Proteolytic Volatile Profile and Electrophoretic Analysis of Casein Composition in Milk and Cheese Derived from Mironutrient-Fed Cows
Abstract
:1. Introduction
2. Results
2.1. Microelements Quantification in Milk and Cheese
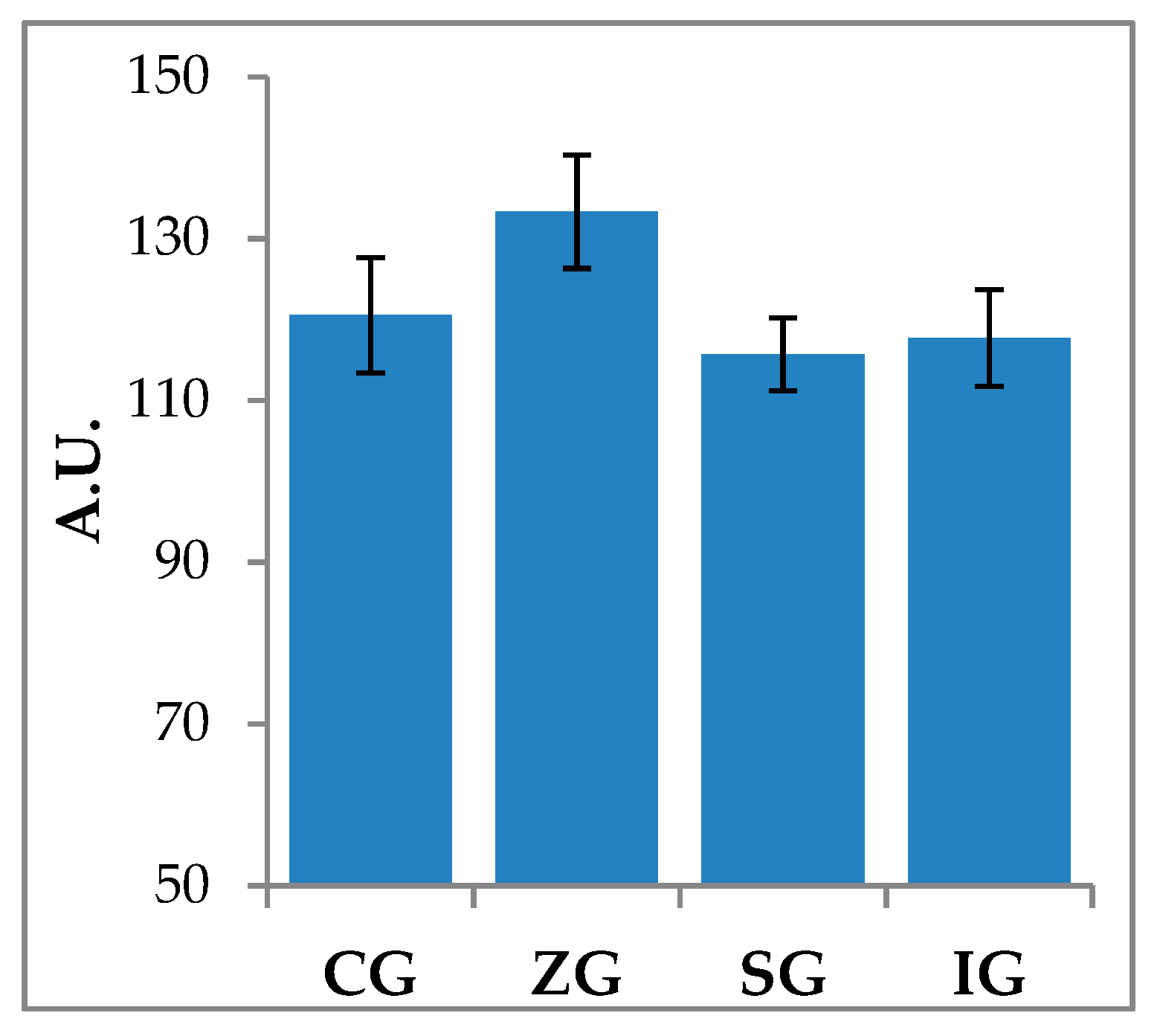
2.2. Zymographic Evaluation of Gelatinolytic and Caseinolytic Activity in Milk
2.3. Caseins Separation by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Identification of Volatile Compounds in Fresh and Ripened Cheese
3. Discussion
4. Materials and Methods
4.1. Experimental Design, Feeding Strategies, Cheesemaking, and Sampling
4.2. Microelements Determination in Milk and Cheese
4.3. Gelatin and Casein Zymography of Milk Samples
4.4. Caseins Extraction and Separation by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.5. Volatile Compounds Extraction and GC-MS Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reffett, J.K.; Spears, J.W.; Brown Jr, T.T. Effect of dietary selenium on the primary and secondary immune response in calves challenged with infectious bovine rhinotracheitis virus. J. Nutr. 1988, 118, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.E. Zinc enzymes. Curr. Opin. Chem. Biol. 1998, 2, 222–234. [Google Scholar] [CrossRef]
- Miller, W.J. Zinc nutrition of cattle: A review. J. Dairy Sci. 1970, 53, 1123–1135. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Yang, W.Z.; Dong, Q.; Yang, X.M.; He, D.C.; Zhang, P.; Dong, K.H.; Huang, Y.X. Effects of selenium yeast on rumen fermentation, lactation performance and feed digestibilities in lactating dairy cows. Livestock Sci. 2009, 126, 239–244. [Google Scholar] [CrossRef]
- Flachowsky, G.; Franke, K.; Meyer, U.; Leiterer, M.; Scho, F. Influencing factors on iodine content of cow milk. Eur. J. Nutr. 2014, 53, 351–365. [Google Scholar] [CrossRef]
- Iannaccone, M.; Elgendy, R.; Ianni, A.; Martino, C.; Palazzo, F.; Giantin, M.; Grotta, L.; Dacasto, M.; Martino, G. Whole-transcriptome profiling of sheep fed with a high iodine-supplemented diet. Animal 2019, 14, 745–752. [Google Scholar] [CrossRef]
- Elgendy, R.; Palazzo, F.; Castellani, F.; Giantin, M.; Grotta, L.; Cerretani, L.; Dacasto, M.; Martino, G. Transcriptome profiling and functional analysis of sheep fed with high zinc-supplemented diet: A nutrigenomic approach. Anim. Feed Sci. Technol. 2017, 234, 195–204. [Google Scholar] [CrossRef]
- Biscarini, F.; Palazzo, F.; Castellani, F.; Masetti, G.; Grotta, L.; Cichelli, A.; Martino, G. Rumen microbiome in dairy calves fed copper and grape-pomace dietary supplementations: Composition and predicted functional profile. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Calamari, L.; Petrera, F.; Bertin, G. Effects of either sodium selenite or Se yeast (Sc CNCM I-3060) supplementation on selenium status and milk characteristics in dairy cows. Livestock Sci. 2010, 128, 154–165. [Google Scholar] [CrossRef]
- Iannaccone, M.; Ianni, A.; Elgendy, R.; Martino, C.; Giantin, M.; Cerretani, L.; Dacasto, M.; Martino, G. Iodine Supplemented Diet Positively Affect Immune Response and Dairy Product Quality in Fresian Cow. Animals 2019, 9, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirone, M.; Tofalo, R.; Perpetuini, G.; Manetta, A.C.; Di Gianvito, P.; Tittarelli, F.; Battistelli, N.; Corsetti, A.; Suzzi, G.; Martino, G. Influence of iodine feeding on microbiological and physico-chemical characteristics and biogenic amines content in a raw ewes’ milk cheese. Foods 2018, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kranenburg, R.; Kleerebezem, M.; Van Hylckama Vlieg, J.; Ursing, B.M.; Boekhorst, J.; Smit, B.A.; Ayad, E.H.E.; Smit, G.; Siezen, R.J. Flavour formation from amino acids by lactic acid bacteria: Predictions from genome sequence analysis. Int. Dairy J. 2002, 12, 111–121. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Yvon, M.; Rijnen, L. Cheese flavor formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–201. [Google Scholar] [CrossRef]
- Ianni, A.; Innosa, D.; Martino, C.; Grotta, L.; Bennato, F.; Martino, G. Zinc supplementation of Friesian cows: Effect on chemical-nutritional composition and aromatic profile of dairy products. J. Dairy Sci. 2019, 102, 2918–2927. [Google Scholar] [CrossRef] [Green Version]
- Ling, K.; Henno, M.; Jõudu, I.; Püssa, T.; Jaakson, H.; Kass, M.; Anton, D.; Ots, M. Selenium supplementation of diets of dairy cows to produce Se-enriched cheese. Int. Dairy. J. 2017, 71, 76–81. [Google Scholar] [CrossRef]
- Pechová, A.; Pavlata, L.; Lokajova, E. Zinc supplementation and somatic cell count in milk of dairy cows. Acta Vet. Brno 2006, 75, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Moschini, M.; Battaglia, M.; Beone, G.M.; Piva, G.; Masoero, F. Iodine and selenium carry over in milk and cheese in dairy cows: Effect of diet supplementation and milk yield. Animal 2010, 4, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Meshref, A.M.; Moselhy, W.A.; Hassan, N.E.H.Y. Heavy metals and trace elements levels in milk and milk products. J. Food Meas. Charact. 2014, 8, 381–388. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manicourt, D.H.; Lefebvre, V. An assay for matrix metalloproteinases and other proteases acting on proteoglycans, casein, or gelatin. Anal. Biochem. 1993, 215, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, N.; Smirnoff, A.L.; Bottini, J.M.; De Simone, E.A. Protease activity and protein profile in milk from healthy dairy cows and cows with different types of mastitis. Int. Dairy J. 2019, 89, 1–5. [Google Scholar] [CrossRef]
- Santos, M.C.L.G.; De Souza, A.P.; Gerlach, R.F.; Trevilatto, P.C.; Scarel-Caminaga, R.M.; Line, S.R.P. Inhibition of human pulpal gelatinases (MMP-2 and MMP-9) by zinc oxide cements. J. Oral Rehabil. 2004, 31, 660–664. [Google Scholar] [CrossRef]
- Costa, F.F.; Brito, M.A.V.P.; Furtado, M.A.M.; Martins, M.F.; De Oliveira, M.A.L.; De Castro Barra, P.M.; Lourdes, A.G.; Dos Santos, A.S.D.O. Microfluidic chip electrophoresis investigation of major milk proteins: Study of buffer effects and quantitative approaching. Anal. Met. 2014, 6, 1666–1673. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, A.J.; Guamis, B.; Carretero, C. Hydrolysis of Bovine and Caprine Caseins by Rennet and Plasmin in Model Systems. J. Agric. Food Chem. 1998, 46, 3066–3072. [Google Scholar] [CrossRef]
- Van Hekken, D.L.; Tunick, M.H.; Soryal, K.A.; Zeng, S.S. Proteolytic and Rheological Properties of Aging Cheddar-Like Caprine Milk Cheeses Manufactured at Different Times during Lactation. J. Food Sci. 2007, 72, E115–E120. [Google Scholar] [CrossRef]
- Zeng, H.; Combs, G.F., Jr. Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef]
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT—Food Sci. Technol. 2011, 44, 2166–2172. [Google Scholar] [CrossRef]
- Ianni, A.; Bennato, F.; Martino, C.; Grotta, L.; Martino, G. Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet. Molecules 2020, 25, 461. [Google Scholar] [CrossRef] [Green Version]
- Suriyaphan, O.; Drake, M.; Chen, X.Q.; Cadwallader, K.R. Characteristic aroma components of British farmhouse Cheddar cheese. J. Agric. Food Chem. 2001, 49, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Smit, B.A.; Engels, W.J.M.; Wouters, J.T.M.; Smit, G. Diversity of L-leucine catabolism in various microorganisms involved in dairy fermentations, and identification of the rate-controlling step in the formation of the potent flavour component 3-methylbutanal. Appl. Microbiol. Biotechnol. 2004, 64, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Maillard, M.B. Production of cheese flavour compounds derived from amino acid catabolism by Propionibacterium freudenreichii. Lait 2002, 82, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Ianni, A.; Martino, C.; Pomilio, F.; Di Luca, A.; Martino, G. Dietary selenium intake in lactating dairy cows modifies fatty acid composition and volatile profile of milk and 30-day-ripened caciotta cheese. Eur. Food Res. Technol. 2019, 245, 2113–2121. [Google Scholar] [CrossRef]
- Martino, C.; Ianni, A.; Grotta, L.; Pomilio, F.; Martino, G. Influence of Zinc Feeding on Nutritional Quality, Oxidative Stability and Volatile Profile of Fresh and Ripened Ewes’ Milk Cheese. Foods 2019, 8, 656. [Google Scholar] [CrossRef] [Green Version]
- Ianni, A.; Iannaccone, M.; Martino, C.; Innosa, D.; Grotta, L.; Bennato, F.; Martino, G. Zinc supplementation of dairy cows: Effects on chemical composition, nutritional quality and volatile profile of Giuncata cheese. Int. Dairy J. 2019, 94, 65–71. [Google Scholar] [CrossRef]
- European Union (2010) Directive 2010/63/EU of the European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010L0063&from=EN (accessed on 25 March 2020).
- European Economic Community (1986) Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31986L0609&from=EN (accessed on 25 March 2020).
- European Commission (2003) Regulation (EC) No. 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003R1831&from=EN (accessed on 25 March 2020).
- European Commission (2014) Regulation No. 121/2014 of the concerning the authorization of L-selenomethionine as a feed additive for all animal species. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0121&from=EN (accessed on 25 March 2020).
- European Commission (2005) Regulation No. 1459/2005 amending the conditions for authorisation of a number of feed additives belonging to the group of trace elements. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:233:0008:0010:EN:PDF (accessed on 25 March 2020).
- NRC. Nutrient Requirements of Dairy Cattle; Natl Acad Press: Washington, DC, USA, 2001. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; Volume 1. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Ianni, A.; Bennato, F.; Martino, C.; Innosa, D.; Grotta, L.; Martino, G. Effects of selenium supplementation on chemical composition and aromatic profiles of cow milk and its derived cheese. J. Dairy Sci. 2019, 102, 6853–6862. [Google Scholar] [CrossRef]
- Nascentes, C.C.; Arruda, M.A.Z.; Nogueira, A.R.A.; Nóbrega, J.A. Direct determination of Cu and Zn in fruit juices and bovine milk by thermospray flame furnace atomic absorption spectrometry. Talanta 2004, 64, 912–917. [Google Scholar] [CrossRef]
- Gerber, N.; Brogioli, R.; Hattendorf, B.; Scheeder, M.R.L.; Wenk, C.; Günther, D. Variability of selected trace elements of different meat cuts determined by ICP-MS and DRC-ICPMS. Animal 2009, 3, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Fecher, P.; Goldmann, I.; Nagengast, A. Determination of iodine in food samples by inductively coupled plasma mass spectrometry after alkaline extraction. J. Anal. At. Spectrom. 1998, 13, 977–982. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook; Humana Press: Totowa, NJ, USA, 2009; pp. 17–24. [Google Scholar]
- Ianni, A.; Celenza, G.; Franceschini, N. Oxaprozin: A new hope in the modulation of matrix metalloproteinase 9 activity. Chem. Biol. Drug Des. 2019, 93, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ Software; National Institutes of Health: Bethesda, MD, USA, 1997. [Google Scholar]
- Bennato, F.; Ianni, A.; Innosa, D.; Grotta, L.; D’Onofrio, A.; Martino, G. Chemical-nutritional characteristics and aromatic profile of milk and related dairy products obtained from goats fed with extruded linseed. Asian-Australas. J. Anim. Sci. 2020, 33, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples analysed during the current study are available from the corresponding author on reasonable request. |


| Microelement | Milk Samples | |||
|---|---|---|---|---|
| CG | ZG | SG | IG | |
| Zinc 1 | 4.18 a ± 0.37 | 5.76 b ± 0.41 | 3.98 a ± 0.33 | 4.04 a ± 0.40 |
| Selenium 1 | 0.036 a ± 0.004 | 0.041 a ± 0.005 | 0.049 b ± 0.005 | 0.039 a ± 0.004 |
| Iodine 1 | 0.12 a ± 0.03 | 0.11 a ± 0.02 | 0.10 a ± 0.02 | 0.17 b ± 0.02 |
| Trace Element | Ripening Time 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| T7 | T120 | |||||||
| CG | ZG | SG | IG | CG | ZG | SG | IG | |
| Zinc 2 | 41.34 a ± 2.03 | 52.61 b ± 2.37 | 42.77 a ± 2.19 | 40.77 a ± 1.98 | 43.21 a ± 2.41 | 54.74 b ± 2.39 | 41.82 a ± 3.09 | 42.91 a ± 2.93 |
| Selenium 2 | 0.21 a ± 0.03 | 0.19 a ± 0.03 | 0.32 b ± 0.04 | 0.22 a ± 0.03 | 0.22 a ± 0.02 | 0.18 a ± 0.03 | 0.31 b ± 0.04 | 0.19 a ± 0.03 |
| Iodine 2 | 0.21 a ± 0.03 | 0.24 a ± 0.03 | 0.19 a ± 0.03 | 0.31 b ± 0.04 | 0.20 a ± 0.03 | 0.22 a ± 0.03 | 0.18 a ± 0.02 | 0.29 b ± 0.04 |
| Protein | CG | ZG | SG | IG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T7 | T120 | p | T7 | T120 | p | T7 | T120 | p | T7 | T120 | p | |
| αS2-CN | 22.93 ± 2.07 | 26.59 ± 2.78 | ns | 20.98 ± 1.87 | 19.60 ± 1.79 | ns | 22.30 ± 2.13 | 21.02 ± 2.04 | ns | 23.80 ± 2.21 | 25.10 ± 2.33 | ns |
| αS1-CN | 33.16 ± 2.88 | 10.82 ± 0.85 | ** | 29.66 ± 2.83 | 8.30 ± 0.78 | ** | 31.82 ± 2.97 | 21.14 ± 1.99 | ** | 29.98 ± 2.83 | 14.66 ± 1.42 | ** |
| β-CN | 26.92 ± 2.54 | 27.94 ± 2.41 | ns | 30.57 ± 2.92 | 30.93 ± 2.82 | ns | 23.39 ± 2.18 | 27.21 ± 2.51 | ns | 31.33 ± 2.86 | 33.60 ± 3.11 | ns |
| Band 1 | 5.04 ± 0.56 | 8.41 ± 0.86 | * | 5.19 ± 0.53 | 10.70 ± 0.96 | ** | 5.48 ± 0.55 | 8.09 ± 0.78 | * | 4.10 ± 0.42 | 6.36 ± 0.61 | * |
| Band 2 | 3.86 ± 0.44 | 6.36 ± 0.67 | ** | 5.18 ± 0.52 | 8.76 ± 0.83 | ** | 4.68 ± 0.47 | 5.96 ± 0.61 | ns | 3.09 ± 0.32 | 6.27 ± 0.62 | ** |
| Band 3 | 3.51 ± 0.39 | 6.77 ± 0.69 | ** | 3.64 ± 0.38 | 8.20 ± 0.77 | ** | 4.39 ± 0.45 | 4.27 ± 0.43 | ns | 2.76 ± 0.29 | 4.30 ± 0.42 | * |
| Band 4 | 1.72 ± 0.23 | 6.70 ± 0.68 | ** | 0.96 ± 0.11 | 6.77 ± 0.66 | ** | 3.11 ± 0.33 | 3.99 ± 0.40 | ns | 2.10 ± 0.22 | 4.50 ± 0.44 | ** |
| Band 5 | 1.87 ± 0.21 | 6.41 ± 0.65 | ** | 3.82 ± 0.39 | 6.74 ± 0.65 | ** | 4.84 ± 0.50 | 8.32 ± 0.81 | ** | 2.84 ± 0.28 | 5.21 ± 0.51 | ** |
| Volatile Compounds | T.I. 2 | Ripening Time 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T7 | T120 | ||||||||||
| CG | ZG | SG | IG | SEM | CG | ZG | SG | IG | SEM | ||
| Phenylalanine | |||||||||||
| Phenylacetaldehyde | 91 | 750 | 862 | 1083 | 480 | 227 | 3015 | 3658 | 5748 | 4334 | 1222 |
| 2-phenylethyl alcohol | 91 | 6499 a | 3125 b | 1364 c | 3037 b | 399 | 9596 a | 6833 b | 7564 a | 14815 c | 2643 |
| Leucine | |||||||||||
| 3-methyl-1-butanol | 55 | 7481 a | 6526 a | 2697 b | 6091 a | 987 | 7648 a | 6327 a | 5921 b | 7597 a | 1179 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianni, A.; Bennato, F.; Martino, C.; Grotta, L.; Franceschini, N.; Martino, G. Proteolytic Volatile Profile and Electrophoretic Analysis of Casein Composition in Milk and Cheese Derived from Mironutrient-Fed Cows. Molecules 2020, 25, 2249. https://doi.org/10.3390/molecules25092249
Ianni A, Bennato F, Martino C, Grotta L, Franceschini N, Martino G. Proteolytic Volatile Profile and Electrophoretic Analysis of Casein Composition in Milk and Cheese Derived from Mironutrient-Fed Cows. Molecules. 2020; 25(9):2249. https://doi.org/10.3390/molecules25092249
Chicago/Turabian StyleIanni, Andrea, Francesca Bennato, Camillo Martino, Lisa Grotta, Nicola Franceschini, and Giuseppe Martino. 2020. "Proteolytic Volatile Profile and Electrophoretic Analysis of Casein Composition in Milk and Cheese Derived from Mironutrient-Fed Cows" Molecules 25, no. 9: 2249. https://doi.org/10.3390/molecules25092249
APA StyleIanni, A., Bennato, F., Martino, C., Grotta, L., Franceschini, N., & Martino, G. (2020). Proteolytic Volatile Profile and Electrophoretic Analysis of Casein Composition in Milk and Cheese Derived from Mironutrient-Fed Cows. Molecules, 25(9), 2249. https://doi.org/10.3390/molecules25092249






