UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles
Abstract
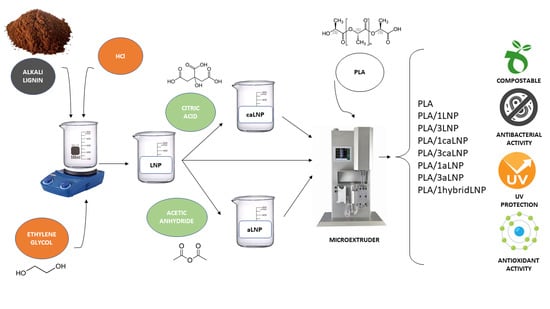
:1. Introduction
2. Results and Discussion
2.1. Lignin Nanoparticles Characterization
2.2. Nanocomposites Characterization
2.2.1. Morphological Analysis and Mechanical Properties
2.2.2. Thermal Properties
2.2.3. Optical Properties
2.2.4. Determination of DPPH Radical Scavenging Activity
2.2.5. Overall Migration
2.2.6. Disintegration in Compost
2.2.7. Antibacterial Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Chemical Modification of Lignin Nanoparticles
3.3. Nanoparticles Characterization
3.4. Nanocomposites Preparation
3.5. Nanocomposites Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| 1st Heating Scan | Cooling | |||||||
|---|---|---|---|---|---|---|---|---|
| Formulation | Tg (°C) | Tcc (°C) | ∆Hcc (J/g) | Tm (°C) | ∆Hm (J/g) | Xc (%) | Tcc (°C) | ∆Hcc (J/g) |
| PLA | 58.9 ± 2.0 | 98.1 ± 0.3 | 27.1 ± 0.3 | 168.9 ± 0.1 | 48.7 ±0.1 | 23.0 ± 0.2 | 98.7 ±1.4 | 7.2 ± 1.4 |
| PLA/1LNP | 58.9 ± 2.0 | 99.3 ±0.1 | 25.0 ± 0.1 | 168.8 ±0.1 | 46.8 ± 0.7 | 23.3 ± 0.7 | 99.1 ± 2.4 | 6.3 ± 2.7 |
| PLA/3LNP | 56.3 ± 1.2 | 99.3 ± 0.6 | 26.6 ± 0.1 | 168.7 ± 0.3 | 47.8 ± 0.4 | 22.6 ± 0.4 | 97.9 ± 0.6 | 6.9 ± 1.7 |
| PLA/1caLNP | 56.4 ± 1.2 | 99.1 ± 0.7 | 27.0 ± 0.1 | 169.4 ± 0.3 | 48.2 ± 2.1 | 22.7 ± 2.1 | 98.3 ± 0.1 | 13.8 ± 1.5 |
| PLA/3caLNP | 56.3 ± 1.3 | 99.5 ± 0.1 | 27.8± 0.9 | 168.6 ± 0.2 | 50.4 ± 0.4 | 24.1 ± 0.5 | 102.1 ± 0.1 | 28.8 ± 0.3 |
| PLA/1aLNP | 55.4 ± 1.4 | 98.1 ± 0.1 | 24.3 ± 5.5 | 168.51 ± 0.4 | 48.9 ± 0.9 | 26.3 ± 6.9 | 96.4 ± 1.7 | 5.2 ± 2.6 |
| PLA/3aLNP | 55.0 ± 2.0 | 98.3 ± 0.3 | 27.2 ± 0.3 | 168.5 ± 0.4 | 50.2 ± 2.1 | 24.5 ± 1.9 | 98.9 ± 0.5 | 7.8 ± 0.3 |
| PLA/1hybridLNP | 58.1 ± 2.7 | 98.1 ± 0.7 | 24.2 ± 4.3 | 169.0 ± 0.4 | 50.3 ± 0.9 | 27.9 ± 5.5 | 98.8 ± 0.5 | 13.0 ± 0.5 |
| 2nd Heating Scan | ||||||||
| Tg (°C) | Tcc (°C) | ∆Hcc (J/g) | Tm (°C) | ∆Hm (J/g) | Xc (%) | |||
| PLA | 59.4 ± 0.6 | 97.1 ± 0.1 | 19.0 ± 1.2 | 168.4 ± 0. 2 | 41.3 ± 0.6 | 30.1 ± 0.6 | ||
| PLA/1LNP | 59.3 ± 0.2 | 97.6 ± 0.5 | 21.3 ±0.3 | 168.6 ± 0.3 | 46.4 ± 0.4 | 26.8 ± 0.8 | ||
| PLA/3LNP | 59.7 ± 0.2 | 99.1 ± 0.1 | 20.7 ±1.5 | 168.5 ± 0.2 | 47.4 ± 0.4 | 28.5 ± 2.1 | ||
| PLA/1caLNP | 59.5 ± 0.3 | 97.1 ± 0.4 | 15.0 ± 0.2 | 168.6 ± 0.2 | 47.2 ± 1.3 | 34.4 ± 1.6 | ||
| PLA/3caLNP | 59.0 ± 0.1 | 95.9 ± 0.2 | 5.2 ± 0.2 | 168.6 ± 0.1 | 47.6 ± 0.6 | 45.3 ± 0.8 | ||
| PLA/1aLNP | 59.4 ± 0.4 | 97.3 ± 0.9 | 21.7 ± 0.7 | 168.6 ± 0.1 | 49.7 ± 0.2 | 29.9 ± 1.0 | ||
| PLA/3aLNP | 59.5 ± 0.3 | 97.4 ± 0.5 | 19.5 ± 0.6 | 168.5 ± 0.3 | 40.5 ± 1.9 | 32.1 ± 2.7 | ||
| PLA/1hybridLNP | 59.1 ± 0.1 | 97.8 ± 0.1 | 14.4 ± 1.2 | 168.5 ± 0.1 | 47.8 ± 0.2 | 35.6 ± 1.5 | ||
References
- Li, J.; He, Y.; Inoue, Y. Thermal and mechanical properties of biodegradable blends of poly(L-lactic acid) and lignin. Polym. Int. 2003, 52, 949–955. [Google Scholar] [CrossRef]
- Henton, D.E.; Gruber, P.; Lunt, J.; Randall, J. Polylactic acid technology. In Natural Fibers, Biopolymers and Biocomposites, 1st ed.; Mohanty, A.K., Misra, M., Drzal, L.T., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 527–577. [Google Scholar]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Akhtar, M.J.; Cleymand, F.; Desobry, S. Structural, mechanical and barrier prop-erties of active PLA–antioxidant films. J. Food Eng. 2012, 110, 380–389. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, L.T.; Rahman, W.A.W.A. Polylactic Acid: PLA Biopolymer Technology and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.; Kenny, J. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Fortunati, E.; Luzi, F.; Yang, W.; Kenny, J.M.; Torre, L.; Puglia, D. Bio-Based Nanocomposites in Food Packaging. In Nanomaterials for Food Packaging; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 71–110. [Google Scholar]
- Yang, W.; Dominici, F.; Fortunati, E.; Kenny, J.; Puglia, D. Effect of lignin nanoparticles and masterbatch procedures on the final properties of glycidyl methacrylate-g-poly (lactic acid) films before and after accelerated UV weathering. Ind. Crop. Prod. 2015, 77, 833–844. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Lopresti, F.; Maio, A.; Sutera, F. Polysaccharide nanocrystals as fillers for PLA based nanocomposites. Cellulose 2017, 24, 447–478. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.; Kenny, J.M.; Puglia, D. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of Antimicrobial Packaging Film Made from Poly(Lactic Acid) Incorporating Titanium Dioxide and Silver Nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef] [Green Version]
- Sharif, A.; Mondal, S.; Hoque, M.E. Polylactic Acid (PLA)-based nanocomposites: Processing and properties. In Bio-Based Polymers and Nanocomposites; Sanyang, M.L., Jawaid, M., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 233–254. [Google Scholar]
- Gordobil, O.; Delucis, R.; Egüés, I.; Labidi, J. Kraft lignin as filler in PLA to improve ductility and thermal properties. Ind. Crop. Prod. 2015, 72, 46–53. [Google Scholar] [CrossRef]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA–Lignin bioplastics properties before and after accelerated weathering. Compos. Part B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.; Kenny, J.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Tanase, C.E. Design, characterization and preliminary biological evaluation of new lignin-PLA biocomposites. Int. J. Biol. Macromol. 2018, 114, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.Y.; Youn, H.J.; Choi, J.W. Utilization of lignin fractions in UV resistant lignin-PLA biocomposites via lig-nin-lactide grafting. Int. J. Biol. Macromol. 2019, 138, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Siddhi, J.; Juikar, N.V. Extraction of nanolignin from coconut fibers by controlled microbial hydrolysis. Ind. Crops Prod. 2017, 109, 420–425. [Google Scholar]
- Zhang, T.; Yang, Y.-L.; Liu, S. Application of biomass by-product lignin stabilized soils as sustainable Geomaterials: A review. Sci. Total. Environ. 2020, 728, 138830. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crop. Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Zhao, W.; Simmons, B.; Singh, S.; Ragauskas, A.; Cheng, G. From lignin association to nano-/micro-particle preparation: Ex-tracting higher value of lignin. Green Chem. 2016, 18, 5693–5700. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin Biopolymers in the Age of Controlled Polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef] [Green Version]
- Bertella, S.; Luterbacher, J.S. Lignin Functionalization for the Production of Novel Materials. Trends Chem. 2020, 2, 440–453. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Chen, F.; Dai, H.; Dong, X.; Yang, J.; Zhong, M. Physical properties of lignin-based polypropylene blends. Polym. Compos. 2011, 32, 1019–1025. [Google Scholar] [CrossRef]
- Sailaja, R.; Deepthi, M. Mechanical and thermal properties of compatibilized composites of polyethylene and esterified lignin. Mater. Des. 2010, 31, 4369–4379. [Google Scholar] [CrossRef]
- Maldhure, A.V.; Ekhe, J.D.; Deenadayalan, E. Mechanical properties of polypropylene blended with esterified and alkylated lignin. J. Appl. Polym. Sci. 2012, 125, 1701–1712. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Kim, Y.; Suhr, J.; Seo, H.-W.; Sun, H.; Kim, S.; Park, I.-K.; Kim, S.-H.; Lee, Y.; Kim, K.-J.; Nam, J. All Biomass and UV Protective Composite Composed of Compatibilized Lignin and Poly (Lactic-acid). Sci. Rep. 2017, 7, 43596. [Google Scholar] [CrossRef] [Green Version]
- Nevárez, L.A.M.; Casarrubias, L.B.; Celzard, A.; Fierro, V.; Muñoz, V.T.; Davila, A.C.; Lubian, J.R.T.; Sánchez, G.G. Bi-opolymer-based nanocomposites: Effect of lignin acetylation in cellulose triacetate films. Sci. Technol. Adv. Mater. 2011, 12, 045006. [Google Scholar] [CrossRef]
- Thulluri, C.; Pinnamaneni, S.R.; Shetty, P.R.; Addepally, U. Synthesis of Lignin-Based Nanomaterials/Nanocomposites: Recent Trends and Future Perspectives. Ind. Biotechnol. 2016, 12, 153–160. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally bio-degradable lignin nanoparticles. Chem. Phys. Chem. 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.A.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and Characterization of Biodegradable Lignin Nanoparticles with Tunable Surface Properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef]
- Beisl, S.; Miltner, A.; Friedl, A. Lignin from Micro- to Nanosize: Production Methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangan, A.; Manchiganti, M.V.; Thilaividankan, R.M.; Kestur, S.G.; Menon, R. Novel method for the preparation of lignin-rich nanoparticles from lignocellulosic fibers. Ind. Crop. Prod. 2017, 103, 152–160. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crop. Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Mattinen, M.-L.; Valle-Delgado, J.J.; Leskinen, T.; Anttila, T.; Rivière, G.; Sipponen, M.H.; Paananen, A.; Lintinen, K.; Kostiainen, M.A.; Österberg, M. Enzymatically and chemically oxidized lignin nanoparticles for biomaterial applications. Enzym. Microb. Technol. 2018, 111, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Rahman, O.U.; Shi, S.; Ding, J.-H.; Wang, D.; Ahmad, S.; Yu, H. Lignin nanoparticles: Synthesis, characterization and corrosion protection performance. New J. Chem. 2018, 42, 3415–3425. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Aliqahtani, A.S.; Al-Thabit, A.; Roni, M.; Syed, R. Novel lignin nanoparticles for oral drug delivery. J. Mater. Chem. B 2019, 7, 4461–4473. [Google Scholar] [CrossRef]
- Henn, A.; Mattinen, M.-L. Chemo-enzymatically prepared lignin nanoparticles for value-added applications. World J. Microbiol. Biotechnol. 2019, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Li, X.; Hegyesi, N.; Zhang, Y.; Mao, Z.; Feng, X.; Wang, B.; Pukánszky, B.; Sui, X.; Zhang, Y. Poly(lactic acid)/lignin blends prepared with the Pickering emulsion template method. Eur. Polym. J. 2019, 110, 378–384. [Google Scholar] [CrossRef] [Green Version]
- Xing, Q.; Buono, P.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W. Biodegradable UV-Blocking Films through Core–Shell Lignin–Melanin Nanoparticles in Poly(butylene adipate-co-terephthalate). ACS Sustain. Chem. Eng. 2019, 7, 4147–4157. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, S. Properties of maleic anhydride-modified lignin nanoparticles/polybutylene adipate-co-terephthalate composites. J. Appl. Polym. Sci. 2020, 137, 49025. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Kenny, J.; Puglia, D. Effect of processing conditions and lignin content on thermal, mechanical and degradative behavior of lignin nanoparticles/polylactic (acid) bionanocomposites prepared by melt extrusion and solvent casting. Eur. Polym. J. 2015, 71, 126–139. [Google Scholar] [CrossRef]
- Eckert, R.C.; Abdullah, Z. A1 Carbon Fibers from Kraft Softwood Lignin. U.S. Patent 2008/0318043, 25 December 2018. [Google Scholar]
- He, X.; Luzi, F.; Yang, W.; Xiao, Z.; Torre, L.; Xie, Y.; Puglia, D. Citric Acid as Green Modifier for Tuned Hydrophilicity of Surface Modified Cellulose and Lignin Nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 9966–9978. [Google Scholar] [CrossRef]
- Hatfield, G.R.; Maciel, G.E.; Erbatur, O.; Erbatur, G. Qualitative and quantitative analysis of solid lignin samples by carbon-13 nuclear magnetic resonance spectrometry. Anal. Chem. 1987, 59, 172–179. [Google Scholar] [CrossRef]
- Jeong, H.; Park, J.; Kim, S.; Lee, J.; Cho, J.W. Use of acetylated softwood kraft lignin as filler in synthetic polymers. Fibers Polym. 2012, 13, 1310–1318. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, L.; Lu, X.; He, C. Biodegradable and renewable poly(lactide)–lignin composites: Synthesis, interface and toughening mechanism. J. Mater. Chem. A 2014, 3, 3699–3709. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Olsson, J.V.; Li, R.J.; Frank, C.W.; Waymouth, R.M.; Billington, S.L.; Sattely, E.S. A Renewable Lignin–Lactide Copolymer and Application in Biobased Composites. ACS Sustain. Chem. Eng. 2013, 1, 1231–1238. [Google Scholar] [CrossRef]
- Ghosh, I.; Jain, R.K.; Glasser, W.G. Blends of biodegradable thermoplastics with lignin esters. In Lignin: Historical, Biological, and Materials Perspectives; Glasser, W.G., Northey, R.A., Schultz, T.P., Eds.; ACS Symposium Series 742; American Chemical Society: Washington, DC, USA, 1999; pp. 331–350. [Google Scholar]
- Thielemans, W.; Wool, R.P. Lignin Esters for Use in Unsaturated Thermosets: Lignin Modification and Solubility Modeling. Biomacromolecules 2005, 6, 1895–1905. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Yetiş, F.; Liu, X.; Sampson, W.W.; Gong, H. Acetylation of lignin containing microfibrillated cellulose and its reinforcing effect for polylactic acid. Eur. Polym. J. 2020, 134, 109803. [Google Scholar] [CrossRef]
- De Oliveira, D.R.; Nogueira, I.D.M.; Maia, F.J.N.; Rosa, M.F.; Mazzetto, S.E.; Lomonaco, D. Ecofriendly modification of ace-tosolv lignin from oil palm biomass for improvement of PMMA thermo-oxidative properties. J. Appl. Polym. Sci. 2017, 134, 45498. [Google Scholar] [CrossRef]
- Bondioli, F.; Dorigato, A.; Fabbri, P.; Messori, M.; Pegoretti, A. Improving the creep stability of high-density polyethylene with acicular titania nanoparticles. J. Appl. Polym. Sci. 2009, 112, 1045–1055. [Google Scholar] [CrossRef]
- Tadano, T.; Zhu, R.; Muroga, Y.; Hoshi, T.; Sasaki, D.; Yano, S.; Sawaguchi, T. A new mechanism for the silica nanoparticle dispersion–agglomeration transition in a poly(methyl methacrylate)/silica hybrid suspension. Polym. J. 2014, 46, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Lepcio, P.; Ondreas, F.; Zarybnicka, K.; Zboncak, M.; Caha, O.; Jancar, J. Bulk polymer nanocomposites with preparation protocol governed nanostructure: The origin and properties of aggregates and polymer bound clusters. Soft Matter 2018, 14, 2094–2103. [Google Scholar] [CrossRef]
- Quyang, W.; Huang, Y.; Luo, H.; Wang, D. Poly(lactic acid) blended with cellulolytic enzyme lignin: Mechanical and thermal properties and morphology evaluation. J. Polym. Environ. 2012, 20, 1–9. [Google Scholar]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly (lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- He, Y.; Wu, T.; Wei, J.; Fan, Z.; Li, S. Morphological investigation on melt crystallized polylactide homo- and stereocopolymers by enzymatic degradation with proteinase K. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 959–970. [Google Scholar] [CrossRef]
- Detyothin, S.; Selke, S.E.; Narayan, R.; Rubino, M.; Auras, R. Reactive functionalization of poly(lactic acid), PLA: Effects of the reactive modifier, initiator and processing conditions on the final grafted maleic anhydride content and molecular weight of PLA. Polym. Degrad. Stab. 2013, 98, 2697–2708. [Google Scholar] [CrossRef]
- Muller, P.; Imre, B.; Bere, J.; Móczó, J.; Pukánszky, B. Physical ageing and molecular mobility in PLA blends and composites. J. Therm. Anal. Calorim. 2015, 122, 1423–1433. [Google Scholar] [CrossRef]
- Kawai, T.; Rahman, N.; Matsuba, G.; Nishida, K.; Kanaya, T.; Nakano, M.; Okamoto, H.; Kawada, J.; Usuki, A.; Honma, N.; et al. Crystallization and Melting Behavior of Poly (l-lactic Acid). Macromolecules 2007, 40, 9463–9469. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, A.H.; Domb§, A.J. Disorder-to-Order Phase Transition and Multiple Melting Behavior of Poly(l-lactide) Investigated by Simultaneous Measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Duncan, S.; Hannah, S. Light-protective packaging materials for foods and beverages. In Emerging Food Packaging Technologies; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 303–322. [Google Scholar]
- Decol, M.; Pachekoski, W.M.; Becker, D. Compatibilization and ultraviolet blocking of PLA/PCL blends via interfacial locali-zation of titanium dioxide nanoparticles. J. Appl. Polym. Sci. 2018, 135, 44849. [Google Scholar] [CrossRef]
- Pan, F.; Chen, L.; Jiang, Y.; Xiong, L.; Min, L.; Xie, J.; Qi, J.; Xiao, H.; Chen, Y.; De Hoop, C.F. Bio-based UV protective films prepared with polylactic acid (PLA) and Phoebe zhennan extractives. Int. J. Biol. Macromol. 2018, 119, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.J.; Sarojini, B.K.; Harshitha, K.R.; Holla, B.S.; Lobo, A. Spectral, morphological and optical studies on bischalcone doped polylactic acid (PLA) thin films as luminescent and UV radiation blocking materials. Opt. Mater. 2019, 90, 145–151. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker-A review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.R.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Lanzalunga, O.; Bietti, M. Photo- and radiation chemical induced degradation of lignin model compounds. J. Photochem. Photobiol. B Biol. 2000, 56, 85–108. [Google Scholar] [CrossRef]
- Barsberg, S.; Elder, T.; Felby, C. Lignin-Quinone Interactions: Implications for Optical Properties of Lignin. Chem. Mater. 2003, 15, 649–655. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Fu, S.; Chen, Y. High-value utilization of kraft lignin: Color reduction and evaluation as sunscreen ingre-dient. Int. J. Biol. Macromol. 2019, 133, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, I.D.M.; Avelino, F.; De Oliveira, D.R.; Souza, N.F.; Rosa, M.F.; Mazzetto, S.E.; Lomonaco, D. Organic solvent fractionation of acetosolv palm oil lignin: The role of its structure on the antioxidant activity. Int. J. Biol. Macromol. 2019, 122, 1163–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhunia, K.; Sablani, S.S.; Tang, J.; A Rasco, B. Migration of Chemical Compounds from Packaging Polymers during Microwave, Conventional Heat Treatment, and Storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef]
- Schmidt, B.; Katiyar, V.; Plackett, D.; Larsen, E.H.; Gerds, N.; Koch, C.B.; Petersen, J.H. Migration of nanosized layered double hydroxide platelets from polylactide nanocomposite films. Food Addit. Contam. Part A 2011, 28, 956–966. [Google Scholar] [CrossRef]
- Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(vinyl alcohol) Films for Food Packaging. Polymers 2019, 11, 1999. [Google Scholar] [CrossRef] [Green Version]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- ISO20200:2005-Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test; BSI: London, UK, 2005.
- Fukushima, K.; Tabuani, D.; Abbate, C.; Arena, M.; Ferreri, L. Effect of sepiolite on the biodegradation of poly(lactic acid) and polycaprolactone. Polym. Degrad. Stab. 2010, 95, 2049–2056. [Google Scholar] [CrossRef]
- Luzi, F.; Dominici, F.; Armentano, I.; Fortunati, E.; Burgos, N.; Fiori, S.; Jiménez, A.; Kenny, J.M.; Torre, L. Combined effect of cellulose nanocrystals, carvacrol and oligomeric lactic acid in PLA_PHB polymeric films. Carbohydr. Polym. 2019, 223, 115131. [Google Scholar] [CrossRef]
- Rahouti, M.; Steiman, R.; Seigle-Murandi, F.; Christov, L.P. Growth of 1044 strains and species of fungi on 7 phenolic lignin model compounds. Chemosphere 1999, 38, 2549–2559. [Google Scholar] [CrossRef]
- Baurhoo, B.; Ruiz-Feria, C.; Zhao, X. Purified lignin: Nutritional and health impacts on farm animals—A review. Anim. Feed. Sci. Technol. 2008, 144, 175–184. [Google Scholar] [CrossRef]
- Qin, L.; Li, W.-C.; Liu, L.; Zhu, J.-Q.; Li, X.; Li, B.-Z.; Yuan, Y.-J. Inhibition of lignin-derived phenolic compounds to cellulase. Biotechnol. Biofuels 2016, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemek, J.; Košíková, B.; Augustín, J.; Joniak, D. Antibiotic properties of lignin components. Folia Microbiol. 1979, 24, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Cazacu, G.; Capranu, M.; Popa, V.I. Advances concerning lignin utilization in new materials. In Advances in Natural Polymers; Thomas, S., Visakh, P.M., Mathew, A.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 18, pp. 278–308. [Google Scholar]
- Dumitriu, S.; Popa, V. Polymeric Biomaterials. Medicinal and Pharmaceutical Applications; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2013; Volume 1. [Google Scholar]
- Yang, W.; Kenny, J.M.; Puglia, D. Structure and properties of biodegradable wheat gluten bionanocomposites containing lignin nanoparticles. Ind. Crop. Prod. 2015, 74, 348–356. [Google Scholar] [CrossRef]
- He, X.; Luzi, F.; Hao, X.; Yang, W.; Torre, L.; Xiao, Z.; Xie, X.; Puglia, D. Thermal, antioxidant and swelling behavior of transparent polyvinyl (alcohol) films in presence of hydrophobic citric acid-modified lignin nanoparticles. Int. J. Biol. Macromol. 2019, 127, 665–676. [Google Scholar] [CrossRef] [PubMed]









| Formulation | PLA | LNP | caLNP | aLNP |
|---|---|---|---|---|
| wt % | wt % | wt % | wt % | |
| PLA | 100 | 0 | 0 | 0 |
| PLA/1LNP | 99 | 1 | 0 | 0 |
| PLA/3LNP | 97 | 3 | 0 | 0 |
| PLA/1caLNP | 99 | 0 | 1 | 0 |
| PLA/3caLNP | 97 | 0 | 3 | 0 |
| PLA/1aLNP | 99 | 0 | 0 | 1 |
| PLA/3aLNP | 97 | 0 | 0 | 3 |
| PLA/1hybridLNP | 99 | 0 | 0.5 | 0.5 |
| Formulation | Tonset (°C) | Tmax (°C) |
|---|---|---|
| PLA | 293 | 345 |
| PLA/1LNP | 290 | 339 |
| PLA/3LNP | 291 | 342 |
| PLA/1caLNP | 289 | 341 |
| PLA/3caLNP | 286 | 334 |
| PLA/1aLNP | 295 | 339 |
| PLA/3aLNP | 293 | 335 |
| PLA/1hybridLNP | 292 | 332 |
| Formulation | Transmittance (%) |
|---|---|
| PLA | 93 |
| PLA/1LNP | 75 |
| PLA/3LNP | 56 |
| PLA/1caLNP | 86 |
| PLA/3caLNP | 74 |
| PLA/1aLNP | 84 |
| PLA/3aLNP | 70 |
| PLA/1hybridLNP | 85 |
| Formulations | Overall Migration | |
|---|---|---|
| Ethanol 10% (v/v) (mg kg−1) @ 10 days 40 °C | Ethanol 50% (v/v) (mg kg−1) @ 10 days 40 °C | |
| PLA | 13.3 ± 2.3 | 13.4 ± 0.3 |
| PLA/1LNP | 13.1 ± 1.4 | 12.5 ± 2.2 |
| PLA/3LNP | 13.9 ± 0.9 | 15.4 ± 1.3 |
| PLA/1caLNP | 9.4 ± 0.9 | 13.4 ± 0.9 |
| PLA/3caLNP | 9.6 ± 0.6 | 13.2 ± 1.3 |
| PLA/1aLNP | 13.7 ± 0.6 | 13.4 ± 0.9 |
| PLA/3aLNP | 12.1 ± 1.7 | 11.9 ± 1.3 |
| PLA/1hybridLNP | 13.1 ± 2.0 | 12.5 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallo, E.; He, X.; Luzi, F.; Dominici, F.; Cerrutti, P.; Bernal, C.; Foresti, M.L.; Torre, L.; Puglia, D. UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles. Molecules 2021, 26, 126. https://doi.org/10.3390/molecules26010126
Cavallo E, He X, Luzi F, Dominici F, Cerrutti P, Bernal C, Foresti ML, Torre L, Puglia D. UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles. Molecules. 2021; 26(1):126. https://doi.org/10.3390/molecules26010126
Chicago/Turabian StyleCavallo, Ema, Xiaoyan He, Francesca Luzi, Franco Dominici, Patricia Cerrutti, Celina Bernal, Maria Laura Foresti, Luigi Torre, and Debora Puglia. 2021. "UV Protective, Antioxidant, Antibacterial and Compostable Polylactic Acid Composites Containing Pristine and Chemically Modified Lignin Nanoparticles" Molecules 26, no. 1: 126. https://doi.org/10.3390/molecules26010126