Insights into the Intraspecific Variability of the above and Belowground Emissions of Volatile Organic Compounds in Tomato
Abstract
1. Introduction
2. Results
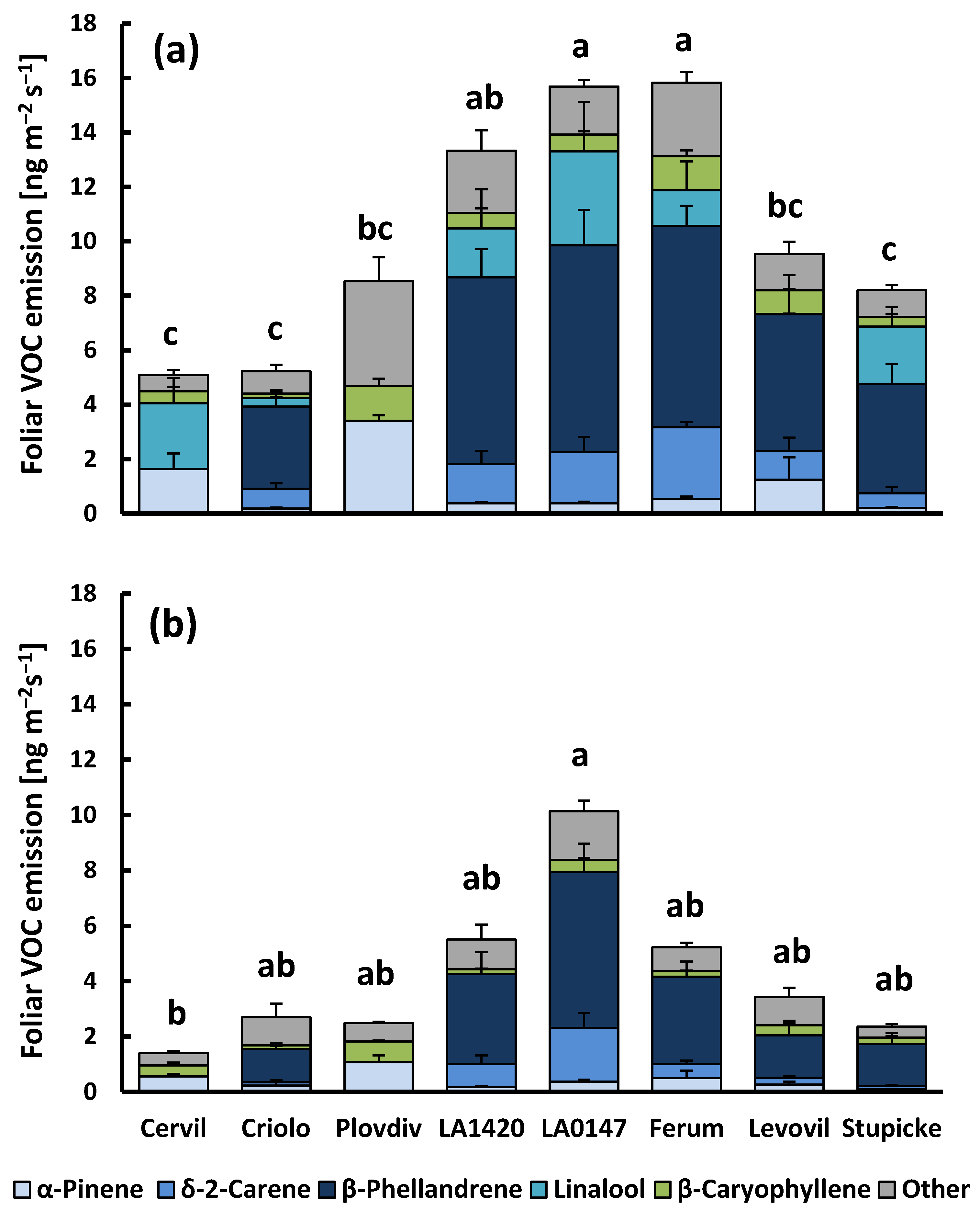
2.1. Aboveground VOC Production and CO2/H2O Gas Exchange
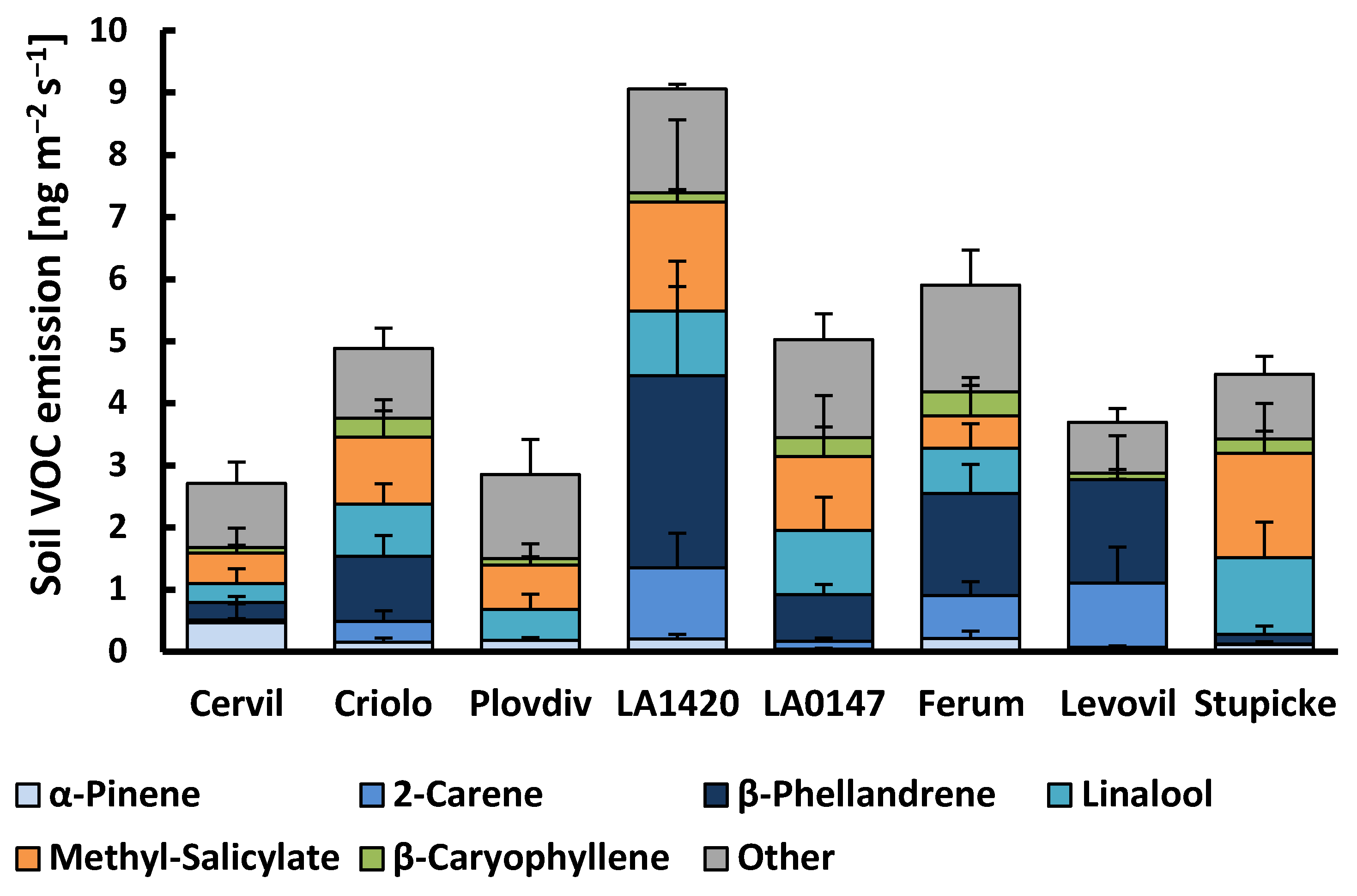
2.2. Belowground VOC Production
2.3. Covariations between Genotypic Differences in above and Belowground VOC Production
3. Discussion
3.1. Aboveground Emissions
3.2. Belowground Emissions
3.3. Implications for Crop Surveillance
4. Materials and Methods
4.1. Plant Material
4.2. Enclosure System and VOC Emission Measurement Protocol
4.3. Solvent Extraction of VOCs Stored in Leaves and Roots
4.4. GC-MS Analysis of VOC Emissions and Contents
4.5. Calculations and Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Kännaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front. Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef] [PubMed]
- Staudt, M.; Jackson, B.; El-Aouni, H.; Buatois, B.; Lacroze, J.-P.; Poessel, J.-L.; Sauge, M.-H. Volatile organic compound emissions induced by the aphid Myzus persicae differ among resistant and susceptible peach cultivars and a wild relative. Tree Physiol. 2010, 30, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Pazouki, L.; Kanagendran, A.; Li, S.; Kännaste, A.; Memari, H.R.; Bichele, R.; Niinemets, Ü. Mono- and sesquiterpene release from tomato (Solanum lycopersicum) leaves upon mild and severe heat stress and through recovery: From gene expression to emission responses. Environ. Exp. Bot. 2016, 132, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Erb, M. Volatiles as inducers and suppressors of plant defense and immunity- origins, specificity, perception and signaling. Curr. Opin. Plant Biol. 2018, 44, 117–121. [Google Scholar] [CrossRef]
- Huang, W.; Gfeller, V.; Erb, M. Root volatiles in plant-plant interactions II: Root volatiles alter root chemistry and plant-herbivore interactions of neighboring plants. Plant Cell Environ. 2019, 42, 1964–1973. [Google Scholar] [CrossRef]
- Crombie, A.T.; Larke-Mejia, N.L.; Emery, H.; Dawson, R.; Pratscher, J.; Murphy, G.P.; McGenity, T.J.; Murrell, J.C. Poplar phyllosphere harbors disparate isoprene-degrading bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 13081–13086. [Google Scholar] [CrossRef]
- Massalha, C.; Korenblum, H.; Tholl, D.; Aharoni, A. Small molecules below-ground: The role of specialized metabolites in the rhizosphere. Plant J. 2017, 90, 788–807. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Berg, M.P.; Staudt, M.; Holmstrup, M.; Glasius, M.; Ellers, J.; Tomiolo, S.; Madsen, R.B.; Slotsbo, S.; Penuelas, J. Plant secondary compounds in soil and their role in belowground species interactions. Trends Ecol. Evol. 2020, 35, 716–730. [Google Scholar] [CrossRef]
- Cui, S.; Inocente, E.A.A.; Acosta, N.; Keener, H.M.; Zhu, H.; Ling, P.P. Development of fast E-nose system for early-stage diagnosis of aphid-stressed Tomato plants. Sensors 2019, 19, 3480. [Google Scholar] [CrossRef] [PubMed]
- Bsaibes, S.; Piel, F.; Gros, V.R.; Truong, F.O.; Lafouge, F.; Ciuraru, R.; Buysse, P.; Kammer, J.; Loubet, B.; Staudt, M. Monoterpene chemical speciation with high time resolution using a fast GC/PTR-MS: Results from the COV3ER experiment on Quercus ilex. Atmosphere 2020, 11, 690. [Google Scholar] [CrossRef]
- Jansen, R.M.C.; Wildt, J.; Kappers, I.F.; Bouwmeester, H.J.; Hofstee, J.W.; van Henten, E.J. Detection of diseased plants by analysis of volatile organic compound emission. Annu. Rev. Phytopathol. 2011, 49, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits—Making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef] [PubMed]
- Niederbacher, B.; Winkler, J.B.; Schnitzler, J.P. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef]
- Jansen, R.M.C.; Hofstee, J.W.; Wildt, J.; Vanthoor, B.H.E.; Verstappen, F.W.A.; Takayama, K.; Bouwmeester, H.J.; van Henten, E.J. Health monitoring of plants by their emitted volatiles: A model to predict the effect of Botrytis cinerea on the concentration of volatiles in a large-scale greenhouse. Biosyst. Eng. 2010, 106, 37–47. [Google Scholar] [CrossRef]
- Jansen, R.M.C.; Miebach, E.; Kleist, E.J.; van Henten, E.J.; Wildt, J. Release of lipoxygenase products and monoterpenes by tomato plants as an indicator of Botrytis cinerea-induced stress. Plant Biol. 2009, 11, 859–868. [Google Scholar] [CrossRef]
- Jansen, R.M.C.; Hofstee, J.W.; Wildt, J.; Verstappen, F.W.A.; Bouwmeester, H.J.; Posthumus, M.A.; Henten, E.J. Health monitoring of plants by their emitted volatiles: Trichome damage and cell membrane damage are detectable at greenhouse scale. Ann. Appl. Biol. 2009, 154, 441–452. [Google Scholar] [CrossRef]
- Takayama, K.; Jansen, R.M.C.; van Henten, E.J.; Verstappen, F.W.A.; Bouwmeester, H.J.; Nishina, H. Emission index for evaluation of volatile organic compounds emitted from tomato plants in greenhouses. Biosyst. Eng. 2012, 113, 220–228. [Google Scholar] [CrossRef]
- Kasal-Slavik, T.; Eschweiler, J.; Kleist, E.; Mumm, R.; Goldbach, H.E.; Schouten, A.; Wildt, J. Early biotic stress detection in tomato (Solanum lycopersicum) by BVOC emissions. Phytochemistry 2017, 144, 180–188. [Google Scholar] [CrossRef]
- Raghava, T.; Ravikumar, P.; Hegde, R.; Kush, A. Spatial and temporal volatile organic compound response of select tomato cultivars to herbivory and mechanical injury. Plant Sci. 2010, 179, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Tomescu, D.; Sumalan, R.; Copolovici, L.; Copolovici, D. The influence of soil salinity on volatile organic compounds emission and photosynthetic parameters of Solanum lycopersicum L. varieties. Open Life Sci. 2017, 12, 135–142. [Google Scholar] [CrossRef]
- Schilmiller, A.; Shi, F.; Kim, J.; Charbonneau, A.L.; Holmes, D.; Daniel Jones, A.; Last, R.L. Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J. 2010, 62, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Galdon-Armero, J.; Arce-Rodriguez, L.; Downie, M.; Li, J.; Martin, C. A Scanning electron micrograph-based resource for identification of loci involved in epidermal development in tomato: Elucidation of a new function for the mixta-like transcription factor in leaves. Plant Cell 2020, 32, 1414–1433. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kuhn, U.; Harley, P.C.; Staudt, M.; Arneth, A.; Cescatti, A.; Ciccioli, P.; Copolovici, L.; Geron, C.; Guenther, A.; et al. Estimation of isoprenoid emission capacity from enclosure studies: Measurements, data processing, quality and standardized measurement protocols. Biogeosciences 2011, 8, 2209–2246. [Google Scholar] [CrossRef]
- Copolovici, L.; Kannaste, A.; Pazouki, L.; Niinemets, Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef]
- Bruce, T.J. Variation in plant responsiveness to defense elicitors caused by genotype and environment. Front. Plant Sci. 2014, 5, 3–6. [Google Scholar] [CrossRef][Green Version]
- Clavijo McCormick, A. Can plant–natural enemy communication withstand disruption by biotic and abiotic factors? Ecol. Evol. 2016, 6, 8569–8582. [Google Scholar] [CrossRef]
- Pascual, L.; Desplat, N.; Huang, B.E.; Desgroux, A.; Bruguier, L.; Bouchet, J.-P.; Le, Q.H.; Chauchard, B.; Verschave, P.; Causse, M. Potential of a tomato MAGIC population to decipher the genetic control of quantitative traits and detect causal variants in the resequencing era. Plant Biotechnol. J. 2015, 13, 565–577. [Google Scholar] [CrossRef]
- Ranc, N.; Muños, S.; Santoni, S.; Causse, M.A. Clarified position for Solanum lycopersicum var. cerasiforme in the evolutionary history of tomatoes (Solanaceae). BMC Plant Biol. 2008, 8, 130. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Schauvinhold, I.; Larson, M.; Xu, R.; Charbonneau, A.L.; Schmidt, A.; Wilkerson, C.; Last, R.L.; Pichersky, E. Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc. Natl. Acad. Sci. USA 2009, 106, 10865–10870. [Google Scholar] [CrossRef] [PubMed]
- Tissier, A.; Morgan, J.A.; Dudareva, N. Plant volatiles: Going ‘In’ but not ‘Out’ of trichome cavities. Trends Plant Sci. 2017, 22, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Balcke, G.U.; Bennewitz, S.; Bergau, N.; Athmer, B.; Henning, A.; Majovsky, P.; Jimenez-Gomez, J.M.; Hoehenwarter, W.; Tissier, A. Multi-omics of tomato glandular trichomes reveals distinct features of central carbon metabolism supporting high productivity of specialized metabolites. Plant Cell. 2017, 29, 960–983. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Pichersky, E. The complete functional characterization of the terpene synthase family in tomato. New Phytol. 2020, 226, 1341–1360. [Google Scholar] [CrossRef] [PubMed]
- Maes, K.; Debergh, P.C. Volatiles emitted from in vitro grown tomato shoots during abiotic and biotic stress. Plant Cell Tissue Organ Cult. 2003, 75, 73–78. [Google Scholar] [CrossRef]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.R.; Ament, K.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004, 135, 483–495. [Google Scholar] [CrossRef]
- Van Schie, C.C.N.; Haring, M.A.; Schuurink, R.C. Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol. Biol. 2007, 64, 251–263. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Ruijgrok, J.; Kim, H.K.; Grosser, K.; Van Dam, N.M.; Klinkhamer, P.G.L.; Leiss, K.A. Light intensity-mediated induction of trichome-associated allelochemicals increases resistance against thrips in tomato. Plant Cell Physiol. 2018, 59, 2462–2475. [Google Scholar] [CrossRef]
- Keskin, N.; Kumral, N.A. Screening tomato varietal resistance against the two-spotted spider mite [Tetranychus urticae (Koch)]. Int. J. Acarol. 2015, 41, 300–309. [Google Scholar] [CrossRef]
- Bennewitz, S.; Bergau, N.; Tissier, A. QTL Mapping of the shape of type VI glandular trichomes in tomato. Front. Plant Sci. 2018, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.; Urban, L.; Bertin, N. The potential of the MAGIC TOM parental accessions to explore the genetic variability in tomato acclimation to repeated cycles of water deficit and recovery. Front. Plant Sci. 2016, 6, 1172. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Gricourt, J.; Bertin, N.; Bonnefoi, J.; Pateyron, S.; Tamby, J.P.; Bitton, F.; Causse, M. Genotype by watering regime interaction in cultivated tomato: Lessons from linkage mapping and gene expression. Theor. Appl. Genet. 2016, 129, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Diouf, I.A.; Derivot, L.; Bitton, F.; Pascual, L.; Causse, M. Water deficit and salinity stress reveal many specific QTL for plant growth and fruit quality traits in tomato. Front. Plant Sci. 2018, 9, 279. [Google Scholar] [CrossRef]
- Diouf, I.; Albert, E.; Duboscq, R.; Santoni, S.; Bitton, F.; Gricourt, J.; Causse, M. Integration of QTL, transcriptome and polymorphism studies reveals candidate genes for water stress response in tomato. Genes 2020, 11, 900. [Google Scholar] [CrossRef]
- Goulet, C.; Mageroy, M.H.; Lam, N.B.; Floystad, A.; Tieman, D.M.; Klee, H.J. Role of an esterase in flavor volatile variation within the tomato clade. Proc. Natl. Acad. Sci. USA 2012, 109, 19009–19014. [Google Scholar] [CrossRef]
- Rambla, J.L.; Tikunov, Y.M.; Monforte, A.J.; Bovy, A.G.; Granell, A. The expanded tomato fruit volatile landscape. J. Exp. Bot. 2014, 65, 4613–4623. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Xu, Y.; Liang, J.; Chang, P.; Yan, F.; Li, M.; Liang, Y.; Zou, Z. Genome-wide association mapping for tomato volatiles positively contributing to tomato flavor. Front. Plant Sci. 2015, 6, 1042. [Google Scholar] [CrossRef]
- Birtić, S.; Ginies, C.; Causse, M.; Renard, C.M.G.C.; Page, D. Changes in volatiles and glycosides during fruit maturation of two contrasted tomato (Solanum lycopersicum) lines. J. Agric. Food Chem. 2009, 57, 591–598. [Google Scholar] [CrossRef]
- Murungi, L.K.; Kirwa, H.; Coyne, D.; Teal, P.E.A.; Beck, J.J.; Torto, B. Identification of key root volatiles signaling preference of Tomato over Spinach by the root knot nematode Meloidogyne incognita. J. Agric. Food Chem. 2018, 66, 7328–7336. [Google Scholar] [CrossRef]
- Gulati, S.; Ballhausen, M.-B.; Kulkarni, P.; Grosch, R.; Garbeva, P. A non-invasive soil-based setup to study tomato root volatiles released by healthy and infected roots. Sci. Rep. 2020, 10, 12704. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic volatile emissions from the soil. Plant Cell Environ. 2014, 37, 1866–1891. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Schurgers, G.; Rinnan, R. 2020. Process understanding of soil BVOC fluxes in natural ecosystems: A review. Rev. Geophys. 2020, 57, 966–986. [Google Scholar] [CrossRef]
- Abis, L.; Loubet, B.; Ciuraru, R.; Lafouge, F.; Houot, S.; Nowak, V.; Tripied, J.; Dequiedt, S.; Maron, P.A.; Sadet-Bourgeteau, S. Reduced microbial diversity induces larger volatile organic compound emissions from soils. Sci. Rep. 2020, 10, 6104. [Google Scholar] [CrossRef] [PubMed]
- Staudt, M.; Byron, J.; Piquemal, K.; Williams, J. Compartment specific chiral pinene emissions identified in a Maritime pine forest. Sci. Total Environ. 2019, 654, 1158–1166. [Google Scholar] [CrossRef]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef]
- Duan, Q.; Bonn, B.; Kreuzwieser, J. Terpenoids are transported in the xylem sap of Norway spruce. Plant Cell Environ. 2020, 43, 1766–1778. [Google Scholar] [CrossRef]
- Ditengou, F.A.; Müller, A.; Rosenkranz, M.; Felten, J.; Lasok, H.; van Doorn, M.M.; Legué, V.; Palme, K.; Schnitzler, J.-P.; Polle, A. Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nat. Commun. 2015, 6, 6279. [Google Scholar] [CrossRef]
- Van Doorn, M.M.; Merl-Pham, J.; Ghirardo, A.; Fink, S.; Polle, A.; Schnitzler, J.-P.; Rosenkranz, M. Root isoprene formation alters lateral root development. Plant Cell Environ. 2020, 43, 2207–2223. [Google Scholar] [CrossRef]
- Falara, V.; Akhtar, T.A.; Nguyen, T.T.H.; Spyropoulou, E.A.; Bleeker, P.M.; Schauvinhold, I.; Matsuba, Y.; Bonini, M.E.; Schilmiller, A.L.; Last, R.L.; et al. The tomato terpene synthase gene family. Plant Physiol. 2011, 157, 770–789. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Williams, J.; Yassaa, N.; Bartenbach, S.; Lelieveld, J. Mirror image hydrocarbons from Tropical and Boreal forests. Atmos. Chem. Phys. 2007, 7, 973–980. [Google Scholar] [CrossRef]
- Gosset, V.; Harmel, N.; Gobel, C.; Francis, F.; Haubruge, E.; Wathelet, J.-P.; du Jardin, P.; Feussner, I.; Fauconnier, M.-L. Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J. Exp. Bot. 2009, 60, 1231–1240. [Google Scholar] [CrossRef]
- Clavijo McCormick, A.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Daussy, J.; Staudt, M. Do future climate conditions change volatile organic compound emissions from Artemisia annua? Elevated CO2 and temperature modulate actual VOC emission rate but not its emission capacity. Atmos. Environ. 2020, 7, 100082. [Google Scholar] [CrossRef]
- Staudt, M.; Bourgeois, I.; Al Halabi, R.; Song, W.; Williams, J. New insights into the parametrization of temperature and light responses of mono- and sesquiterpene emissions from Aleppo pine and rosemary. Atmos. Environ. 2017, 152, 212–221. [Google Scholar] [CrossRef]
- Von Caemmerer, S.F.; Farquhar, G.D. Some relationships between the biogeochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]


| VOC | Cervil | Criolo | Plovdiv | LA1420 | LA0147 | Ferum | Levovil | Stupicke | p |
|---|---|---|---|---|---|---|---|---|---|
| α-Pinene | 3.9 ± 1.9 | 10.1 ± 1.7 | 1.0 ± 0.4 | 8.4 ± 3.2 | 9.0 ± 4.9 | 3.9 ± 1.2 | 6.6 ± 2.7 | 1.4 ± 0.6 | 0.101 $ |
| δ-2-Carene | 10.4 ± 1.8 | 1.0 ± 0.6 | 2.3±2.4 | 6.7 ± 3.5 | 7.6 ± 3.8 | 1.4 ± 1.1 | 10.6±4.6 | 7.3 ± 5.9 | 0.323 $ |
| β-Phellandrene | 12.9 ± 2.7 a | 13.5 ± 4.3 ab | 13.9 ± 4.3 ab | 52.2 ± 13.2 ab | 30.0 ± 10.3 ab | 42.9 ± 6.5 ab | 57.6 ± 11.8 b | 22.3 ± 4.8 ab | 0.002 £ |
| β-Caryophyllene | 13.1 ± 2.3 | 5.9 ± 1.0 | 22.4 ± 9.5 | 5.8 ± 2.1 | 27.1 ± 7.8 | 14.3 ± 6.4 | 15.5 ± 4.4 | 13.4 ± 3.5 | 0.240 $ |
| Sum major VOCs | 40.3 ± 4.0 a | 30.6 ± 4.5 a | 39.6 ± 14.0 a | 73.1 ± 14.7 a | 73.8 ± 14.3 a | 62.5 ± 6.2 a | 90.3 ± 16.7 a | 44.4 ± 14.5 a | 0.045 £ |
| Sum all VOCs | 44.7 ± 5.3 a | 43.5 ± 5.6 a | 47.5 ± 13.2 a | 85.3 ± 16.8 a | 95.6 ± 18.1 a | 89.1 ± 9.3 a | 95.0 ± 16.2 a | 53.6 ± 15.9a | 0.037 £ |
| VOC | Cervil | Criolo | Plovdiv | LA1420 | LA0147 | Ferum | Levovil | Stupicke |
|---|---|---|---|---|---|---|---|---|
| β-Phellandrene | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.10 ± 0.02 | 0.08 ± 0.07 | 0.04 ± 0.02 | 0.01 ± 0.00 | 0.17 ± 0.02 | 0.00 ± 0.00 |
| Geraniol | 0.35 ± 0.13 | 0.09 ± 0.06 | 0.00 ± 0.00 | 0.16 ± 0.14 | 0.10 ± 0.09 | 0.08 ± 0.08 | 0.05 ± 0.02 | 0.03 ± 0.02 |
| Methyl-salicylate | 0.66 ± 0.09 | 1.04 ± 0.15 | 0.25 ± 0.14 | 0.50 ± 0.06 | 0.64 ± 0.29 | 0.66 ± 0.31 | 2.43 ± 0.59 | 0.50 ± 0.03 |
| Phenyl-acetaldehyde | 1.22 ± 0.16 | 1.47 ± 0.20 | 0.10 ± 0.01 | 0.99 ± 0.21 | 2.39 ± 0.96 | 1.18 ± 0.64 | 2.96 ± 0.88 | 0.75 ± 0.05 |
| Unknown aldehyde | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.01 ± 0.01 | 0.17 ± 0.14 | 0.15 ± 0.05 | 0.06 ± 0.03 | 0.12 ± 0.05 | 0.08 ± 0.04 |
| Guaiacol | 0.07 ± 0.01 | 0.13 ± 0.02 | 0.06 ± 0.02 | 0.08 ± 0.04 | 0.12 ± 0.03 | 0.04 ± 0.02 | 0.14 ± 0.07 | 0.10 ± 0.01 |
| Sum of VOCs | 2.36 ± 0.30 | 2.79 ± 0.59 | 0.52 ± 0.15 | 1.97 ± 0.66 | 3.43 ± 0.50 | 2.03 ± 1.04 | 5.87 ± 1.44 | 1.46 ± 0.09 |
| Sum of All VOCs | |||
|---|---|---|---|
| Foliar emission | 0.73 (0.95 Levovil) | 0.36 | 0.00 |
| 0.57 (0.89 Levovil) | Foliar content | 0.25 | 0.30 (0.16 Levovil) |
| 0.34 (0.61 LA0147) | 0.31 | Soil emission | 0.00 |
| 0.04 | 0.43 (0.10 Levovil) | 0.03 (0.23 LA1420) | Root content |
| Sum of major VOCs | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehimeche, N.; Buatois, B.; Bertin, N.; Staudt, M. Insights into the Intraspecific Variability of the above and Belowground Emissions of Volatile Organic Compounds in Tomato. Molecules 2021, 26, 237. https://doi.org/10.3390/molecules26010237
Dehimeche N, Buatois B, Bertin N, Staudt M. Insights into the Intraspecific Variability of the above and Belowground Emissions of Volatile Organic Compounds in Tomato. Molecules. 2021; 26(1):237. https://doi.org/10.3390/molecules26010237
Chicago/Turabian StyleDehimeche, Nafissa, Bruno Buatois, Nadia Bertin, and Michael Staudt. 2021. "Insights into the Intraspecific Variability of the above and Belowground Emissions of Volatile Organic Compounds in Tomato" Molecules 26, no. 1: 237. https://doi.org/10.3390/molecules26010237
APA StyleDehimeche, N., Buatois, B., Bertin, N., & Staudt, M. (2021). Insights into the Intraspecific Variability of the above and Belowground Emissions of Volatile Organic Compounds in Tomato. Molecules, 26(1), 237. https://doi.org/10.3390/molecules26010237






