Structural Diversity of Peptoids: Tube-Like Structures of Macrocycles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of Cyclic Peptoids
2.2. Topology of Cyclic Peptoids
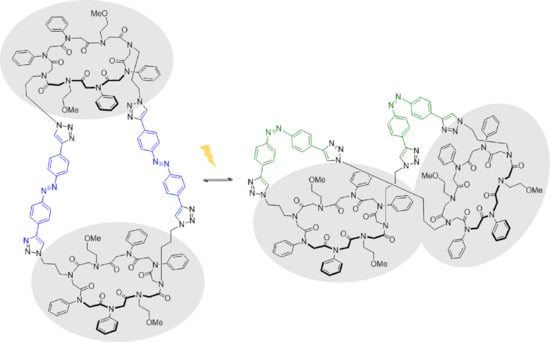
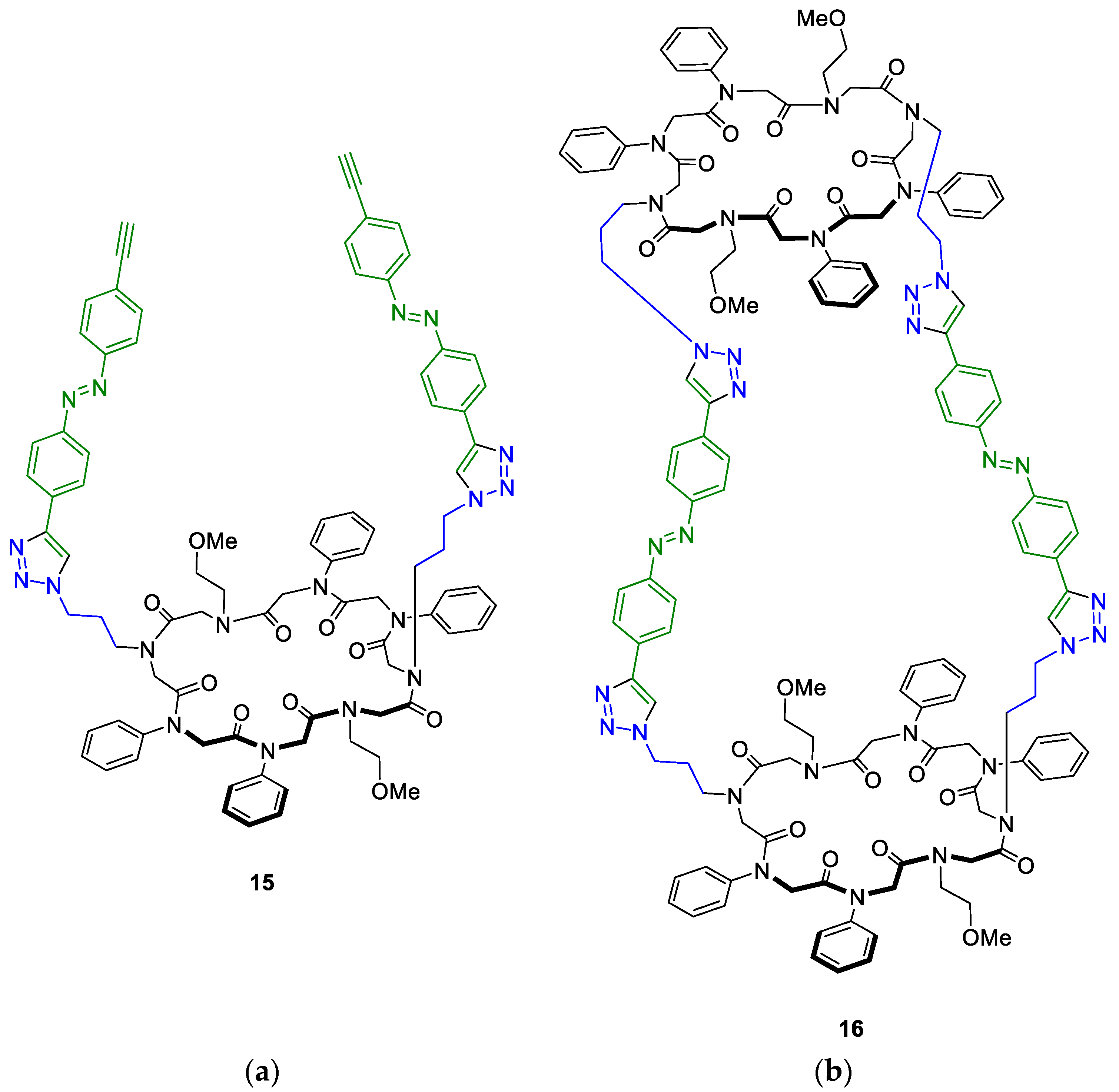
2.3. CuAAC of Two Cyclic Peptoids
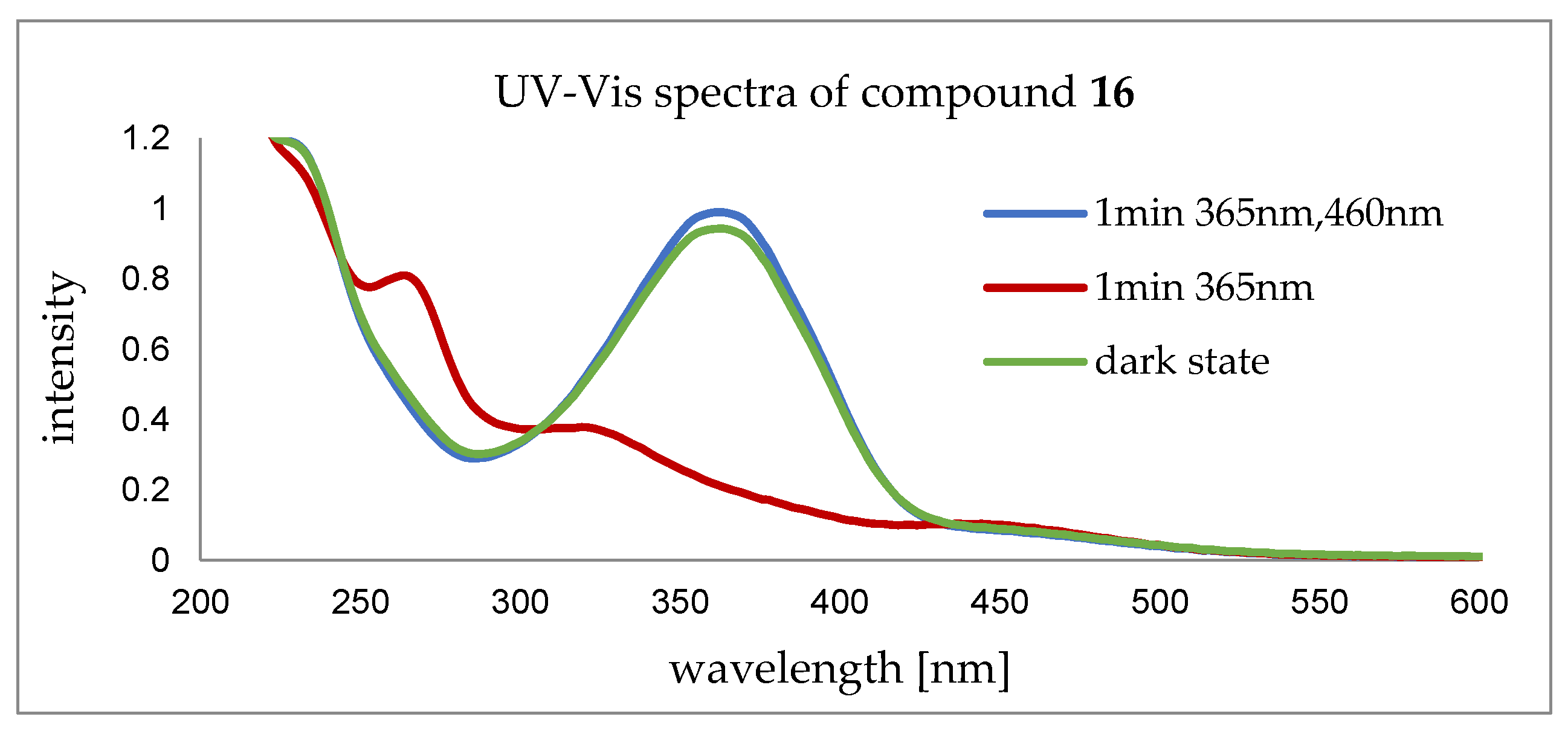
2.4. CuAAC of Cyclic Peptoids and Small Molecules
3. Materials and Methods
Crystal Structure Determinations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shin, S.B.; Yoo, B.; Todaro, L.J.; Kirshenbaum, K. Cyclic peptoids. J. Am. Chem. Soc. 2007, 129, 3218–3225. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Shin, S.B.Y.; Huang, M.L.; Kirshenbaum, K. Peptoid macrocycles: Making the rounds with peptidomimetic oligomers. Chem. Eur. J. 2010, 16, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.M.; Cobb, S.L. Recent advances in the synthesis of peptoid macrocycles. Chem. Eur. J. 2018, 24, 7560–7573. [Google Scholar] [CrossRef]
- Baldauf, C.; Günther, R.; Hofmann, H.-J. Helices in peptoids of α-and β-peptides. Phys. Biol. 2006, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, T.J.; Wu, C.W.; Zuckermann, R.N.; Barron, A.E. Extreme stability of helices formed by water-soluble poly-n-substituted glycines (polypeptoids) with α-chiral side chains. Biopolymers 2002, 63, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Stringer, J.R.; Crapster, J.A.; Guzei, I.A.; Blackwell, H.E. Extraordinarily robust polyproline type i peptoid helices generated via the incorporation of α-chiral aromatic n-1-naphthylethyl side chains. J. Am. Chem. Soc. 2011, 133, 15559–15567. [Google Scholar] [CrossRef] [Green Version]
- Mannige, R.V.; Haxton, T.K.; Proulx, C.; Robertson, E.J.; Battigelli, A.; Butterfoss, G.L.; Zuckermann, R.N.; Whitelam, S. Peptoid nanosheets exhibit a new secondary-structure motif. Nature 2015, 526, 415–420. [Google Scholar] [CrossRef]
- Robertson, E.J.; Oliver, G.K.; Qian, M.; Proulx, C.; Zuckermann, R.N.; Richmond, G.L. Assembly and molecular order of two-dimensional peptoid nanosheets through the oil-water interface. Proc. Natl. Acad. Sci. USA 2014, 111, 13284–13289. [Google Scholar] [CrossRef] [Green Version]
- Kudirka, R.; Tran, H.; Sanii, B.; Nam, K.T.; Choi, P.H.; Venkateswaran, N.; Chen, R.; Whitelam, S.; Zuckermann, R.N. Folding of a single-chain, information-rich polypeptoid sequence into a highly ordered nanosheet. Biopolymers 2011, 96, 586–595. [Google Scholar] [CrossRef]
- Sanii, B.; Kudirka, R.; Cho, A.; Venkateswaran, N.; Olivier, G.K.; Olson, A.M.; Tran, H.; Harada, R.M.; Tan, L.; Zuckermann, R.N. Shaken, not stirred: Collapsing a peptoid monolayer to produce free-floating, stable nanosheets. J. Am. Chem. Soc. 2011, 133, 20808–20815. [Google Scholar] [CrossRef]
- Smith, P.T.; Huang, M.L.; Kirshenbaum, K. Osmoprotective polymer additives attenuate the membrane pore-forming activity of antimicrobial peptoids. Biopolymers 2015, 103, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Shin, S.B.Y.; Benson, M.A.; Torres, V.J.; Kirshenbaum, K. A comparison of linear and cyclic peptoid oligomers as potent antimicrobial agents. ChemMedChem 2012, 7, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Chongsiriwatana, N.P.; Patch, J.A.; Czyzewski, A.M.; Dohm, M.T.; Ivankin, A.; Gidalevitz, D.; Zuckermann, R.N.; Barron, A.E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 2794–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolt, H.L.; Eggimann, G.A.; Jahoda, C.A.B.; Zuckermann, R.N.; Sharples, G.J.; Cobb, S.L. Exploring the links between peptoid antibacterial activity and toxicity. MedChemComm 2017, 8, 886–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojsoska, B.; Jenssen, H. Peptides and peptidomimetics for antimicrobial drug design. Pharmaceuticals 2015, 8, 366–415. [Google Scholar] [CrossRef]
- Mojsoska, B.; Zuckermann, R.N.; Jenssen, H. Structure-activity relationship study of novel peptoids that mimic the structure of antimicrobial peptides. Antimicrob. Agents Chemother. 2015, 59, 4112–4120. [Google Scholar] [CrossRef] [Green Version]
- Czyzewski, A.M.; Jenssen, H.; Fjell, C.D.; Waldbrook, M.; Chongsiriwatana, N.P.; Yuen, E.; Hancock, R.E.; Barron, A.E. In vivo, in vitro, and in silico characterization of peptoids as antimicrobial agents. PLoS ONE 2016, 11, e0135961. [Google Scholar] [CrossRef] [Green Version]
- Jahnsen, R.D.; Frimodt-Moller, N.; Franzyk, H. Antimicrobial activity of peptidomimetics against multidrug-resistant Escherichia coli: A comparative study of different backbones. J. Med. Chem. 2012, 55, 7253–7261. [Google Scholar] [CrossRef]
- Mojsoska, B.; Carretero, G.; Larsen, S.; Mateiu, R.V.; Jenssen, H. Peptoids successfully inhibit the growth of gram negative E. coli causing substantial membrane damage. Sci. Rep. 2017, 7, 42332. [Google Scholar] [CrossRef] [Green Version]
- Bicker, K.L.; Cobb, S.L. Recent advances in the development of anti-infective peptoids. Chem. Comm. 2020, 56, 11158–11168. [Google Scholar] [CrossRef]
- Molchanova, N.; Nielsen, J.E.; Sørensen, K.B.; Prabhala, B.K.; Hansen, P.R.; Lund, R.; Barron, A.E.; Jenssen, H. Halogenation as a tool to tune antimicrobial activity of peptoids. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Choi, J.; Kumar, S.D.; Nielsen, J.E.; Kyeong, M.; Wang, S.; Kang, D.; Lee, Y.; Lee, J.; Yoon, M.-H. Helicity modulation improves the selectivity of antimicrobial peptoids. ACS Infect. Dis. 2020, 6, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Khara, J.S.; Mojsoska, B.; Mukherjee, D.; Langford, P.R.; Robertson, B.D.; Jenssen, H.; Ee, P.L.R.; Newton, S.M. Ultra-short antimicrobial peptoids show propensity for membrane activity against multi-drug resistant mycobacterium tuberculosis. Front. Microbol. 2020, 11, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohm, M.T.; Kapoor, R.; Barron, A.E. Peptoids: Bio-inspired polymers as potential pharmaceuticals. Curr. Pharm. Des. 2011, 17, 2732–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuckermann, R.N.; Kodadek, T. Peptoids as potential therapeutics. Curr. Opin. Mol. Ther. 2009, 11, 299–307. [Google Scholar]
- Rhodes, C.A.; Pei, D. Bicyclic peptides as next-generation therapeutics. Chem. Eur. J. 2017, 23, 12690–12703. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y.; Wang, M.; Jian, T.; Ding, S.; Mu, P.; Liao, Z.; Shi, Q.; Cai, X.; Jin, H. Bioinspired peptoid nanotubes for targeted tumor cell imaging and chemo-photodynamic therapy. Small 2019, 15, 1902485. [Google Scholar] [CrossRef]
- Maayan, G.; Ward, M.D.; Kirshenbaum, K. Folded biomimetic oligomers for enantioselective catalysis. Proc. Natl. Acad. Sci. USA 2009, 106, 13679–13684. [Google Scholar] [CrossRef] [Green Version]
- Drapaneni, C.; Ghosh, P.; Ghosh, T.; Maayan, G. Unique β-turn peptoid structures and their application as asymmetric catalysts. Chem. Eur. J. 2020, 26, 9573–9579. [Google Scholar] [CrossRef]
- Olivier, G.K.; Cho, A.; Sanii, B.; Connolly, M.D.; Tran, H.; Zuckermann, R.N. Antibody-mimetic peptoid nanosheets for molecular recognition. ACS Nano 2013, 7, 9276–9286. [Google Scholar] [CrossRef]
- Liu, J.; Cai, B.; Cui, L.; Chen, C.-L. Peptoid-based hierarchically-structured biomimetic nanomaterials: Synthesis, characterization and applications. Sci. China Mater. 2020, 63, 1099–1112. [Google Scholar] [CrossRef] [Green Version]
- Battigelli, A.; Kim, J.H.; Dehigaspitiya, D.C.; Proulx, C.; Robertson, E.J.; Murray, D.J.; Rad, B.; Kirshenbaum, K.; Zuckermann, R.N. Glycosylated peptoid nanosheets as a multivalent scaffold for protein recognition. ACS Nano 2018, 12, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Kim, S.C.; Kline, M.A.; Grzincic, E.M.; Tresca, B.W.; Cardiel, J.; Karbaschi, M.; Dehigaspitiya, D.C.; Chen, Y.; Udumula, V. Discovery of stable and selective antibody mimetics from combinatorial libraries of polyvalent, loop-functionalized peptoid nanosheets. ACS Nano 2019, 14, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Song, Y.; Mu, P.; Cai, X.; Lin, Y.; Chen, C.-L. Peptoid-based programmable 2d nanomaterial sensor for selective and sensitive detection of h2s in live cells. ACS Appl. Bio Mater. 2020, 3, 6039–6048. [Google Scholar] [CrossRef]
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.H.; Moos, W.H. Efficient method for the preparation of peptoids [oligo(n-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Butterfoss, G.L.; Renfrew, P.D.; Kuhlman, B.; Kirshenbaum, K.; Bonneau, R. A preliminary survey of the peptoid folding landscape. J. Am. Chem. Soc. 2009, 131, 16798–16807. [Google Scholar] [CrossRef]
- Vollrath, S.B.; Bräse, S.; Kirshenbaum, K. Twice tied tight: Enforcing conformational order in bicyclic peptoid oligomers. Chem. Sci. 2012, 3, 2726–2731. [Google Scholar] [CrossRef]
- Vollrath, S.B.; Hu, C.; Bräse, S.; Kirshenbaum, K. Peptoid nanotubes: An oligomer macrocycle that reversibly sequesters water via single-crystal-to-single-crystal transformations. Chem. Comm. 2013, 49, 2317–2319. [Google Scholar] [CrossRef]
- Shah, N.H.; Butterfoss, G.L.; Nguyen, K.; Yoo, B.; Bonneau, R.; Rabenstein, D.L.; Kirshenbaum, K. Oligo(n-aryl glycines): A new twist on structured peptoids. J. Am. Chem. Soc. 2008, 130, 16622–16632. [Google Scholar] [CrossRef]
- Wijaya, A.W.; Nguyen, A.I.; Roe, L.T.; Butterfoss, G.L.; Spencer, R.K.; Li, N.K.; Zuckermann, R.N. Cooperative intramolecular hydrogen bonding strongly enforces cis-peptoid folding. J. Am. Chem. Soc. 2019, 141, 19436–19447. [Google Scholar] [CrossRef]
- Knight, A.S.; Zhou, E.Y.; Francis, M.B.; Zuckermann, R.N. Sequence programmable peptoid polymers for diverse materials applications. Adv. Mater. 2015, 27, 5665–5691. [Google Scholar] [CrossRef] [PubMed]
- Kolaskar, A.; Lakshminarayanan, A.; Sarathy, K.; Sasisekharan, V. The nonplanar peptide unit iii. Quantum chemical calculations for related compounds and experimental x-ray diffraction data. Biopolymers 1975, 14, 1081–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amato, A.; Pierri, G.; Tedesco, C.; Della Sala, G.; Izzo, I.; Costabile, C.; De Riccardis, F. Reverse turn and loop secondary structures in stereodefined cyclic peptoid scaffolds. J. Org. Chem. 2019, 84, 10911–10928. [Google Scholar] [CrossRef] [PubMed]
- De Riccardis, F. The challenge of conformational isomerism in cyclic peptoids. Eur. J. Org. Chem. 2020, 2020, 2981–2994. [Google Scholar] [CrossRef]
- D’Amato, A.; Della Sala, G.; Izzo, I.; Costabile, C.; Masuda, Y.; De Riccardis, F. Cyclic octamer peptoids: Simplified isosters of bioactive fungal cyclodepsipeptides. Molecules 2018, 23, 1779. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, C.; Erra, L.; Izzo, I.; De Riccardis, F. Solid state assembly of cyclic α-peptoids. CrystEngComm 2014, 16, 3667–3687. [Google Scholar] [CrossRef]
- Schettini, R.; Costabile, C.; Della Sala, G.; Iuliano, V.; Tedesco, C.; Izzo, I.; De Riccardis, F. Cation-induced molecular switching based on reversible modulation of peptoid conformational states. J. Org. Chem. 2018, 83, 12648–12663. [Google Scholar] [CrossRef]
- Yoo, B.; Kirshenbaum, K. Peptoid architectures: Elaboration, actuation, and application. Curr. Opin. Chem. Biol. 2008, 12, 714–721. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide–alkyne cycloadditions (cuaac): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(i)-catalyzed alkyne-azide cycloaddition (cuaac) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Holub, J.M.; Jang, H.; Kirshenbaum, K. Fit to be tied: Conformation-directed macrocyclization of peptoid foldamers. Org. Lett. 2007, 9, 3275–3278. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, R.; Holub, J.M.; Bollinger, M.; Kirshenbaum, K.; Finn, M. Peptide cyclization and cyclodimerization by cui-mediated azide− alkyne cycloaddition. J. Org. Chem. 2009, 74, 2964–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordel, C.; Popeney, C.S.; Haag, R. Photoresponsive amphiphiles based on azobenzene-dendritic glycerol conjugates show switchable transport behavior. Chem. Comm. 2011, 47, 6584–6586. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-X.; Stellacci, F.; McGrath, D.V. Photoswitchable flexible and shape-persistent dendrimers: Comparison of the interplay between a photochromic azobenzene core and dendrimer structure. J. Am. Chem. Soc. 2004, 126, 2181–2185. [Google Scholar] [CrossRef] [PubMed]
- Zeitouny, J.; Aurisicchio, C.; Bonifazi, D.; De Zorzi, R.; Geremia, S.; Bonini, M.; Palma, C.-A.; Samorì, P.; Listorti, A.; Belbakra, A. Photoinduced structural modifications in multicomponent architectures containing azobenzene moieties as photoswitchable cores. J. Mater. Chem. 2009, 19, 4715–4724. [Google Scholar] [CrossRef]
- Shah, N.H.; Kirshenbaum, K. Photoresponsive peptoid oligomers bearing azobenzene side chains. Org. Biomol. Chem. 2008, 6, 2516–2521. [Google Scholar] [CrossRef]
- Kimoto, A.; Iwasaki, K.; Abe, J. Formation of photoresponsive gold nanoparticle networks via click chemistry. Photochem. Photobiol. Sci. 2010, 9, 152–156. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with shelxl. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H. Precise absolute-structure determination in light-atom crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 2004, 60, 61. [Google Scholar] [CrossRef] [Green Version]











 | ||||
|---|---|---|---|---|
| Macrocycle | R1 | R2 | Yield | Purity |
| 7a | propargyl | H | 11% | 99% |
| 7b | propylazide | H | 25% | 98% |
| 7c | 2-methoxyethyl | ethynyl | 8.0% | 96% |
| 7d | 2-methoxyethyl | N3 | 9.0% | 87% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herlan, C.N.; Sommer, K.; Weis, P.; Nieger, M.; Bräse, S. Structural Diversity of Peptoids: Tube-Like Structures of Macrocycles. Molecules 2021, 26, 150. https://doi.org/10.3390/molecules26010150
Herlan CN, Sommer K, Weis P, Nieger M, Bräse S. Structural Diversity of Peptoids: Tube-Like Structures of Macrocycles. Molecules. 2021; 26(1):150. https://doi.org/10.3390/molecules26010150
Chicago/Turabian StyleHerlan, Claudine Nicole, Katharina Sommer, Patrick Weis, Martin Nieger, and Stefan Bräse. 2021. "Structural Diversity of Peptoids: Tube-Like Structures of Macrocycles" Molecules 26, no. 1: 150. https://doi.org/10.3390/molecules26010150
APA StyleHerlan, C. N., Sommer, K., Weis, P., Nieger, M., & Bräse, S. (2021). Structural Diversity of Peptoids: Tube-Like Structures of Macrocycles. Molecules, 26(1), 150. https://doi.org/10.3390/molecules26010150