The purpose of this study was to inhibit the recrystallization of the commercial B535.0 (Al–7Mg–0.15Ti) aluminum alloy and enhance its mechanical properties. Certainly, the above designed and experimental alloys could not fully or satisfactorily replace the functions of the B535.0 alloy. For one thing, the tensile test could not elucidate all the mechanical properties. For another, the chemical and physical properties of the alloys require further investigation. However, this study might offer a valuable reference for future researchers.
2.1. Microstructure Analysis
Figure 1a shows the as-cast optical microstructure of Alloy A (0Mn), and the crystal grains appeared to be equiaxed. The diameter of the crystal grains measured by the intercept method was about 180 μm, and it was found that there were many dendrites produced by non-equilibrium cooling in the crystal grains. The microstructures of Alloy A after the one-stage homogenization and the two-stage homogenization processes, respectively, are shown in
Figure 1b,c. The dendrites in the as-cast state were clearly observed. Whether the alloy was subjected to the one-stage homogenization or the two-stage homogenization process, the dendrites were eliminated. The grain size after the homogenization did not differ greatly from that of the as-cast grain.
Figure 1d shows the as-cast optical microstructure of the Mn-containing Alloy B (0.8Mn). The crystal grains were equiaxed dendritic grains with a diameter of about 98 µm. The microstructures after the homogenization are shown in
Figure 1e,f. The dendrites in the as-cast state were fully eliminated after both homogenization processes. The grain size after the homogenizations was also similar to the size of the as-cast grain.
It is evident that the grains of Alloy B (0.8Mn) were smaller than those of Alloy A (0Mn), whether in the as-cast state or after the homogenization. Therefore, the addition of Mn resulted in grain refinement of the casting grains in the aluminum–magnesium alloys [
3]. In addition, the Al
4Mn dispersed phase that precipitated during the homogenization process suppressed the growth of crystal grains. Similarly, the two kinds of homogenization heat treatments effectively eliminated the micro-segregation present in the as-cast alloy and achieved homogenization.
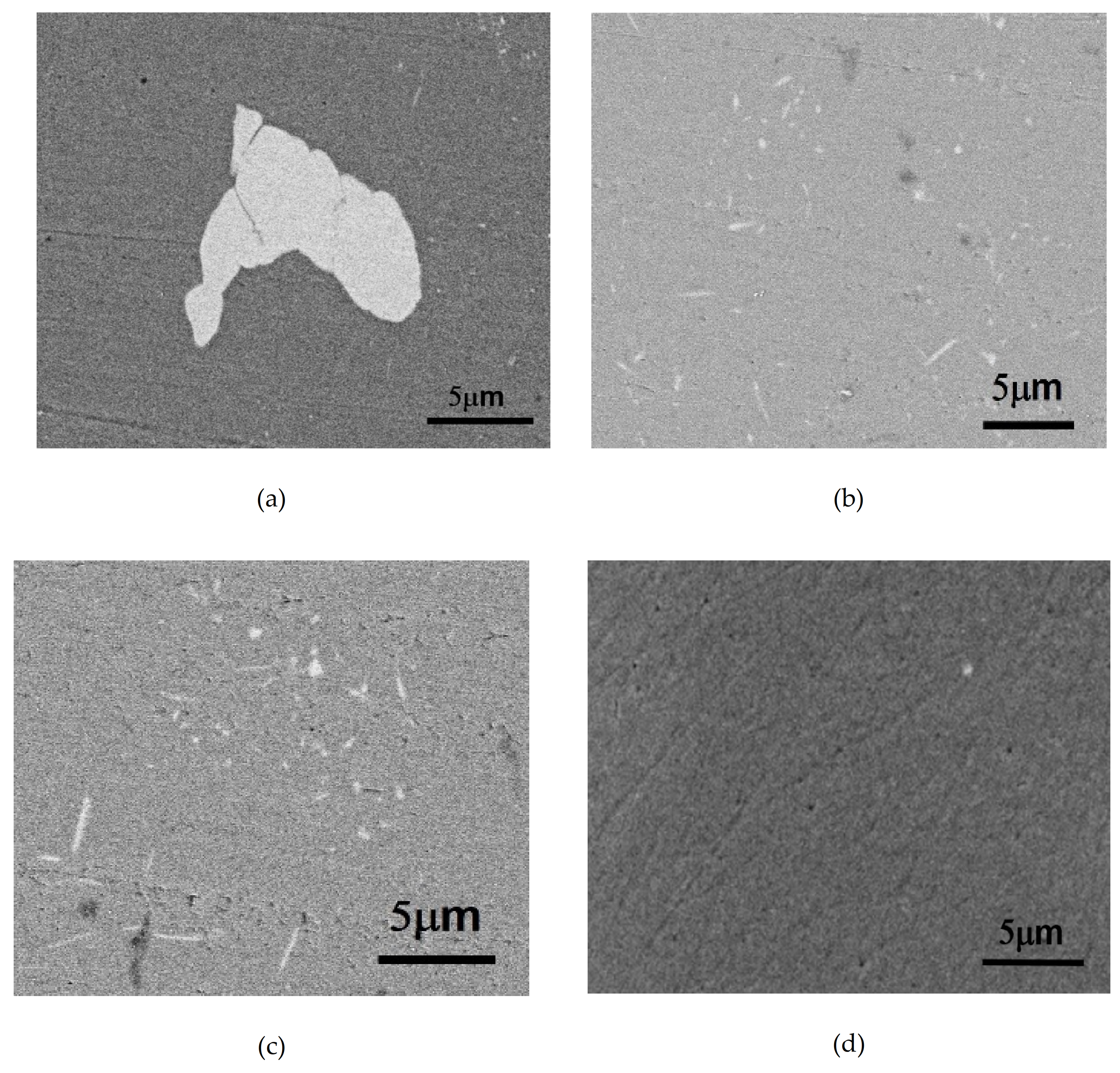
The Mn-containing as-cast Alloy B (0.8Mn) was observed with SEM-BEI (Backscattering Electron Image of Scanning Electron Microscope). As shown in
Figure 2a, the Fe-containing Al
6Mn crystallized phase existed at the grain boundary [
3]. However, no other crystalline phases were observed in the aluminum matrix. After Alloy B (0.8Mn) was subjected to the one-stage homogenization heat treatment (530 °C for 12 h) and the two-stage homogenization heat treatment (430 °C for 8 h + 530 °C for 10 h), respectively, many dispersoids were precipitated in the grains, as shown in
Figure 2b,c. By contrast,
Figure 2d shows that after the two-stage homogenization heat treatment of Alloy A (0Mn), no dispersoids were observed in the grains.
These precipitates in the grains of Alloy B (0.8Mn) after the homogenization heat treatment were analyzed by TEM diffraction analysis. They were hexagonal close-packed Al
4Mn particles of the high-temperature stable dispersoids. As shown in
Figure 3a,b, their sizes were approximately 400 and 250 nm, respectively. The high-temperature stable precipitated dispersoids of Alloy B (0.8Mn) after the two-stage homogenization heat treatment were smaller and denser than those after the one-stage homogenization heat treatment. That is, in the one-stage homogenization heat treatment (530 °C for 12 h), the resulting nucleated Al
4Mn dispersoids were coarser due to the higher temperature. Comparatively, in the first stage (the low-temperature homogenization heat treatment process (430 °C for 8 h)) of the two-stage homogenization (430 °C for 8 h + 530 °C for 10 h), a large amount of smaller Al
4Mn particles nucleated and precipitated. Despite the high temperature (530 °C) used in the second stage of the homogenization heat treatment process, it did not cause obvious coarsening of the Al
4Mn particles [
11].
Figure 3c shows the as-cast Alloy B (0.8Mn) without any homogenization treatment. The phase containing Al
4Mn particles was present in small amounts in the as-cast aluminum matrix. The coarse crystallized Al
6Mn particles during casting were observed only at the grain boundaries. As for Alloy A (0Mn), after the homogenization heat treatment, no precipitated phases were observed in the aluminum matrix, as shown in
Figure 3d.
As shown in
Figure 4a, the microstructure of Alloy A (0Mn), after the one-stage homogenization heat treatment, 420 °C hot rolling (80%), and 400 °C full annealing for 2.5 h, consisted of fully recrystallized equiaxed grains. The size of the grain, as calculated by the intercept method, was approximately 60 µm. The microstructure of Alloy A (0Mn) after the two-stage homogenization heat treatment and full annealing showed no difference from that in
Figure 4b. In
Figure 4b, the residual direction of hot rolling could also be observed roughly, mainly because the amount of hot working per pass was less than 10%. Before each pass of the hot rolling process, the alloy was preheated at 420 °C for 5 min, so the accumulated stored energy in the alloy was very low during the process. Throughout the annealing, the orientation of the crystals after hot rolling was retained.
Figure 4b,c show the microstructures of Alloy B (0.8Mn) after the one-stage and the two-stage homogenization heat treatments and full annealing, respectively. Similarly, both had a completely recrystallized equiaxed grain morphology. However, the crystal grains of Alloy B (0.8Mn) were evidently finer than those of Alloy A (0Mn). Their grain sizes were approximately 38 and 29 µm, respectively. This was due to the Al
4Mn dispersoids in Alloy B. The findings of previous studies [
5,
9] confirmed that Al
4Mn dispersoids became nucleation points for annealing recrystallization. That is, Al
4Mn dispersoids promoted recrystallization. As shown in
Figure 3a,b, for Alloy B (0.8Mn) after the two-stage homogenization heat treatment, the high-temperature stable precipitated Al
4Mn dispersoids were denser than those after the one-stage homogenization heat treatment and became the second crystal nucleation points. Moreover, the finer and denser Al
4Mn dispersoids more effectively limited the movement of the grain boundaries. As a result, the grains of Alloy B (0.8Mn) after the two-stage homogenization heat treatment were the finest, while those of Alloy A (0Mn) were the coarsest.
Figure 3c shows the grains of Alloy B (0.8Mn) after the two-stage homogenization heat treatment. The Al
4Mn dispersoids were shown-using TEM to exist at the annealed grain boundaries, indicating their inhibition of grain boundary movement.
After the two-stage homogenization heat treatment, the Al–7Mg–0.15Ti alloys were hot rolled, fully annealed, and then subjected to 60% room-temperature cold rolling. The microstructures of Alloy A (0Mn) and Alloy B (0.8Mn) are shown in
Figure 5a and
Figure 5b, respectively. The alloys had plastic deformation after cold working at room temperature, so the crystal grains all had a slender, plastically deformed structure. After careful observation, it was found that Alloy B (0.8Mn) was denser than Alloy A (0Mn). It showed that the presence of Al
4Mn dispersoids suppressed dislocations and increased the grain strengthening in Alloy B (0.8Mn). When the alloy was subjected to the one-stage homogenization heat treatment, the 60% cold-rolled microstructures of Alloy A (0Mn) and Alloy B (0.8Mn) were very similar, as shown in
Figure 5a and
Figure 5b, respectively. It was reasonable to speculate that after the one-stage homogenization, the density of the 60% cold-rolled Alloy B (0.8Mn) structure should be slightly lower than that after the two-stage homogenization; however, the difference could not be observed under the optical microscope (OM, Olympus BX60M, Shinjuku, Tokyo, Japan).
Figure 6 shows the microstructures of the Al–7Mg–0.15Ti alloy after cold working and annealing at 400 °C by means of EBSD analysis. Alloy A (0Mn), after the one-stage homogenization heat treatment, was completely recrystallized in only 0.5 h. As shown in
Figure 6a, the crystal grains had an equiaxed spherical structure. By means of EBSD software analysis, the grain size was approximately 45 µm. As the annealing time increased to one hour, the crystal grains grew significantly. Their size was approximately 53 µm, as shown in
Figure 6b. In addition, when Alloy A (0Mn) underwent the two-stage homogenization heat treatment, its degree of recrystallization was the same as that obtained after the one-stage homogenization heat treatment. The alloy grains also grew significantly as the annealing time increased. After the homogenization heat treatment of Alloy A (0Mn), because there were no Al
4Mn dispersoids precipitated in the aluminum matrix, annealing at 400 °C neither promoted recrystallization nucleation nor inhibited grain growth. Therefore, when the annealing times were the same, there was no difference in the sizes of the crystal grains whether the alloys underwent the one-stage or two-stage homogenization heat treatment. As shown in
Figure 6b,d, the crystal grains had normal grain growth with the increase in the recrystallization time, and were equiaxed, as shown in
Figure 6a,b.
Figure 7 shows the microstructures of Alloy B (0.8Mn) after cold working and annealing at 400 °C by means of EBSD analysis. Similarly, Alloy B (0.8Mn) that underwent the one-stage homogenization heat treatment was completely recrystallized in 0.5 h. As shown in
Figure 7a, average grain size was only approximately 30 µm, as determined by EBSD software analysis. As the annealing time increased to 1 h, the recrystallized grains had no obvious growth, as shown in
Figure 7b. In addition, after the two-stage homogenization heat treatment, the degree of recrystallization was the same as that after the one-stage homogenization heat treatment; however, the average grain size was smaller (only approximately 26 μm) as shown in
Figure 7c,d, and the crystal grains did not grow with the increase in the annealing time.
By measuring the change in conductivity (%IACS), the microstructures and precipitation states of the aluminum alloys were determined. The concentration of point defects had the greatest influence on the conductivity of the alloys. The way in which point defects occurred in the aluminum–magnesium alloys was mainly because of crystal lattice distortion caused by the solid dissolution of Mg and Mn atoms in the aluminum matrix and the dislocation pile-up caused during the processing.
Table 1 shows the electrical conductivities of the alloys after various processing conditions.
After 60% cold rolling of Alloy A (0Mn), the crystal grains became the fibrous structure due to plastic deformation, as shown in
Figure 6a. Large numbers of point defects and dislocations accumulated inside the crystal grains, resulting in a lower conductivity of the alloy in the cold-rolled state than in the recrystallized state. Even after different homogenization heat treatments, the total amount of solid solution elements were similar, so the electrical conductivity did not significantly differ. It can be seen from
Table 1 that the conductivity (%IACS) of the cold-worked Alloy A (0Mn) was approximately 26.4%. By contrast, the conductivity of manganese (6.9 × 105 Ω
−1 m
−1) was not as good as that of aluminum (3.8 × 107 Ω
−1 m
−1), so the conductivity of Alloy B (0.8Mn) was lower than that of Alloy A (0Mn). The conductivity of Alloy B (0.8Mn) was approximately 23.3. There was a difference of approximately 12% between the two alloys.
After the cold-rolled alloys were recrystallized and annealed, large numbers of dislocations and point defects were eliminated, resulting in an increase in the conductivity of the alloys. The electrical conductivity (%IACS) of Alloy A (0Mn) and Alloy B (0.8Mn) were approximately 27.4 and 24, respectively. It can also be seen from
Table 1 that when the annealing time was increased to 1 h, the conductivity of the alloys did not change significantly. This means that after annealing for 0.5 h, all the point defects and dislocations in the alloys were almost in equilibrium. The factors affecting the conductivity of the alloys were fully eliminated, so even if the alloys were annealed for a longer time, the conductivity of the alloy could not be improved further. This result was consistent with the above-mentioned micro-structural observation in
Figure 6 and
Figure 7.
Table 1 also shows that although the homogenization had little effect on the conductivity of Alloy B (0.8Mn) in the recrystallized state, with a change of approximately 1%, it was still higher than that of Alloy A (0Mn). This might be because the two-stage homogenization heat treatment precipitated denser Al
4Mn high-temperature stable dispersoids than those of the one-stage homogenization heat treatment. As a result, the alloys that underwent the two-stage homogenization heat treatment had lower conductivities.
2.2. Mechanical Properties Tests
The results of the mechanical properties tests of Al–7Mg–0.15Ti alloys in different states are summarized in
Table 2 and
Figure 8. It can be seen from
Table 2 and
Figure 8a that whether Alloy B (0.8Mn) was subjected to the one-stage or two-stage homogenization heat treatment, the hardness, strength, and ductility were significantly improved to approximately 5.9% to 30% higher, respectively, than those of the Alloy A (0Mn). It is worth noting that after the two-stage homogenization, the values of the hardness, strength, and ductility of Alloy B (0.8Mn) were approximately 2.1–6.4% higher than the corresponding values after the one-stage homogenization. However, Alloy A (0Mn) had no such differences. This result can be explained by the changes in the microstructures discussed in the previous section. That is, Alloy B (0.8Mn) had the finest and densest Al
4Mn thermally stable phase dispersoids after the two-stage homogenization. As a result, it had the best grain dispersion strengthening and the highest processing strengthening, as shown in
Figure 5b. Furthermore, it had the highest mechanical strength and hardness in the 60% cold-working condition.
Despite the homogenization heat treatment that the recrystallized Alloy A (0Mn) was subjected to, if the annealing time was the same, the mechanical properties were similar. After annealing at 400 °C for 0.5 h as shown in
Figure 8b, work hardening was fully eliminated. As a result, the hardness and strength of the alloy were significantly reduced for the 20% cold-rolled state compared to those of the 60% cold-rolled state, but the ductility greatly increased from approximately 7% to 20%. Similarly, as the annealing time increased to 1 h, it can be seen from the microstructures in
Figure 6 that the alloy grains grew and became coarser. Consequently, the grain strengthening effect was reduced, and the mechanical properties of the alloy also changed slightly. The strength of the alloy decreased by approximately 1.7%, but its ductility increased from approximately 20.5% to 22.5%.
After the different homogenizations and annealing at 400 °C for 0.5 h, the processing strengthening of the recrystallized Alloy B (0.8Mn) was also fully eliminated as with that of Alloy A (0Mn). Its strength was greatly reduced by approximately 20%, but its ductility was greatly increased from approximately 8% to about 18%, compared to the strength and ductility in the 60% cold-rolled state. However, unlike Alloy A (0Mn), when the annealing time was increased to 1 h as shown
Figure 8c, the mechanical properties of the alloy differed little from those when the annealing time was 0.5 h. These phenomena might be due to the fact that the dispersion strengthening of Al
4Mn’s high-temperature stable dispersoids and the fine grain strengthening of the crystal grains did not change significantly despite the increased annealing time.
In addition, it can be seen in
Table 2 and
Figure 8d that the hardness and strength of the recrystallized Alloy B (0.8Mn) after the two-stage homogenization were approximately 2.2% and 6.8% higher than the corresponding values after the one-stage homogenization. This was also due to the dispersion strengthening of the fine and dense Al
4Mn dispersoids of Alloy B (0.8Mn) through the two-stage homogenization, as shown in
Figure 5b.
Table 2 shows the comparison of the changes in the ductility (EL%) of Alloy A (0Mn) and Alloy B (0.8Mn) in each state. It can be found that in the recrystallized state at 400 °C, the ductility of the alloy decreased, while its strength increased. That was a common characteristic of the material. That is, in comparison with Alloy A (0Mn), the ductility of Alloy B (0.8Mn) decreased when its strength increased. However, the ductility of the 60% cold-rolled alloy was different. Its ductility increased with the increase in its strength, which was different from the characteristics of the general materials. This result might be related to the strain hardening rate of the alloy during the tensile test. According to Considère’s criterion and Hart’s criterion, if the alloy has a high strain hardening rate, the ductility of the alloy will increase as its strength increases. With the above description of the strengthening mechanism of the Al–7Mg–0.15Ti alloy, it was shown that the 60% cold-rolled Alloy B (0.8Mn) had more strengthening mechanisms than those in the recrystallized state. The Al–7Mg–0.15Ti alloy also benefited from solid solution, dispersion, and processing strengthening, resulting in a higher work hardening rate during the tensile test.