soc. Rev. 2014, 43, 6325–6369. [Google Scholar] [CrossRef]
- Deng, Q.H.; Melen, R.L.; Gade, L.H. Anionic chiral tridentate N-donor pincer ligands in asymmetric catalysis. Acc. Chem. Res. 2014, 47, 3162–3173. [Google Scholar] [CrossRef]
- Murugesan, S.; Kirchner, K. Non-precious metal complexes with an anionic PCP pincer architecture. Dalton Trans. 2016, 45, 416–439. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Key factors in pincer ligand design. Chem. Soc. Rev. 2018, 27, 1959–1968. [Google Scholar] [CrossRef]
- Liu, J.K.; Gong, J.F.; Song, M.P. Chiral palladium pincer complexes for asymmetric catalytic reactions. Org. Biomol. Chem. 2019, 17, 6069–6098. [Google Scholar] [CrossRef]
- Luconi, L.; Rossin, A.; Motta, A.; Tuci, G.; Giambastiani, G. Group IV organometallic compounds based on dianionic pincer ligands: Synthesis, characterization, and catalytic activity in intramolecular hydroamination reactions. Chem. Eur. J. 2013, 19, 4906–4921. [Google Scholar] [CrossRef]
- Agapie, T.; Day, M.W.; Bercaw, J.E. Synthesis and reactivity of tantalum complexes supported by bidentate X2 and tridentate LX2 ligand with two phenolates linked to pyridine, thiophene, furan, and benzene connectors: Mechanistic studies of the formation of a tantalum benzylidene and insertion chemistry for tantalum−carbon bonds. Organometallics 2008, 27, 6123–6142. [Google Scholar]
- Winston, M.S.; Bercaw, J.E. A novel bis(phosphido)pyridine [PNP]2− pincer ligand and its potassium and bis(dimethylamido)zirconium(IV) complexes. Organometallics 2010, 29, 6408–6416. [Google Scholar] [CrossRef]
- Lenton, T.N.; VanderVelde, D.G.; Bercaw, J.E. Synthesis of a bis(thiophenolate)pyridine ligand and its titanium, zirconium, and tantalum complexes. Organometallics 2012, 31, 7492–7499. [Google Scholar] [CrossRef]
- Komiyama, Y.; Kuwabara, J.; Kanbara, T. Deprotonation-induced structural changes in SNS-pincer ruthenium complexes with secondary thioamide groups. Organometallics 2014, 33, 885–891. [Google Scholar] [CrossRef]
- Suzuki, T.; Kajita, Y.; Masuda, H. Deprotonation/protonation-driven change of the σ-donor ability of a sulfur atom in iron(II) complexes with a thioamide SNS pincer type ligand. Dalton Trans. 2014, 43, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Smith, M.D.; Peryshkov, D.V. Sterically encumbered dianionic dicarboranyl pincer ligand (C5H3N)(C2B10H11)2 and its CNC nickel(II) complex. J. Organomet. Chem. 2018, 867, 208–2013. [Google Scholar] [CrossRef]
- Kubo, K.; Jones, N.D.; Ferguson, M.J.; McDonald, R.; Cavell, R.G. Chelate and pincer carbene complexes of rhodium and platinum derived from hexaphenylcarbodiphosphorane, Ph3=C=PPh3. J. Am. Chem. Soc. 2005, 127, 5314–5315. [Google Scholar] [CrossRef]
- Petz, W.; Neumüller, B. New platinum complexes with carbodiphosphorane as pincer ligand via ortho phenyl metallation. Polyhedron 2011, 30, 1779–1784. [Google Scholar] [CrossRef]
- Kubo, K.; Okitsu, H.; Miwa, H.; Kume, S.; Cavell, R.G. Carbon(0)-bridged Pt/Ag dinuclear and tetranuclear complexes based on a cyclometalated pincer carbodiphosphorane platform. Organometallics 2017, 36, 266–274. [Google Scholar] [CrossRef]
- Liddle, S.T.; Edworthy, I.S.; Arnold, P.L. Anionic tethered N-heterocyclic carbene chemistry. Chem. Soc. Rev. 2007, 36, 1732–1744. [Google Scholar] [CrossRef]
- Zhang, D.; Zi, G. N-heterocyclic carbene (NHC) complexes of group 4 transition metals. Chem. Soc. Rev. 2015, 44, 1898–1921. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Antisymbiosis and the trans effect. Inorg. Chem. 1973, 12, 712–713. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Arnold, P.L.; Liddle, S.T. F-block N-heterocyclic carbenes. Chem. Commun. 2006, 38, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Fliedel, C.; Schnee, G.; Avilés, T.; Dagorne, S. Group 13 metal (Al, Ga, In, Tl) complexes supported by heteroatom-bonded carbene ligands. Coord. Chem. Rev. 2014, 275, 63–86. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S.; Dagorne, S. Group 1 and 2 and early transition metal complexes bearing N-heterocyclic carbene ligands: Coordination chemistry, reactivity and applications. Chem. Rev. 2014, 114, 8747–8774. [Google Scholar] [CrossRef]
- Hameury, S.; de Frémont, P.; Braunstein, P. Metal complexes with oxygen-functionalized NHC ligands: Synthesis and applications. Chem. Soc. Rev. 2017, 46, 632–733. [Google Scholar] [CrossRef]
- Guérin, V.; Ménard, A.; Guernon, H.; Moutounet, O.; Legault, C.Y. From chelating to bridging ligands: N-sulfonyliminoimidazolium ylides as precursors to anionic N-heterocyclic carbene ligands. Organometallics 2019, 38, 409–416. [Google Scholar] [CrossRef]
- Bartoszewicz, A.; Marcos, R.; Sahoo, S.; Inge, A.K.; Zou, X.; Martin-Matute, B. A highly active bifunctional iridium complex with an alcohol/alkoxide-tethered N-heterocyclic carbene for alkylation of amines with alcohols. Chem. Eur. J. 2012, 18, 14510–14519. [Google Scholar] [CrossRef]
- Pape, F.; Teichert, J.F. Dealing at arm’s length: Catalysis with N-Heterocyclic carbene ligands bearing anionic tethers. Eur. J. Org. Chem. 2017, 2017, 4206–4229. [Google Scholar] [CrossRef]
- Evans, K.J.; Campbell, C.L.; Haddow, M.F.; Luz, C.; Morton, P.A.; Mansell, S.M. Lithium complexes with bridging and terminal NHC ligands: The decisive influence of an anionic tether. Eur. J. Inorg. Chem. 2019, 2019, 4894–4901. [Google Scholar] [CrossRef]
- Aihara, H.; Matsuo, T.; Kawaguchi, H. Titanium N-heterocyclic carbene complexes incorporating an imidazolium-linked bis(phenol). Chem. Commun. 2003, 17, 2204–2205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Aihara, H.; Watanabe, T.; Matsuo, T.; Kawaguchi, H. Zirconium complexes of the tridentate bis(aryloxide)-N-heterocyclic-carbene ligand: Chloride and alkyl functionalized derivatives. J. Organomet. Chem. 2007, 692, 234–242. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, N. Titanium complexes bearing bisaryloxy-N-heterocyclic carbenes: Synthesis, reactivity, and ethylene polymerization study. Organometallics 2009, 28, 499–505. [Google Scholar] [CrossRef]
- Zhang, D. Dinuclear titanium(IV) complexes bearing phenoxide-tethered N-heterocyclic carbene ligands with cisoid conformation through control of hydrolysis. Eur. J. Inorg. Chem. 2007, 2007, 4839–4845. [Google Scholar] [CrossRef]
- Zhang, D.; GengShi, G.; Wang, J.; Yue, Q.; Zheng, W.; Weng, L. Macrocyclic hexanuclear zirconium(IV) complex bearing a bisaryloxyl N-heterocyclic-carbene ligand: Synthesis, structure, and catalytic properties. Inorg. Chem. Commun. 2010, 13, 433–435. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Ni, X.; Shen, Z. Bis(phenolate) N-heterocyclic carbene rare earth metal complexes: Synthesis, characterization and applications in the polymerization of n-hexyl isocyanate. RSC Adv. 2015, 5, 83295–83303. [Google Scholar] [CrossRef]
- Romain, C.; Brelot, L.; Bellemin-Laponnaz, S.; Dagorne, S. Synthesis and structural characterization of a novel family of titanium complexes bearing a tridentate bis-phenolate-N-heterocyclic carbene dianionic ligand and their use in the controlled ROP of rac-lactide. Organometallics 2010, 29, 1191–1198. [Google Scholar] [CrossRef]
- Dagorne, S.; Bellemin-Laponnaz, S.; Romain, C. Neutral and cationic N-heterocyclic carbene zirconium and hafnium benzyl complexes: Highly regioselective oligomerization of 1-hexene with a preference for trimer formation. Organometallics 2013, 32, 2736–2743. [Google Scholar] [CrossRef]
- Romain, C.; Choua, S.; Collin, J.P.; Heinrich, M.; Bailly, C.; Karmazin-Brelot, L.; Bellemin-Laponnaz, S.; Dagorne, S. Redox and luminescent properties of robust and air-stable N-heterocyclic carbene group 4 metal complexes. Inorg. Chem. 2014, 53, 7371–7376. [Google Scholar] [CrossRef]
- Quadri, C.C.; Le Roux, E. Copolymerization of cyclohexene oxide with CO2 catalyzed by tridentate N-heterocyclic carbene titanium(IV) complexes. Dalton Trans. 2014, 43, 4242–4246. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Miqueu, K.; Sotiropoulos, J.M.; Bellemin-Laponnaz, S.; Dagorne, S. Non-innocent behavior of a tridentate NHC chelating ligand coordinated onto a zirconium(IV) center. Angew. Chem. Int. Ed. 2010, 49, 2198–2201. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Specklin, D.; Miqueu, K.; Sotiropoulos, J.M.; Fliedel, C.; Bellemin-Laponnaz, S.; Dagorne, S. Unusual benzyl migration reactivity in NHC-bearing group 4 metal chelates: Synthesis, characterization, and mechanistic investigations. Organometallics 2015, 34, 4854–4863. [Google Scholar] [CrossRef]
- Despagnet-Ayoub, E.; Takase, M.K.; Labinger, J.A.; Bercaw, J.E. Reversible 1,2-alkyl migration to carbene and ammonia activation in an N-heterocyclic carbene−zirconium complex. J. Am. Chem. Soc. 2015, 137, 10500–10503. [Google Scholar] [CrossRef] [PubMed]
- Bellemin-Laponnaz, S.; Welter, R.; Brelot, L.; Dagorne, S. Synthesis and structure of V(V) and Mn(III) NHC complexes supported by a tridentate bis-aryloxide-N-heterocyclic carbene ligand. J. Organomet. Chem. 2009, 694, 604–606. [Google Scholar] [CrossRef]
- Baltrun, M.; Watt, F.A.; Schoch, R.; Hohloch, S. Dioxo-, oxo-imido-, and bis-imido-molybdenum(VI) complexes with a bis-phenolate-NHC ligand. Organometallics 2019, 38, 3719–3729. [Google Scholar] [CrossRef]
- Weinberg, D.R.; Hazari, N.; Labinger, J.A.; Bercaw, J.E. Iridium(I) and iridium(III) complexes supported by a diphenolate imidazolyl-carbene ligand. Organometallics 2010, 29, 89–100. [Google Scholar] [CrossRef]
- Harris, C.F.; Bayless, M.B.; van Leest, N.P.; Bruch, Q.J.; Livesay, B.N.; Basca, J.; Hardcastle, K.I.; Shores, M.P.; de Bruin, B.; Soper, J.D. Redox-active bis(phenolate) N-heterocyclic carbene [OCO] pincer ligands support cobalt electron transfer series spannning four oxidation states. Inorg. Chem. 2017, 56, 12421–12435. [Google Scholar] [CrossRef]
- Harris, C.F.; Kuehner, C.S.; Basca, J.; Soper, J.D. Photoinduced cobalt(III)–trifluoromethyl bond activation enables arene C−H trifluoromethylation. Angew. Chem. Int. Ed. 2018, 57, 1311–1315. [Google Scholar] [CrossRef]
- Borré, E.; Dahm, G.; Aliprandi, A.; Mauro, M.; Dagorne, S.; Bellemin-Laponnaz, S. Tridentate complexes of group 10 bearing bis-aryloxide N-heterocyclic carbene ligands: Synthesis, structural, spectroscopic, and computational characterization. Organometallics 2014, 33, 4374–4384. [Google Scholar] [CrossRef]
- Romain, C.; Fliedel, C.; Bellemin-Laponnaz, S.; Dagorne, S. NHC bis-phenolate aluminium chelates: Synthesis, structure, and use in lactide and trimethylene carbonate polymerization. Organometallics 2014, 33, 5370–5379. [Google Scholar] [CrossRef]
- Baltrun, M.; Watt, F.A.; Schoch, R.; Wölper, C.; Neuba, A.G.; Hohloch, S. A new bis-phenolate mesoionic carbene ligand for early transition metal chemistry. Dalton Trans. 2019, 48, 14611–14625. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Rodden, M.; Davis, K.M.; Scarisbrick, A.C.; Blake, A.J.; Wilson, C. Asymmetric lithium(I) and copper(II) alkoxy-N-heterocyclic carbene complexes; crystallographic characterization and Lewis acid catalysis. Chem. Commun. 2004, 14, 1612–1613. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Wilson, C. Sterically demanding bi- and tridentate alkoxy-N-heterocyclic carbenes. Inorg. Chim. Acta 2007, 360, 190–196. [Google Scholar] [CrossRef]
- Arnold, P.L.; Casely, I.J.; Turner, Z.R.; Carmichael, C.D. Functionalized saturated-backbone carbene ligands: Yttrium and uranyl alkoxy-carbene complexes and bicyclic carbene-alcohol adducts. Chem. Eur. J. 2008, 14, 10415–10422. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Dolpin, J.S.; Gurau, G.; Marshall, W.J.; Nelson, J.C.; Petrov, V.A.; Runyon, J.W. Synthesis and complexes of fluoroalkoxy carbenes. Angew. Chem. Int. Ed. 2013, 52, 5110–5114. [Google Scholar] [CrossRef]
- Matsumura, N.; Kawano, J.I.; Fukunishi, N.; Inoue, H. Synthesis of new transition metal carbene complexes from π-sulfurane compounds: Reaction of 10-S-3 tetraazapentalene derivatives with Pd(PPh3)4 and RhCl(PPh3)3. J. Am. Chem. Soc. 1995, 117, 3623–3624. [Google Scholar] [CrossRef]
- Iwasaki, F.; Manabe, N.; Nishiyama, H.; Takada, K.; Yasui, M.; Kusamiya, M.; Matsumura, N. Crystal and molecular structures of hypervalent thia/selena pentalenes. Bull. Chem. Soc. Jpn. 1997, 70, 1267–1275. [Google Scholar] [CrossRef]
- Iwasaki, F.; Nishiyama, H.; Yasui, M.; Kusamiya, M.; Matsumura, N. Structures and formation mechanism of novel metal-carbene complexes derived from hypervalent thiadiselenadiazapentalenes. Bull. Chem. Soc. Jpn. 1997, 70, 1277–1287. [Google Scholar] [CrossRef]
- Sellmann, D.; Allmann, C.; Heinemann, F.; Knoch, F.; Sutter, J. Ubergangsmetallkomplexe mit schwefelliganden CXXIV. J. Organomet. Chem. 1997, 541, 291–305. [Google Scholar] [CrossRef]
- Spencer, L.P.; Winston, S.; Fryzuk, M.D. Tridentate amido carbene ligands in early-transition-metal coordination chemistry. Organometallics 2004, 23, 3372–3374. [Google Scholar] [CrossRef]
- Spencer, L.P.; Fryzuk, M.D. Synthesis and reactivity of zirconium and hafnium complexes incorporating chelating diamido-N-heterocyclic-carbene ligands. J. Organomet. Chem. 2005, 690, 5788–5803. [Google Scholar] [CrossRef]
- Spencer, L.P.; Beddie, C.; Hall, M.B.; Fryzuk, M.D. Synthesis, reactivity, and DFT studies of tantalum complexes incorporating diamido-N-heterocyclic carbene ligands. Facile endocyclic C–H bond activation. J. Am. Chem. Soc. 2006, 128, 12531–12543. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Chan, K.T.; Zeng, J.Y.; Hu, C.H.; Tu, C.Y.; Lee, H.M. Nonchelate and chelate complexes of palladium(II) with N-heterocyclic carbene ligands of amido functionality. Organometallics 2007, 26, 1692–1702. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminoly, 2nd ed.; (The Gold Book) Online Corrected Version 2006 η (eta or hapto) in Inorganic Nomenclature; McNaught, A.D., Wilkinson, A., Eds.; RSC: Cambridge, UK, 1997. [Google Scholar]
- Schmidbaur, H. Phosphorus ylides in the coordination sphere of transition metals: An inventory. Angew. Chem. Int. Ed. 1983, 22, 907–927. [Google Scholar] [CrossRef]
- Chauvin, R.; Canac, Y. Late Transition Metals of Neutral η1-Carbon Ligands; Springer: Berlin/Heidelberg, Germany, 2010; Volume 30, pp. 1–252. [Google Scholar]
- Abdalilah, M.; Canac, Y.; Lepetit, C.; Chauvin, R. Towards the stability limit of cyclic diphosphonium bis-ylides. C. R. Chimie 2010, 3, 1091–1098. [Google Scholar] [CrossRef]
- Canac, Y.; Duhayon, C.; Chauvin, R. A diaminocarbene-phosphonium ylide: Direct access to C,C-chelating ligands. Angew. Chem. Int. Ed. 2007, 46, 6313–6315. [Google Scholar] [CrossRef]
- Abdellah, I.; Debono, N.; Canac, Y.; Duhayon, C.; Chauvin, R. Atropochiral (C,C)-chelating NHC-ylide ligands: Synthesis and resolution of palladium(II) complexes thereof. Dalton Trans. 2009, 35, 7196–7202. [Google Scholar] [CrossRef]
- Canac, Y.; Chauvin, R. Atropochiral C,X- and C,C-chelating carbon ligands. Eur. J. Inorg. Chem. 2010, 16, 2325–2335. [Google Scholar] [CrossRef]
- Maaliki, C.; Abdalilah, M.; Barthes, C.; Duhayon, C.; Canac, Y.; Chauvin, R. Bis-ylide ligands from acyclic proximal diphosphonium precursors. Eur. J. Inorg. Chem. 2012, 2012, 4057–4064. [Google Scholar] [CrossRef]
- Benaissa, I.; Taakili, R.; Lugan, N.; Canac, Y. A convenient access to N-phosphonio-substituted NHC metal complexes [M = Ag(I), Rh(I), Pd(II)]. Dalton Trans. 2017, 46, 12293–12305. [Google Scholar] [CrossRef] [PubMed]
- Barthes, C.; Bijani, C.; Lugan, N.; Canac, Y. A palladium(II) complex of a C4 chelating bis(NHC) diphosphonium bis(ylide) ligand. Organometallics 2018, 37, 673–678. [Google Scholar] [CrossRef]
- Taakili, R.; Lepetit, C.; Duhayon, C.; Valyaev, D.A.; Lugan, N.; Canac, Y. Palladium(II) pincer complexes of a C,C,C-NHC, diphosphonium bis(ylide) ligand. Dalton Trans. 2019, 48, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- Canac, Y. Carbeniophosphines versus phosphoniocarbenes: The role of the positive charge. Chem. Asian. J. 2018, 13, 1872–1887. [Google Scholar] [CrossRef]
- Despagnet-Ayoub, E.; Miqueu, K.; Sotiropoulos, J.M.; Henling, L.M.; Day, M.W.; Labinger, J.A.; Bercaw, J.E. Unexpected rearrangements in the synthesis of an unsymmetrical tridentate dianionic N-heterocyclic carbene. Chem. Sci. 2013, 4, 2117–2121. [Google Scholar] [CrossRef]
- Despagnet-Ayoub, E.; Henling, L.M.; Labinger, J.A.; Bercaw, J.E. Group 4 transition-metal complexes of an aniline-carbene-phenol ligand. Organometallics 2013, 32, 2934–2938. [Google Scholar] [CrossRef]
- Manabe, N.; Yasui, M.; Nishiyama, H.; Shimamoto, S.; Matsumura, N.; Iwasaki, F. Crystal and molecular structures of novel metal–carbene complexes IV. Effect of carbonyl groups and formation mechanism. Bull. Chem. Soc. Jpn. 1996, 69, 2771–2780. [Google Scholar] [CrossRef]
- Taakili, R.; Barthes, C.; Goëffon, A.; Lepetit, C.; Duhayon, C.; Valyaev, D.A.; Canac, Y. NHC Core Phosphonium Ylide-based Palladium(II) Pincer Complexes: The Second Ylide Extremity Makes the Difference. Inorg. Chem. 2020. [Google Scholar] [CrossRef]
- Canac, Y.; Lepetit, C.; Abdalilah, M.; Duhayon, C.; Chauvin, R. Diaminocarbene and phosphonium ylide ligands: A systematic comparison of their donor character. J. Am. Chem. Soc. 2008, 130, 8406–8413. [Google Scholar] [CrossRef]
- Canac, Y.; Lepetit, C. Classification of the electronic properties of chelating ligands in cis-[LL′Rh(CO)2] complexes. Inorg. Chem. 2017, 56, 667–675. [Google Scholar] [CrossRef]
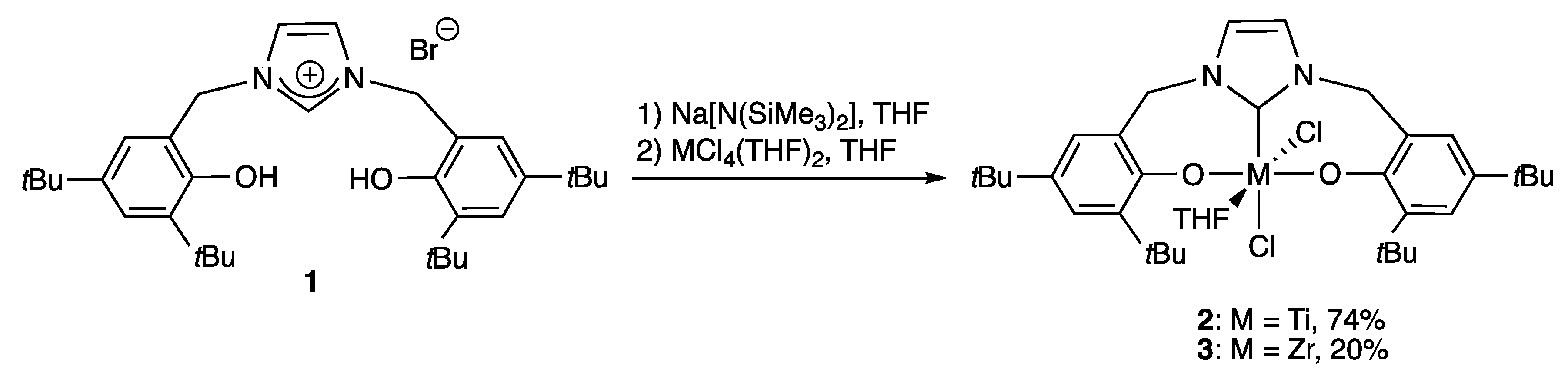





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taakili, R.; Canac, Y. NHC Core Pincer Ligands Exhibiting Two Anionic Coordinating Extremities. Molecules 2020, 25, 2231. https://doi.org/10.3390/molecules25092231
Taakili R, Canac Y. NHC Core Pincer Ligands Exhibiting Two Anionic Coordinating Extremities. Molecules. 2020; 25(9):2231. https://doi.org/10.3390/molecules25092231
Chicago/Turabian StyleTaakili, Rachid, and Yves Canac. 2020. "NHC Core Pincer Ligands Exhibiting Two Anionic Coordinating Extremities" Molecules 25, no. 9: 2231. https://doi.org/10.3390/molecules25092231
APA StyleTaakili, R., & Canac, Y. (2020). NHC Core Pincer Ligands Exhibiting Two Anionic Coordinating Extremities. Molecules, 25(9), 2231. https://doi.org/10.3390/molecules25092231






