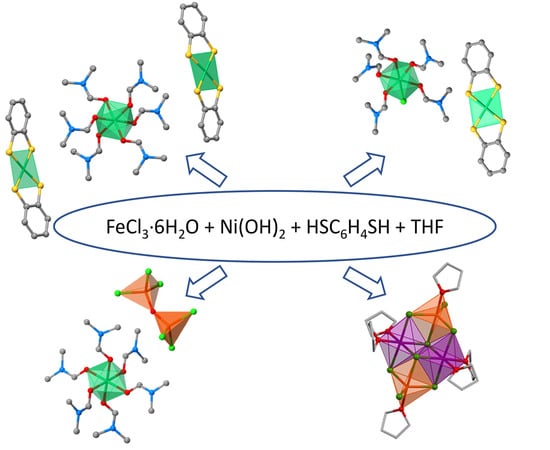
Structural Study of the Compounds Formed in the Reactions of FeCl3·6H2O with Ni(OH)2 in the Presence of Dithiolenes HSRSH (R = C6H2Cl2 or C6H4)
Abstract
1. Introduction
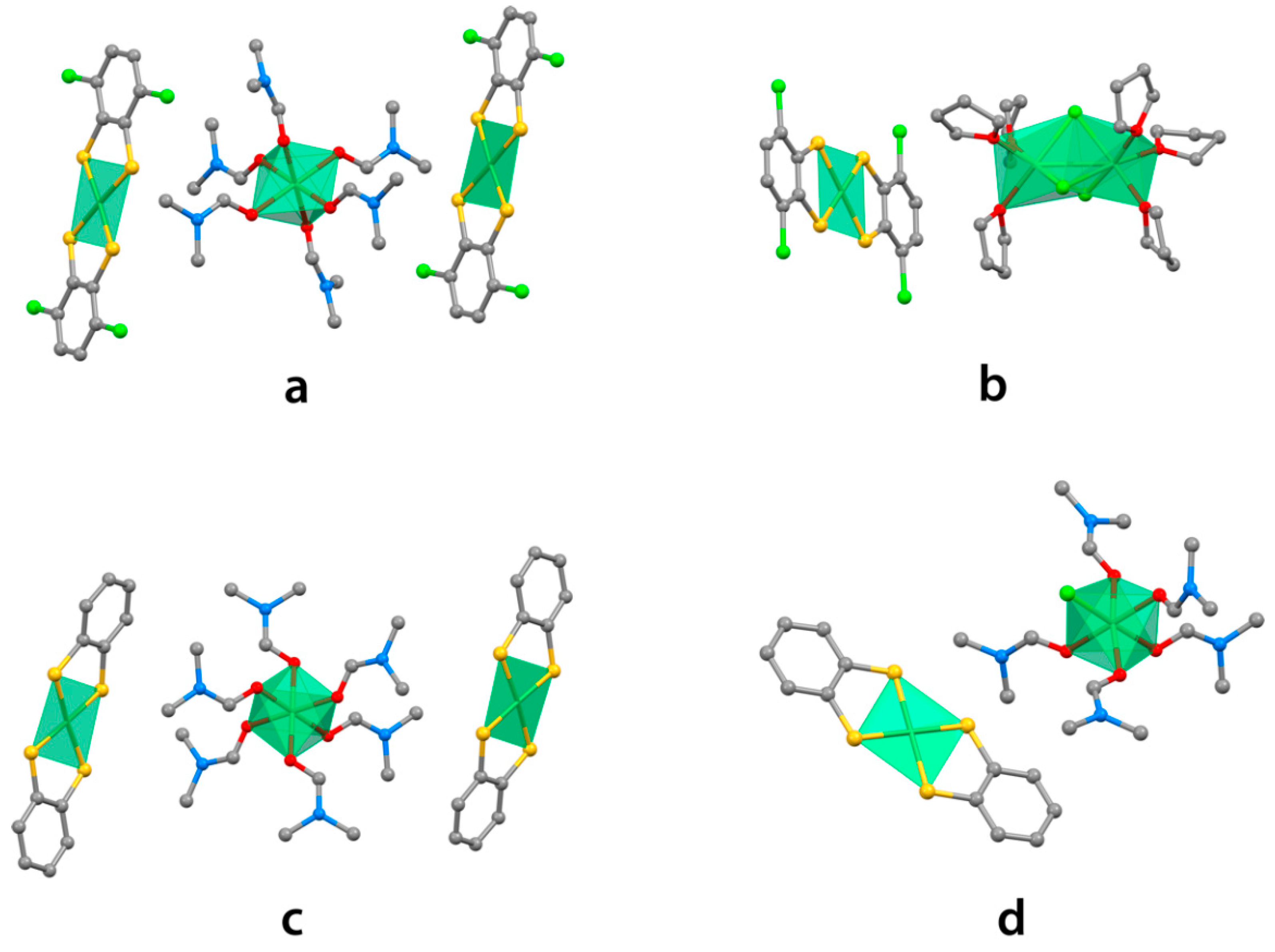
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.1.1. Reaction of FeCl3·6H2O and Ni(OH)2 in the presence of HSC6H2Cl2SH
3.1.2. Reaction of FeCl3·6H2O and Ni(OH)2 in the presence of HSC6H4SH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robertson:, N.; Cronin, L. Metal bis-1,2-dithiolene complexes in conducting or magnetic crystalline assemblies. Coord. Chem. Rev. 2002, 227, 93–127. [Google Scholar] [CrossRef]
- Rauchfuss, T.B. Dithiolene Chemistry: Synthesis, Properties and applications. In Progress in Inorganic Chemistry; John Wiley & Sons: New York, NY, USA, 2004; Volume 52, pp. 1–54. [Google Scholar]
- Müller-Westerhoff, U.T.; Vance, B. Dithiolenes and Related Species. In Comprehensive Coordination Chemistry; Pergamon Press: Oxford, UK, 1987; Volume 2, pp. 595–631. [Google Scholar]
- Clemenson, P.I. The Chemistry and Solid State Properties of Nickel, Palladium and Platinum Bis(Maleonitriledithiolate) Compounds. Coord. Chem. Rev. 1990, 106, 171–203. [Google Scholar] [CrossRef]
- Ezzaher, S.; Gogoll, A.; Bruhn, C.; Ott, S. Directing protonation in [FeFe] hydrogenase active site models by modifications in their second coordination sphere. Chem. Commun. 2010, 46, 5775–5777. [Google Scholar] [CrossRef] [PubMed]
- Alcácer, L.; Novais, H. Linear Chain 1,2-Dithiolene Complexes. In Extended Linear Chain Compounds; Miller, J.S., Ed.; Springer: New York, NY, USA, 1983; Volume 6, pp. 319–351. [Google Scholar]
- Cassoux, P.; Valade, L.; Kobayashi, H.; Kobayashi, A.; Clark, R.A.; Underhill, A.E. Molecular metals and superconductors derived from metal complexes of 1,3-dithiol-2-thione-4,5-dithiolate (dmit). Coord. Chem. Rev. 1991, 110, 115–160. [Google Scholar] [CrossRef]
- Sproules, S.; Wieghardt, K. o-Dithiolene and o-aminothiolate chemistry of iron: Synthesis, structure and reactivity. Coord. Chem. Rev. 2010, 254, 1358–1382. [Google Scholar] [CrossRef]
- Bonneval, B.G.D.; Ching, K.I.M.-C.; Alary, F.; Bui, T.-T.; Valade, L. Neutral d8 metal bis-dithiolene complexes: Synthesis, electronic properties and applications. Coord. Chem. Rev. 2010, 254, 1457–1467. [Google Scholar] [CrossRef]
- Alvarez, S.; Vicente, R.; Hoffmann, R. Dimerization and stacking in transition-metal bisdithiolenes and tetrathiolates. J. Am. Chem. Soc. 1985, 107, 6253–6277. [Google Scholar] [CrossRef]
- Takaishi, S.; Hosoda, M.; Kajiwara, T.; Miyasaka, H.; Yamashita, M.; Nakanishi, Y.; Kitagawa, Y.; Yamaguchi, K.; Kobayashi, A.; Kitagawa, H. Electroconductive Porous Coordination Polymer Cu[Cu(pdt)2] Composed of Donor and Acceptor Building Units. Inorg. Chem. 2009, 48, 9048–9050. [Google Scholar] [CrossRef]
- Ribas, X.; Dias, J.C.; Morgado, J.; Wusrt, K.; Santos, I.C.; Almeida, M.; Vidal-Gancedo, J.; Veciana, J.; Rovira, C. Alkaline Side-Coordination Strategy for the Design of Nickel(II) and Nickel(III) Bis(1,2-diselenolene) Complex Based Materials. Inorg. Chem. 2004, 43, 3631–3641. [Google Scholar] [CrossRef]
- Llusar, R.; Uriel, S.; Vicent, C.; Clemente-Juan, J.; Coronado, E.; Gómez-García, C.J.; Braïda, B.; Canadell, E. Single-Component Magnetic Conductors Based on Mo3S7 Trinuclear Clusters with Outer Dithiolate Ligands. J. Am. Chem. Soc. 2004, 126, 12076–12083. [Google Scholar] [CrossRef]
- Llusar, R.; Triguero, S.; Polo, V.; Vicent, C.; Gómez-García, C.J.; Jeannin, O.; Fourmigué, M. Trinuclear Mo3S7 Clusters Coordinated to Dithiolate or Diselenolate Ligands and Their Use in the Preparation of Magnetic Single Component Molecular Conductors. Inorg. Chem. 2008, 47, 9400–9409. [Google Scholar] [CrossRef] [PubMed]
- Gushchin, A.L.; Llusar, R.; Vicent, C.; Abramov, P.A.; Gómez-Garcia, C.J. Mo3Q7 (Q = S, Se) Clusters Containing Dithiolate/Diselenolate Ligands: Synthesis, Structures, and Their Use as Precursors of Magnetic Single-Component Molecular Conductors. Eur. J. Inorg. Chem. 2013, 2013, 2615–2622. [Google Scholar] [CrossRef]
- Delgado, E.; Gómez-García, C.J.; Hernández, D.; Hernández, E.; Martín, A.; Zamora, F. Unprecedented layered coordination polymers of dithiolene group 10 metals: Magnetic and electrical properties. Dalton Trans. 2016, 45, 6696–6701. [Google Scholar] [CrossRef] [PubMed]
- Belo, D.; Almeida, M. Transition metal complexes based on thiophene-dithiolene ligands. Coord. Chem. Rev. 2010, 254, 1479–1492. [Google Scholar] [CrossRef]
- Benmansour, S.; Delgado, E.; Gómez-García, C.J.; Hernández, D.; Hernández, E.; Martín, A.; Perles, J.; Zamora, F. Coordination Polymers Based on Diiron Tetrakis(dithiolato) Bridged by Alkali Metals, Electrical Bistability around Room Temperature, and Strong Antiferromagnetic Coupling. Inorg. Chem. 2015, 54, 2243–2252. [Google Scholar] [CrossRef]
- Castillo, O.; Delgado, E.; Hernández, D.; Hernández, E.; Martín, A.; Martín, I.; Zamora, F. Structural Diversity of Compounds Based on Iron-Dithiolene with Sodium or Potassium Complexes. Cryst. Growth Des. 2016, 16, 5466–5478. [Google Scholar] [CrossRef]
- Delgado, E.; Hernández, D.; Hernández, E.; Martín, A.; Zamora, F. Synthesis and Structural Characterization of Transition Metal Dithiolene Derivatives Containing Divalent Metals as Counter-Cations. Cryst. Eng. Commun. 2019, 21, 1423–1432. [Google Scholar]
- Martinez-Martin, P.; Perles, J.; Rodriguez-Ubis, J.C. Structure Dependence of the Energy Transfer from Tb(III) to Yb(III) in Metal–Organic Frameworks Based in Bispyrazolylpyridines. Crystals 2020, 10, 69. [Google Scholar] [CrossRef]
- Madhu, V.; Das, S.K. New Series of Asymmetrically Substituted Bis(1,2-dithiolato)-Nickel(III) Complexes Exhibiting Near IR Absorption and Structural Diversity. Inorg. Chem. 2008, 47, 5055–5070. [Google Scholar] [CrossRef]
- Keefer, C.E.; Purrington, S.T.; Bereman, R.D.; Boyle, P.D. The First Systematic Synthesis of Heterobimetallic Dithiolene-Bridged Complexes. Synthesis and Characterization of Metal Complexes of 4-(1’,2’-Ethylenedithiolate)-1,3-dithiole-2-one and Dimeric Metal Complexes of 1,2,3,4-Butadienetetrathiolate. Inorg. Chem. 1999, 38, 5437–5442. [Google Scholar] [CrossRef]
- Qu, L.; Guo, Y.; Luo, H.; Zhong, C.; Yu, G.; Liu, Y.; Qin, J. A simple nickel bis(dithiolene) complex as an excellent n-type molecular semiconductor for field-effect transistors. Chem. Commun. 2012, 48, 9965–9967. [Google Scholar] [CrossRef] [PubMed]
- Curreli, S.; Deplano, P.; Mercuri, M.L.; Pilia, L.; Serpe, A.; Bigoli, F.; Pellinghelli, M.A.; Coronado, E.; Gómez-Garcıa, C.J.; Canadell, E. A New Conducting Molecular Solid Based on the Magnetic [Ni(dmf)6]2+ Cation on [Ni(dsit)2]22- (dsit = 1,3-dithiole-2-thione-4,5-diselenolate) Showing an Unprecedent Anion Packing. J. Solid State Chem. 2002, 168, 653–660. [Google Scholar] [CrossRef]
- Karlheinz, S.; Dietmar, R. Coordination Chemistry of Polynitriles, I. Syntheses and Crystal Structures of [Ag(PCC)(DMF)], [Ni(DMF)6](PCC)2 and [Co(DMF)6](PCC)2 (PCC = [C5(CN)5]−, DMF = N,N-Dimethylformamide). Z. Nat. B 2013, 68, 546–550. [Google Scholar]
- Famengo, A.; Pinero, D.; Jeannin, O.; Guizouarn, T.; Fourmigue, M. Paramagnetic dithiolene complexes as metallo-ligands: Ether/thioether coordination. Dalton Trans. 2012, 41, 1441–1443. [Google Scholar] [CrossRef]
- Janas, Z.; Lis, T.; Sobota, P. Chloride ion abstraction from cobalt and nickel chlorides by SnCI4. Crystal structure of [Ni2(µ-Cl3)(THF)6][SnCl5(THF)]. Polyhedron 1992, 11, 3019. [Google Scholar] [CrossRef]
- Zebrowski, J.P.; Hayashi, R.K.; Dahl, L.F. A new family of icosahedral cages with transition metal and main group IV (14) atoms: Synthesis and structural-bonding analysis of the [Ni11(SnR)2(CO)18]2- dianions (R = Bu, Me) containing nickel-centered icosahedral Ni10Sn2 cages and of their unusual [Ni(SnRCl2)4(CO)]2- precursors containing a trigonal-bipyramidal d8 nickel(II) configuration. J. Am. Chem. Soc. 1993, 115, 1142–1144. [Google Scholar]
- McLauchlan, C.; Robowski, S.; Ibers, J.A. Syntheses and Characterization of the Metal Maleonitrilediselenolates [K([2.2.2]-cryptand)]2[M(Se2C2(CN)2)2] (M = Ni, Pd, Pt) and [Ni(dmf)5Cl]2[Ni(Se2C2(CN)2)2]. Inorg. Chem. 2001, 40, 1372. [Google Scholar] [CrossRef]
- Chygorin, E.N.; Petrusenko, S.R.; Kokozay, V.N.; Smal, Y.O.; Omelchenko, I.V.; Shishkin, O.V. Hexakis(dimethylformamide-κO)-manganese(II) μ-oxido-bis[trichloridoferrate(III)]. Acta Cryst. 2011, 67, m1563–m1564. [Google Scholar] [CrossRef]
- Girma, K.B.; Lorenz, V.; Blaurock, S.; Edelmann, F.T. Coordination chemistry of acrylamide: Formation and structural characterization of [Fe(O-OC(NH2)CHCH2)6][Fe2Cl6O]. Inorg. Chim. Acta 2008, 361, 346–348. [Google Scholar] [CrossRef]
- Yana, B.; Chen, Z.-D.; Wang, S.-X. Synthesis, Crystal Structure and Magnetic Property of Diiron Complex [Fe(phen)3][Fe2Cl6O].2CH3CN. J. Chin. Chem. Soc. 2000, 47, 1211–1214. [Google Scholar] [CrossRef]
- Wei, J.; Ju, G.; You, X. Study on magnetic properties for (μ-oxo)bis[trichloroferrate(III)] dimer [Fe2Cl6O]2− by local spin theory. Chem. Phys. Lett. 2004, 391, 226–233. [Google Scholar] [CrossRef]
- Liu, T.; Shao, Y.; Li, G.; Gu, M.; Hu, J.; Xu, S.; Nie, Z.; Chen, X.; Wang, C.; Liu, J. A facile approach using MgCl2 to formulate high performance Mg2+ electrolytes for rechargeable Mg batteries. J. Mater. Chem. A 2014, 2, 3430–3438. [Google Scholar] [CrossRef]
- Calderazzo, F.; De Benedetto, G.E.; Pampaloni, G.; Mossmer, C.M.; Strahle, J.; Wurst, K. Bis(arene)vanadium(O) complexes as a source of vanadium(II) derivatives by both disproportionation of the [V(η6-arene)2]+ cations and oxidation. of [V(η6-arene)2]. J. Organomet. Chem. 1993, 73, 451. [Google Scholar] [CrossRef]
- Cotton, F.-A.; Luck, R.-L.; Son, K.-A. New polynuclear compounds of iron(II) chloride with oxygen donor ligands Part I. Fe4Cl8(THF)6: Synthesis and a single crystal X-ray structure determination. Inorg. Chim. Acta 1991, 179, 11–15. [Google Scholar] [CrossRef]
- Zhao, H.; Clérac, R.; Sun, J.-S.; Ouyang, X.; Clemente-Juan, J.M.; Gómez-García, C.J.; Coronado, E.; Dunbar, K.R. Comparative Structural and Magnetic Study of Three Compounds Based on the Cluster Unit M4Cl8(THF)6 (M = Mn, Fe, Co). J. Solid State Chem. 2001, 159, 281–292. [Google Scholar] [CrossRef]
- Sobota, P.; Olejnik, Z.; Utko, J.; Lis, T. Synthesis, magnetic properties and structure of the [Co4(μ3-Cl)2(μ2-Cl)4Cl2(THF)6] complex. Polyhedron 1993, 12, 613. [Google Scholar] [CrossRef]
- Bkouche-Waksman, I. Données cristallographiques sur les solvates des halogénures de CoII et NiII avec le méthanol et l’éthanol. J. Inorg. Nucl. Chem. 1976, 38, 1871. [Google Scholar] [CrossRef]
- Luo, F.; Zheng, J.-M.; Kurmoo, M. One-Pot Synthesis of Schiff-Base-Containing Ni8 Clusters: Solvothermal Synthesis, Structure, and Magnetic Properties. Inorg. Chem. 2007, 46, 8448–8450. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–7 are available from the authors. |


| 1 | 2A | 2B | 3 | |
|---|---|---|---|---|
| Formula | C48H64Cl8N8Ni3O8S8 | C9H21Cl3FeN3Ni0.50O3.50 | C18H42Cl6Fe2N6NiO7 | C12H4Cl4FeNiS4 |
| Space group | P21/n | R-3 | P-1 | P21/c |
| a/Å | 8.730(8) | 14.193(2) | 9.1594(3) | 12.999(1) |
| b/Å | 17.144(6) | 14.193(2) | 9.3113(3) | 12.1054(8) |
| c/Å | 23.789(8) | 15.147(3) | 22.1594(8) | 23.509(2) |
| α/° | 90 | 90 | 82.911(2) | 90 |
| β/° | 101.06(4) | 90 | 80.829(2) | 90.899 |
| ɣ/° | 90 | 120 | 87.782(2) | 90 |
| V/Å3 | 3500(4) | 2642.4(9) | 1851.1(1) | 3698.7(5) |
| Z | 2 | 6 | 2 | 4 |
| d calc/g·cm−3 | 1.515 | 1.579 | 1.503 | 1.534 |
| μ/mm−1 | 1.394 | 1.839 | 1.750 | 1.933 |
| R indices (I > 2σ(I)) | R1 = 0.0687 wR2 = 0.1325 | R1 = 0.0362 wR2 = 0.0738 | R1 = 0.0535 wR2 = 0.1222 | R1 = 0.0569 wR2 = 0.1663 |
| GooF on F2 | 1.085 | 1.006 | 1.059 | 1.119 |
| 4 | 5 | 6 | 7 | |
|---|---|---|---|---|
| Formula | C36H52Cl7FeNi2O6S4 | C48H72N8Ni3O8S8 | C27H43ClN5Ni2O5S4 | C24H48Cl8Fe2.6Ni1.4O6 |
| Space group | P-1 | P21/c | P-1 | P-1 |
| a/Å | 9.7565(3) | 8.4611(4) | 8.718(1) | 9.99511(6) |
| b/Å | 14.2853(5) | 18.882(1) | 12.212(1) | 10.4813(5) |
| c/Å | 18.0305(6) | 19.522(1) | 17.889(2) | 10.8907(7) |
| α/° | 96.762(2) | 90 | 101.414(1) | 62.650(2) |
| β/° | 90.792(2) | 90.197(3) | 97.572(4) | 67.991(3) |
| ɣ/° | 109.047(2) | 90 | 99.593(5) | 82.248(3) |
| V/Å3 | 2355.2(1) | 3118.9(3) | 1813.8(4) | 934.4(1) |
| Z | 2 | 2 | 2 | 1 |
| d calc/g·cm−3 | 1.598 | 1.407 | 1.463 | 1.677 |
| μ/mm−1 | 1.802 | 1.217 | 1.383 | 2.290 |
| R indices (I > 2σ(I)) | R1 = 0.0326 wR2 = 0.0834 | R1 = 0.0297 wR2 = 0.0688 | R1 = 0.0312 wR2 = 0.0758 | R1 = 0.0310 wR2 = 0.0772 |
| GooF on F2 | 1.070 | 1.086 | 1.091 | 1.031 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, E.; Hernández, E.; Pérez, M.; Perles, J.; Zamora, F. Structural Study of the Compounds Formed in the Reactions of FeCl3·6H2O with Ni(OH)2 in the Presence of Dithiolenes HSRSH (R = C6H2Cl2 or C6H4). Molecules 2020, 25, 2240. https://doi.org/10.3390/molecules25092240
Delgado E, Hernández E, Pérez M, Perles J, Zamora F. Structural Study of the Compounds Formed in the Reactions of FeCl3·6H2O with Ni(OH)2 in the Presence of Dithiolenes HSRSH (R = C6H2Cl2 or C6H4). Molecules. 2020; 25(9):2240. https://doi.org/10.3390/molecules25092240
Chicago/Turabian StyleDelgado, Esther, Elisa Hernández, María Pérez, Josefina Perles, and Félix Zamora. 2020. "Structural Study of the Compounds Formed in the Reactions of FeCl3·6H2O with Ni(OH)2 in the Presence of Dithiolenes HSRSH (R = C6H2Cl2 or C6H4)" Molecules 25, no. 9: 2240. https://doi.org/10.3390/molecules25092240
APA StyleDelgado, E., Hernández, E., Pérez, M., Perles, J., & Zamora, F. (2020). Structural Study of the Compounds Formed in the Reactions of FeCl3·6H2O with Ni(OH)2 in the Presence of Dithiolenes HSRSH (R = C6H2Cl2 or C6H4). Molecules, 25(9), 2240. https://doi.org/10.3390/molecules25092240