Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes
Abstract
:1. Introduction
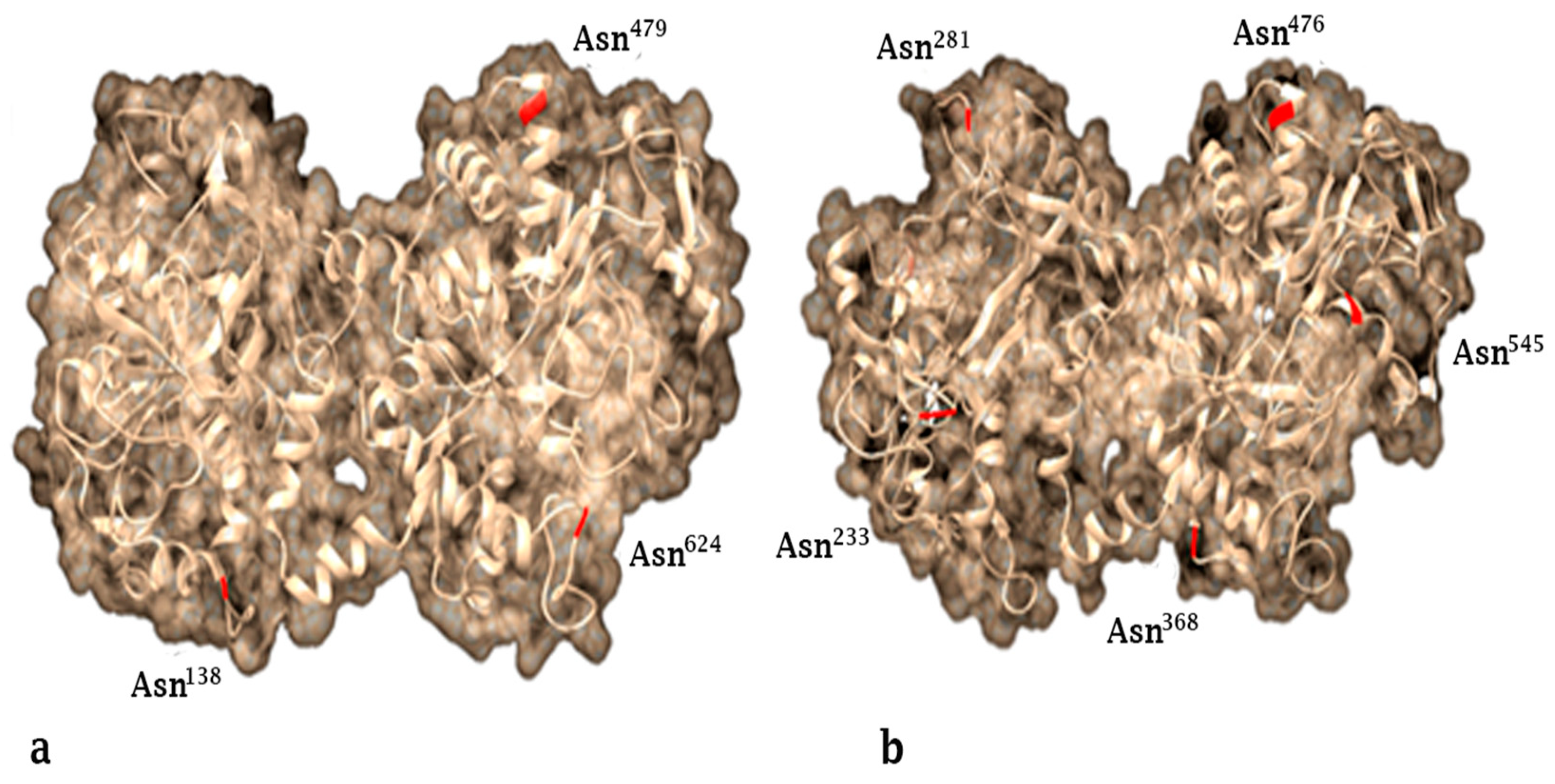
2. Lactoferrin Structure and Production
3. Immunomodulatory and Anti-Inflammatory Activity
4. Iron-Mediated Lactoferrin in Neuropathies
5. Anticancer Activity
5.1. Lactoferrin in Breast Cancer
5.2. Lactoferrin in Leukemia
5.3. Lactoferrin in Cervical Cancer
6. Antimicrobial Activity
6.1. Antibacterial Activity
6.2. Antiviral Activity
6.3. Antifungal Activity
6.4. Antiparasitic Activity
6.5. Antimicrobial Activity in Domestic Animals
7. Other Applications of Lactoferrin
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Groves, M.L. The Isolation of a Red Protein from Milk2. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar] [CrossRef]
- Nuijens, J.H.; Van Berkel, P.H.C.; Schanbacher, F.L. Structure and biological actions of lactoferrin. J. Mammary Gland. Biol. Neoplasia 1996, 1, 285–295. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M.; Kidd, R.D. Lactoferrin and transferrin: Functional variations on a common structural framework. Biochem. Cell Biol. 2002, 80, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Figueroa, B.F.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Rascón-Cruz, Q. Lactoferrin as a nutraceutical protein from milk, an overview. Int. Dairy J. 2019, 89, 37–41. [Google Scholar] [CrossRef]
- García-Montoya, I.; Salazar-Martínez, J.; Arévalo-Gallegos, S.; Sinagawa-García, S.; Rascón-Cruz, Q. Expression and characterization of recombinant bovine lactoferrin in E. coli. BioMetals 2012, 26, 113–122. [Google Scholar] [CrossRef] [PubMed]
- A Moore, S.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional structure of diferric bovine lactoferrin at 2.8 Å resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef]
- Adlerova, L.; Bartoskova, A.; Faldyna, M. Lactoferrin: A review. Veterinární Med. 2008, 53, 457–468. [Google Scholar] [CrossRef] [Green Version]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef] [Green Version]
- Redwan, E.M.; Uversky, V.N.; El-Fakharany, E.M.; Al-Mehdar, H. Potential lactoferrin activity against pathogenic viruses. C. R. Biol. 2014, 337, 581–595. [Google Scholar] [CrossRef]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a Pleiotropic Protein in Health and Disease. Antioxid. Redox Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2017, 59, 580–596. [Google Scholar] [CrossRef]
- Latorre, D.; Puddu, P.; Valenti, P.; Gessani, S. Reciprocal Interactions between Lactoferrin and Bacterial Endotoxins and Their Role in the Regulation of the Immune Response. Toxins 2010, 2, 54–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Actor, J.K.; Hwang, S.-A.; Kruzel, M.L. Lactoferrin as a Natural Immune Modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Embleton, N.; Berrington, J.; McGuire, W.; Stewart, C.J.; Cummings, S.P.; Stewart, C.J. Lactoferrin: Antimicrobial activity and therapeutic potential. Semin. Fetal Neonatal Med. 2013, 18, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y. Lactoferrin and Its Role in Wound Healing; Springer Science and Business Media LLC: New York, NY, USA, 2012. [Google Scholar]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- A Kelley, L.; Mezulis, S.; Yates, C.M.; Wass, M.N.; E Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Drago-Serrano, M.E.; Campos-Rodriguez, R.; Carrero, J.C.; De La Garza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef] [Green Version]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Lozano, S.; Valk-Weeber, R.L.; Akkerman, R.; Abdulahad, W.; Van Leeuwen, S.S.; Dijkhuizen, L.; De Vos, P. Inhibitory Effects of Dietary N-Glycans from Bovine Lactoferrin on Toll-Like Receptor 8; Comparing Efficacy with Chloroquine. Front. Immunol. 2020, 11, 790. [Google Scholar] [CrossRef]
- Valk-Weeber, R.L.; Ruiter, T.E.-D.; Dijkhuizen, L.; Van Leeuwen, S.S. Dynamic Temporal Variations in Bovine Lactoferrin Glycan Structures. J. Agric. Food Chem. 2019, 68, 549–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hu, X.; Long, K.; Gao, C.; Dong, H.-L.; Zhong, Q.; Gao, X.-M.; Gong, F.-Y. Extraordinarily potent proinflammatory properties of lactoferrin-containing immunocomplexes against human monocytes and macrophages. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Panella, G.; Le Boffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisgrill, L.; Wessely, I.; Spittler, A.; Förster-Waldl, E.; Berger, A.; Sadeghi, K. Human lactoferrin attenuates the proinflammatory response of neonatal monocyte-derived macrophages. Clin. Exp. Immunol. 2018, 192, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.B.; Deng, Y.Q.; Ren, J.; Xiao, B.K.; Chen, Z.; Tao, Z.Z. Lactoferrin Administration into the Nostril Alleviates Murine Allergic Rhinitis and its Mechanisms. Scand. J. Immunol. 2013, 78, 507–515. [Google Scholar] [CrossRef] [Green Version]
- MacManus, C.F.; Collins, C.B.; Nguyen, T.T.; Alfano, R.W.; Jedlicka, P.; De Zoeten, E.F. VEN-120, a Recombinant Human Lactoferrin, Promotes a Regulatory T Cell [Treg] Phenotype and Drives Resolution of Inflammation in Distinct Murine Models of Inflammatory Bowel Disease. J. Crohn’s Coliti 2017, 11, 1101–1112. [Google Scholar] [CrossRef]
- Alexander, D.B.; Iigo, M.; Abdelgied, M.; Ozeki, K.; Tanida, S.; Joh, T.; Takahashi, S.; Tsuda, H. Bovine lactoferrin and Crohn’s disease: A case study. Biochem. Cell Biol. 2017, 95, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Roberta, G.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID -19. Diabetes/Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012, 11, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Doughty, C.T.; Sadjadi, R. Approach to Peripheral Neuropathy for the Primary Care Clinician. Am. J. Med. 2018, 131, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Sorlie, P.; Paulose-Ram, R.; Gu, Q.; Eberhardt, M.S.; Wolz, M.; Burt, V.; Curtin, L.; Engelgau, M.; Geiss, L. Prevalence of lower-extremity disease in the US adult population ≥40 years of age with and without diabetes: 1999–2000 National Health and Nutrition Examination Survey. Diabetes Care 2004, 27, 1591–1597. [Google Scholar] [CrossRef] [Green Version]
- Bruni, A.C. Neurodegenerative diseases: Complexity of clinical phenotypes in genetic models of alzheimer’s disease and frontotemporal dementia. BMC Geriatr. 2010, 10, A89-1. [Google Scholar] [CrossRef] [Green Version]
- Logroscino, G.; Tortelli, R.; Saba, L. Epidemiology of Neurodegenerative Diseases; Oxford University Press (OUP): Oxford, UK, 2015; pp. 3–19. [Google Scholar]
- Smith, A.G. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J. Peripher. Nerv. Syst. 2012, 17, 15–21. [Google Scholar] [CrossRef]
- Brannagan, T.H., III. Current issues in peripheral neuropathy. J. Peripher. Nerv. Syst. 2012, 17 (Suppl. 2), 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Bogulavsky, J.; Larsen, K.E.; Behr, G.; Karatekin, E.; Kleinman, M.H.; Turro, N.; Krantz, D.; Edwards, R.H.; Greene, L.A.; et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. USA 2000, 97, 11869–11874. [Google Scholar] [CrossRef] [Green Version]
- Duvigneau, J.C.; Piskernik, C.; Haindl, S.; Kloesch, B.; Hartl, R.T.; Hüttemann, M.; Lee, I.; Ebel, T.; Moldzio, R.; Gemeiner, M.; et al. A novel endotoxin-induced pathway: Upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab. Investig. 2008, 88, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; I Rojo, A. Heme Oxygenase-1 as a Therapeutic Target in Neurodegenerative Diseases and Brain Infections. Curr. Pharm. Des. 2008, 14, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-G.; Jeong, J.-K.; Lee, J.-H.; Lee, Y.; Seol, J.-W.; Kim, S.-J.; Hur, T.-Y.; Jung, Y.-H.; Kang, S.-J.; Park, S. Lactoferrin protects against prion protein-induced cell death in neuronal cells by preventing mitochondrial dysfunction. Int. J. Mol. Med. 2012, 31, 325–330. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Sato, H.; Konishi, Y.; Walker, D.G.; Beach, T.G.; Rogers, J.; Tooyama, I. Expression and localization of lactotransferrin messenger RNA in the cortex of Alzheimer’s disease. Neurosci. Lett. 2009, 452, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, E.; Michel, P.P.; Hirsch, E.C. The Iron-Binding Protein Lactoferrin Protects Vulnerable Dopamine Neurons from Degeneration by Preserving Mitochondrial Calcium Homeostasis. Mol. Pharmacol. 2013, 84, 888–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zheng, Z.; Zhu, X.; Shi, Y.; Tian, D.; Zhao, F.; Liu, N.; Hüppi, P.S.; Troy, F.A.; Wang, B. Lactoferrin Promotes Early Neurodevelopment and Cognition in Postnatal Piglets by Upregulating the BDNF Signaling Pathway and Polysialylation. Mol. Neurobiol. 2015, 52, 256–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B. Molecular Determinants of Milk Lactoferrin as a Bioactive Compound in Early Neurodevelopment and Cognition. J. Pediatr. 2016, 173, S29–S36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Loly, M.M.; Mahfouz, M.B. Lactoferrin in Relation to Biological Functions and Applications: A Review. Int. J. Dairy Sci. 2011, 6, 79–111. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chen, H.-L.; Yen, C.-C.; Lee, P.-Y.; Tsai, H.-C.; Lin, M.-F.; Chen, C.-M. Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor. J. Dairy Sci. 2013, 96, 2095–2106. [Google Scholar] [CrossRef] [Green Version]
- Zemann, N.; Klein, P.; Wetzel, E.; Huettinger, F.; Huettinger, M. Lactoferrin induces growth arrest and nuclear accumulation of Smad-2 in HeLa cells. Biochimie 2010, 92, 880–884. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Fillebeen, C.; Ruchoux, M.-M.; Mitchell, V.; Vincent, S.; Benaïssa, M.; Pierce, A. Lactoferrin is synthesized by activated microglia in the human substantia nigra and its synthesis by the human microglial CHME cell line is upregulated by tumor necrosis factor α or 1-methyl-4-phenylpyridinium treatment. Mol. Brain Res. 2001, 96, 103–113. [Google Scholar] [CrossRef]
- Faucheux, B.A.; Nillesse, N.; Damier, P.; Spik, G.; Mouatt-Prigent, A.; Pierce, A.; Leveugle, B.; Kubis, N.; Hauw, J.J.; Agid, Y. Expression of lactoferrin receptors is increased in the mesencephalon of patients with Parkinson disease. Proc. Natl. Acad. Sci. USA 1995, 92, 9603–9607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, B.B.; Devos, S.L.; Kfoury, N.; Li, M.; Jacks, R.; Yanamandra, K.; Ouidja, M.O.; Brodsky, F.M.; Marasa, J.; Bagchi, D.P.; et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA 2013, 110, E3138–E3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toulorge, D.; Guerreiro, S.; Hirsch, E.C.; Michel, P.P. KATP channel blockade protects midbrain dopamine neurons by repressing a glia-to-neuron signaling cascade that ultimately disrupts mitochondrial calcium homeostasis. J. Neurochem. 2010, 114, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Grau, A.J.; Willig, V.; Fogel, W.; Werle, E. Assessment of plasma lactoferrin in Parkinson’s disease. Mov. Disord. 2001, 16, 131–134. [Google Scholar] [CrossRef]
- Somm, E.; Larvaron, P.; Van De Looij, Y.; Toulotte, A.; Chatagner, A.; Faure, M.; Métairon, S.; Mansourian, R.; Raymond, F.; Gruetter, R.; et al. Protective effects of maternal nutritional supplementation with lactoferrin on growth and brain metabolism. Pediatr. Res. 2013, 75, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Looij, Y.; Ginet, V.; Chatagner, A.; Toulotte, A.; Somm, E.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during lactation protects the immature hypoxic-ischemic rat brain. Ann. Clin. Transl. Neurol. 2014, 1, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Cancer, World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 13 June 2020).
- Mph, K.D.M.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lima, C.F.; Rodrigues, L.R. Anticancer effects of lactoferrin: Underlying mechanisms and future trends in cancer therapy. Nutr. Rev. 2014, 72, 763–773. [Google Scholar] [CrossRef] [Green Version]
- De Mejía, E.G.; Dia, V.P. The role of nutraceutical proteins and peptides in apoptosis, angiogenesis, and metastasis of cancer cells. Cancer Metastasis Rev. 2010, 29, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Figueroa, B.; Siqueiros-Cendón, T.S.; Gutierrez, D.A.; Aguilera, R.J.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Varela-Ramirez, A.; Rascón-Cruz, Q. Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells. Apoptosis 2019, 24, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Di Patti, M.C.B.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, S.T.; Lundy, F.T.; Nelson, J.; Lockhart, D.; Irwin, C.R.; Cowan, C.G.; Marley, J.J. The cytotoxic effects of human neutrophil peptide-1 (HNP1) and lactoferrin on oral squamous cell carcinoma (OSCC) in vitro. Oral Oncol. 2006, 42, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nicolau, A.; Lima, C.F.; Rodrigues, L.R. Bovine Lactoferrin Induces Cell Cycle Arrest and Inhibits Mtor Signaling in Breast Cancer Cells. Nutr. Cancer 2014, 66, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Patel, Y.S.; Roy, K.; Kanwar, R.K.; Rajkhowa, R.; Wang, X. Biodegradable Eri silk nanoparticles as a delivery vehicle for bovine lactoferrin against MDA-MB-231 and MCF-7 breast cancer cells. Int. J. Nanomed. 2015, 11, 25–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura-Bencomo, S.; Gutierrez, D.A.; Robles-Escajeda, E.; Iglesias-Figueroa, B.; Siqueiros-Cendón, T.S.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Aguilera, R.J.; Rascón-Cruz, Q.; Varela-Ramirez, A. Recombinant human lactoferrin carrying humanized glycosylation exhibits antileukemia selective cytotoxicity, microfilament disruption, cell cycle arrest, and apoptosis activities. Investig. New Drugs 2020. [Google Scholar] [CrossRef]
- Kazan, H.H.; Urfali-Mamatoglu, C.; Gunduz, U. Iron metabolism and drug resistance in cancer. BioMetals 2017, 30, 629–641. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F. Iron homeostasis and tumorigenesis: Molecular mechanisms and therapeutic opportunities. Protein Cell 2014, 6, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Gopal, S.H.; Das, S.K. Role of Lactoferrin in the Carcinogenesis of Triple-Negative Breast Cancer. J. Cancer Clin. Trials 2016, 1, e105. [Google Scholar] [PubMed]
- McCann, K.E.; Hurvitz, S.A.; McAndrew, N. Advances in Targeted Therapies for Triple-Negative Breast Cancer. Drugs 2019, 79, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.; Guedes, J.P.; Gonçalves, M.; Loureiro, L.; Castro, L.; Gerós, H.; Rodrigues, L.R.; Côrte-Real, M. Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget 2016, 7, 62144. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.A.; Kanwar, J.R.; Kanwar, R.K. Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer 2015, 15, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Townley, A. Investigating the Role of Survivin in Mitochondrial Health. Ph.D. Thesis, University of Nottingham, Nottingham, UK, July 2017. [Google Scholar]
- Kanwar, J.R.; Mahidhara, G.; Kanwar, R.K.; Roy, K. Oral administration of iron-saturated bovine lactoferrin–loaded ceramic nanocapsules for breast cancer therapy and influence on iron and calcium metabolism. Int. J. Nanomed. 2015, 10, 4081–4098. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Gao, C.-H.; Hong, C.; Zhong, Q.; Dong, H.-L.; Gao, X.-M. Expression, purification, and breast cancer cell inhibiting effect of recombinant human lactoferrin C-lobe. Biosci. Biotechnol. Biochem. 2015, 80, 257–263. [Google Scholar] [CrossRef]
- Bhakta, N.; Force, L.M.; Allemani, C.; Atun, R.; Bray, F.; Coleman, M.P.; Steliarova-Foucher, E.; Frazier, A.L.; Robison, L.L.; Rodriguez-Galindo, C.; et al. Childhood cancer burden: A review of global estimates. Lancet Oncol. 2019, 20, e42–e53. [Google Scholar] [CrossRef] [Green Version]
- Eryılmaz, E.; Canpolat, C. Novel agents for the treatment of childhood leukemia: An update. OncoTargets Ther. 2017, 10, 3299–3306. [Google Scholar] [CrossRef] [Green Version]
- Gibson, T.M.; Ehrhardt, M.J.; Ness, K.K. Obesity and Metabolic Syndrome Among Adult Survivors of Childhood Leukemia. Curr. Treat. Options Oncol. 2016, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Hwang, H.-M.; Pyo, C.-W.; Hahm, D.H.; Choi, S.-Y. E2F1-directed activation of Bcl-2 is correlated with lactoferrin-induced apoptosis in Jurkat leukemia T lymphocytes. BioMetals 2010, 23, 507–514. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, T.-F.; Shi, Y.; Zhou, H.-W.; Chen, Q.; Wei, B.-Y.; Wang, X.; Yang, T.-X.; Chinn, Y.E.; Kang, J.; et al. PFR peptide, one of the antimicrobial peptides identified from the derivatives of lactoferrin, induces necrosis in leukemia cells. Sci. Rep. 2016, 6, 20823. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.; De Antueno, R.; Duncan, R.; Hoskin, D.W. Intracellular delivery of bovine lactoferricin’s antimicrobial core (RRWQWR) kills T-leukemia cells. Biochem. Biophys. Res. Commun. 2009, 388, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Hilchie, A.L.; Haney, E.F.; Bolscher, J.G.; Hyndman, M.E.; Hancock, R.E.; Vogel, H.J. Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem. Cell Biol. 2017, 95, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Onishi, J.; Roy, M.K.; Juneja, L.R.; Watanabe, Y.; Tamai, Y. A lactoferrin-derived peptide with cationic residues concentrated in a region of its helical structure induces necrotic cell death in a leukemic cell line (HL-60). J. Pept. Sci. 2008, 14, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Amitay, E.L.; Keinan-Boker, L. Breastfeeding and childhood leukemia incidence: A meta-analysis and systematic review. JAMA Pediatr. 2015, 169, e151025. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, T.P.; Metayer, C.; Wiemels, J.L.; Singer, A.W.; Miller, M.D. Childhood Leukemia and Primary Prevention. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 317–352. [Google Scholar] [CrossRef] [Green Version]
- Mehta, F.F.; Baik, S.; Chung, S.-H. Recurrence of cervical cancer and its resistance to progestin therapy in a mouse model. Oncotarget 2016, 8, 2372–2380. [Google Scholar] [CrossRef]
- Adewumi, K.; Oketch, S.Y.; Choi, Y.; Huchko, M.J. Female perspectives on male involvement in a human-papillomavirus-based cervical cancer-screening program in western Kenya. BMC Women’s Health 2019, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cai, H.; Xiao, Z.-X.; Wang, H.; Yang, P. Effect of radiotherapy on the survival of cervical cancer patients. Medicine 2019, 98, e16421. [Google Scholar] [CrossRef]
- Luzi, C.; Brisdelli, F.; Iorio, R.; Bozzi, A.; Carnicelli, V.; Di Giulio, A.; Lizzi, A.R. Apoptotic effects of bovine apo-lactoferrin on HeLa tumor cells. Cell Biochem. Funct. 2017, 35, 33–41. [Google Scholar] [CrossRef]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Roy, K.; Patel, Y.; Zhou, S.-F.; Singh, M.R.; Singh, D.; Nasir, M.; Sehgal, R.; Sehgal, A.; Singh, R.S.; et al. Multifunctional Iron Bound Lactoferrin and Nanomedicinal Approaches to Enhance Its Bioactive Functions. Molecules 2015, 20, 9703–9731. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Cole, N.; Dutta, D.; Kumar, N.; Willcox, M.D. Antimicrobial activity of immobilized lactoferrin and lactoferricin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Karav, S. Selective deglycosylation of lactoferrin to understand glycans’ contribution to antimicrobial activity of lactoferrin. Cell. Mol. Biol. 2018, 64, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Figueroa, B.; Valdiviezo-Godina, N.; Siqueiros-Cendón, T.; Sinagawa-García, S.R.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. High-Level Expression of Recombinant Bovine Lactoferrin in Pichia pastoris with Antimicrobial Activity. Int. J. Mol. Sci. 2016, 17, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Hu, Y.; Du, C.; Piao, J.; Yang, L.; Yang, X. The effect of recombinant human lactoferrin from the milk of transgenic cows on Salmonella enterica serovar typhimurium infection in mice. Food Funct. 2016, 7, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Inhibitory effects of bovine lactoferrin and lactoferricin B on Enterobacter sakazakii. Biocontrol Sci. 2008, 13, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Fulgione, A.; Nocerino, N.; Iannaccone, M.; Roperto, S.; Capuano, F.; Roveri, N.; Lelli, M.; Crasto, A.; Calogero, A.; Pilloni, A.P.; et al. Lactoferrin Adsorbed onto Biomimetic Hydroxyapatite Nanocrystals Controlling—In Vivo—The Helicobacter pylori Infection. PLoS ONE 2016, 11, e0158646. [Google Scholar] [CrossRef] [Green Version]
- Sijbrandij, T.; Ligtenberg, A.J.; Nazmi, K.; Keijbus, P.A.M.V.D.; Veerman, E.C.I.; Bolscher, J.G.M.; Bikker, F.J. LFchimera protects HeLa cells from invasion by Yersinia spp. in vitro. BioMetals 2018, 31, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Morici, P.; Florio, W.; Rizzato, C.; Ghelardi, E.; Tavanti, A.; Rossolini, G.M.; Lupetti, A. Synergistic activity of synthetic N-terminal peptide of human lactoferrin in combination with various antibiotics against carbapenem-resistant Klebsiella pneumoniae strains. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1739–1748. [Google Scholar] [CrossRef]
- Morita, Y.; Ishikawa, K.; Nakano, M.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Ooka, T.; Hironaka, S. Effects of lactoferrin and lactoperoxidase-containing food on the oral hygiene status of older individuals: A randomized, double blinded, placebo-controlled clinical trial. Geriatr. Gerontol. Int. 2016, 17, 714–721. [Google Scholar] [CrossRef] [PubMed]
- García-Borjas, K.A.; Ceballos-Olvera, I.; Luna-Castro, S.; Peña-Avelino, Y.; Alejandra, G.-B.K.; Ivonne, C.-O.; Sarahí, L.-C.; Yosahandy, P.-A. Bovine lactoferrin can decrease the in vitro biofilm production or shown synergy with antibiotics against Listeria and Escherichia coli isolates. Protein Pept. Lett. 2020, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sijbrandij, T.; Ligtenberg, A.J.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Bikker, F.J. Effects of lactoferrin derived peptides on simulants of biological warfare agents. World J. Microbiol. Biotechnol. 2016, 33, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drago-Serrano, M.E.; De La Garza-Amaya, M.; Luna, J.S.; Campos-Rodríguez, R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.A.; Maga, E.A.; Murray, J.D. Production of human lactoferrin and lysozyme in the milk of transgenic dairy animals: Past, present, and future. Transgenic Res. 2015, 24, 605–614. [Google Scholar] [CrossRef]
- Ciccaglione, A.F.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Cellini, L.; Marzio, L. Bovine lactoferrin enhances the efficacy of levofloxacin-based triple therapy as first-line treatment of Helicobacter pylori infection: An in vitro and in vivo study. J. Antimicrob. Chemother. 2019, 74, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Ammendolia, M.G.; Agamennone, M.; Pietrantoni, A.; Lannutti, F.; Siciliano, R.A.; De Giulio, B.; Amici, C.; Superti, F. Bovine lactoferrin-derived peptides as novel broad-spectrum inhibitors of influenza virus. Pathog. Glob. Health 2012, 106, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Oda, H.; Kolawole, A.; Mirabelli, C.; Wakabayashi, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Wobus, C.E. Antiviral Effects of Bovine Lactoferrin on Human Norovirus. Biochem. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Scala, M.C.; Sala, M.; Pietrantoni, A.; Spensiero, A.; Di Micco, S.; Agamennone, M.; Bertamino, A.; Novellino, E.; Bifulco, G.; Gomez-Monterrey, I.M.; et al. Lactoferrin-derived Peptides Active towards Influenza: Identification of Three Potent Tetrapeptide Inhibitors. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.A.M.; Casseb, S.M.M.; Gonçalves, R.B.; Silva, E.V.P.; Gomes, A.M.O.; Vasconcelos, P.F.C. Bovine lactoferrin activity against Chikungunya and Zika viruses. J. Gen. Virol. 2017, 98, 1749–1754. [Google Scholar] [CrossRef]
- Carthagena, L.; Becquart, P.; Hocini, H.; Kazatchkine, M.D.; Bouhlal, H.; Belec, L. Modulation of HIV binding to epithelial cells and HIV transfer from immature dendritic cells to CD4 T lymphocytes by human lactoferrin and its major exposed LF-33 peptide. Open Virol. J. 2011, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, N.; Saffari, M.; Faizi, M. Optimized Transferosomal Bovine Lactoferrin (BLF) as a Promising Novel Non-Invasive Topical Treatment for Genital Warts Caused by Human Papiluma Virus (HPV). Iran. J. Pharm. Res. IJPR 2018, 17, 12–23. [Google Scholar] [PubMed]
- Cegolon, L.; Javanbakht, M.; Mastrangelo, G. Nasal disinfection for the prevention and control of COVID-19: A scoping review on potential chemo-preventive agents. Int. J. Hyg. Environ. Health 2020, 230, 113605. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef]
- Peroni, D.G.; Fanos, V. Lactoferrin is an important factor when breastfeeding and COVID-19 are considered. Acta Paediatr. 2020, 109, 2139–2140. [Google Scholar] [CrossRef]
- Dierick, M.; Vanrompay, D.; Devriendt, B.; Cox, E. Minireview: Lactoferrin, a versatile natural antimicrobial glycoprotein which modulates host innate immunity. Biochem. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Skalickova, S.; Heger, Z.; Krejcova, L.; Pekarik, V.; Bastl, K.; Janda, J.; Kostolansky, F.; Varečková, E.; Zitka, O.; Adam, V.; et al. Perspective of Use of Antiviral Peptides against Influenza Virus. Viruses 2015, 7, 5428–5442. [Google Scholar] [CrossRef] [Green Version]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Lozano, S.; Valk-Weeber, R.L.; Van Leeuwen, S.S.; Dijkhuizen, L.; De Vos, P. Dietary N-Glycans from Bovine Lactoferrin and TLR Modulation. Mol. Nutr. Food Res. 2018, 62, 1700389. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Dalmaroni, M.J.; Gerswhin, M.E.; Adamopoulos, I.E. The critical role of toll-like receptors—From microbial recognition to autoimmunity: A comprehensive review. Autoimmun. Rev. 2016, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pristov, K.; A Ghannoum, M. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassoun, L.A.; Sivamani, R.K. A systematic review of lactoferrin use in dermatology. Crit. Rev. Food Sci. Nutr. 2017, 57, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Ellepola, A.N.B.; Dassanayake, R.S.; Khan, Z. Impact of Brief Exposure to Drugs with Antifungal Properties on the Susceptibility of Oral Candida dubliniensis Isolates to Lysozyme and Lactoferrin. Med Princ. Pr. 2018, 27, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Weeks, K.; Carter, D.A. Lactoferrin Is Broadly Active against Yeasts and Highly Synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Liu, S.; Wang, H.; Su, H.; Liu, Z. Enhanced antifungal activity of bovine lactoferrin-producing probiotic Lactobacillus casei in the murine model of vulvovaginal candidiasis. BMC Microbiol. 2019, 19, 7. [Google Scholar] [CrossRef]
- Machado, R.; Da Costa, A.; Silva, D.M.; Gomes, A.C.; Casal, M.; Sencadas, V. Antibacterial and Antifungal Activity of Poly(Lactic Acid)-Bovine Lactoferrin Nanofiber Membranes. Macromol. Biosci. 2018, 18, 1700324. [Google Scholar] [CrossRef]
- Frontera, L.S.; Moyano, S.; Quassollo, G.; Lanfredi-Rangel, A.; Rópolo, A.S.; Touz, M.C. Lactoferrin and lactoferricin endocytosis halt Giardia cell growth and prevent infective cyst production. Sci. Rep. 2018, 8, 18020. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- López-Soto, F.; León-Sicairos, N.; Nazmi, K.; Bolscher, J.G.; de la Garza, M. Microbicidal effect of the lactoferrin peptides lactoferricin17–30, lactoferrampin265–284, and lactoferrin chimera on the parasite Entamoeba histolytica. BioMetals 2010, 23, 563–568. [Google Scholar] [CrossRef]
- Leboffe, L.; Giansanti, F.; Antonini, G. Antifungal and antiparasitic activities of lactoferrin. Antiinfect. Agents Med. Chem. 2009, 8, 114–127. [Google Scholar] [CrossRef]
- Anand, N.; Kanwar, R.K.; Sehgal, R.; Kanwar, J.R. Antiparasitic and immunomodulatory potential of oral nanocapsules encapsulated lactoferrin protein against Plasmodium berghei. Nanomedicine 2016, 11, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Anand, N.; Sehgal, R.; Kanwar, R.K.; Dubey, M.L.; Vasishta, R.K. Oral administration of encapsulated bovine lactoferrin protein nanocapsules against intracellular parasite Toxoplasma gondii. Int. J. Nanomed. 2015, 10, 6355–6369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikadai, H.; Tanaka, T.; Igarashi, I.; Oyamada, T.; Matsuu, A.; Kudo, N.; Shimazaki, K.-I.; Shibahara, N.; Tanaka, H. Inhibitory effect of lactoferrin on in vitro growth of Babesia caballi. Am. J. Trop. Med. Hyg. 2005, 73, 710–712. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Shan, T.; Xu, Z.-R.; Feng, J.; Wang, Z.-Q. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed. Sci. Technol. 2007, 135, 263–272. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, T.; Xu, Z.; Liu, J.; Feng, J. Effect of lactoferrin on the growth performance, intestinal morphology, and expression of PR-39 and protegrin-1 genes in weaned piglets1. J. Anim. Sci. 2006, 84, 2636–2641. [Google Scholar] [CrossRef]
- Tang, Z.; Yin, Y.; Zhang, Y.; Huang, R.; Sun, Z.; Li, T.; Chu, W.; Kong, X.; Li, L.; Geng, M.; et al. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin–lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2008, 101, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Watson, M.; Callon, K.E.; Tuari, D.; Dray, M.S.; Naot, D.; Amirapu, S.; Munro, J.T.; Cornish, J.; Musson, D.S. Local application of lactoferrin promotes bone regeneration in a rat critical-sized calvarial defect model as demonstrated by micro-CT and histological analysis. J. Tissue Eng. Regen. Med. 2017, 12, e620–e626. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhu, S.; Hu, J. Bone Regeneration Is Promoted by Orally Administered Bovine Lactoferrin in a Rabbit Tibial Distraction Osteogenesis Model. Clin. Orthop. Relat. Res. 2015, 473, 2383–2393. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Ren, F.; Xiong, L.; Zhao, L.; Guo, H. Bovine lactoferrin suppresses high-fat diet induced obesity and modulates gut microbiota in C57BL/6J mice. J. Funct. Foods 2016, 22, 189–200. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Cell Line | Cancer Type | SCI | Ref. |
|---|---|---|---|
| MDA-MB-231 | Human triple negative breast cancer MDA-MB-231 cell line; non metastatic | 11.68 | [65] |
| MDA-MB-231-LM2-4 | Lung metastatic (LM) variant derived from MDA-MB-231 cells | 13.99 | [65] |
| CCRF-CEM | Peripheral blood-derived leukemia cells, from a 4-year-old female | 23.25 | [70] |
| HeLa | Tumor’s epithelial cells derived from an adult with cervical adenocarcinoma | 9.59 | [70] |
| Sup-T1 | T-lymphoblast from an 8-year-old male with T-cell lymphoblastic lymphoma | 6.12 | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rascón-Cruz, Q.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Nakamura-Bencomo, S.I.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules 2021, 26, 205. https://doi.org/10.3390/molecules26010205
Rascón-Cruz Q, Espinoza-Sánchez EA, Siqueiros-Cendón TS, Nakamura-Bencomo SI, Arévalo-Gallegos S, Iglesias-Figueroa BF. Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules. 2021; 26(1):205. https://doi.org/10.3390/molecules26010205
Chicago/Turabian StyleRascón-Cruz, Quintín, Edward A. Espinoza-Sánchez, Tania S. Siqueiros-Cendón, Sayuri I. Nakamura-Bencomo, Sigifredo Arévalo-Gallegos, and Blanca F. Iglesias-Figueroa. 2021. "Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes" Molecules 26, no. 1: 205. https://doi.org/10.3390/molecules26010205
APA StyleRascón-Cruz, Q., Espinoza-Sánchez, E. A., Siqueiros-Cendón, T. S., Nakamura-Bencomo, S. I., Arévalo-Gallegos, S., & Iglesias-Figueroa, B. F. (2021). Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules, 26(1), 205. https://doi.org/10.3390/molecules26010205







