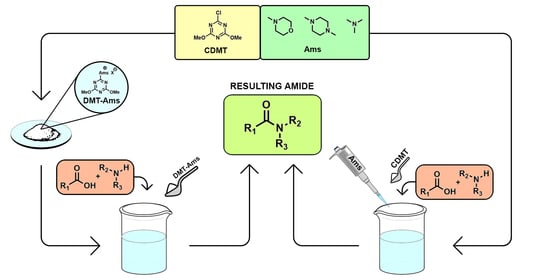
Sustainable Triazine-Based Dehydro-Condensation Agents for Amide Synthesis
Abstract
:1. Introduction
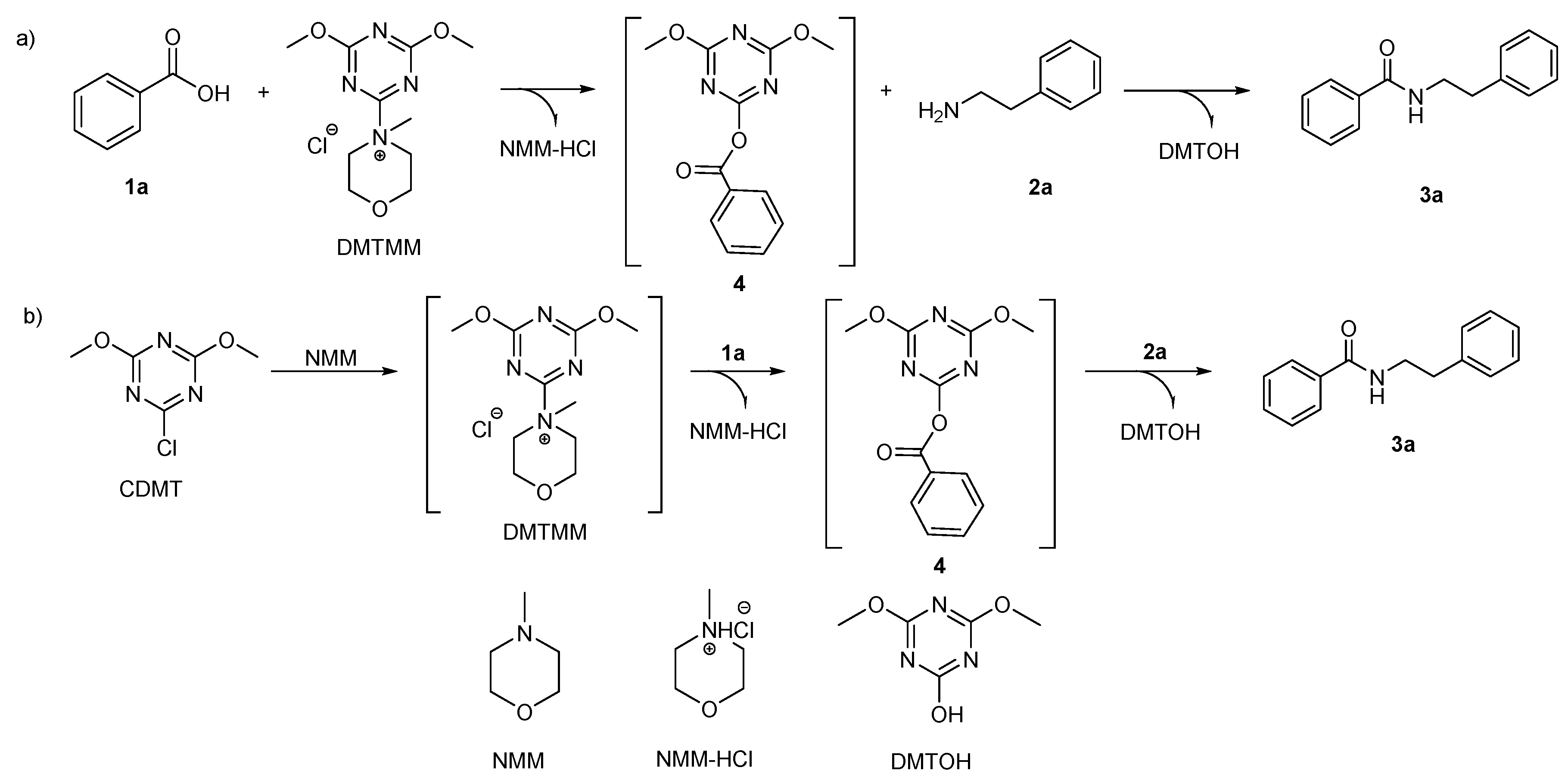
2. Results and Discussion
2.1. Influence of the Solvent on Dehydro-Condensation Reactions
2.2. Influence of the Tert-Amine on Dehydro-Condensation Reactions
2.3. CDMT/Tert-Amine Activity for the Dehydro-Condensation of Various Primary Amines and Carboxylic Acids
3. Materials and Methods
3.1. Synthesis of DMTTMA(ClO4)
3.2. General Procedure for Dehydro-Condensation Reactions with CDMT/Tert-Amine System
3.3. General Procedure for Dehydro-Condensation Reaction with Isolated DMTMM
NMR Characterization of Products 3a–3i
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Albericio, F.; El-Faham, A. Choosing the right coupling reagent for peptides: A twenty-five-year journey. Org. Process Res. Dev. 2018, 22, 760–772. [Google Scholar] [CrossRef]
- Rajput, P.; Sharma, A. Synthesis and biological importance of amide analogues. J. Pharmacol. Med. Chem. 2018, 2, 22–31. [Google Scholar]
- Krause, T.; Baader, S.; Erb, B.; Goossen, L.J. Atom-economic catalytic amide synthesis from amines and carboxylic acids activated in situ with acetylenes. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanigan, R.M.; Sheppard, T.D. Recent developments in amide synthesis: Direct amidation of carboxylic acids and transamidation reactions. Eur. J. Org. Chem. 2013, 33, 7453–7465. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.Y.; Gang, H.Z.; Zhou, L.; Liu, J.F.; Mu, B.Z.; Yang, S.Z. A high yield method for the direct amidation of long-chain fatty acids. Int. J. Chem. Kinet. 2020, 52, 99–108. [Google Scholar] [CrossRef]
- El-Faham, A.; Albericio, F. Peptide couplig reagents, more than a letter soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef]
- Zarecki, A.P.; Kolanowski, J.L.; Markiewicz, W.T. Microwave-assisted catalytic method for a green synthesis of amides directly from amines and carboxylic acids. Molecules 2020, 25, 1761. [Google Scholar] [CrossRef]
- Guo, L.; Jia, S.; Diercks, C.S.; Yang, X.; Alshmimri, S.A.; Yaghi, O.M. Amidation, esterification, and thioesterification of a carboxyl-functionalized covalent organic framework. Angew. Chem. Int. Ed. 2020, 59, 2023–2027. [Google Scholar] [CrossRef]
- Philipova, I.; Stavrakov, G.; Dimitrov, V.; Vassilev, N. Galantamine derivatives: Synthesis, NMR study, DFT calculations and application in asymmetric catalysis. J. Mol. Struct. 2020, 1219, 128568. [Google Scholar] [CrossRef]
- El-Faham, A.; Albericio, F. Morpholine-based immonium and halogenoamidinium salts as coupling reagents in peptide synthesis. J. Org. Chem. 2008, 73, 2731–2737. [Google Scholar] [CrossRef]
- Massolo, E.; Pirola, M.; Benaglia, M. Amide bond formation strategies: Latest advances on a dateless transformation. Eur. J. Org. Chem. 2020, 30, 4641–4651. [Google Scholar] [CrossRef]
- Sharma, S.; Das, J.; Braje, W.M.; Dash, A.; Handa, S. A Glimpse into green chemistry practices in the pharmaceutical industry. ChemSusChem 2020, 13, 2859–2875. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide bond bioisosteres: Strategies, synthesis, and successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef] [PubMed]
- Sole, R.; Bortoluzzi, M.; Spannenberg, A.; Tin, S.; Beghetto, V.; de Vries, J.G. Synthesis, characterization and catalytic activity of novel ruthenium complexes bearing NNN click based ligands. Dalton Trans. 2019, 48, 13580–13588. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L.; Zhang, J.; Gong, H. Metal–and solvent-free synthesis of amides using substitute formamides as an amino source under mild conditions. Sci. Rep. 2019, 9, 2787. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green chemistry and resource efficiency: Towards a green economy. Green Chem. 2016, 18, 3180–3183. [Google Scholar] [CrossRef]
- Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Greening Fmoc/tBu solid-phase peptide synthesis. Green Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Sharma, S.; Buchbinder, N.W.; Braje, W.M.; Handa, S. Fast amide couplings in water: Extraction, column chromatography, and crystallization not required. Org. Lett. 2020, 22, 5737–5740. [Google Scholar] [CrossRef]
- Treitler, D.S.; Marriott, A.S.; Chadwick, J.; Quirk, E. Mutagenic impurities in 1-hydroxybenzotriazole (HOBt). Org. Process Res. Dev. 2019, 23, 2562–2566. [Google Scholar] [CrossRef]
- D’Este, M.; Eglin, D.; Alini, M. A systematic analysis of DMTMM vs EDC/NHS for ligation of amines to Hyaluronan in water. Carbohydr. Polym. 2014, 108, 239–246. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Conca, S.; Bardella, N.; Scrivanti, A. Polyamidoamide dendrimers and cross-linking agents for stabilized bioenzymatic resistant metal-free bovine collagen. Molecules 2019, 24, 3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Borke, T.; Winnik, F.M.; Tenhu, H.; Hietala, S. Optimized triazine-mediated amidation for efficient and controlled functionalization of hyaluronic acid. Carbohydr. Polym. 2015, 116, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.T.; Pharmar, T.H.; Sangani, C.B.; Shah, A.S.; Ameta, R.K. 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo-[4,5-b] pyridinium3-oxide Hexafluorophosphate (HATU)/Hydroxybenzotriazole (HOBT)-based one-pot cyclization of N-substituted 2-arylbenzimidazole derivatives. Russ. J. Org. Chem. 2020, 56, 856–862. [Google Scholar] [CrossRef]
- Albericio, F. Developments in peptide and amide synthesis. Curr. Opin. Chem. Biol. 2004, 8, 211–221. [Google Scholar] [CrossRef]
- Vokkaliga, S.; Jeong, J.; LaCourese, R.; Kalivretenos, A. Synthesis of amide libraries with immobilized HOBt. Tetrahedron Lett. 2011, 52, 2722–2724. [Google Scholar] [CrossRef]
- Itoh, H.; Miura, K.; Kamiya, K.; Yamashita, T.; Inoue, M. Solid-phase total synthesis of Yaku’amide B enabled by traceless staudinger ligation. Angew. Chem. 2020, 132, 4594–4601. [Google Scholar] [CrossRef]
- Carpino, L.A.; Xia, J.; Zhang, C.; El-Faham, A. Organophosphorus and nitro-substituted sulfonate esters of 1-hydroxy-7-azabenzotriazole as highly efficient fast-acting peptide coupling reagents. J. Org. Chem. 2004, 69, 62–67. [Google Scholar] [CrossRef]
- Carpino, L.A.; El-Faham, A. The diisopropylcarbodiimide/1-hydroxy-7-azabenzotriazole system: Segment coupling and stepwise peptide assembly. Tetrahedron 1999, 55, 6813–6830. [Google Scholar] [CrossRef]
- Carpino, L.A.; Imazumi, H.; El-Faham, A.; Ferrer, F.J.; Zhang, C.; Lee, Y.; Foxman, B.M.; Henklein, P.; Hanay, C.; Mügge, C.; et al. The uronium/guanidinium peptide coupling reagents: Finally the true uronium salts. Angew. Chem. Int. Ed. 2002, 41, 441–445. [Google Scholar] [CrossRef]
- Hou, X.M.; Liang, T.M.; Guo, Z.Y.; Wang, C.Y.; Shao, C.L. Discovery, absolute assignments, and total synthesis of asperversiamides A–C and their potent activity against mycobacterium marinum. Chem. Commun. 2019, 55, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Nakamura, M.; Ohno, A.; Tanaka, T.; Kobayashi, A.; Ishihara, M.; Fujita, M.; Tsuchida, A.; Mizuno, M.; Shoda, S. A dimethoxytriazine type glycosyl donor enables a facile chemo-enzymatic route toward α-linked N-acetylglucosaminyl-galactose disaccharide unit from gastric mucin. Chem. Commun. 2012, 48, 5560–5562. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, Z.J. Triazine-based condensing reagents. Biopolymers 2000, 55, 140–164. [Google Scholar] [CrossRef]
- Kitamura, M.; Sasaki, S.; Nishikawa, R.; Yamada, K.; Kunishima, M. Imido-substituted triazines as dehydrative condensing reagents for the chemoselective formation of amides in the presence of free hydroxy groups. RSC Adv. 2018, 8, 22482–22489. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, M.; Komine, S.; Yamada, K.; Kunishima, M. Triazine-based dehydrative condensation reagents bearing carbon-substituents. Tetrahedron 2020, 76, 130900. [Google Scholar] [CrossRef]
- Scrivanti, A.; Bortoluzzi, M.; Sole, R.; Beghetto, V. Synthesis and characterization of yttrium, europium, terbium and dysprosium complexes containing a novel type of triazolyl–oxazoline ligand. Chem. Pap. 2018, 72, 799–808. [Google Scholar] [CrossRef]
- Kunishima, M.; Hioki, K.; Wada, A.; Kobayashi, H.; Tani, S. Approach to green chemistry of DMT-MM: Recovery and recycle of coproduct to chloromethane-free DMT-MM. Tetrahedron Lett. 2002, 43, 3323–3326. [Google Scholar] [CrossRef]
- Beghetto, V.; Agostinis, L.; Gatto, V.; Samiolo, R.; Scrivanti, A. Sustainable use of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride as metal free tanning agent. J. Clean. Prod. 2019, 220, 864–872. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Conca, S.; Bardella, N.; Buranello, C.; Gasparetto, G.; Sole, R. Development of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride cross-linked carboxymethyl cellulose films. Carbohydr. Polym. 2020, 249, 116810. [Google Scholar] [CrossRef]
- Scrivanti, A.; Sole, R.; Bortoluzzi, M.; Beghetto, V.; Bardella, N.; Dolmella, A. Synthesis of new triazolyl-oxazoline chiral ligands and study of their coordination to Pd (II) metal centers. Inorg. Chim. Acta 2019, 498, 119129. [Google Scholar] [CrossRef]
- Petta, D.; Eglin, D.; Grijpma, D.W.; D’Este, M. Enhancing hyaluronan pseudoplasticity via 4-(4, 6-dimethoxy-1, 3, 5-triazin-2-yl)-4-methylmorpholinium chloride-mediated conjugation with short alkyl moieties. Carbohydr. Polym. 2016, 151, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.M.E.; Tran, W.; Nagalingam, G.; Cheung, C.Y.; Giltrap, A.M.; Cook, G.M.; Britton, W.J.; Payne, R.J. Total synthesis and antimycobacterial activity of ohmyungsamycin A, deoxyecumicin, and ecumicin. Chem. Eur. J. 2020, 66, 15200–15205. [Google Scholar] [CrossRef] [PubMed]
- Kunishima, M.; Ujigawa, T.; Nagaoka, Y.; Kawachi, C.; Hioki, K.; Shiro, M. Study on 1,3,5-Triazine chemistry in dehydrocondensation: Gauche effect on the generation of active triazinylammonium species. Chem. Eur. J. 2012, 18, 15856–15867. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, Z.J.; Kolesińska, B.; Kamińska, J.E.; Góra, J. A novel generation of coupling reagents. Enantiodifferentiating coupling reagents prepared in situ from 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) and chiral tertiary amines. J. Org. Chem. 2001, 66, 6276–6281. [Google Scholar] [CrossRef]
- Kamiński, Z.J.; Paneth, P.; Rudziński, J. A study on the activation of carboxylic acids by means of 2-chloro-4,6-dimethoxy-1,3,5-triazine and 2-chloro-4,6-diphenoxy-1,3,5-triazine. J. Org. Chem. 1998, 63, 4248–4255. [Google Scholar] [CrossRef]
- Garret, C.E.; Jiang, X.; Prasad, K.; Repič, O. New observations on peptide bond formation using CDMT. Tetrahedron Lett. 2002, 43, 4161–4165. [Google Scholar] [CrossRef]
- Barnett, C.J.; Wilson, T.M.; Kobierski, M.E. A practical synthesis of multitargeted antifolate LY231514. Org. Process Res. Dev. 1999, 3, 184–188. [Google Scholar] [CrossRef]
- Walker, A.J.; Adolph, S.; Connell, R.B.; Laue, K.; Roeder, M.; Rueggeberg, C.J.; Hahn, D.U.; Voegtli, K.; Watson, J. Implementation of a High-Temperature Claisen Approach for Early Phase Delivery of a Benzopyran Derivative. Org. Process Res. Dev. 2010, 14, 85–91. [Google Scholar] [CrossRef]
- Villahuer, E.B.; Shieh, W.C.; Du, Z.; Vargas, K.; Ciszewki, L.; Lu, Y.; Girgis, M.; Lin, M.; Prashad, M. Facile and practical synthesis of a cannabinoid-1 antagonist viaregio-and stereoselective ring-opening of an aziridinium ion. Tetrahedron 2009, 65, 9067–9074. [Google Scholar] [CrossRef]
- Kallman, N.J.; Liu, C.; Yates, M.H.; Linder, R.J.; Ruble, J.C.; Kogut, E.F.; Patterson, L.E.; Laird, D.L.T.; Hansen, M.M. Route design and development of a MET kinase inhibitor: A copper-catalyzed preparation of an N1-methylindazole. Org. Process Res. Dev. 2014, 18, 501–510. [Google Scholar] [CrossRef]
- Paganelli, S.; Alam, M.M.; Beghetto, V.; Scrivanti, A.; Amadio, E.; Bertoldini, M.; Matteoli, U. A pyridyl-triazole ligand for ruthenium and iridium catalyzed C = C and C = O hydrogenations in water/organic solvent biphasic systems. Appl. Catal. A 2015, 503, 20–25. [Google Scholar] [CrossRef]
- Beghetto, V.; Scrivanti, A.; Bertoldini, M.; Aversa, M.; Zancanaro, A.; Matteoli, U. A practical, enantioselective synthesis of the fragrances canthoxal, and silvial, and evaluation of their olfactory activity. Synthesis 2015, 47, 272–278. [Google Scholar] [CrossRef]
- Matteoli, U.; Beghetto, V.; Scrivanti, A.; Aversa, M.; Bertoldini, M.; Bovo, S. An alternative stereoselective synthesis of (R)-and (S)-rosaphen via asymmetric catalytic hydrogenation. Chirality 2011, 23, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Sole, R.; Taddei, L.; Franceschi, C.; Beghetto, V. Efficient chemo-enzymatic transformation of animal biomass waste for eco-friendly leather production. Molecules 2019, 24, 2979. [Google Scholar] [CrossRef] [Green Version]
- Raw, S.A. An improved process for the synthesis of DMTMM-based coupling reagents. Tetrahedron Lett. 2009, 50, 946–948. [Google Scholar] [CrossRef]
- Kunishima, M.; Kawachi, C.; Morita, J.; Terao, K.; Iwasaki, F.; Tani, S. 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride: An efficient condensing agent leading to the formation of amides and esters. Tetrahedron 1999, 55, 13159–13170. [Google Scholar] [CrossRef]
- Kamiński, Z.J. 2-Chloro-4,6-disubstituted-1,3,5-triazines a novel group of condensing reagents. Tetrahedron Lett. 1985, 26, 2901–2904. [Google Scholar] [CrossRef]
- Kunishima, M.; Kawachi, C.; Hioki, K.; Terao, K.; Tani, S. Formation of carboxamides by direct condensation of carboxylic acids and amines in alcohols using a new alcohol- and watersoluble condensing agent: DMT-MM. Tetrahedron 2001, 57, 1551–1558. [Google Scholar] [CrossRef]
- Hirano, N.; Saijyo, M. (Tokuyama Corporation), Method for Storing Quaternary Ammonium Salt. EP1178043A1, 6 February 2002. [Google Scholar]
- Kameta, N.; Shiroishi, H. PEG-nanotube liquid crystals as templates for construction of surfactant-free gold nanorods. Chem. Commun. 2018, 54, 4665–4668. [Google Scholar] [CrossRef]
- Loebel, C.; D’Este, M.; Alini, M.; Zenobi-Wong, M.; Eglin, D. Precise tailoring of tyramine-based hyaluronan hydrogel properties using DMTMM conjugation. Carbohydr. Polym. 2015, 115, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, Z.J. 2-Chloro-4,6-dimethoxy-1,3,5-triazine. A new coupling reagent for Peptide Synthesis. Synthesis 1987, 10, 917–920. [Google Scholar] [CrossRef]
- Yamada, K.; Kota, M.; Takahashi, K.; Fujita, H.; Kitamura, M.; Kunishima, M. Development of triazinone-based condensing reagents for amide formation. J. Org. Chem. 2019, 84, 15042–15051. [Google Scholar] [CrossRef] [PubMed]
- Beghetto, V. (Crossing Srl), Method for the Industrial Production of 2-halo-4,6-dialkoxy-1,3,5-triazine and Their Use in the Presence of Amines. EP 3237390B1, 8 May 2019. [Google Scholar]
- Daa Funder, E.; Trads, J.B.; Gothelf, K.V. Oxidative activation of dihydropyridine amides to reactive acyl donors. Org. Biomol. Chem. 2015, 13, 185–198. [Google Scholar] [CrossRef]
- Katarzyna, J.; Małolepsza, J.; Kusy, D.; Maniukiewicz, W.; Błażewska, K.M. The McKenna reaction–Avoiding side reactions in phosphonate deprotection. Beilstein J. Org. Chem. 2020, 16, 1436–1446. [Google Scholar]
- Wu, J.W.; Wu, Y.D.; Dai, J.J.; Xu, H.J. Benzoic acid-catalyzed transamidation reactions of carboxamides, phthalimide, ureas and thioamide with amines. Adv. Synth. Catal. 2014, 356, 2429–2436. [Google Scholar] [CrossRef]
- Fraczyk, J.; Kaminski, Z.J.; Katarzynska, J.; Kolesinska, B. 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium Toluene-4-sulfonate (DMT/NMM/TsO) universal coupling reagent for synthesis in solution. Helv. Chim. Acta 2018, 101, e1700187. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Cho, E.J. Visible-light-mediated synthesis of amides from aldehydes and amines via in situ acid chloride formation. J. Org. Chem. 2016, 81, 1905–1911. [Google Scholar] [CrossRef]
- Li, J.; He, S.; Fu, H.; Chen, X.; Tang, M.; Zhang, D.; Wang, B. An efficient procedure for chemoselective amidation from carboxylic acid and amine (ammonium salt) under mild conditions. Res. Chem. Intermed. 2018, 44, 2289–2303. [Google Scholar] [CrossRef]
- Álvarez-Pérez, A.; Esteruelas, M.A.; Izquierdo, S.; Varela, J.A.; Saá, C. Ruthenium-catalyzed oxidative amidation of alkynes to amides. Org. Lett. 2019, 21, 5346–5350. [Google Scholar] [CrossRef] [PubMed]







| Entry | Solvent | Coupling Agent [a] | Yield (%) [b] (15/60 min) |
|---|---|---|---|
| 1 | THF | CDMT/NMM | 74/78 |
| 2 | THF | DMTMM | 80/89 |
| 3 | CH3OH | CDMT/NMM | 93/95 |
| 4 | CH3OH | DMTMM | 98/99 |
| 5 | EtOH | CDMT/NMM | 92/95 |
| 6 | EtOH | DMTMM | 98/99 |
| 7 | CH3CN | CDMT/NMM | 78/82 |
| 8 | CH3CN | DMTMM | 58/66 |
| 9 | Acetone | CDMT/NMM | 82/83 |
| 10 | Acetone | DMTMM | 73/97 |
| 11 | CH2Cl2 | CDMT/NMM | 86/93 |
| 12 | CH2Cl2 | DMTMM | 85/92 |
| 13 | Toluene | CDMT/NMM | 69/90 |
| 14 | Toluene | DMTMM | 65/72 |
| 15 | H2O | CDMT/NMM | 45/52 [c] |
| 16 | H2O | DMTMM | 49/53 [c] |
| Entry | Coupling Agent [a] | Counter Anion [b] | Yield (%) [c] (15/60 min) |
|---|---|---|---|
| 1 | CDMT/NMM | Cl− | 93/95 |
| 2 | DMTMM | Cl− | 98/99 |
| 3 | CDMT/NMM | ClO4− | 82/83 |
| 4 | DMTMM | ClO4− | 38/47 |
| 5 | CDMT/TMA | Cl− | 93/96 |
| 6 | DMTTMA [d] | Cl− | n.d. |
| 7 | CDMT/TMA | ClO4− | 87/91 |
| 8 | DMTTMA | ClO4− | 79/81 |
| 9 | CDMT/NMP | Cl− | 49/52 |
| 10 | DMTMP [d] | Cl− | n.d. |
| 11 | CDMT/NMP | ClO4− | 56/60 |
| 12 | DMTMP | ClO4− | 86/90 |
| 13 | CDMT/MPD | Cl− | 65/77 |
| 14 | DMTMPD [d] | Cl− | n.d. |
| 15 | CDMT/MPD | ClO4− | 68/80 |
| 16 | DMTMPD | ClO4− | 80/85 |
| 17 | CDMT/NNDP | Cl− | 93/96 |
| 18 | CDMT/NNDP [e] | Cl− | 92/95 |
| 19 | CDMT/NNDP [f] | Cl− | 79/90 |
| 20 | DMTDP [d] | Cl− | n.d. |
| 21 | CDMT/NNDP | ClO4− | 77/88 |
| 22 | DMTDP [d] | ClO4− | n.d. |
| Entry | Acid | Coupling Agent [a] | Amide | Yield (%) [b] (15/60 min) |
|---|---|---|---|---|
| 1 | 1a | CDMT/NMM | 3a | 93/95 |
| 2 | CDMT/NNDP | 92/95 | ||
| 3 | CDMT/TMA | 93/96 | ||
| 4 | 1b | CDMT/NMM | 3b | 82/87 |
| 5 | CDMT/NNDP | 69/71 | ||
| 6 | CDMT/TMA | 45/51 | ||
| 7 | 1c | CDMT/NMM | 3c | 71/75 |
| 8 | CDMT/NNDP | 65/74 | ||
| 9 | CDMT/TMA | 12/15 | ||
| 10 | 1d | CDMT/NMM | 3d | 88/92 |
| 11 | CDMT/NNDP | 70/86 | ||
| 12 | CDMT/TMA | 18/21 | ||
| 13 | 1e | CDMT/NMM | 3e | 34/38 |
| Entry | Amine | Coupling Agent [a] | Amide | Yield (%) [b] (15/60 min) |
|---|---|---|---|---|
| 1 | 2b | CDMT/NMM | 58/74 | |
| 2 | CDMT/NNDP | 3f | 49/65 | |
| 3 | CDMT/TMA | 52/67 | ||
| 4 | 2c | CDMT/NMM | 77/93 | |
| 5 | CDMT/NNDP | 3g | 59/64 | |
| 6 | CDMT/TMA | 72/78 | ||
| 7 | 2d | CDMT/NMM | 61/70 | |
| 8 | CDMT/NNDP | 3h | 55/58 | |
| 9 | CDMT/TMA | 19/30 | ||
| 10 | 2e | CDMT/NMM | 56/66 | |
| 11 | CDMT/NNDP | 3i | 50/55 | |
| 12 | CDMT/TMA | 34/35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sole, R.; Gatto, V.; Conca, S.; Bardella, N.; Morandini, A.; Beghetto, V. Sustainable Triazine-Based Dehydro-Condensation Agents for Amide Synthesis. Molecules 2021, 26, 191. https://doi.org/10.3390/molecules26010191
Sole R, Gatto V, Conca S, Bardella N, Morandini A, Beghetto V. Sustainable Triazine-Based Dehydro-Condensation Agents for Amide Synthesis. Molecules. 2021; 26(1):191. https://doi.org/10.3390/molecules26010191
Chicago/Turabian StyleSole, Roberto, Vanessa Gatto, Silvia Conca, Noemi Bardella, Andrea Morandini, and Valentina Beghetto. 2021. "Sustainable Triazine-Based Dehydro-Condensation Agents for Amide Synthesis" Molecules 26, no. 1: 191. https://doi.org/10.3390/molecules26010191