7?-(3-Ethyl-cis-crotonoyloxy)-1?-(2-methylbutyryloxy)-3,14-dehydro-Z Notonipetranone Attenuates Neuropathic Pain by Suppressing Oxidative Stress, Inflammatory and Pro-Apoptotic Protein Expressions
Abstract
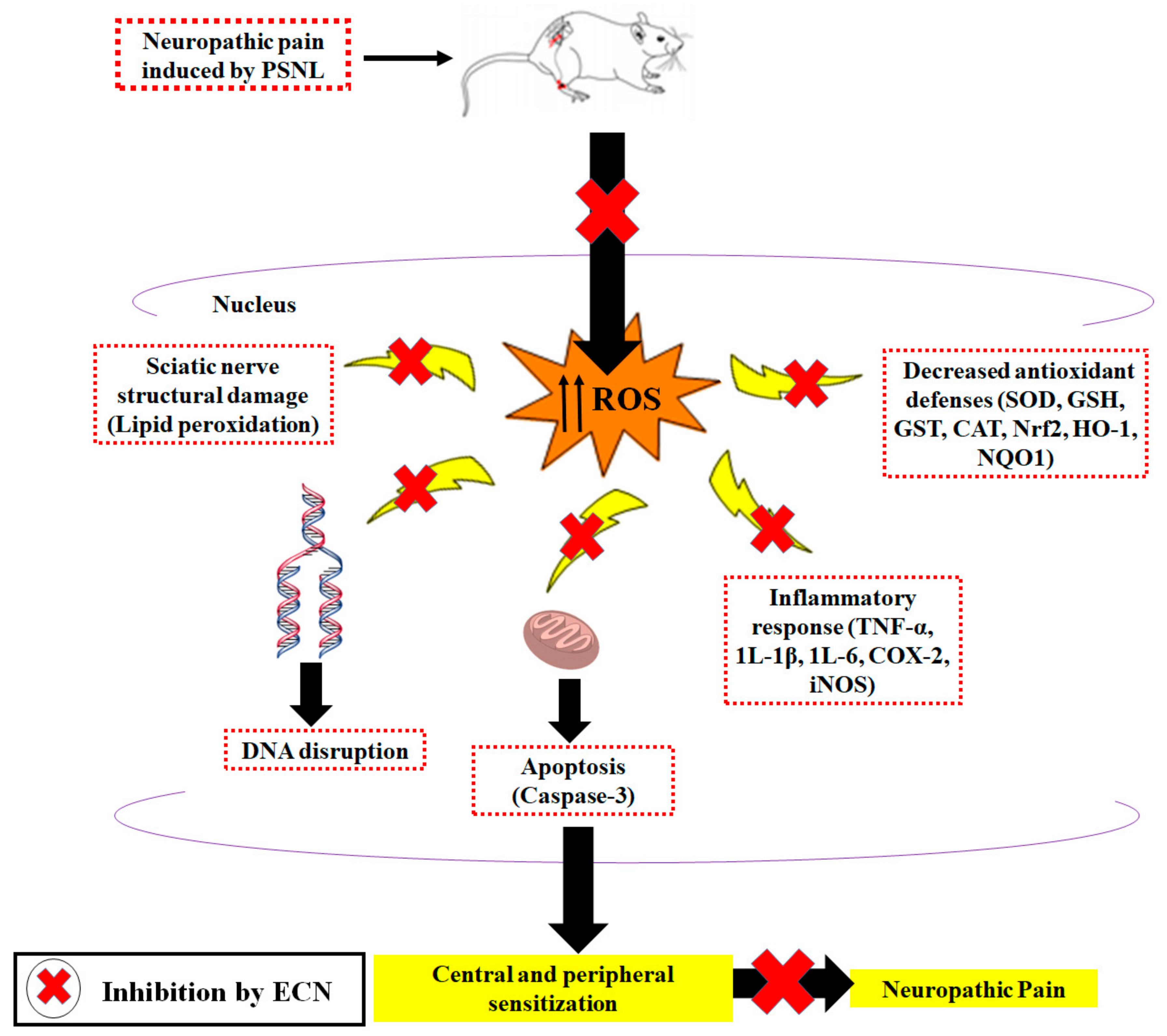
:1. Introduction
2. Results
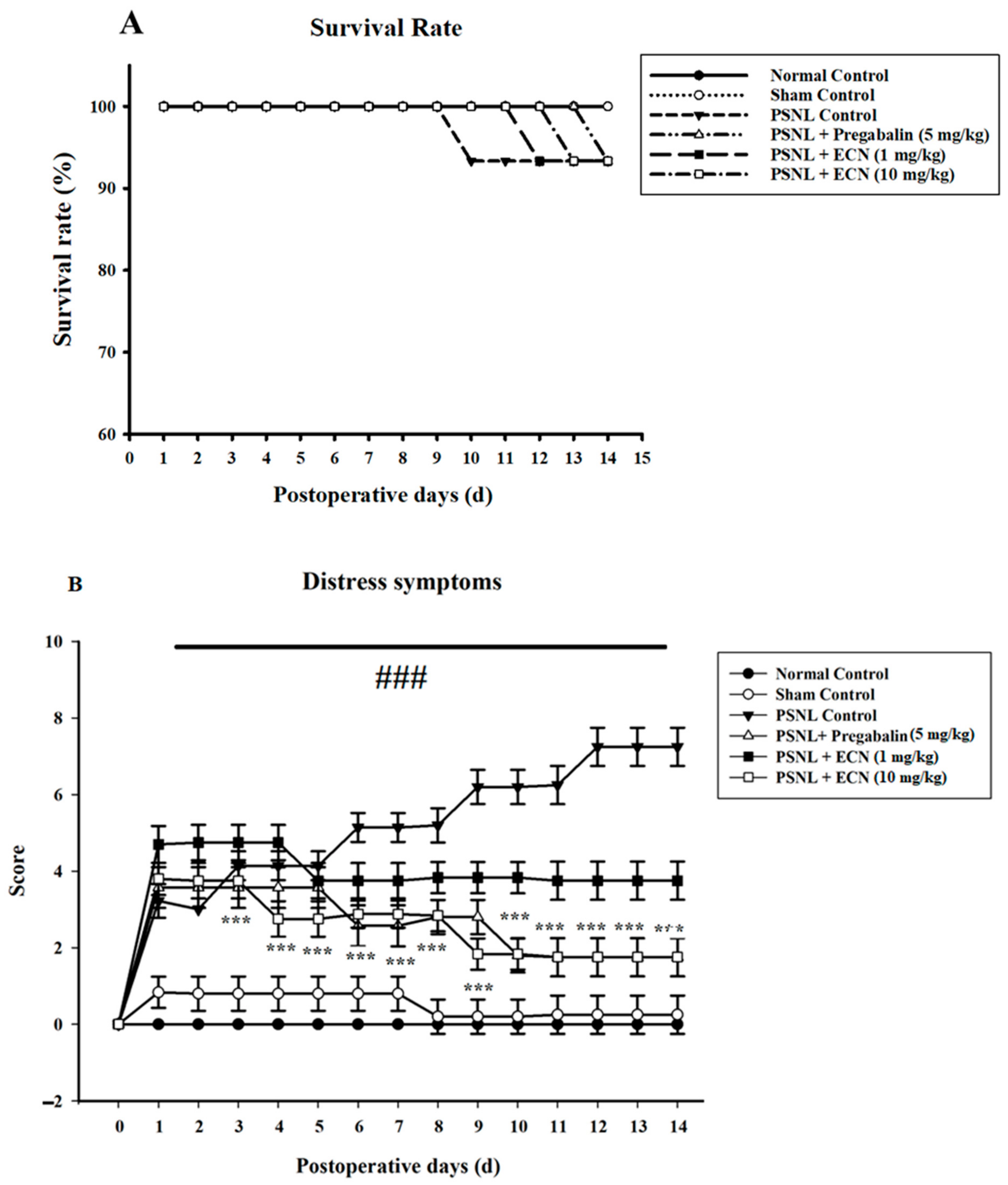
2.1. Effect of ECN on Distress Symptoms and Survival Rate
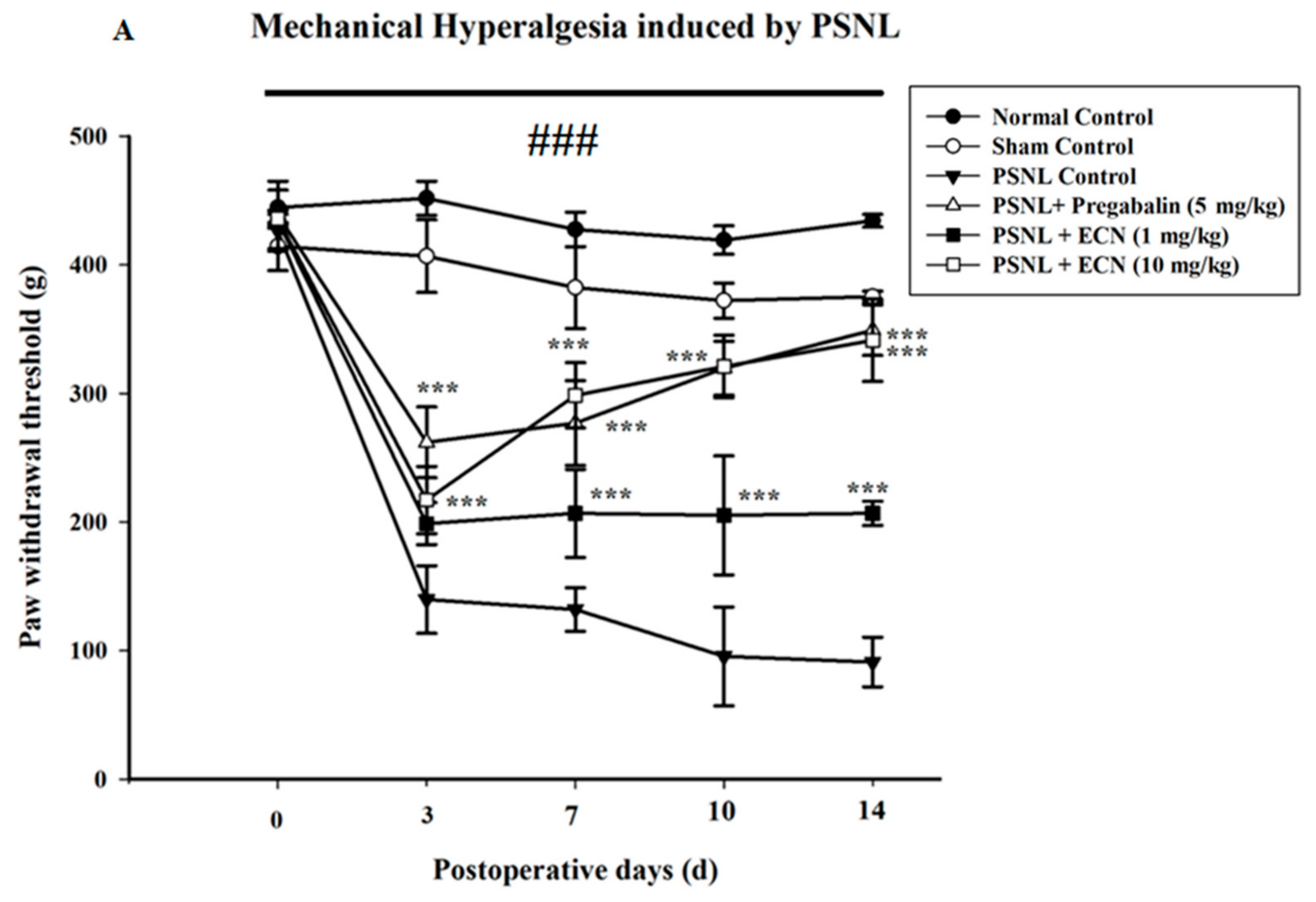
2.2. Effect of ECN on Neuropathic Mechanical Hyperalgesia, Thermal Hyperalgesia, Mechanical Allodynia and Cold Allodynia
2.3. Effect of ECN on Neuropathic Muscle and Motor Coordination
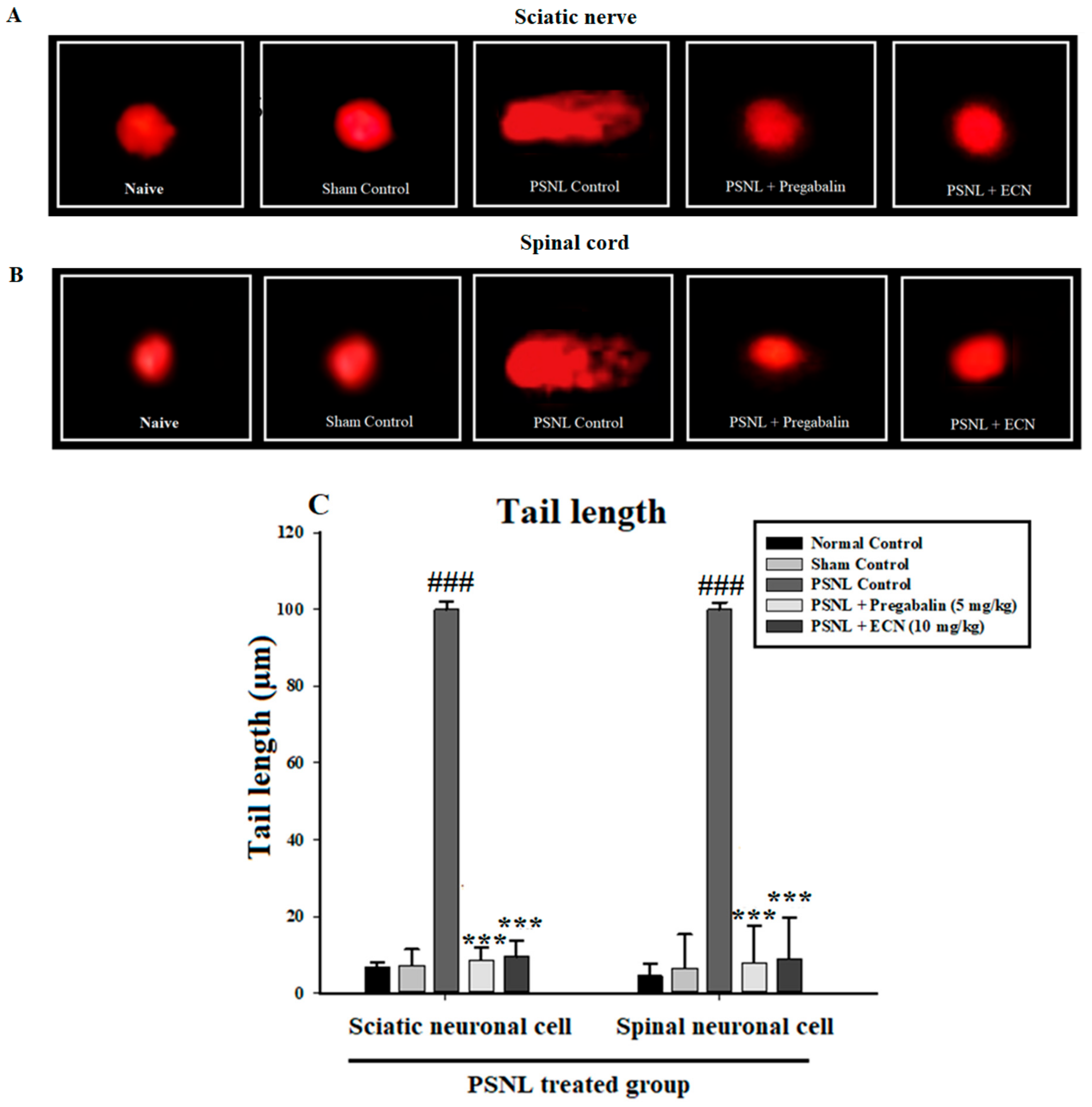
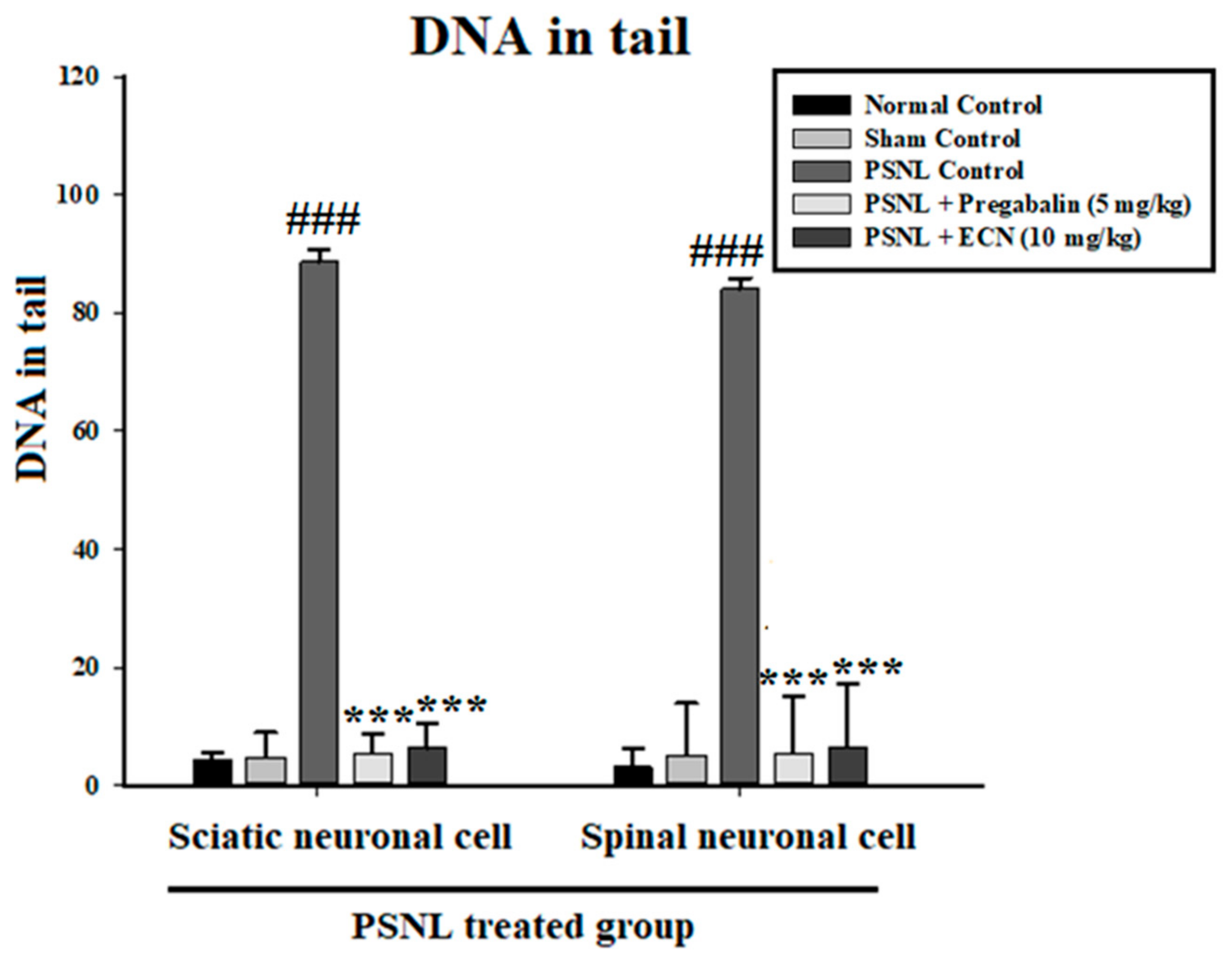
2.4. Effect of ECN on DNA Disruption
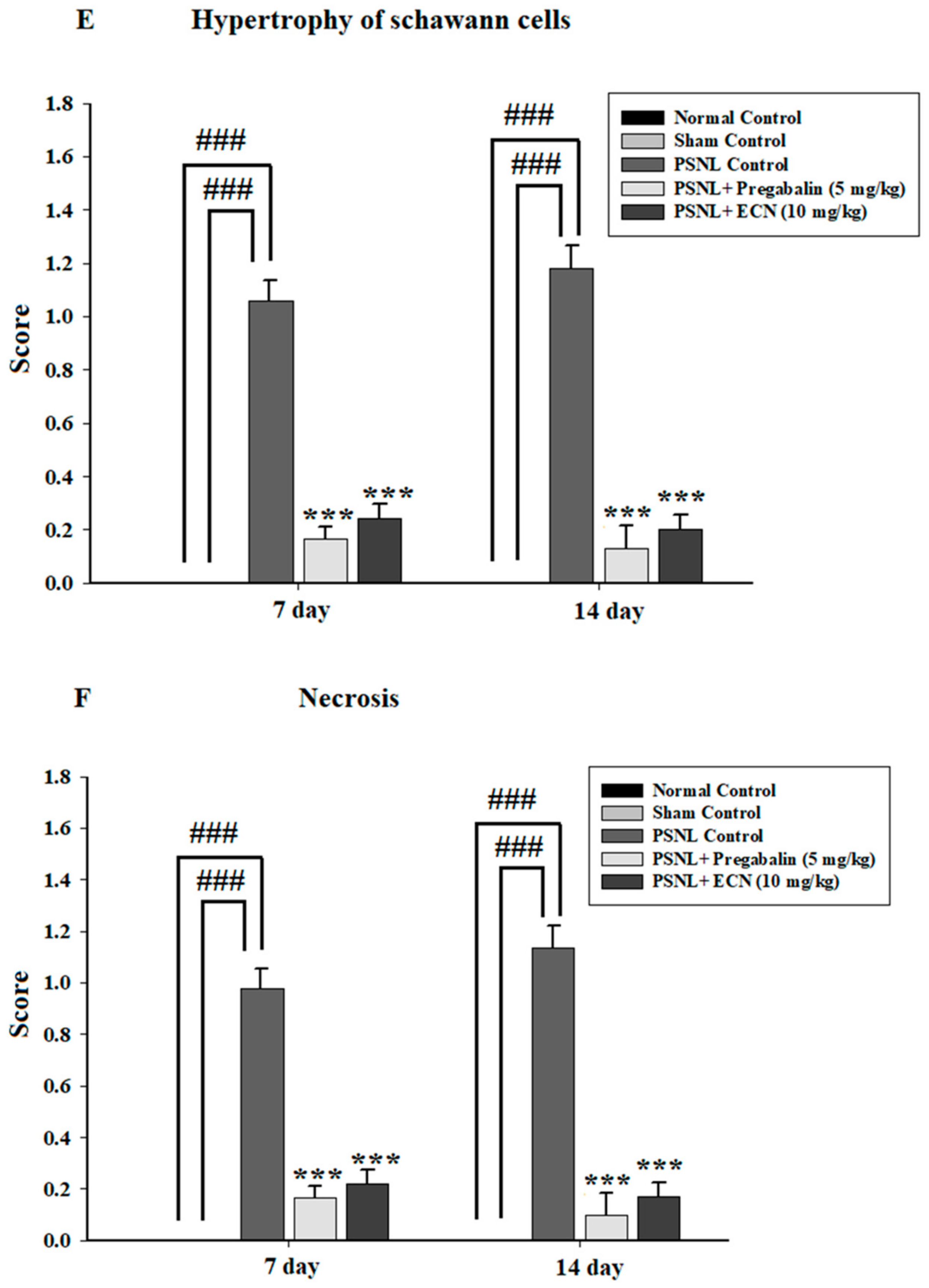
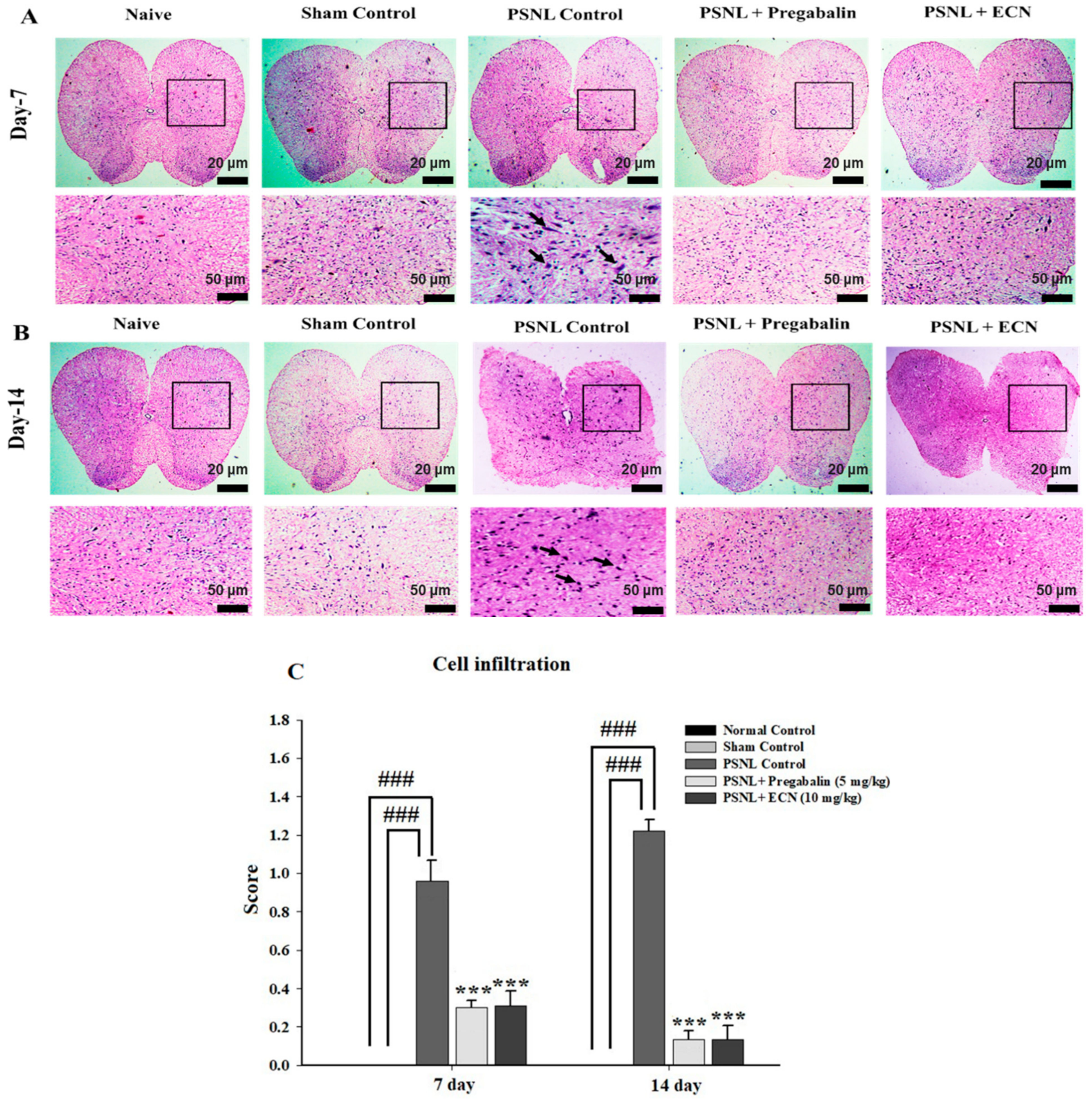
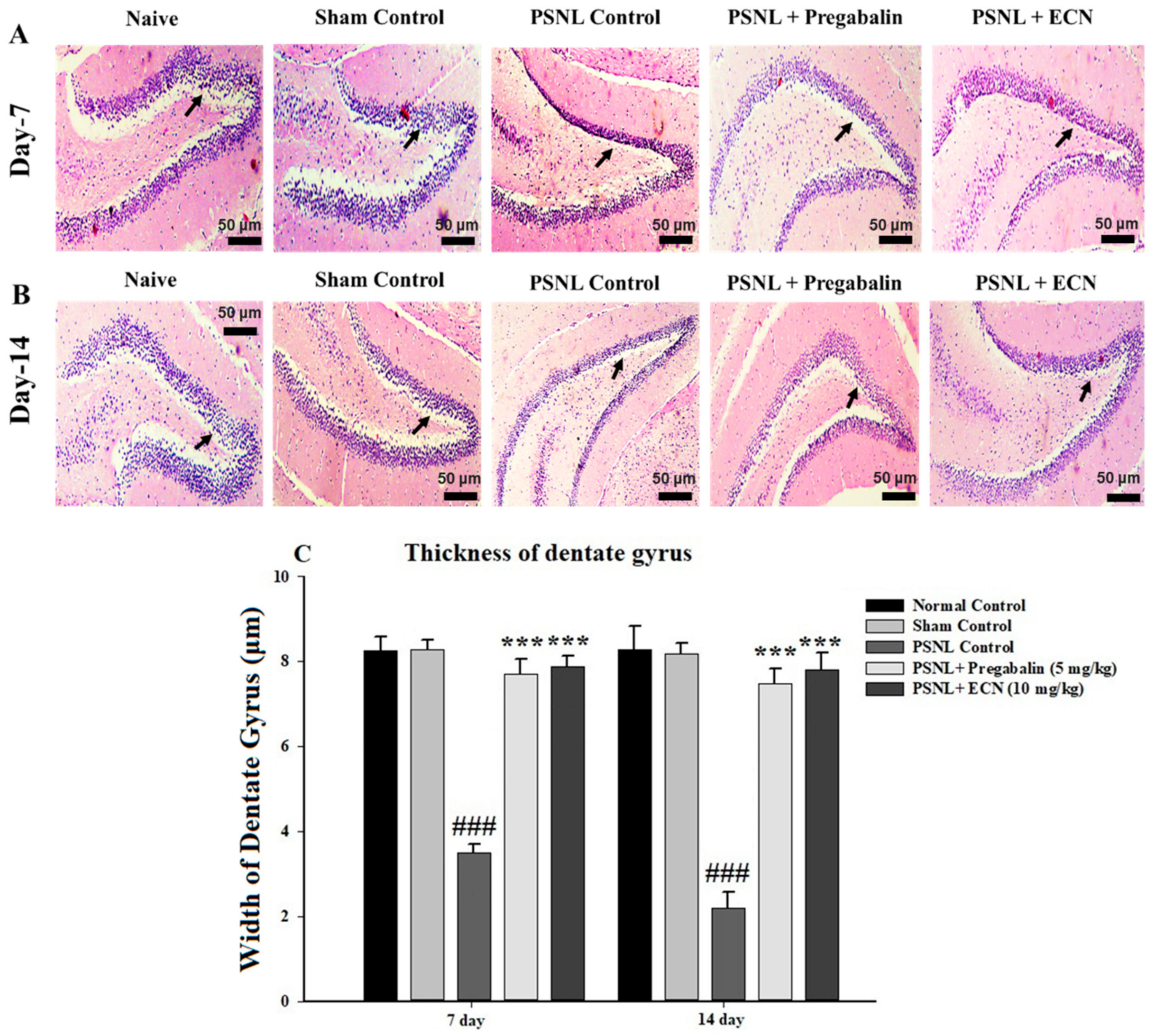
2.5. Effect of ECN on Histological Examination
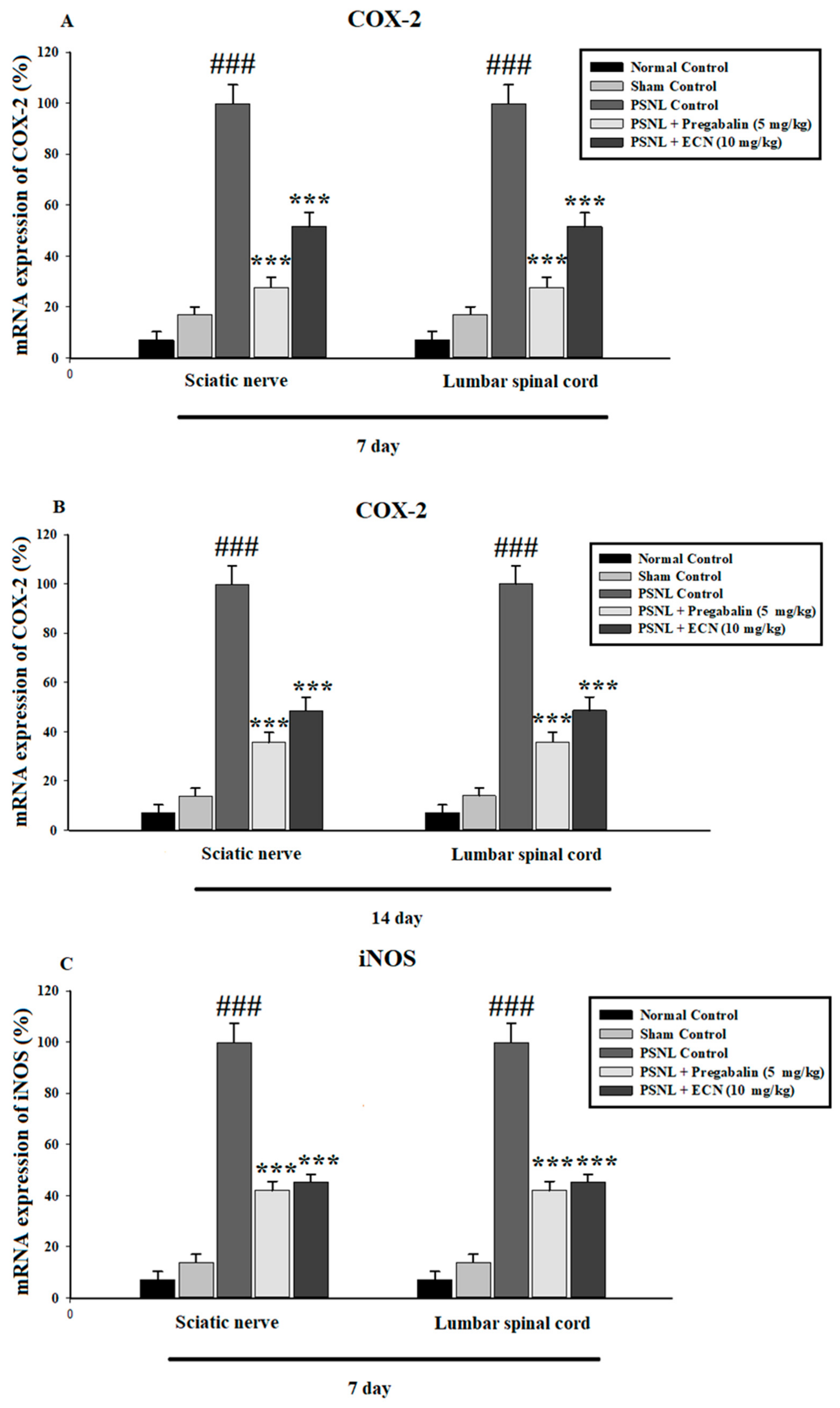
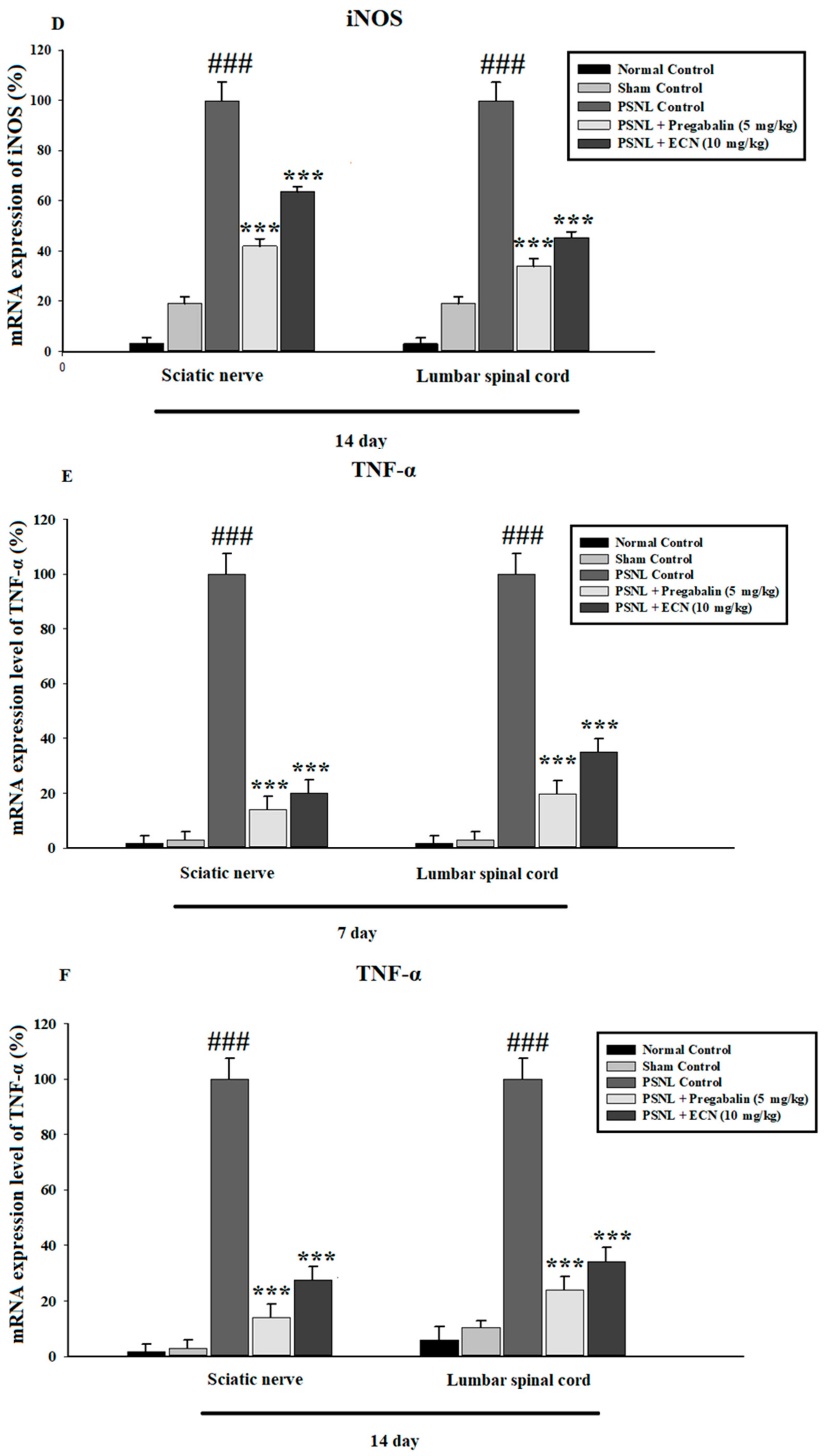
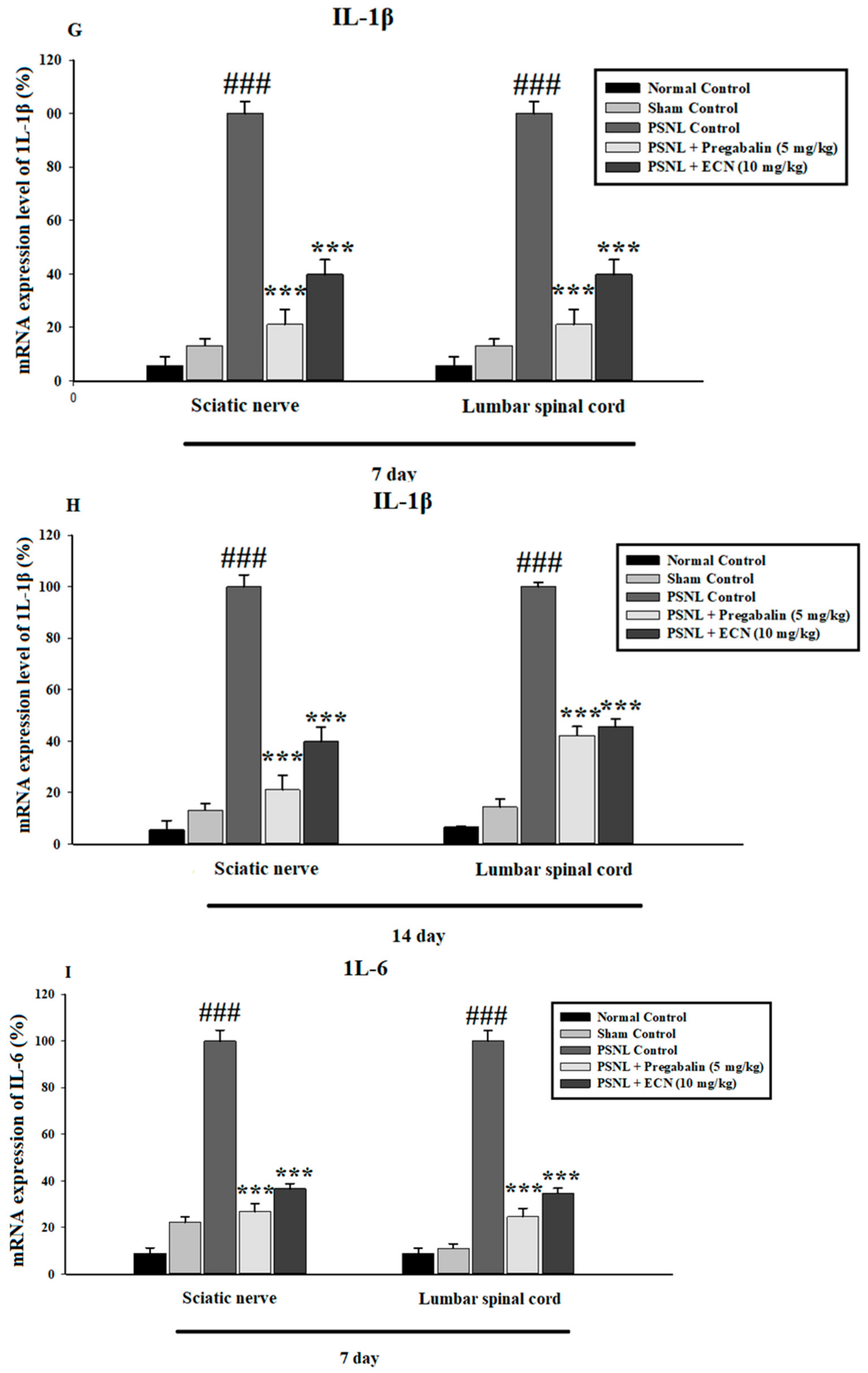
2.6. Effect of ECN on Inflammatory Cytokines Expression
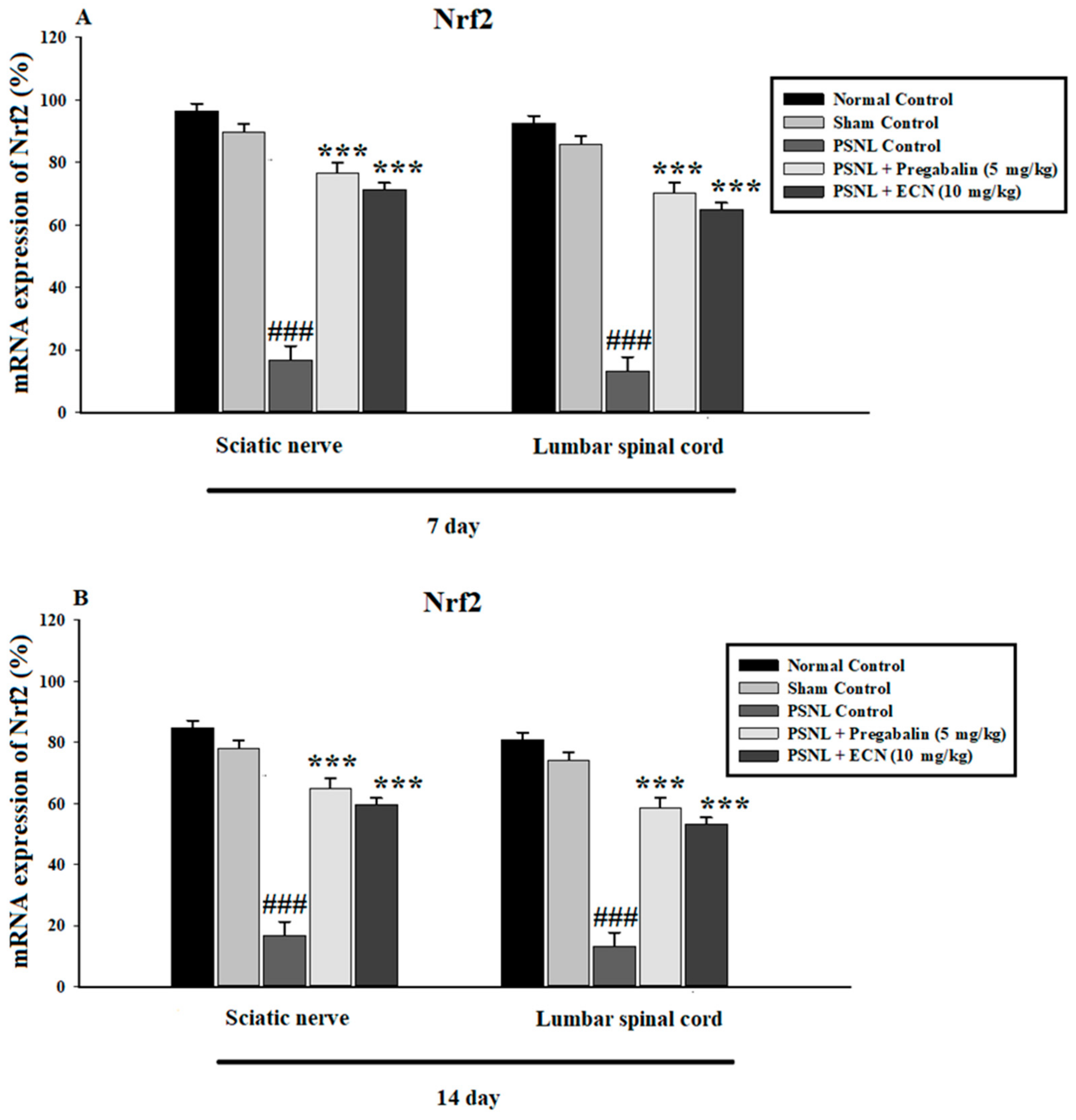
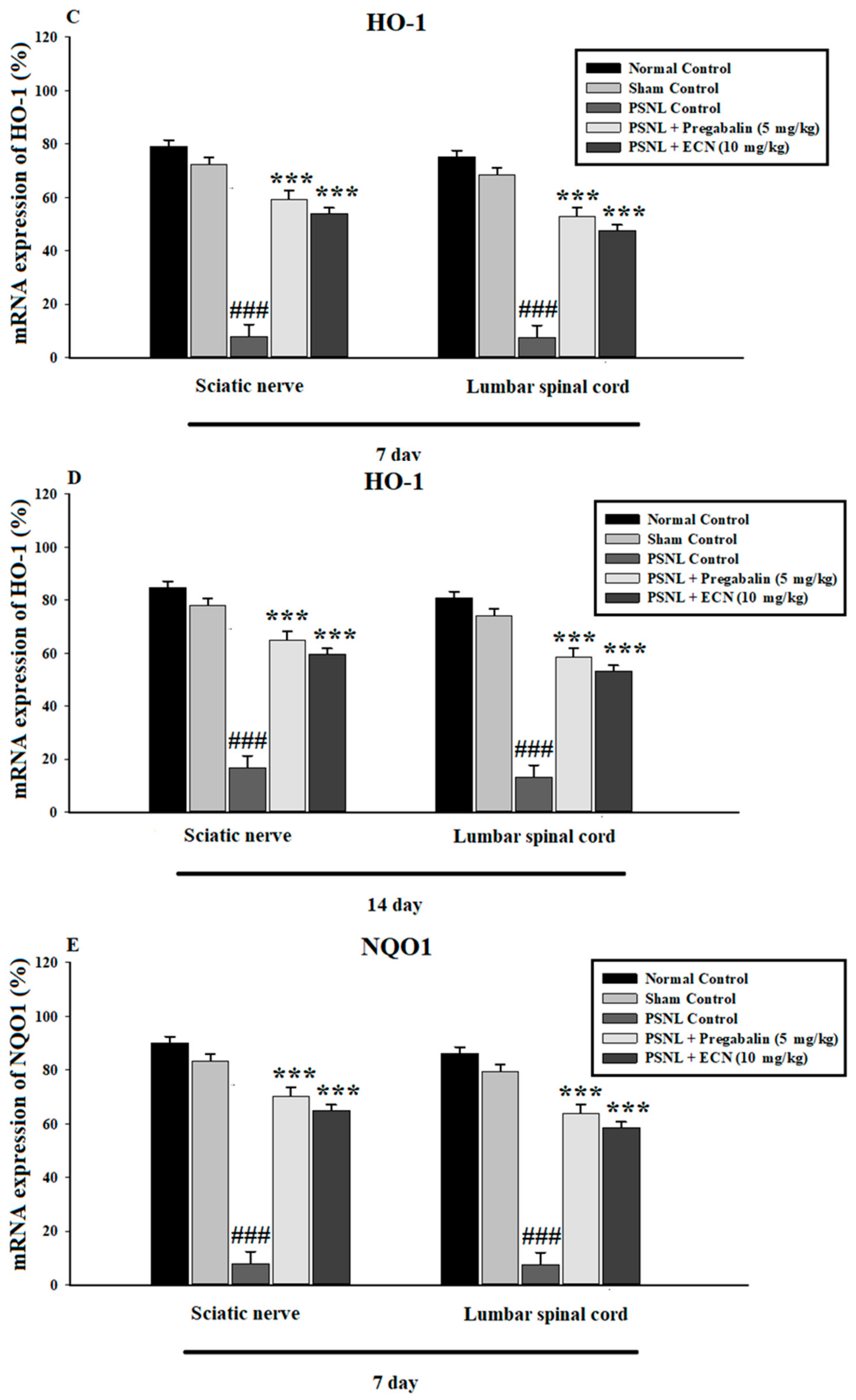
2.7. Effect of ECN on Anti-Oxidants Expression
2.8. Effect of ECN on MDA Production
2.9. Effect of ECN on NO Production
2.10. Effect of ECN on SOD
2.11. Effect of ECN on GSH
2.12. Effect of ECN on GST
2.13. Effect of ECN on Catalase
2.14. Effect of PSNL on Kidney and Liver Functions
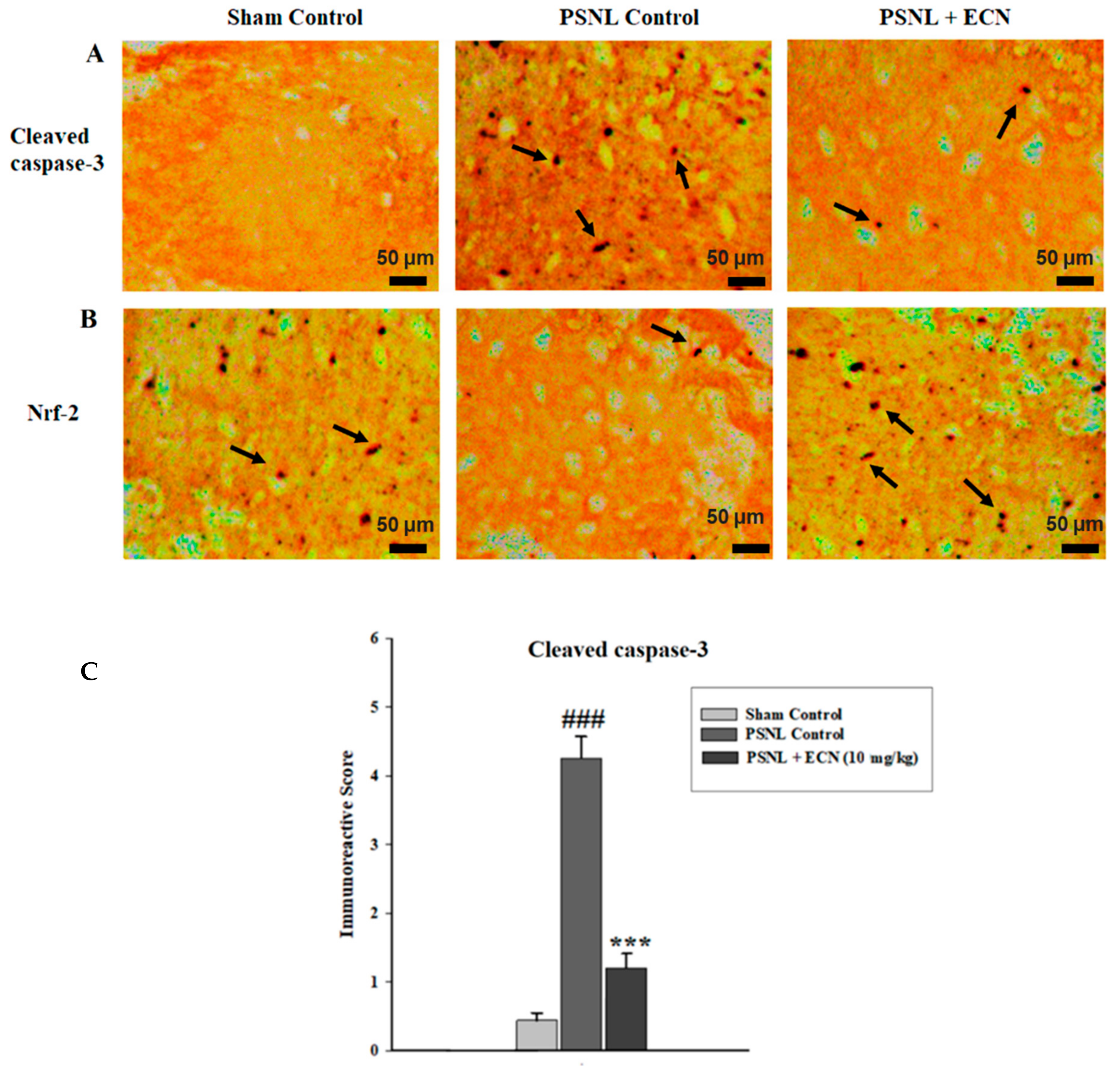
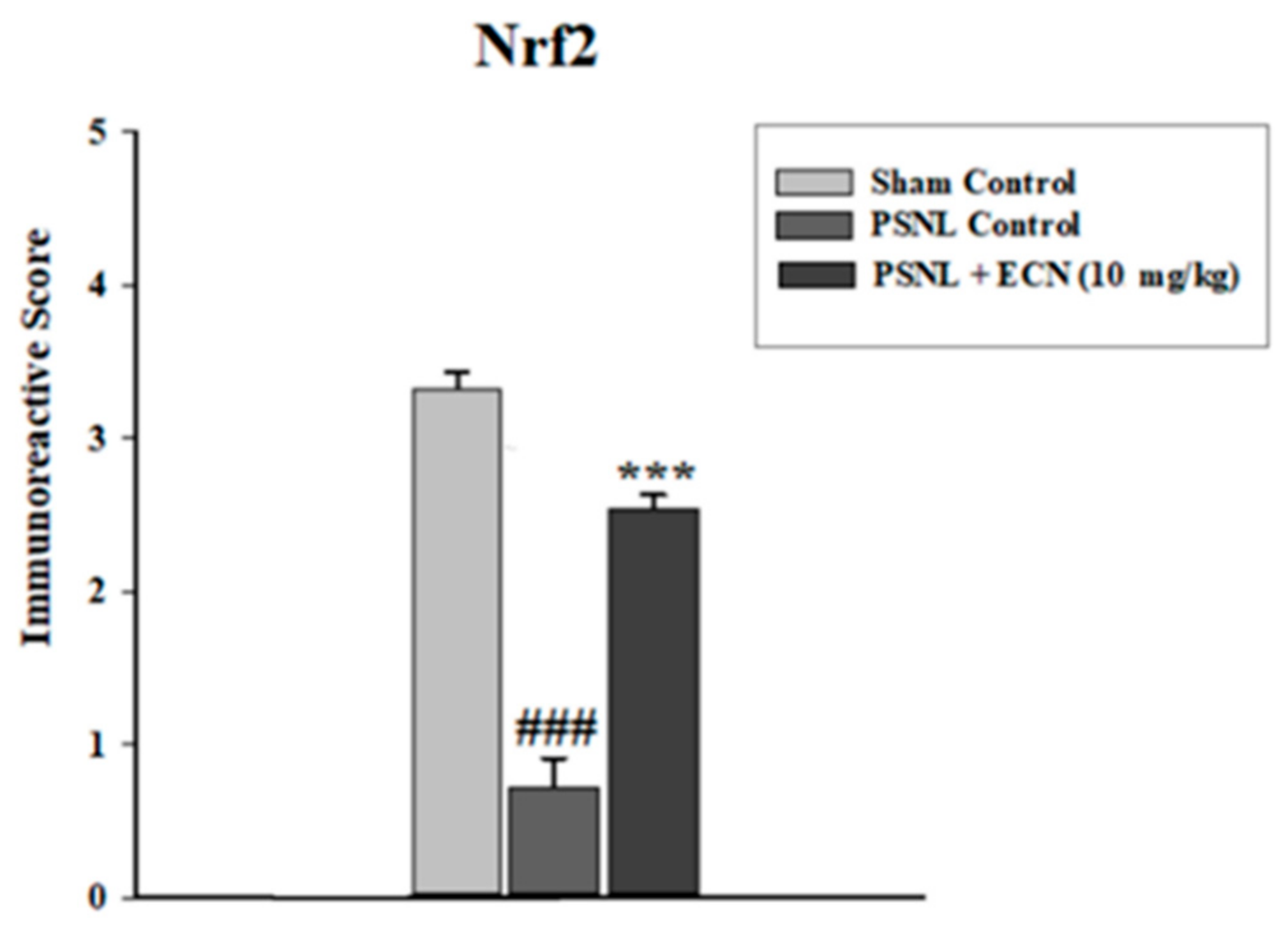
2.15. Effect of ECN on Nrf2 and Caspase-3 Expression
2.16. Effect of ECN on Myelin Sheath of Sciatic Nerve
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Reagents
4.3. Animals and Surgical Procedure
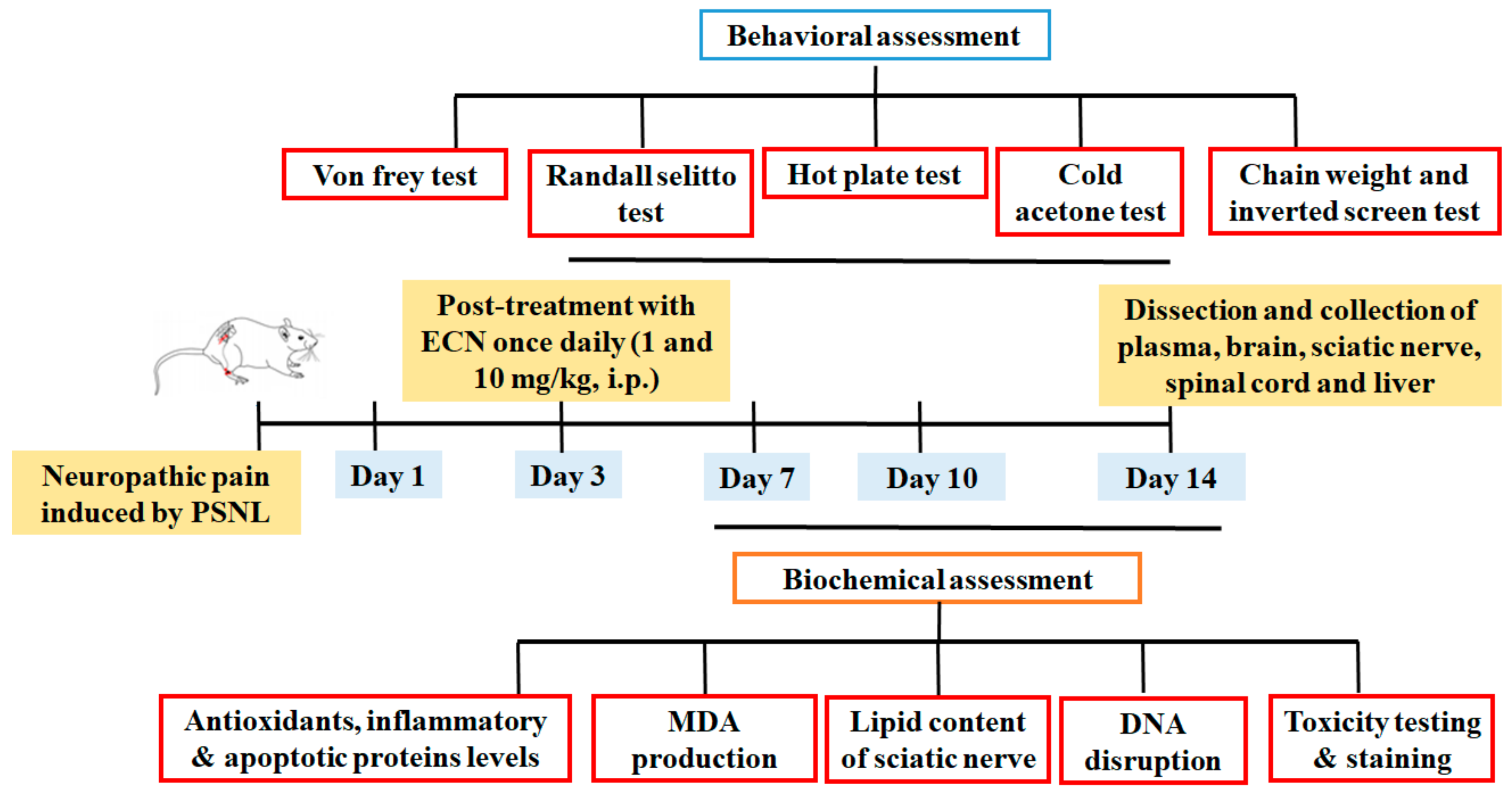
4.4. Drug Treatment and PSNL Model
- Group 1:
- Normal (naïve mice; did not undergo any surgical procedure)
- Group 2:
- Sham control (sciatic nerve exposure without nerve ligation)
- Group 3:
- PSNL control (sciatic nerve exposure with nerve ligation)
- Group 4:
- PSNL+ pregabalin (5 mg/kg) (sciatic exposure with nerve ligation and pregabalin (5 mg/kg) administered)
- Group 5:
- PSNL+ECN (1 mg/kg) (sciatic exposure with nerve ligation and ECN (mg/kg) administered)
- Group 6:
- PSNL+ECN (10 mg/kg) (sciatic exposure with nerve ligation and ECN (10 mg/kg) administered)
4.5. Behavior Test
4.5.1. Distress Symptoms and Survival Rate
4.5.2. Mechanical Hyperalgesia
4.5.3. Thermal Hyperalgesia
4.5.4. Mechanical Allodynia
4.5.5. Cold Allodynia
4.5.6. Muscle Coordination and Motor Coordination
4.6. Biochemical Experiments
4.6.1. Comet Assay
4.6.2. Histopathological Analysis
4.6.3. Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction
4.6.4. Determination of Malondialdehyde (MDA)
4.6.5. Determination of Nitric Oxide (NO)
4.6.6. Determination of Superoxide Dismutase (SOD)
4.6.7. Determination of Glutathione (GSH)
4.6.8. Determination of GST
4.6.9. Determination of Catalase (CAT)
4.6.10. Analysis of Renal and Hepatotoxicity
4.6.11. Immunohistochemistry
4.6.12. Determination of Sciatic Nerve Structural Damage
4.6.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alles, S.R.; Smith, P.A. Etiology and pharmacology of neuropathic pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, Z.; Sun, W.; Jiang, C.; Ba, X.; Zhou, Q.; Xiong, D.; Xiao, L.; Deng, Q.; Hao, Y. The antiviral alkaloid berberine ameliorates neuropathic pain in rats with peripheral nerve injury. Acta Neurol. Belg. 2020, 120, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Bai, L.; Li, S.; Li, L.; Dou, Z.; Huang, Y.; Li, Y.; Lv, Y. Dexmedetomidine Alleviates CCI-Induced Neuropathic Pain via Inhibiting HMGB1-Mediated Astrocyte Activation and the TLR4/NF-κB Signaling Pathway in Rats. Neurotox. Res. 2020, 38, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B. Neuropathic pain. Nat. Rev. Dis. Prim. 2017, 3, 17002. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Khan, S.; Kim, Y.S. Insight into Pain Modulation: Nociceptors Sensitization and Therapeutic Targets. Curr. Drug Targets 2019, 20, 775–788. [Google Scholar] [CrossRef]
- Brell, J.M. Animal models of peripheral neuropathy: Modeling what we feel, understanding what they feel. ILAR J. 2014, 54, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.M.; Kim, Y.S. Reviews: Molecular mechanism of inflammatory signaling and predominant role of Saposhnikovia divaricata as anti-inflammatory potential. Nat. Prod. Sci. 2013, 19, 120–126. [Google Scholar]
- Hall, O.M.; Broussard, A.; Range, T.; Turpin, M.A.C.; Ellis, S.; Lim, V.M.; Cornett, E.M.; Kaye, A. Novel Agents in Neuropathic Pain, the Role of Capsaicin: Pharmacology, Efficacy, Side Effects, Different Preparations. Curr. Pain Headache Rep. 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Singh, H.; Kaur, J.; Arora, R.; Mannan, R.; Buttar, H.S.; Arora, S.; Singh, B. Ameliorative potential of Argyreia speciosa against CCI-induced neuropathic pain in rats: Biochemical and histopathological studies. J. Ethnopharmacol. 2020, 249, 112399. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, H.-Q.; Liu, H.; Liu, M.; Huang, Z.-Y.; Yang, J.; Su, Y.-P.; Yu, C.-X. Effects of koumine, an alkaloid of Gelsemium elegans Benth., on inflammatory and neuropathic pain models and possible mechanism with allopregnanolone. Pharmacol. Biochem. Behav. 2012, 101, 504–514. [Google Scholar] [CrossRef]
- Khan, S.; Choi, R.J.; Lee, J.; Kim, Y. Attenuation of neuropathic pain and neuroinflammatory responses by a pyranocoumarin derivative, anomalin in animal and cellular models. Eur. J. Pharmacol. 2016, 774, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Rezq, S.; Alsemeh, A.E.; D’Elia, L.; El-Shazly, A.M.; Monti, D.M.; Sobeh, M.; Mahmoud, M. Thymus algeriensis and Thymus fontanesii exert neuroprotective effect against chronic constriction injury-induced neuropathic pain in rats. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Safakhah, H.A.; Damghanian, F.; Bandegi, A.-R.; Miladi-Gorji, H. Effect of crocin on morphine tolerance and serum BDNF levels in a rat model of neuropathic pain. Pharmacol. Rep. 2020, 72, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Harvey, V.L.; Dickenson, A.H.J.M.p. Behavioural and electrophysiological characterisation of experimentally induced osteoarthritis and neuropathy in C57Bl/6 mice. Mol. Pain 2009, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, P.A.; de Paula Werner, M.F.; Oliveira, E.C.; Burgos, L.; Pereira, P.; da Silva Brum, L.F.; Story, G.M.; Santos, A. The antinociceptive effect of (-)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J. Pain 2010, 11, 1222–1229. [Google Scholar] [CrossRef]
- Sakhaee, M.H.; Sayyadi, S.A.H.; Sakhaee, N.; Sadeghnia, H.R.; Hosseinzadeh, H.; Nourbakhsh, F.; Forouzanfar, F. Cedrol protects against chronic constriction injury-induced neuropathic pain through inhibiting oxidative stress and inflammation. Metab. Brain Dis. 2020, 35, 1119–1126. [Google Scholar] [CrossRef]
- Li, R.; Dang, S.; Yao, M.; Zhao, C.; Zhang, W.; Cui, J.; Wang, J.; Wen, A.J.A. Osthole alleviates neuropathic pain in mice by inhibiting the P2Y1-receptor-dependent JNK signaling pathway. Aging 2020, 12, 7945. [Google Scholar] [CrossRef]
- Negi, G.; Kumar, A.; Sharma, S.S. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr. Neurovascular Res. 2011, 8, 294–304. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, Z.; Wang, X.; Sun, D.; Wang, D.; Li, Y.; Pei, B.; Ye, M.; Xu, J.; Yue, X.J.B.; et al. Curcumin alleviates oxaliplatin-induced peripheral neuropathic pain through inhibiting oxidative stress-mediated activation of NF-κB and mitigating inflammation. Biol. Pharm. Bull. 2020, 43, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Kiasalari, Z.; Rahmani, T.; Mahmoudi, N.; Baluchnejadmojarad, T.; Roghani, M.J.B. Pharmacotherapy. Diosgenin ameliorates development of neuropathic pain in diabetic rats: Involvement of oxidative stress and inflammation. Biomed. Pharmacother. 2017, 86, 654–661. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Darabi, S.; Hasanvand, A.; Amini-Khoei, H.; Abbasnezhad, A.; Choghakhori, R.; Aaliehpour, A. Minocycline through attenuation of oxidative stress and inflammatory response reduces the neuropathic pain in a rat model of chronic constriction injury. Iran. J. Basic Med. Sci. 2018, 21, 138. [Google Scholar] [PubMed]
- Li, Y.; Li, H.; Han, J. Sphingosine-1-phosphate receptor 2 modulates pain sensitivity by suppressing the ROS-RUNX3 pathway in a rat model of neuropathy. J. Cell. Physiol. 2020, 235, 3864–3873. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zuo, Z.; Yan, W.; Liu, W.; Zheng, Q.; Liu, X. Berberine ameliorates diabetic neuropathic pain in a rat model: Involvement of oxidative stress, inflammation, and μ-opioid receptors. Naunyn Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Gaudet, A.D.; Staikopoulos, V.; Maier, S.F.; Hutchinson, M.R.; Salvemini, D.; Watkins, L.R. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci. 2016, 39, 862–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Liu, D.; Chen, S.; Chen, N.; Sun, J.; Wang, X.; Cao, F.; Tian, Y.; Ye, D. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol. Sin. 2020, 41, 1041–1048. [Google Scholar] [CrossRef]
- Shan, W.; Liao, X.; Tang, Y.; Liu, J. Dexmedetomidine alleviates inflammation-induced neuropathic pain by suppressing NLRP3 via activation of Nrf2. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-Q.; Liu, D.-Q.; Chen, S.-P.; Chen, N.; Sun, J.; Wang, X.-M.; Li, D.-Y.; Tian, Y.-K.; Ye, D.-W. Pharmacotherapy. PPARγ activation mitigates mechanical allodynia in paclitaxel-induced neuropathic pain via induction of Nrf2/HO-1 signaling pathway. Biomed. Pharmacother. 2020, 129, 110356. [Google Scholar] [CrossRef]
- Younis, N.S.; Abduldaium, M.S.; Mohamed, M.E. Protective Effect of Geraniol on Oxidative, Inflammatory and Apoptotic Alterations in Isoproterenol-Induced Cardiotoxicity: Role of the Keap1/Nrf2/HO-1 and PI3K/Akt/mTOR Pathways. Antioxidants 2020, 9, 977. [Google Scholar] [CrossRef]
- Ali, H.; Khan, A.; Ali, J.; Ullah, H.; Khan, A.; Ali, H.; Irshad, N.; Khan, S. Attenuation of LPS-induced acute lung injury by continentalic acid in rodents through inhibition of inflammatory mediators correlates with increased Nrf2 protein expression. BMC Pharmacol. Toxicol. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, A.M.; Khan, A.; Shal, B.; Aziz, A.; Ahmed, M.N.; Khan, S.J.C.-B.I. The newly synthesized compounds (NCHDH and NTHDH) attenuates LPS-induced septicemia and multi-organ failure via Nrf2/HO1 and HSP/TRVP1 signaling in mice. Chem. Biol. Interact. 2020, 329, 109220. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Seo, E.-J.; Klauck, S.M.; Efferth, T. Adaptogens in chemobrain (part IV): Adaptogenic plants prevent the chemotherapeutics-induced imbalance of redox homeostasis by modulation of expression of genes encoding Nrf2-mediated signaling proteins and antioxidant, metabolizing, detoxifying enzymes in neuroglia cells. Longhua Chin. Med. 2020, 3, 4. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.; Moon, I.S. Neuroprotection against oxidative stress: Phytochemicals targeting TrkB signaling and the Nrf2-ARE antioxidant system. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, X.; Wang, Z.; Wang, P.; Zhang, Z.; Dong, J.; Zhuang, R.; Zhou, Y.; Ma, G.; Cai, W.; et al. Alphalipoic acid prevents oxidative stress and peripheral neuropathy in nab-paclitaxel-treated rats through the Nrf2 signalling pathway. Oxidative Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, K.; Chen, Y.; Wang, Y.; Wang, Y.; Lian, N.; Zhang, K.; Yu, Y.J.I.I. Nrf2/HO-1 signaling pathway participated in the protection of hydrogen sulfide on neuropathic pain in rats. Int. Immunopharmacol. 2019, 75, 105746. [Google Scholar] [CrossRef] [PubMed]
- Rahbardar, M.G.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Rosmarinic acid attenuates development and existing pain in a rat model of neuropathic pain: An evidence of anti-oxidative and anti-inflammatory effects. Phytomedicine 2018, 40, 59–67. [Google Scholar] [CrossRef]
- Amin, B.; Abnous, K.; Motamedshariaty, V.; Hosseinzadeh, H. Attenuation of oxidative stress, inflammation and apoptosis by ethanolic and aqueous extracts of Crocus sativusL. stigma after chronic constriction injury of rats. Anais Acad. Bras. Ciênc. 2014, 86, 1821–1832. [Google Scholar] [CrossRef] [Green Version]
- Jamali-Raeufy, N.; Baluchnejadmojarad, T.; Roghani, M. Isorhamnetin exerts neuroprotective effects in STZ-induced diabetic rats via attenuation of oxidative stress, inflammation and apoptosis. J. Chem. Neuroanat. 2019, 102, 101709. [Google Scholar] [CrossRef]
- Clark, A.K.; Old, E.A.; Malcangio, M. Neuropathic pain and cytokines: Current perspectives. J. Pain Res. 2013, 6, 803. [Google Scholar]
- Khan, A.; Khan, S.; Ali, H.; Shah, K.U.; Ali, H.; Shehzad, O.; Onder, A.; Kim, Y.S. Anomalin attenuates LPS-induced acute lungs injury through inhibition of AP-1 signaling. Int. Immunopharmacol. 2019, 73, 451–460. [Google Scholar] [CrossRef]
- Nadeau, S.; Filali, M.; Zhang, J.; Kerr, B.J.; Rivest, S.; Soulet, D.; Iwakura, Y.; de Rivero Vaccari, J.P.; Keane, R.W.; Lacroix, S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: Implications for neuropathic pain. J. Neurosci. 2011, 31, 12533–12542. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Wang, L.; Song, X.-Y.J.B. Mitoquinone alleviates vincristine-induced neuropathic pain through inhibiting oxidative stress and apoptosis via the improvement of mitochondrial dysfunction. Biomed. Pharmacother. 2020, 125, 110003. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-L.; Yang, J.-L.; Shi, Y.-P. Sesquiterpenoids and other constituents from the flower buds of Tussilago farfara. J. Asian Nat. Prod. Res. 2011, 13, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Nho, C.W.; Ha, I.J.; Kim, Y. Cellular Target Proteome in Breast Cancer Cells of an Oplopane Sesquiterpenoid Isolated from Tussilago farfara. J. Nat. Prod. 2020, 83, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-Q.; Sun, J.; Yu, J.-H.; Zhang, J.-S.; Bao, J.; Zhang, H. Prenylated indole alkaloids and lignans from the flower buds of Tussilago farfara. Fitoterapia 2020, 146, 104729. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, J.W.; Lee, C.; Jin, Q.; Choi, J.Y.; Lee, D.; Han, S.B.; Kim, Y.; Hong, J.T.; Lee, M.K. Sesquiterpenoids from Tussilago farfara inhibit LPS-induced nitric oxide production in macrophage RAW 264.7 cells. Arch. Pharmacal Res. 2016, 39, 127–132. [Google Scholar] [CrossRef]
- Hwangbo, C.; Lee, H.S.; Park, J.; Choe, J.; Lee, J.-H. The anti-inflammatory effect of tussilagone, from Tussilago farfara, is mediated by the induction of heme oxygenase-1 in murine macrophages. Int. Immunopharmacol. 2009, 9, 1578–1584. [Google Scholar] [CrossRef]
- Cho, J.; Kim, H.M.; Ryu, J.-H.; Jeong, Y.S.; Lee, Y.S.; Jin, C. Neuroprotective and antioxidant effects of the ethyl acetate fraction prepared from Tussilago farfara L. Biol. Pharm. Bull. 2005, 28, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Park, H.R.; Yoo, M.Y.; Seo, J.H.; Kim, I.S.; Kim, N.Y.; Kang, J.Y.; Cui, L.; Lee, C.-S.; Lee, C.-H.; Lee, H.S. Sesquiterpenoids isolated from the flower buds of Tussilago farfara L. inhibit diacylglycerol acyltransferase. J. Agric. Food Chem. 2008, 56, 10493–10497. [Google Scholar] [CrossRef]
- Lee, J.; Song, K.; Huh, E.; Oh, M.S.; Kim, Y.S. Neuroprotection against 6-OHDA toxicity in PC12 cells and mice through the Nrf2 pathway by a sesquiterpenoid from Tussilago farfara. Redox Biol. 2018, 18, 6–15. [Google Scholar] [CrossRef]
- Lim, H.J.; Dong, G.-Z.; Lee, H.J.; Ryu, J.-H. In vitro neuroprotective activity of sesquiterpenoids from the flower buds of Tussilago farfara. J. Enzym. Inhib. Med. Chem. 2015, 30, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Ko, H.; Song, K.; Kim, Y.S. A sesquiterpenoid from Farfarae Flos induces apoptosis of MDA-MB-231 human breast cancer cells through inhibition of JAK–STAT3 signaling. Biomolecules 2019, 9, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, M.J.; McCanney, G.A.; Willison, H.J.; Barnett, S.C. MyelinJ: An ImageJ macro for high throughput analysis of myelinating cultures. Bioinformatics 2019, 35, 4528–4530. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.J. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C.J.B. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129, 3249–3269. [Google Scholar] [CrossRef]
- Mane, D.R.; Kale, A.D.; Belaldavar, C. Validation of immunoexpression of tenascin-C in oral precancerous and cancerous tissues using ImageJ analysis with novel immunohistochemistry profiler plugin: An immunohistochemical quantitative analysis. J. Oral Maxillofac. Pathol. 2017, 21, 211. [Google Scholar] [CrossRef] [Green Version]
- Sysel, A.M.; Valli, V.E.; Nagle, R.B.; Bauer, J.A. Immunohistochemical quantification of the vitamin B12 transport protein (TCII), cell surface receptor (TCII-R) and Ki-67 in human tumor xenografts. Anticancer. Res. 2013, 33, 4203–4212. [Google Scholar]
- Severcan, F.; Sahin, I.; Kazancı, N. Melatonin strongly interacts with zwitterionic model membranes—evidence from Fourier transform infrared spectroscopy and differential scanning calorimetry. Biochim. Biophys. Acta (BBA) Biomembr. 2005, 1668, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Mantsch, H.H. Biological applications of Fourier transform infrared spectroscopy: A study of phase transitions in biomembranes. J. Mol. Struct. 1984, 113, 201–212. [Google Scholar] [CrossRef]
- Severcan, F.; Toyran, N.; Kaptan, N.; Turan, B. Fourier transform infrared study of the effect of diabetes on rat liver and heart tissues in the C-H region. Talanta 2000, 53, 55–59. [Google Scholar] [CrossRef]
- Severcan, F.; Kaptan, N.; Turan, B. Fourier transform infrared spectroscopic studies of diabetic rat heart crude membranes. Spectroscopy 2003, 17, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Severcan, F. Vitamin E decreases the order of the phospholipid model membranes in the gel phase: An FTIR study. Biosci. Rep. 1997, 17, 231–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozek, N.S.; Sara, Y.; Onur, R.; Severcan, F. Low dose simvastatin induces compositional, structural and dynamic changes in rat skeletal extensor digitorum longus muscle tissue. Biosci. Rep. 2010, 30, 41–50. [Google Scholar]
- Garip, S.; Severcan, F. Determination of simvastatin-induced changes in bone composition and structure by Fourier transform infrared spectroscopy in rat animal model. J. Pharm. Biomed. Anal. 2010, 52, 580–588. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Backonja, M.; Rowbotham, M.C.; Allen, R.R.; Argoff, C.R.; Bennett, G.J.; Bushnell, M.C.; Farrar, J.T.; Galer, B.S.; Haythornthwaite, J.A. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch. Neurol. 2003, 60, 1524–1534. [Google Scholar] [CrossRef] [Green Version]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Khan, S.; Shehzad, A.; Shehzad, O.; Al-Suhaimi, E.A. Inpatient antibiotics pharmacology and physiological use in Hayatabad medical complex, Pakistan. Int. J. Physiol. Pathophysiol. Pharmacol. 2013, 5, 120. [Google Scholar]
- Zhao, W.X.; Wang, P.F.; Song, H.G.; Sun, N. Diosgenin attenuates neuropathic pain in a rat model of chronic constriction injury. Mol. Med. Rep. 2017, 16, 1559–1564. [Google Scholar] [CrossRef]
- Khan, S.; Choi, R.J.; Shehzad, O.; Kim, H.P.; Islam, M.N.; Choi, J.S.; Kim, Y.S. Molecular mechanism of capillarisin-mediated inhibition of MyD88/TIRAP inflammatory signaling in in vitro and in vivo experimental models. J. Ethnopharmacol. 2013, 145, 626–637. [Google Scholar] [CrossRef]
- Joseph, E.K.; Levine, J.D. Caspase signalling in neuropathic and inflammatory pain in the rat. Eur. J. Neurosci. 2004, 20, 2896–2902. [Google Scholar] [CrossRef]
- Olmos, G.; Lladó, J. Tumor necrosis factor alpha: A link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Sacerdote, P.; Trovato, A.E.; Colleoni, M. Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: Anti-inflammatory and antioxidant activity. J. Neurochem. 2008, 107, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, Y.-Q. Spinal glial activation contributes to pathological pain states. Neurosci. Biobehav. Rev. 2008, 32, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Scheid, T.; Moraes, M.S.; Henriques, T.P.; Riffel, A.P.K.; Belló-Klein, A.; Poser, G.L.V.; Ethur, E.M.; Partata, W.A. Effects of Methanol Fraction from Leaves of Schinus terebinthifolius Raddi on Nociception and Spinal-Cord Oxidative Biomarkers in Rats with Neuropathic Pain. Evid. Based Complement. Altern. Med. 2018, 2018, 5783412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulahoum, H.; Boumaza, B.M.A.; Ferrat, M.; Nagy, A.-L.; Olteanu, D.E.; Bounaama, A.; Clichici, S. Aberrant crypt foci are regionally affected by zinc treatment in a 1, 2-dimethylhydrazine induced colon carcinogenesis model. J. Trace Elem. Med. Biol. 2018, 47, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M.; Dikici, D.M.; Dursun, Ş. Role of oxidative stress and Ca 2+ signaling on molecular pathways of neuropathic pain in diabetes: Focus on TRP channels. Neurochem. Res. 2012, 37, 2065–2075. [Google Scholar] [CrossRef]
- Chinnaiyan, A.M.; Orth, K.; O’Rourke, K.; Duan, H.; Poirier, G.G.; Dixit, V.M. Molecular Ordering of the Cell Death Pathway Bcl-2 AND Bcl-x FUNCTION UPSTREAM OF THE CED-3-LIKE APOPTOTIC PROTEASES. J. Biol. Chem. 1996, 271, 4573–4576. [Google Scholar]
- Maione, S.; Siniscalco, D.; Galderisi, U.; de Novellis, V.; Uliano, R.; Di Bernardo, G.; Berrino, L.; Cascino, A.; Rossi, F. Apoptotic genes expression in the lumbar dorsal horn in a model neuropathic pain in rat. Neuroreport 2002, 13, 101–106. [Google Scholar] [CrossRef]
- Wu, F.; Miao, X.; Chen, J.; Sun, Y.; Liu, Z.; Tao, Y.; Yu, W. Down-regulation of GAP-43 by inhibition of caspases-3 in a rat model of neuropathic pain. Int. J. Clin. Exp. Pathol. 2012, 5, 948. [Google Scholar]
- Shal, B.; Khan, A.; Naveed, M.; Khan, N.U.; AlSharari, S.D.; Kim, Y.S.; Khan, S. Effect of 25-methoxy hispidol A isolated from Poncirus trifoliate against bacteria-induced anxiety and depression by targeting neuroinflammation, oxidative stress and apoptosis in mice. Biomed. Pharmacother. 2019, 111, 209–223. [Google Scholar] [CrossRef]
- Song, K.; Lee, K.J.; Kim, Y.S. Development of an efficient fractionation method for the preparative separation of sesquiterpenoids from Tussilago farfara by counter-current chromatography. J. Chromatogr. A 2017, 1489, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.-W.; Lee, J.-Y.; Song, K.; Kim, M.-H.; Yoon, S.; Nguyen, D.-T.; Kim, S.; Kim, Y.S.; Kim, D.-D. Development and Validation of Liquid Chromatography-Tandem Mass Spectrometry Method for Pharmacokinetic Evaluation of 7β-(3-Ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3, 14-dehydro-Z-notonipetranon in Rats. Molecules 2020, 25, 1774. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, A.S.; Jain, V.; Singh, N. Animal models of neuropathic pain. Fundam. Clin. Pharmacol. 2011, 25, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Biswas, T. How to calculate sample size for different study designs in medical research? Indian J. Psychol. Med. 2013, 35, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atiq, A.; Shal, B.; Naveed, M.; Khan, A.; Ali, J.; Zeeshan, S.; Al-Sharari, S.D.; Kim, Y.S.; Khan, S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur. J. Pharmacol. 2019, 843, 292–306. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, A.; Baig, M.W.; Ullah, N.; Ahmed, N.; Tipu, M.K.; Ali, H.; Khan, S. Poncirin attenuates CCL4-induced liver injury through inhibition of oxidative stress and inflammatory cytokines in mice. BMC Complement. Med. Ther. 2020, 20, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Khan, S.Z.; Zeeshan, S.; Khan, A.; Shal, B.; Atiq, A.; Ali, H.; Ullah, R.; Khan, S. A new cationic palladium (II) dithiocarbamate exhibits anti-inflammatory, analgesic, and antipyretic activities through inhibition of inflammatory mediators in in vivo models. Naunyn Schmiedeberg’s Arch. Pharmacol. 2019, 392, 961–977. [Google Scholar] [CrossRef]
- Khan, S.M.; Choi, R.J.; Lee, D.U.; Kim, Y.S. Sesquiterpene derivatives isolated from Cyperus rotundus L. inhibit inflammatory signaling mediated by NF-κB. Nat. Prod. Sci. 2011, 17, 250–255. [Google Scholar]
- Shahid, M.; Subhan, F.; Ahmad, N.; Ali, G.; Akbar, S.; Fawad, K.; Sewell, R.D.E. Topical gabapentin gel alleviates allodynia and hyperalgesia in the chronic sciatic nerve constriction injury neuropathic pain model. Eur. J. Pain 2017, 21, 668–680. [Google Scholar] [CrossRef]
- Khan, A.; Ullah, M.Z.; Afridi, R.; Rasheed, H.; Khalid, S.; Ullah, H.; Ali, H.; AlSharari, S.D.; Kim, Y.S.; Khan, S. Antinociceptive properties of 25-methoxy hispidol A, a triterpinoid isolated from Poncirus trifoliata (Rutaceae) through inhibition of NF-κB signalling in mice. Phytotherapy Res. 2019, 33, 327–341. [Google Scholar] [CrossRef]
- Khalid, S.; Khan, A.; Shal, B.; Ali, H.; Kim, Y.S.; Khan, S. Suppression of TRPV1 and P2Y nociceptors by honokiol isolated from Magnolia officinalis in 3rd degree burn mice by inhibiting inflammatory mediators. Biomed. Pharmacother. 2019, 114, 108777. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khan, A.; Ali, H.; Kim, Y.S.; Khan, S. Antihyperalgesic properties of honokiol in inflammatory pain models by targeting of NF-κB and Nrf2 signaling. Front. Pharmacol. 2018, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shehzad, O.; Chun, J.; Kim, Y.S. Mechanism underlying anti-hyperalgesic and anti-allodynic properties of anomalin in both acute and chronic inflammatory pain models in mice through inhibition of NF-κB, MAPKs and CREB signaling cascades. Eur. J. Pharmacol. 2013, 718, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, L.; Wang, J.; Wang, C.; Yang, Z.; Wang, C.; Zhu, Y.; Zhang, J. Paeoniflorin and albiflorin attenuate neuropathic pain via mapk pathway in chronic constriction injury rats. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Shehzad, O.; Chun, J.; Choi, R.J.; Park, S.; Islam, M.N.; Choi, J.S.; Kim, Y.S. Anti-hyperalgesic and anti-allodynic activities of capillarisin via suppression of inflammatory signaling in animal model. J. Ethnopharmacol. 2014, 152, 478–486. [Google Scholar] [CrossRef]
- Afridi, R.; Khan, A.U.; Khalid, S.; Shal, B.; Rasheed, H.; Ullah, M.Z.; Shehzad, O.; Kim, Y.S.; Khan, S. Anti-hyperalgesic properties of a flavanone derivative Poncirin in acute and chronic inflammatory pain models in mice. BMC Pharmacol. Toxicol. 2019, 20, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Contet, C.; Rawlins, J.N.P.; Deacon, R.M. A comparison of 129S2/SvHsd and C57BL/6JOlaHsd mice on a test battery assessing sensorimotor, affective and cognitive behaviours: Implications for the study of genetically modified mice. Behav. Brain Res. 2001, 124, 33–46. [Google Scholar] [CrossRef]
- Rasheed, H.; Afridi, R.; Khan, A.U.; Ullah, M.Z.; Khalid, S.; Atiq, A.; Kashif, H.; Ahmed, M.N.; Kim, Y.S.; Khan, S. Anti-inflammatory, anti-rheumatic and analgesic activities of 2-(5-mercapto-1, 3, 4-oxadiazol-2-yl)-N-propylbenzenesulphonamide (MOPBS) in rodents. Inflammopharmacology 2018, 26, 1037–1049. [Google Scholar] [CrossRef]
- Deacon, R.M.; Croucher, A.; Rawlins, J.N.P. Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav. Brain Res. 2002, 132, 203–213. [Google Scholar] [CrossRef]
- Khan, A.; Shal, B.; Naveed, M.; Nasir, B.; Irshad, N.; Ali, H.; Khan, S. Matrine alleviates neurobehavioral alterations via modulation of JNK-mediated caspase-3 and BDNF/VEGF signaling in a mouse model of burn injury. Psychopharmacology 2020, 237, 2327–2343. [Google Scholar] [CrossRef]
- Shal, B.; Khan, A.; Naveed, M.; Ali, H.; Seo, E.K.; Choi, H.; Khan, S. Neuroprotective effect of 25-Methoxyhispidol A against CCl4-induced behavioral alterations by targeting VEGF/BDNF and caspase-3 in mice. Life Sci. 2020, 253, 117684. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shin, E.M.; Choi, R.J.; Jung, Y.H.; Kim, J.; Tosun, A.; Kim, Y.S. Suppression of LPS-induced inflammatory and NF-κB responses by anomalin in RAW 264.7 macrophages. J. Cell. Biochem. 2011, 112, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shehzad, O.; Cheng, M.-S.; Li, R.-J.; Kim, Y.S. Pharmacological mechanism underlying anti-inflammatory properties of two structurally divergent coumarins through the inhibition of pro-inflammatory enzymes and cytokines. J. Inflamm. 2015, 12, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Shal, B.; Naveed, M.; Shah, F.A.; Atiq, A.; Khan, N.U.; Kim, Y.S.; Khan, S. Matrine ameliorates anxiety and depression-like behaviour by targeting hyperammonemia-induced neuroinflammation and oxidative stress in CCl4 model of liver injury. Neurotoxicology 2019, 72, 38–50. [Google Scholar] [CrossRef]
- Khan, S.; Shehzad, O.; Lee, K.J.; Tosun, A.; Kim, Y.S. Anti-inflammatory properties of samidin from Seseli resinosum through suppression of NF-κB and AP-1-mediated-genes in LPS-stimulated RAW 264.7 cells. Arch. Pharm. Res. 2014, 37, 1496–1503. [Google Scholar] [CrossRef]
- Khan, S.; Shehzad, O.; Jin, H.-G.; Woo, E.-R.; Kang, S.S.; Baek, S.W.; Kim, J.; Kim, Y.S. Anti-inflammatory mechanism of 15, 16-epoxy-3α-hydroxylabda-8, 13 (16), 14-trien-7-one via inhibition of LPS-induced multicellular signaling pathways. J. Nat. Prod. 2012, 75, 67–71. [Google Scholar] [CrossRef]
- Stuart, B.H. Biological Applications of Infrared Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Kazmi, Z.; Zeeshan, S.; Khan, A.; Malik, S.; Shehzad, A.; Seo, E.K.; Khan, S. Anti-epileptic activity of daidzin in PTZ-induced mice model by targeting oxidative stress and BDNF/VEGF signaling. NeuroToxicology 2020, 79, 150–163. [Google Scholar] [CrossRef]






| Parameters | ALT (IU/L) | AST (IU/L) | Creatinine (mg/dL) |
|---|---|---|---|
| Naive | 41.1 ± 2.9 | 45.5 ± 3.3 | 1.2 ± 0.25 |
| PSNL Control | 46.4 ± 5.3 | 48.9 ± 4.5 | 1.6 ± 0.31 |
| ECN (10 mg/kg) | 44.6 ± 4.2 | 47.1 ± 5 | 1.41 ± 0.27 |
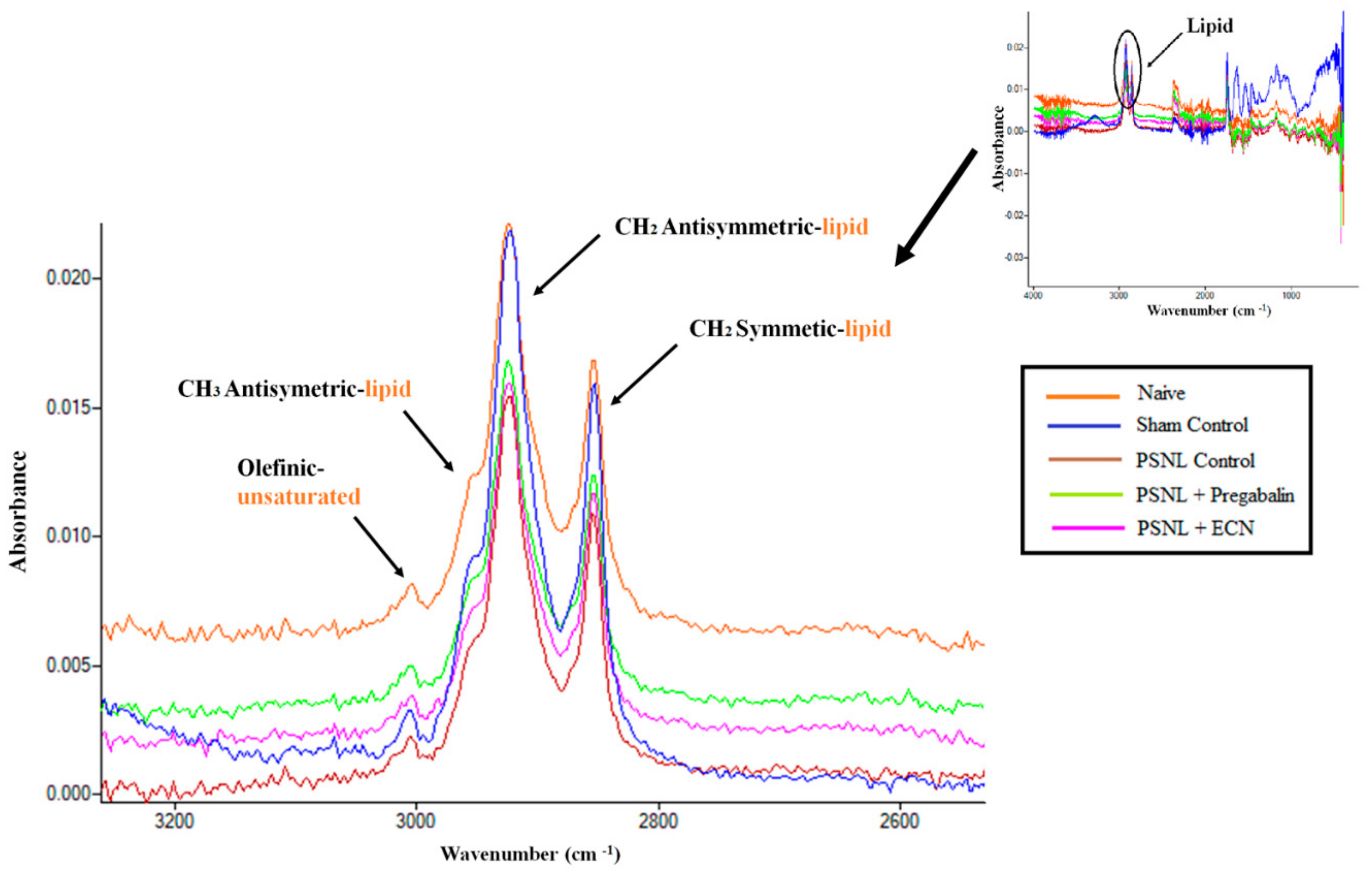
| Bands | Naive | Sham Control | PSNL Control | PSNL + Pregabalin | PSNL + ECN | P |
|---|---|---|---|---|---|---|
| Olefinic | 3008.9 ± 0.04 | 3004.2 ± 1.96 | 2999.3 ± 0.57 | 3003.2 ± 0.39 | 3002.2 ± 0.39 | *** |
| CH2 antisymmetric | 2926.9 ± 1.59 | 2924.5 ± 2.11 | 2918.0 ± 0.90 | 2921.2 ± 0.35 | 2920.3 ± 0.34 | *** |
| CH2 symmetric | 2857.23 ± 1.14 | 2854.6 ± 2.46 | 2848.0 ± 0.88 | 2853.3 ± 0.4 | 2852.1 ± 0.26 | *** |
| CH3 antisymmetric | 2958.7 ± 0.13 | 2955.6 ± 3.14 | 2851.2 ± 1.07 | 2950.4 ± 0.51 | 2948.1 ± 1.01 | *** |
| Wavenumber (cm−1) | Definition of the Spectral Assignment |
|---|---|
| 3014 | Olefinic=CH stretching vibration: unsaturated lipids, cholesterol esters. |
| 2962 | CH3 antisymmetric stretching: equal contribution of lipids, and proteins, carbohydrates, nucleic acids. |
| 2929 | CH2 antisymmetric stretching: mainly lipids, with the little contribution from proteins, carbohydrates, nucleic acids |
| 2855 | CH2 symmetric stretching: mainly lipids, with the little contribution from proteins, carbohydrates, nucleic acids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Khan, A.; Khalid, S.; Shal, B.; Kang, E.; Lee, H.; Laumet, G.; Seo, E.K.; Khan, S. 7?-(3-Ethyl-cis-crotonoyloxy)-1?-(2-methylbutyryloxy)-3,14-dehydro-Z Notonipetranone Attenuates Neuropathic Pain by Suppressing Oxidative Stress, Inflammatory and Pro-Apoptotic Protein Expressions. Molecules 2021, 26, 181. https://doi.org/10.3390/molecules26010181
Khan A, Khan A, Khalid S, Shal B, Kang E, Lee H, Laumet G, Seo EK, Khan S. 7?-(3-Ethyl-cis-crotonoyloxy)-1?-(2-methylbutyryloxy)-3,14-dehydro-Z Notonipetranone Attenuates Neuropathic Pain by Suppressing Oxidative Stress, Inflammatory and Pro-Apoptotic Protein Expressions. Molecules. 2021; 26(1):181. https://doi.org/10.3390/molecules26010181
Chicago/Turabian StyleKhan, Amna, Adnan Khan, Sidra Khalid, Bushra Shal, Eunwoo Kang, Hwaryeong Lee, Geoffroy Laumet, Eun Kyoung Seo, and Salman Khan. 2021. "7?-(3-Ethyl-cis-crotonoyloxy)-1?-(2-methylbutyryloxy)-3,14-dehydro-Z Notonipetranone Attenuates Neuropathic Pain by Suppressing Oxidative Stress, Inflammatory and Pro-Apoptotic Protein Expressions" Molecules 26, no. 1: 181. https://doi.org/10.3390/molecules26010181
APA StyleKhan, A., Khan, A., Khalid, S., Shal, B., Kang, E., Lee, H., Laumet, G., Seo, E. K., & Khan, S. (2021). 7?-(3-Ethyl-cis-crotonoyloxy)-1?-(2-methylbutyryloxy)-3,14-dehydro-Z Notonipetranone Attenuates Neuropathic Pain by Suppressing Oxidative Stress, Inflammatory and Pro-Apoptotic Protein Expressions. Molecules, 26(1), 181. https://doi.org/10.3390/molecules26010181








