Identification of Phlorotannins in the Brown Algae, Saccharina latissima and Ascophyllum nodosum by Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
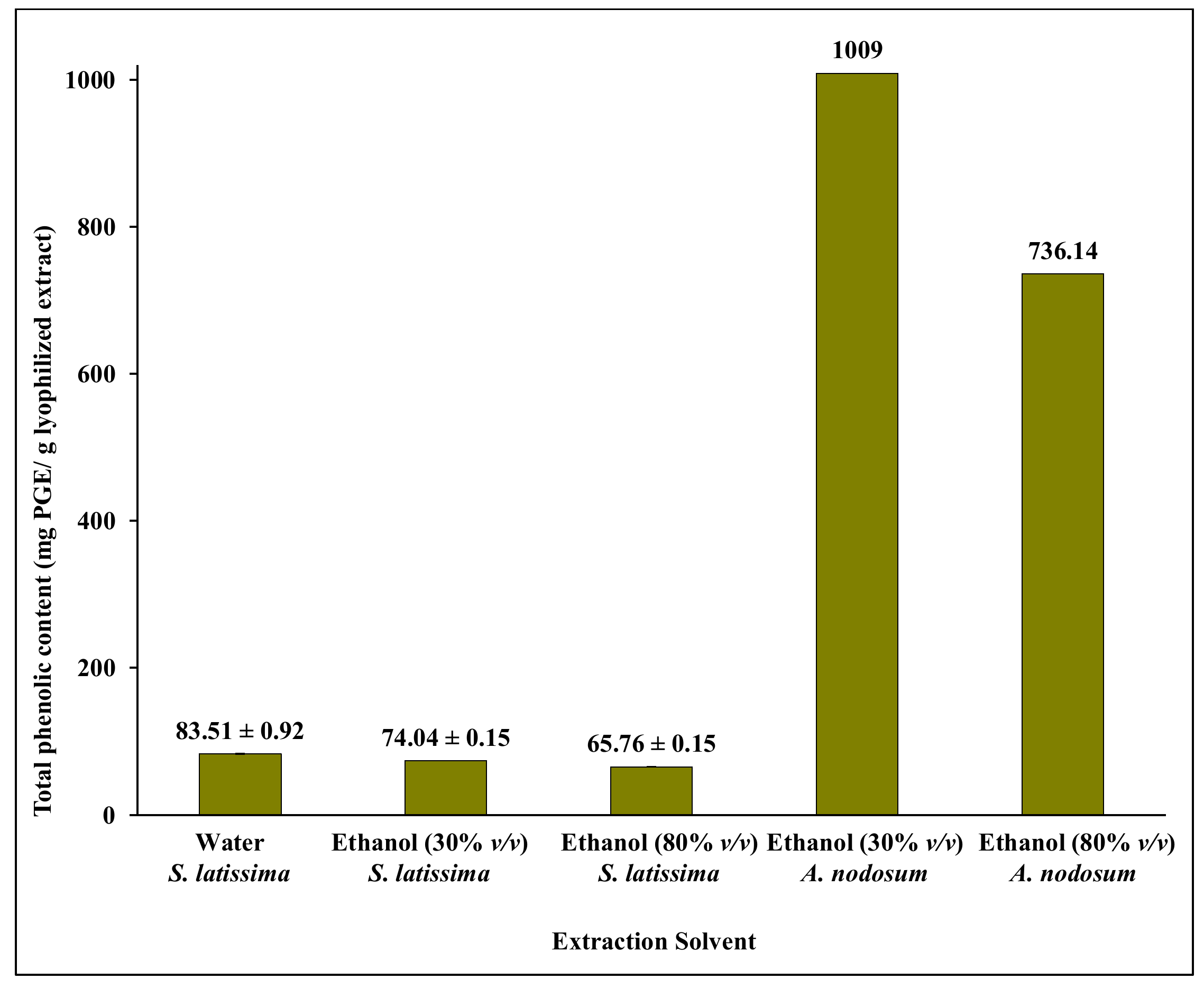
2.1. Single Step Ethanol/Water Extraction from S. latissima
2.2. Ethanol/Water Extraction from S. latissima and A. nodosum Combined with a Two-Step Purification by Solid Phase Extraction (SPE)
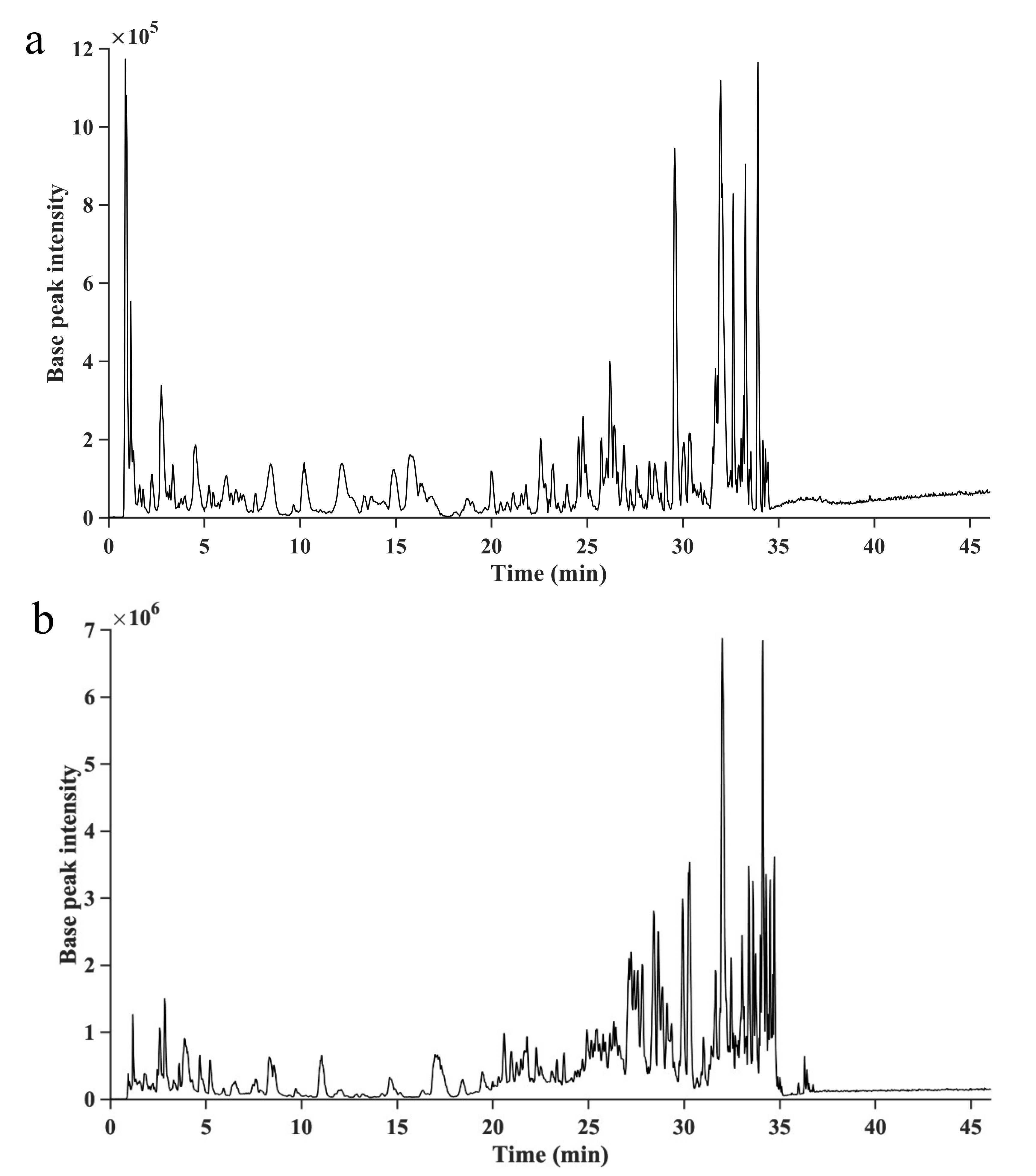
2.3. Identification of Phlorotannins by UHPLC/HRMS2
3. Materials and Methods
3.1. Materials
3.2. Solvent Extraction
3.2.1. Extraction of Phlorotannins by Water
3.2.2. Extraction of Phlorotannins by Ethanol
3.3. Two-Step Purification of Phlorotannins by Solid Phase Extraction (SPE)
3.4. Analytical Procedures
3.4.1. Determination of Total Phenolic Content (TPC)
3.4.2. Qualitative Analysis of Phlorotannins by UHPLC-UV/Vis
3.4.3. Sugar Analysis
3.4.4. Analysis of Phlorotannins by UHPLC/MS2
3.4.5. Identification of Phlorotannins from the UHPLC/MS2 Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.E. Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Ann. Rev. Mar. Sci. 2009, 1, 193. [Google Scholar] [CrossRef] [PubMed]
- Potin, P. Oxidative burst and related responses in biotic interactions of algae. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 245–271. [Google Scholar]
- De la Coba, F.; Aguilera, J.; Figueroa, F.; De Gálvez, M.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Fernando, I.S.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef]
- Freile-Pelegrin, Y.; Robledo, D. Bioactive phenolic compounds from algae. Bioact. Compd. Mar. Foods Plant Anim. Sources 2014, 113–129. [Google Scholar] [CrossRef]
- Werner, T. Presence of Phenolic Compounds in Acetone Extracts from Saccharina latissima and Their Antibacterial and Ferrous Ion-Chelating Activities. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 2013. [Google Scholar]
- Steevensz, A.J.; MacKinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melanson, J.E. Profiling phlorotannins in brown macroalgae by liquid chromatography–high resolution mass spectrometry. Phytochem. Anal. 2012, 23, 547–553. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MS(n): Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240 (Suppl. C), 904–909. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Ciocalteu, V.J.J. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar]
- Li, Y.; Lee, S.-H.; Le, Q.-T.; Kim, M.-M.; Kim, S.-K. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Lee, S.H.; Yoon, N.Y.; Jung, W.K.; Jeon, Y.J.; Kim, S.K.; Lee, M.S.; Kim, Y.M. α-Glucosidase- and α-amylase-inhibitory activities of phlorotannins from Eisenia bicyclis. J. Sci. Food Agric. 2012, 92, 2084–2090. [Google Scholar] [CrossRef]
- Tierney, M.S.; Soler-Vila, A.; Rai, D.K.; Croft, A.K.; Brunton, N.P.; Smyth, T.J. UPLC-MS profiling of low molecular weight phlorotannin polymers in Ascophyllum nodosum, Pelvetia canaliculata and Fucus spiralis. Metabolomics 2014, 10, 524–535. [Google Scholar] [CrossRef]
- Forbord, S.; Matsson, S.; Brodahl, G.E.; Bluhm, B.A.; Broch, O.J.; Handå, A.; Metaxas, A.; Skjermo, J.; Steinhovden, K.B.; Olsen, Y. Latitudinal, seasonal and depth-dependent variation in growth, chemical composition and biofouling of cultivated Saccharina latissima (Phaeophyceae) along the Norwegian coast. J. Appl. Phycol. 2020, 32, 2215–2232. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Marinho, G.S.; Sørensen, A.-D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant content and activity of the seaweed Saccharina latissima: A seasonal perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Okolie, C. The Structure-Function Relationship between Ascophyllum nodosum Polysaccharides and In Vitro Prebiotic Activity: An Assessment of the Impact of Extraction Technologies. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, 2018. [Google Scholar]
- Marinho, G.; Holdt, S.; Angelidaki, I. Seasonal variations in the amino acid profile and protein nutritional value of Saccharina latissima cultivated in a commercial IMTA system. J. Appl. Phycol. 2015, 27, 1991–2000. [Google Scholar] [CrossRef]
- Sharma, S.; Neves, L.; Funderud, J.; Mydland, L.T.; Øverland, M.; Horn, S.J. Seasonal and depth variations in the chemical composition of cultivated Saccharina latissima. Algal Res. 2018, 32, 107–112. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Gall, E.J.P.A. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A.J.A.M. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Rajauria, G. Optimization and validation of reverse phase HPLC method for qualitative and quantitative assessment of polyphenols in seaweed. J. Pharm. Biomed. Anal. 2018, 148, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Boi, V.N.; Cuong, D.X.; Vinh, P.T.K. Effects of extraction conditions over the phlorotannin content and antioxidant activity of extract from brown algae Sargassum serratum (Nguyen Huu Dai 2004). Free Radic. Antioxid. 2016, 7, 115–122. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]




| Species | Detected [M − H]− | Theoretical [M − H]− | Mass Difference (mDa) | RT (min) | Chemical Formula (Neutral) | RDB | Detected MS2 Fragments and Proposed Neutral Losses | Identification Confidence Level |
|---|---|---|---|---|---|---|---|---|
| A. nodosum | 389.0509 | 389.0501 | −0.8 | 21.03 | C18H14O10 | 12.0 | 343.04 (−CO, −H2O); 315.02 (−2 × CO, −H2O); 264.02 (−C6H5O3); 249.02 (−C6H4O4) | 2 |
| 497.0719 | 497.0720 | 0.1 | 9.72 | C24H18O12 | 16.0 | 479.20 (−H2O); 413.02 (−3 × CO); 373.07 (−C6H4O3); 371.03 (−C6H6O3); 353.01 (−C6H6O3, −H2O); 265.01 (−C12H8O5); 229.13 (−C12H8O5, −2 × H2O) | 2 | |
| 621.0875 | 621.0881 | −0.6 | 19.56 | C30H22O15 | 20.0 | 495.10 (−C6H6O3); 371.04 (−C12H10O6) | 2 * | |
| 745.1026 | 745.1041 | 1.5 | 12.17 | C36H26O18 | 24.0 | 4 | ||
| S. latissima | 265.0352 | 265.0348 | −0.4 | 6.43 | C12H10O7 | 8.0 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardari, R.R.R.; Prothmann, J.; Gregersen, O.; Turner, C.; Nordberg Karlsson, E. Identification of Phlorotannins in the Brown Algae, Saccharina latissima and Ascophyllum nodosum by Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry. Molecules 2021, 26, 43. https://doi.org/10.3390/molecules26010043
Sardari RRR, Prothmann J, Gregersen O, Turner C, Nordberg Karlsson E. Identification of Phlorotannins in the Brown Algae, Saccharina latissima and Ascophyllum nodosum by Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry. Molecules. 2021; 26(1):43. https://doi.org/10.3390/molecules26010043
Chicago/Turabian StyleSardari, Roya R. R., Jens Prothmann, Olavur Gregersen, Charlotta Turner, and Eva Nordberg Karlsson. 2021. "Identification of Phlorotannins in the Brown Algae, Saccharina latissima and Ascophyllum nodosum by Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry" Molecules 26, no. 1: 43. https://doi.org/10.3390/molecules26010043
APA StyleSardari, R. R. R., Prothmann, J., Gregersen, O., Turner, C., & Nordberg Karlsson, E. (2021). Identification of Phlorotannins in the Brown Algae, Saccharina latissima and Ascophyllum nodosum by Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry. Molecules, 26(1), 43. https://doi.org/10.3390/molecules26010043





