Water-in-Oil-in-Water Nanoemulsions Containing Temulawak (Curcuma xanthorriza Roxb) and Red Dragon Fruit (Hylocereus polyrhizus) Extracts
Abstract
:1. Introduction
2. Results and Discussion
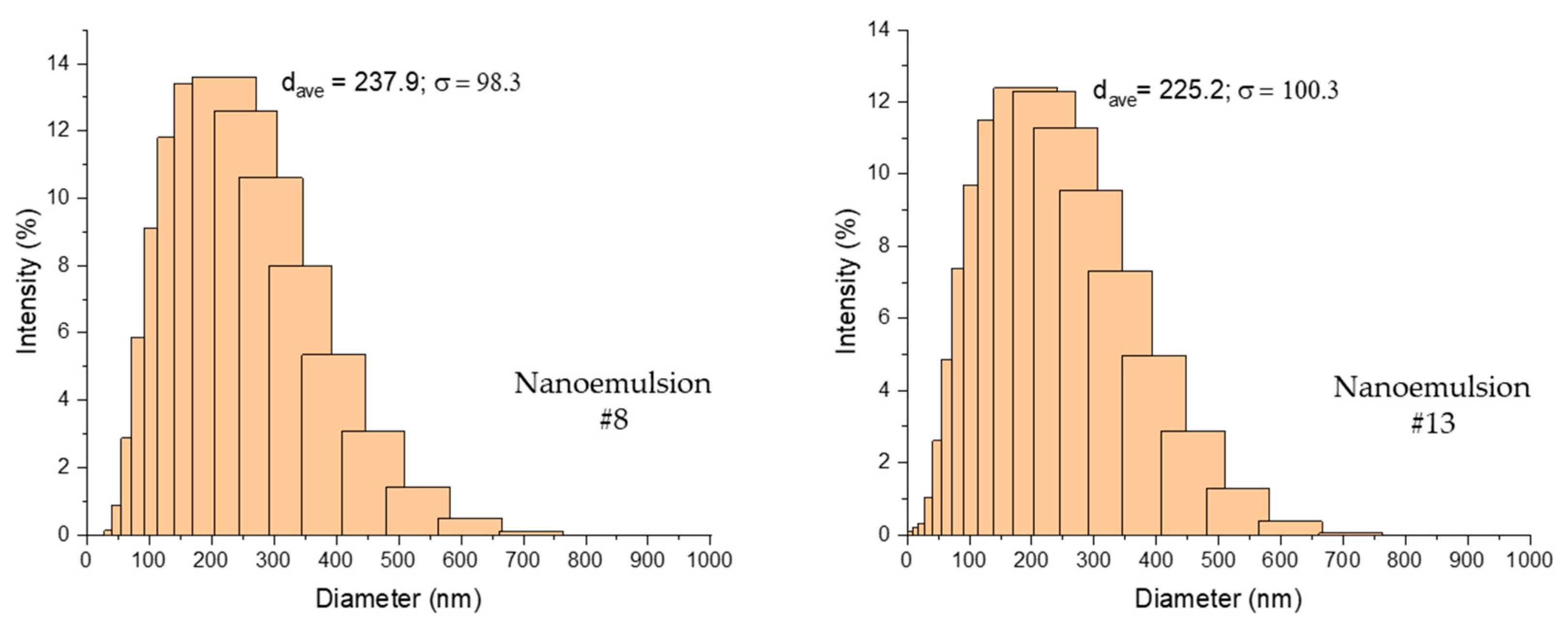
2.1. MDD and PDI
2.2. Stability Study
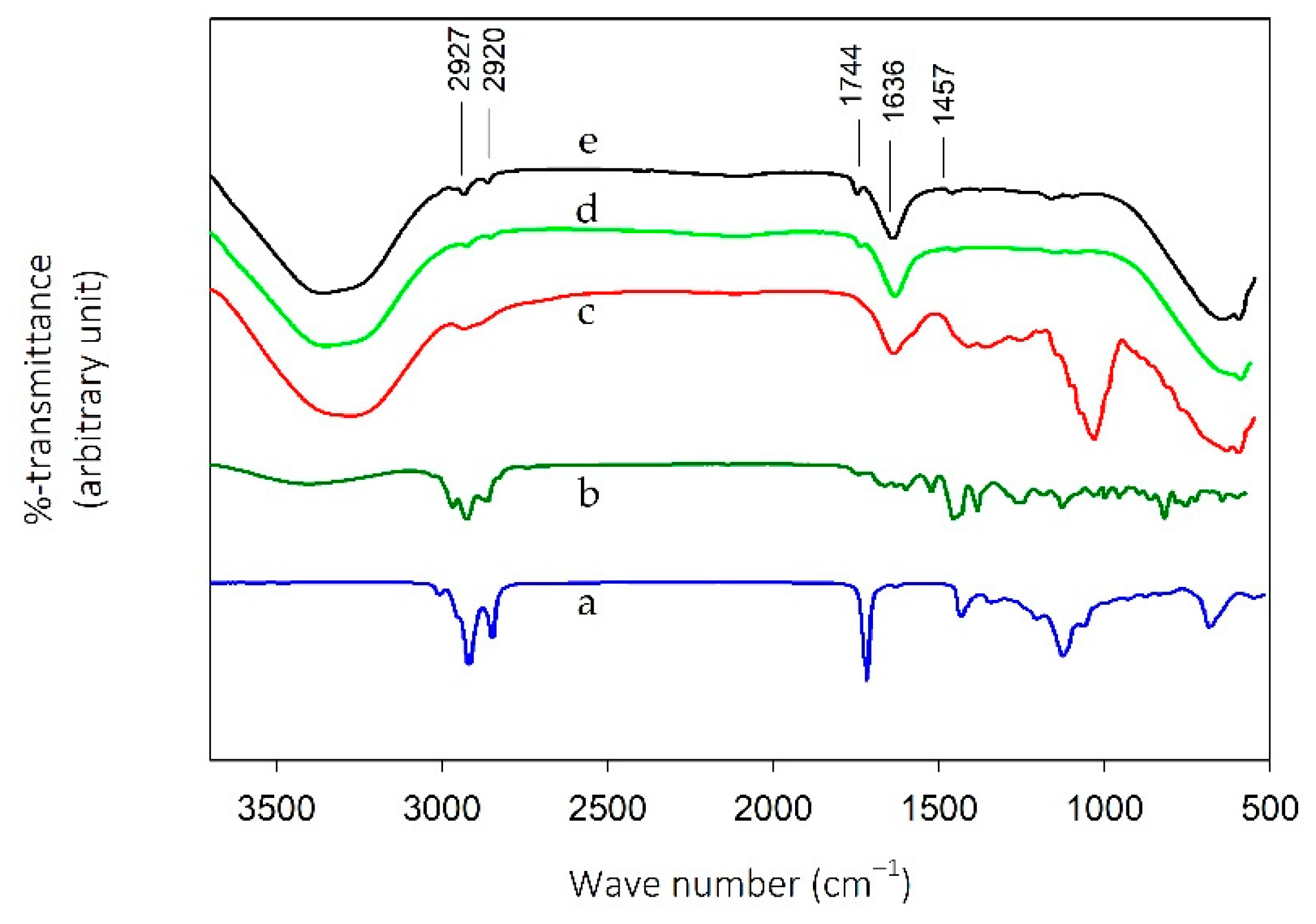
2.3. FTIR Spectroscopy
2.4. Zeta Potential
2.5. Antioxidant Activity
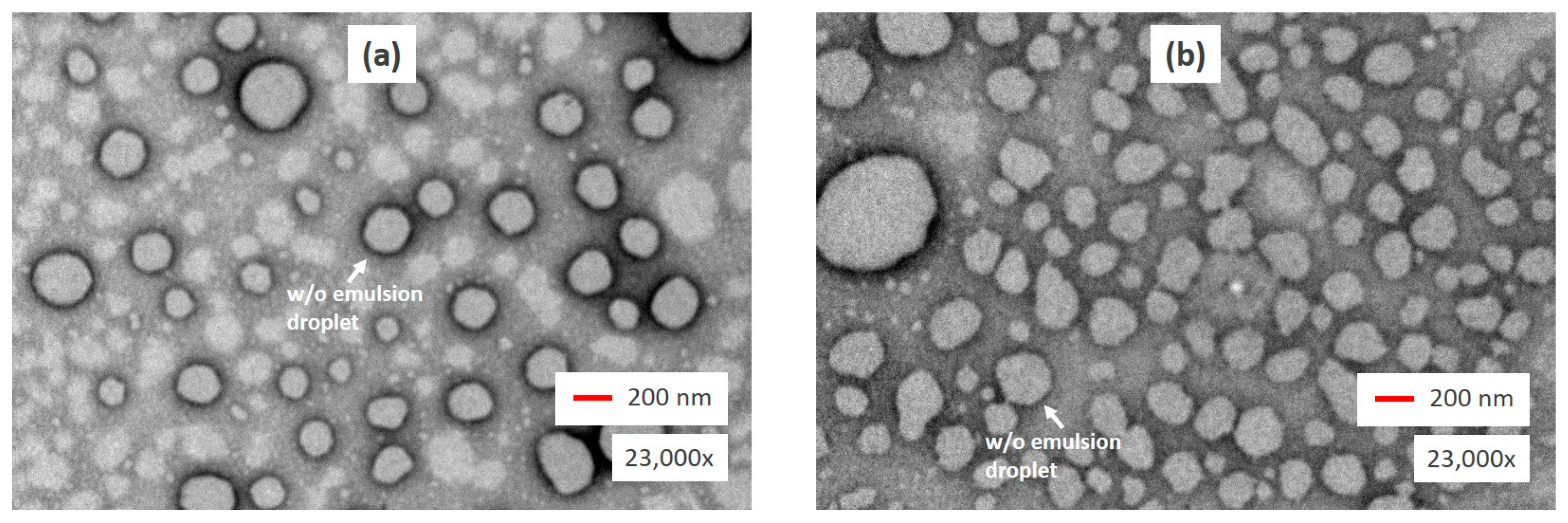
2.6. Morphology of W/O/W Nanoemulsions
3. Materials and Methods
3.1. Materials
3.2. Preparation of Temulawak and Red Dragon Fruit Extracts
3.3. Fabrication of w/o/w Nanoemulsions
3.3.1. Primary w/o Emulsion
3.3.2. Final w/o/w Nanoemulsion
3.4. Stability Study
3.5. Droplet Size Measurement
3.6. Zeta Potential Measurement
3.7. Antioxidant Activity
3.8. FTIR Spectra
3.9. TEM Images
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aditya, N.P.; Macedo, A.S.; Doktorovova, S.; Souto, E.B.; Kim, S.; Chang, P.-S.; Ko, S. Development and evaluation of lipid nanocarriers for quercetin delivery: A comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). LWT—Food Sci. Technol. 2014, 59, 115–121. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Athira, G.K.; Jyothi, A.N. Preparation and characterization of curcumin loaded cassava starch nanoparticles with improved cellular absorption. Int. J. Pharm. Pharm. Sci. 2014, 6, 171–176. [Google Scholar]
- Malik, P.; Ameta, R.K.; Singh, M. Preparation and characterization of bionanoemulsions for improving and modulating the antioxidant efficacy of natural phenolic antioxidant curcumin. Chem. Biol. Interact 2014, 222, 77–86. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Betacyanins in fruits from red-purple pitaya, Hylocereus polyrhizus (Weber) Britton & Rose. Food Chem. 2002, 77, 101–106. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Stability of betacyanin from red pitahaya (Hylocereus polyrhizus) and its potential application as a natural colourant in milk. Int. J. Food Sci. Technol. 2016, 51, 427–434. [Google Scholar] [CrossRef]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef]
- Tang, C.S.; Norziah, M.H. Stability of betacyanin pigments from red purple pitaya fruit (Hylocereuspolyrhizus): Influence of pH, temperature, metal ions and ascorbic acid. Indones. J. Chem. 2007, 7, 327–331. [Google Scholar] [CrossRef]
- Kaimainen, M.; Marze, S.; Järvenpää, E.; Anton, M.; Huopalahti, R. Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT—Food Sci. Technol. 2015, 60, 899–904. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.Q.; Niu, J.; Liu, W.; Peng, S.F.; Liu, C.M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Campos, L.; Valle-Guadarrama, S.; Martinez-Bustos, F.; Salinas-Moreno, Y.; Lobato-Calleros, C.; Calvo-Lopez, A.D. Encapsulation and pigmenting potential of betalains of pitaya (Stenocereus pruinosus) fruit. J. Food Sci. Technol. 2018, 55, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems: Preparation, Stability, and Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press Taylor and Francis Group LLC: New York, NY, USA, 2016. [Google Scholar]
- Mahmood, T.; Akhtar, N.; Manickam, S. Interfacial film stabilized W/O/W nano multiple emulsions loaded with green tea and lotus extracts: Systematic characterization of physicochemical properties and shelf-storage stability. J. Nanobiotechnol. 2014, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Sigward, E.; Mignet, N.; Rat, P.; Dutot, M.; Muhamed, S.; Guigner, J.M.; Scherman, D.; Brossard, D.; Crauste-Manciet, S. Formulation and cytotoxicity evaluation of new self-emulsifying multiple W/O/W nanoemulsions. Int. J. Nanomed. 2013, 8, 611–625. [Google Scholar] [CrossRef] [Green Version]
- Sigward, E.; Corvis, Y.; Doan, B.-T.; Kindsiko, K.; Seguin, J.; Scherman, D.; Brossard, D.; Mignet, N.; Espeau, P.; Crauste-Manciet, S. Preparation and evaluation of multiple nanoemulsions containing gadolinium (III) chelate as a potential magnetic resonance imaging (MRI) contrast agent. Pharm. Res. 2015, 32, 2983–2994. [Google Scholar] [CrossRef]
- Zhang, J.; Reineccius, G.A. Preparation and stability of W/O/W emulsions containing sucrose as weighting agent. Flavour Fragr. J. 2016, 31, 51–56. [Google Scholar] [CrossRef]
- Ding, S.; Anton, N.; Akram, S.; Er-Rafik, M.; Anton, H.; Klymchenko, A.; Yu, W.; Vandamme, T.F.; Serra, C.A. A new method for the formulation of double nanoemulsions. Soft Matter 2017, 13, 1660–1669. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.-J.; Kim, H.W.; Park, S.O.; Lee, J.; Ko, S. Curcumin and catechin co-loaded water-in-oil-in-water emulsion and its beverage application. J. Funct. Foods 2015, 15, 35–43. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Al-Dhfyan, A.; Alanazi, F.K.; Alsarra, I.A. Double w/o/w nanoemulsion of 5-fluorouracil for self-nanoemulsifying drug delivery system. J. Mol. Liq. 2014, 200, 183–190. [Google Scholar] [CrossRef]
- Xiao, J.; Lu, X.; Huang, Q. Double emulsion derived from kafirin nanoparticles stabilized Pickering emulsion: Fabrication, microstructure, stability and in vitro digestion profile. Food Hydrocol. 2017, 62, 230–238. [Google Scholar] [CrossRef]
- McClements, D.; Jafari, S. Historical perspective: Improving emulsion formation, stability and performance using mixed emulsifier. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Lamba, H.; Sathish, K.; Sabikhi, L. Double Emulsions: Emerging Delivery System for Plant Bioactives. Food Bioprocess Technol. 2015, 8, 709–728. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Martínez-Rey, L.; Iglesias, O.; Pazos, C. Encapsulation of resveratrol using food-grade concentrated double emulsions: Emulsion characterization and rheological behaviour. J. Food Eng. 2018, 226, 73–81. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, Y.; Jia, X.; Li, J.; Zhang, M.; Yin, L. Review on the stability mechanism and application of water-in-oil emulsions encapsulating various additives. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1660–1675. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, T.; Dobler, D.; Nissing, C.; Runkel, F. Influence of hydrophilic surfactants on the properties of multiple W/O/W emulsions. J. Colloid Interface Sci. 2009, 338, 184–192. [Google Scholar] [CrossRef]
- Ghosh, S.; Coupland, J.N. Factors affecting the freeze–thaw stability of emulsions. Food Hydrocolloids 2008, 22, 105–111. [Google Scholar] [CrossRef]
- Ariyaprakai, S.; Tananuwong, K. Freeze–thaw stability of edible oil-in-water emulsions stabilized by sucrose esters and Tweens. J. Food Eng. 2015, 152, 57–64. [Google Scholar] [CrossRef]
- Márquez, A.L.; Medrano, A.; Panizzolo, L.A.; Wagner, J.R. Effect of calcium salts and surfactant concentration on the stability of water-in-oil (w/o) emulsions prepared with polyglycerol polyricinoleate. J. Colloid Interface Sci. 2010, 341, 101–108. [Google Scholar]
- Rohman, A.; Che Man, Y.B. Quantification and classification of corn and sunflower oils as adulterants in olive oil using chemometrics and FTIR spectra. Sci. World J. 2012, 2012, 250795. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.N.; Angell, C.L.; Ito, T.; Smith, R.J.D. The Carbonyl Stretching Bands in the Infrared Spectra of Unsaturated Lactones. Can. J. Chem. 1959, 37, 2007–2022. [Google Scholar] [CrossRef]
- Rohaeti, E.; Rafi, M.; Syafitri, U.D.; Heryanto, R. Fourier transform infrared spectroscopy combined with chemometrics for discrimination of Curcuma longa, Curcuma xanthorrhiza and Zingiber cassumunar. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Rohman, A.; Sudjadi; Devi; Ramadhani, D.; Nugroho, A. Analysis of Curcumin in Curcuma longa and Curcuma xanthorriza Using FTIR Spectroscopy and Chemometrics. Res. J. Med. Plant 2015, 9, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.; Mohamad, A.B.; Kadhum, A.A.; Ludin, N.A. Effect of solvents on the extraction of natural pigments and adsorption onto TiO2 for dye-sensitized solar cell applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 130–137. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 2). Trop. J. Pharm. Res. 2013. [Google Scholar] [CrossRef]
- Wang, D.Y.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-Based Antimicrobial Delivery-Systems for the Treatment of Bacterial Infections. Front Chem. 2020, 7, 872. [Google Scholar] [CrossRef]
- Gupta, V.; Trivedi, P. Chapter 15—In vitro and in vivo characterization of pharmaceutical topical nanocarriers containing anticancer drugs for skin cancer treatment. In Lipid Nanocarriers for Drug Targeting; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 563–627. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Shah, D.O. Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J. Colloid Interface Sci 2002, 256, 201–207. [Google Scholar] [CrossRef]
- Nurcholis, W.; Ambarsari, L.; Luh, N.; Eka, P.; Sari, K.; Darusman, L.K. Curcuminoid Contents, Antioxidant and Anti-Inflammatory Activities of Curcuma xanthorrhiza RoxB. and Curcuma domestica Val. Promising Lines from Sukabumi of Indonesia. Pros. Semin. Nas. Kim. Unesa 2012, 284, 292. [Google Scholar]
- Rosidi, A.; Khomsan, A.; Setiawan, B.; Riyadi, H.; Briawan, D. Antioxidant Potential of Temulawak (Curcuma xanthorriza roxb). Pakistan J. Nutr. 2016, 15, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. Chemical composition and in vitro evaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem. Cent. J. 2014, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicentini, F.T.; Vaz, M.M.; Fonseca, Y.M.; Bentley, M.V.; Fonseca, M.J. Characterization and stability study of a water-in-oil microemulsion incorporating quercetin. Drug Dev. Ind. Pharm. 2011, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Mao, L.; Yang, J.; Zhang, C.; Miao, S.; Gao, Y. Effect of Oil Content and Emulsifier Type on the Properties and Antioxidant Activity of Sea Buckthorn Oil-in-Water Emulsions. J. Food Qual. 2020, 2020, 1540925. [Google Scholar] [CrossRef]
- Perez-Roses, R.; Risco, E.; Vila, R.; Penalver, P.; Canigueral, S. Antioxidant activity of Tween-20 and Tween-80 evaluated through different in-vitro tests. J. Pharm. Pharmacol. 2015, 67, 666–672. [Google Scholar] [CrossRef]
- Eisinaite, V.; Duque Estrada, P.; Schroen, K.; Berton-Carabin, C.; Leskauskaite, D. Tayloring W/O/W emulsion composition for effective encapsulation: The role of PGPR in water transfer-induced swelling. Food Res. Int. 2018, 106, 722–728. [Google Scholar] [CrossRef]
- Kubo, I.; Masuoka, N.; Xiao, P.; Haraguchi, H. Antioxidant activity of dodecyl gallate. J. Agric. Food Chem. 2002, 50, 3533–3539. [Google Scholar] [CrossRef]
| Emulsion # 1 | w/o Emulsion (% w/w) | Tween 80 (% w/w) | MDD 2 (nm) | PDI 2 | Zeta Potential (mV) | Oil Phase Separation 3 (% v/v) |
|---|---|---|---|---|---|---|
| 1 | 22.5 | 1.25 | 222 ± 3 | 0.19 | −37.0 ± 2.5 | 15 |
| 2 | 22.5 | 1.25 | 207 ± 1 | 0.17 | −34.2 ± 1.4 | 15 |
| 3 | 22.5 | 0.89 | 243 ± 2 | 0.22 | −38.2 ± 4.0 | 15 |
| 4 | 33.1 | 1.25 | 216 ± 1 | 0.15 | −33.2 ± 1.2 | 20 |
| 5 | 22.5 | 1.60 | 180 ± 1 | 0.15 | −34.0 ± 2.5 | 15 |
| 6 | 22.5 | 1.25 | 195 ± 1 | 0.17 | −32.7 ± 0.9 | 15 |
| 7 | 11.9 | 1.25 | 191 ± 2 | 0.22 | −35.0 ± 0.9 | 10 |
| 8 | 30.0 | 1.50 | 209 ± 2 | 0.17 | −34.2 ± 0.9 | 20 |
| 9 | 30.0 | 1.00 | 247 ± 1 | 0.20 | −35.1 ± 3.3 | 15 |
| 10 | 22.5 | 1.25 | 197 ± 1 | 0.15 | −32.6 ± 0.7 | 15 |
| 11 | 22.5 | 1.25 | 204 ± 1 | 0.16 | −32.3 ± 0.6 | 15 |
| 12 | 15.0 | 1.00 | 203 ± 2 | 0.19 | −34.5 ± 2.1 | 10 |
| 13 | 15.0 | 1.50 | 189 ± 2 | 0.16 | −32.0 ± 1.1 | 5 |
| 14 | 22.5 | 1.25 | 219 ± 2 | 0.19 | −34.2 ± 1.5 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harimurti, N.; Nasikin, M.; Mulia, K. Water-in-Oil-in-Water Nanoemulsions Containing Temulawak (Curcuma xanthorriza Roxb) and Red Dragon Fruit (Hylocereus polyrhizus) Extracts. Molecules 2021, 26, 196. https://doi.org/10.3390/molecules26010196
Harimurti N, Nasikin M, Mulia K. Water-in-Oil-in-Water Nanoemulsions Containing Temulawak (Curcuma xanthorriza Roxb) and Red Dragon Fruit (Hylocereus polyrhizus) Extracts. Molecules. 2021; 26(1):196. https://doi.org/10.3390/molecules26010196
Chicago/Turabian StyleHarimurti, Niken, Mohammad Nasikin, and Kamarza Mulia. 2021. "Water-in-Oil-in-Water Nanoemulsions Containing Temulawak (Curcuma xanthorriza Roxb) and Red Dragon Fruit (Hylocereus polyrhizus) Extracts" Molecules 26, no. 1: 196. https://doi.org/10.3390/molecules26010196
APA StyleHarimurti, N., Nasikin, M., & Mulia, K. (2021). Water-in-Oil-in-Water Nanoemulsions Containing Temulawak (Curcuma xanthorriza Roxb) and Red Dragon Fruit (Hylocereus polyrhizus) Extracts. Molecules, 26(1), 196. https://doi.org/10.3390/molecules26010196








