The Effects of Statins on Neurotransmission and Their Neuroprotective Role in Neurological and Psychiatric Disorders
Abstract
:1. Introduction
2. Statins–Structure and Permeability
3. Statins and Dopaminergic Neurotransmission
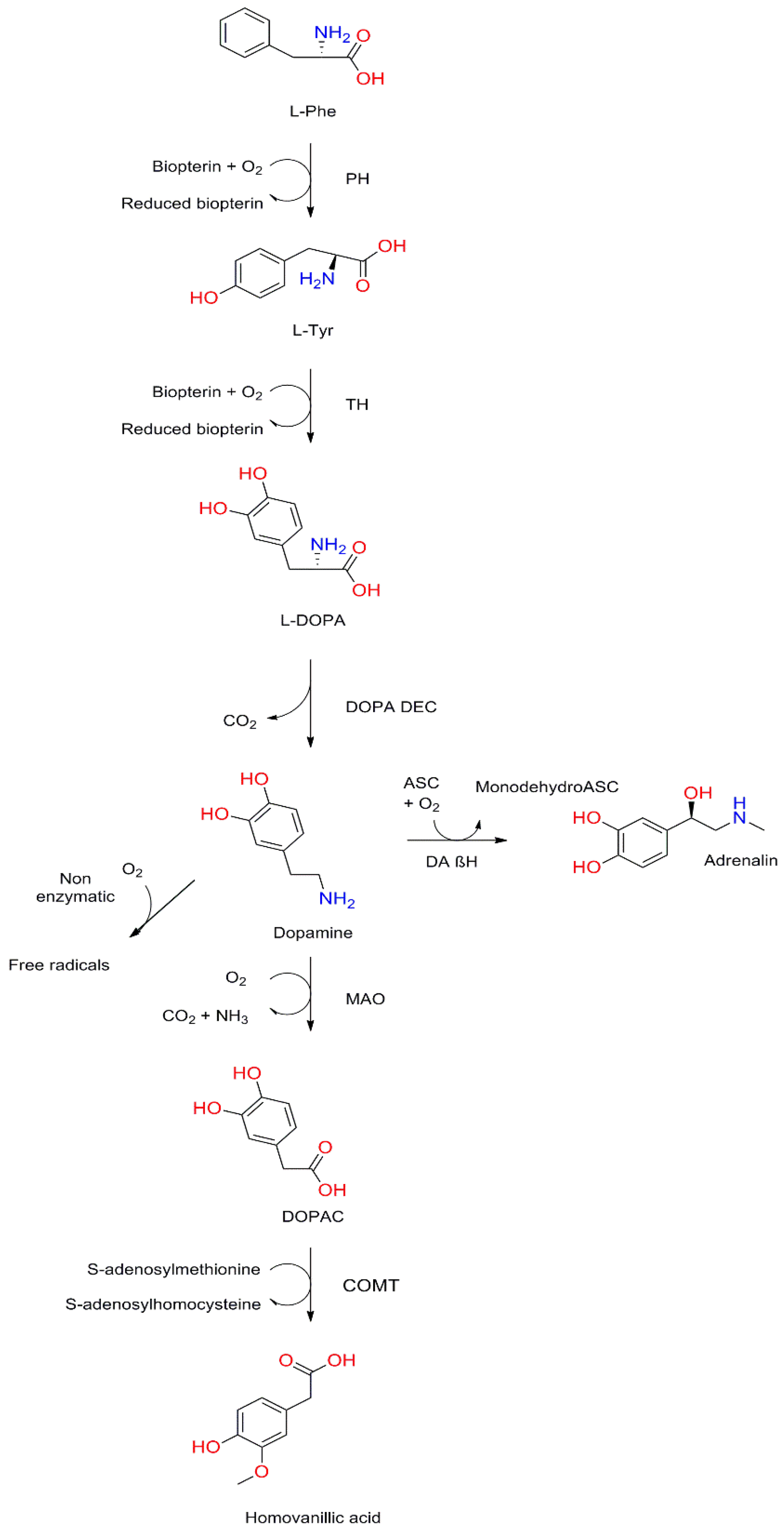
3.1. Structure and Synthesis of Dopamine
3.2. Dopamine Receptors
3.3. Cholesterol and Dopaminergic Transmission
3.4. Influence of Statins on Dopaminergic Transmission
4. Statins and Cholinergic Neurotransmission
4.1. Cholinergic Transmission in Pathogenesis of Vascular Dementia
4.2. Influence of Statins on Cholinergic Transmission
5. Statins and Glutamatergic Neurotransmission
5.1. Structure and Synthesis of Glutamate
5.2. N-Methyl-D-Aspartate Receptor
5.3. Role of Glutamatergic Transmission in the Pathogenesis of Stroke
5.4. Influence of Statins on Glutamatergic Transmission and Their Neuroprotective Effect
6. Statins and Serotoninergic Neurotransmission
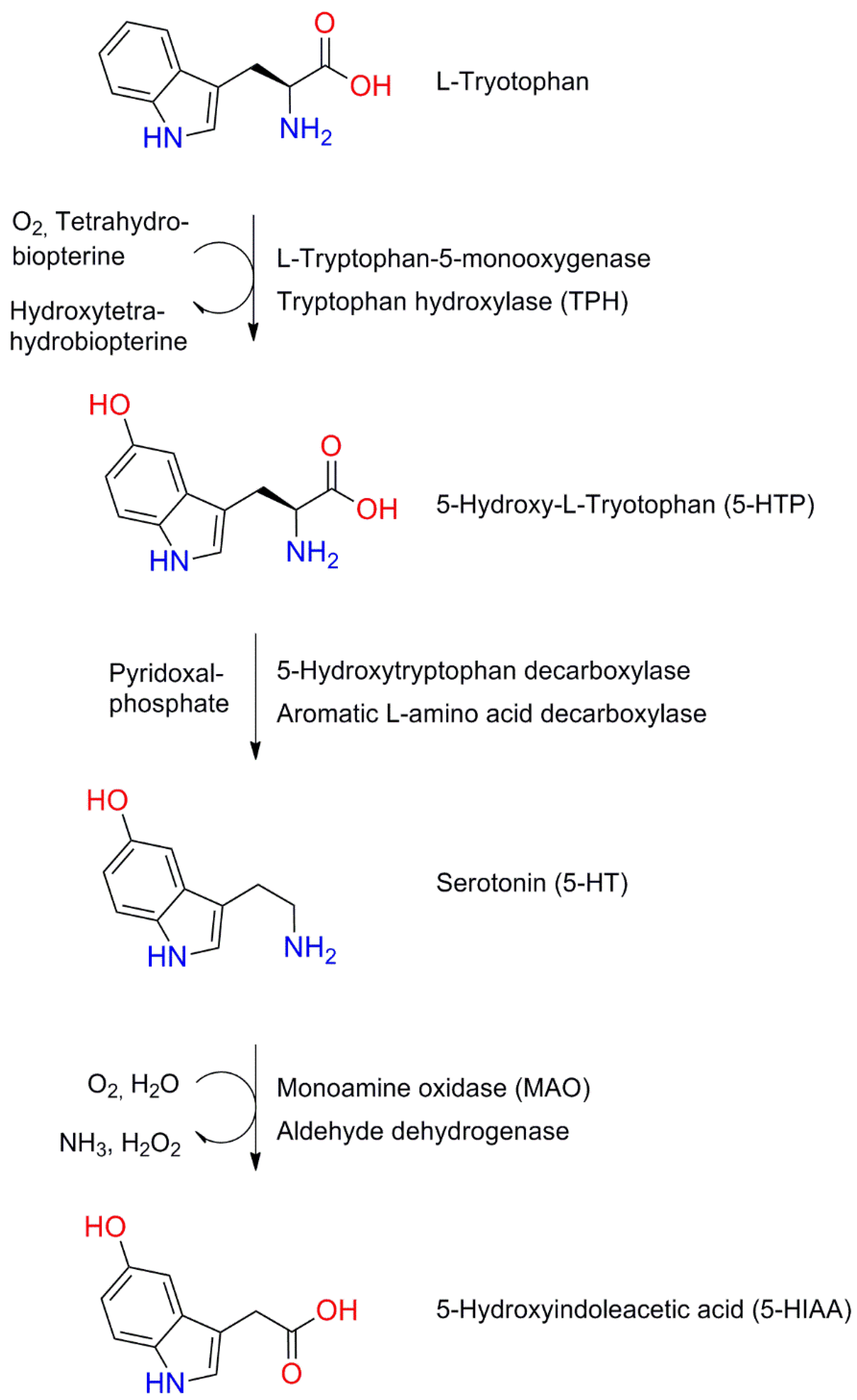
6.1. Structure and Synthesis of Serotonin
6.2. Serotonin Receptors and Transporters
6.3. Influence of Statins on Serotoninergic Transmission
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koushki, K.; Shahbaz, S.K.; Mashayekhi, K.; Sadeghi, M.; Zayeri, Z.D.; Taba, M.Y.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Anti-inflammatory Action of Statins in Cardiovascular Disease: The Role of Inflammasome and Toll-Like Receptor Pathways. Clin. Rev. Allergy Immunol. 2021, 60, 175–199. [Google Scholar] [CrossRef]
- Rabar, S.; Harker, M.; O’Flynn, N.; Wierzbicki, A.S.; On behalf of the Guideline Development Group. Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: Summary of updated NICE guidance. BMJ 2014, 349, g4356. [Google Scholar] [CrossRef]
- Altaf, A.; Qu, P.; Zhao, Y.; Wang, H.; Lou, D.; Niu, N. NLRP3 inflammasome in peripheral blood monocytes of acute coronary syndrome patients and its relationship with statins. Coron. Artery Dis. 2015, 26, 409–421. [Google Scholar] [CrossRef]
- de Bont, N.; Netea, M.G.; Rovers, C.; Smilde, T.; Demacker, P.N.; van der Meer, J.W.; Stalenhoef, A.F. LPS-induced cytokine pro-duction and expression of LPS-receptors by peripheral blood mononuclear cells of patients with familial hypercholesterolemia and the effect of HMG-CoA reductase inhibitors. Atherosclerosis 1998, 139, 147–152. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Manuck, S.B.; Matthews, K.A. Lowering cholesterol concentrations and mortality: A quantitative review of primary prevention trials. BMJ 1990, 301, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.-L.; Wu, S.-C.; Chiang, Y.-S.; Chen, J.-F. Correlation between serum lipid, lipoprotein concentrations and anxious state, depressive state or major depressive disorder. Psychiatry Res. 2003, 118, 147–153. [Google Scholar] [CrossRef]
- Vevera, J.; Zukov, I.; Morcinek, T.; Papezová, H. Cholesterol concentrations in violent and non-violent women suicide attempters. Eur. Psychiatry 2003, 18, 23–27. [Google Scholar] [CrossRef]
- Parsaik, A.K.; Singh, B.; Hassan, M.M.; Singh, K.; Mascarenhas, S.S.; Williams, M.D.; Lapid, M.I.; Richardson, J.W.; West, C.P.; Rummans, T.A. Statins use and risk of depression: A systematic review and meta-analysis. J. Affect. Disord. 2014, 160, 62–67. [Google Scholar] [CrossRef]
- Yatham, M.S.; Yatham, K.S.; Ravindran, A.V.; Sullivan, F. Do statins have an effect on depressive symptoms? A systematic review and meta-analysis. J. Affect. Disord. 2019, 257, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, F.; Van Rhijn, A.; Ijomah, G.; McIntyre, F.; Skinner, E.; Horrobin, D.; Ward, N. Tin and fatty acids in dementia. Prostaglandins, Leukot. Essent. Fat. Acids. 1991, 43, 229–238. [Google Scholar] [CrossRef]
- Dexter, D.T.; Holley, A.E.; Flitter, W.D.; Slater, T.F.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: An HPLC and ESR study. Mov. Disord. 1994, 9, 92–97. [Google Scholar] [CrossRef]
- Kivipelto, M.; Solomon, A. Cholesterol as a risk factor for Alzheimer’s disease—Epidemiological evidence. Acta Neurol. Scand. Suppl. 2006, 185, 50–57. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, H.; Tian, Y.; Xu, F.; Chen, X.; Wang, K. Reduced Serum Levels of Triglyceride, Very Low Density Lipoprotein Cholesterol and Apolipoprotein B in Parkinson’s Disease Patients. PLoS ONE 2013, 8, e75743. [Google Scholar] [CrossRef] [Green Version]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2004, 19, 117–125. [Google Scholar] [CrossRef]
- Davidson, M.H. Rosuvastatin: A highly efficacious statin for the treatment of dyslipidaemia. Expert Opin. Investig. Drugs. 2002, 11, 125–141. [Google Scholar] [CrossRef]
- Irwin, J.C.; Fenning, A.S.; Vella, R.K. Statins with different lipophilic indices exert distinct effects on skeletal, cardiac and vascular smooth muscle. Life Sci. 2020, 242, 117225. [Google Scholar] [CrossRef]
- Fuxe, K.; Borroto-Escuela, D.O. Volume transmission and receptor-receptor interactions in heteroreceptor complexes: Understanding the role of new concepts for brain communication. Neural Regen. Res. 2016, 11, 1220–1223. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F.; Marcoli, M.; Borroto-Escuela, D.O. Volume Transmission in Central Dopamine and Noradrenaline Neurons and Its Astroglial Targets. Neurochem. Res. 2015, 40, 2600–2614. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Torri, C.; Ferrari, R.; Jansson, A.; Zini, I.; Fuxe, K.; Agnati, L.F. The emergence of the volume transmission concept. Brain Res. Rev. 1998, 26, 136–147. [Google Scholar] [CrossRef]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019, 176, 21–141. [Google Scholar]
- Ricci, A.; Mignini, F.; Tomassoni, D.; Amenta, F. Dopamine receptor subtypes in the human pulmonary arterial tree. Auton. Autacoid Pharmacol. 2006, 26, 361–369. [Google Scholar] [CrossRef]
- Hussain, T.; Lokhandwala, M.F. Renal Dopamine Receptors and Hypertension. Exp. Biol. Med. 2003, 228, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Aslanoglou, D.; Bertera, S.; Sánchez-Soto, M.; Benjamin Free, R.; Lee, J.; Zong, W.; Xue, X.; Shrestha, S.; Brissova, M.; Logan, R.W.; et al. Dopamine regulates pancreatic glucagon and insulin secretion via adrenergic and dopaminergic receptors. Transl. Psychiatry 2021, 11, 59. [Google Scholar] [CrossRef]
- Kranzler, H.R.; Edenberg, H.J. Pharmacogenetics of Alcohol and Alcohol Dependence Treatment. Curr. Pharm. Des. 2010, 16, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Le Foll, B.; Gallo, A.; Le Strat, Y.; Lu, L.; Gorwood, P. Genetics of dopamine receptors and drug addiction: A comprehensive review. Behav. Pharmacol. 2009, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Watson, M.; Gates, S.; Ball, D.; Foxcroft, D. Meta-Analysis of the Association of the Taq1A Polymorphism with the Risk of Alcohol Dependency: A HuGE Gene-Disease Association Review. Am. J. Epidemiol. 2007, 167, 125–138. [Google Scholar] [CrossRef]
- Tyndale, R.F. Genetics of alcohol and tobacco use in humans. Ann. Med. 2003, 35, 94–121. [Google Scholar] [CrossRef]
- Franco, N.; Franco, R. Understanding the Added Value of G-Protein-Coupled Receptor Heteromers. Scientifica 2014, 2014, 362937. [Google Scholar] [CrossRef]
- Franco, R.; Casadó, V.; Cortés, A.; Ferrada, C.; Mallol, J.; Woods, A.; Lluís, C.; Canela, E.I.; Ferre, S. Basic Concepts in G-Protein-Coupled Receptor Homo- and Heterodimerization. Sci. World J. 2007, 7, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Marcellino, D.; Ferré, S.; Casadó, V.; Cortés, A.; Le Foll, B.; Mazzola, C.; Drago, F.; Saur, O.; Stark, H.; Soriano, A.; et al. Identification of dopamine D1-D3 receptor heteromers: Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J. Biol. Chem. 2008, 283, 26016–26025. [Google Scholar] [CrossRef] [Green Version]
- Scarselli, M.; Novi, F.; Schallmach, E.; Lin, R.; Baragli, A.; Colzi, A.; Griffon, N.; Corsini, G.U.; Sokoloff, P.; Levenson, R.; et al. D2/D3 Dopamine Receptor Heterodimers Exhibit Unique Functional Properties. J. Biol. Chem. 2001, 276, 30308–30314. [Google Scholar] [CrossRef] [Green Version]
- Hasbi, A.; Fan, T.; Alijaniaram, M.; Nguyen, T.; Perreault, M.L.; O’Dowd, B.F.; George, S.R. Calcium signaling cascade links do-pamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. USA 2009, 106, 21377–21382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroto-Escuela, D.O.; Van Craenenbroeck, K.; Romero-Fernandez, W.; Guidolin, D.; Woods, A.S.; Rivera, A.; Haegeman, G.; Agnati, L.F.; Tarakanov, A.O.; Fuxe, K. Dopamine D2 and D4 receptor heteromerization and its allosteric receptor–receptor inter-actions. Biochem. Biophys. Res. Commun. 2011, 404, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Ginés, S.; Hillion, J.; Torvinen, M.; Le Crom, S.; Casadó, V.; Canela, E.I.; Rondin, S.; Lew, J.Y.; Watson, S.; Zoli, M.; et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. USA 2000, 97, 8606–8611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillion, J.; Canals, M.; Torvinen, M.; Casadó, V.; Scott, R.; Terasmaa, A.; Hansson, A.; Watson, S.; Olah, M.E.; Mallol, J.; et al. Coaggregation, Cointernalization, and Codesensitization of Adenosine A2A Receptors and Dopamine D2Receptors. J. Biol. Chem. 2002, 277, 18091–18097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrada, C.; Moreno, E.; Casadó, V.; Bongers, G.; Cortés, A.; Mallol, J.; Canela, E.I.; Leurs, R.; Ferré, S.; Lluís, C.; et al. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br. J. Pharmacol. 2009, 157, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Ferrada, C.; Ferré, S.; Casadó, V.; Cortés, A.; Justinova, Z.; Barnes, C.; Canela, E.I.; Goldberg, S.R.; Leurs, R.; Lluis, C.; et al. Inter-actions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology 2008, 55, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Borroto-Escuela, D.O.; Brito, I.; Romero-Fernandez, W.; Di Palma, M.; Oflijan, J.; Skieterska, K.; Duchou, J.; Van Craenenbroeck, K.; Suárez-Boomgaard, D.; Rivera, A.; et al. The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub com-ponents. Int. J. Mol. Sci. 2014, 15, 8570–8590. [Google Scholar] [CrossRef]
- Gonzalez, S.; Moreno-Delgado, D.; Moreno, E.; Pérez-Capote, K.; Franco, R.; Mallol, J.; Cortés, A.; Casadó, V.; Lluis, C.; Ortiz, J.; et al. Circadian-Related Heteromerization of Adrenergic and Dopamine D4 Receptors Modulates Melatonin Synthesis and Release in the Pineal Gland. PLoS Biol. 2012, 10, e1001347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, G.; Borroto-Escuela, D.O.D.O.; Fuxe, K.; Franco, R. Purinergic signaling in Parkinson’s disease. Relevance for treatment. Neuropharmacology 2015, 104, 161–168. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.; Jacobsen, K.; Hillion, J.; Canals, M.; Torvinen, M.; Tinner-Staines, B.; Staines, W.; Rosin, D.; Terasmaa, A.; et al. Receptor heteromerization in adenosine A2A receptor signaling: Relevance for striatal function and Parkinson’s disease. Neurology 2003, 61, S19–S23. [Google Scholar] [CrossRef] [PubMed]
- Zeppelin, T.; Ladefoged, L.K.; Sinning, S.; Periole, X.; Schiøtt, B. A direct interaction of cholesterol with the dopamine transporter prevents its out-to-inward transition. PLoS Comput. Biol. 2018, 14, e1005907. [Google Scholar] [CrossRef] [Green Version]
- Orłowski, A.; Grzybek, M.; Bunker, A.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Männistö, P.T.; Róg, T. Strong preferences of dopamine and l-dopa towards lipid head group: Importance of lipid composition and implication for neurotransmitter metabolism. J. Neurochem. 2012, 122, 681–690. [Google Scholar] [CrossRef]
- Zhuge, W.; Wen, F.; Ni, Z.; Zheng, Z.; Zhu, X.; Lin, J.; Wang, J.; Zhuge, Q.; Ding, S. Dopamine Burden Triggers Cholesterol Overload Following Disruption of Synaptogenesis in Minimal Hepatic Encephalopathy. Neuroscience 2019, 410, 1–15. [Google Scholar] [CrossRef]
- Björkhem, I.; Lövgren-Sandblom, A.; Leoni, V.; Meaney, S.; Brodin, L.; Salveson, L.; Winge, K.; Pålhagen, S.; Svenningsson, P. Oxysterols and Parkinson’s disease: Evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci. Lett. 2013, 555, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Kim, W.S.; Garner, B. Regulation of α-synuclein expression by liver X receptor ligands in vitro. NeuroReport 2008, 19, 1685–1689. [Google Scholar] [CrossRef] [PubMed]
- Schommer, J.; Marwarha, G.; Schommer, T.; Flick, T.; Lund, J.; Ghribi, O. 27-Hydroxycholesterol increases α-synuclein protein levels through proteasomal inhibition in human dopaminergic neurons. BMC Neurosci. 2018, 19, 17. [Google Scholar] [CrossRef]
- Marwarha, G.; Rhen, T.; Schommer, T.; Ghribi, O. The oxysterol 27-hydroxycholesterol regulates α-synuclein and tyrosine hy-droxylase expression levels in human neuroblastoma cells through modulation of liver X receptors and estrogen receptors--relevance to Parkinson’s disease. J. Neurochem. 2011, 119, 1119–1136. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Mori, F.; Tanji, K.; Miki, Y.; Yamada, M.; Kakita, A.; Takahashi, H.; Utsumi, J.; Sasaki, H.; Wakabayashi, K. Iso-pentenyl diphosphate isomerase, a cholesterol synthesizing enzyme, is localized in Lewy bodies. Neuropathology 2015, 35, 432–440. [Google Scholar] [CrossRef]
- Scott, D.; Roy, S. α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci. 2012, 32, 10129–10135. [Google Scholar] [CrossRef] [Green Version]
- Krüger, R.; Vieira-Saecker, A.M.; Kuhn, W.; Berg, D.; Müller, T.; Kühnl, N.; Fuchs, G.A.; Storch, A.; Hungs, M.; Woitallam, D.; et al. Increased susceptibility to sporadic Parkinson’s disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann. Neurol. 1999, 45, 611–617. [Google Scholar] [CrossRef]
- Fantini, J.; Carlus, D.; Yahi, N. The fusogenic tilted peptide (67–78) of α-synuclein is a cholesterol binding domain. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 2343–2351. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, J.-H.T.; Halliday, G.M.; Kim, W.S. α-Synuclein Regulates Neuronal Cholesterol Efflux. Molecules 2017, 22, 1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Y.-T.; Bullock, K.M.; Erickson, M.A.; Zhang, J.; Banks, W. Alpha synuclein is transported into and out of the brain by the blood–brain barrier. Peptides 2014, 62, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barceló-Coblijn, G.; Golovko, M.Y.; Weinhofer, I.; Berger, J.; Murphy, E.J. Brain neutral lipids mass is increased in α-synuclein gene-ablated mice. J. Neurochem. 2006, 101, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Van Maarschalkerweerd, A.; Vetri, V.; Vestergaard, B. Cholesterol facilitates interactions between α-synuclein oligomers and charge-neutral membranes. FEBS Lett. 2015, 589, 2661–2667. [Google Scholar] [CrossRef] [Green Version]
- Emamzadeh, F.N.; Aojula, H.; McHugh, P.C.; Allsop, D. Effects of different isoforms of apoE on aggregation of the α-synuclein protein implicated in Parkinson’s disease. Neurosci Lett. 2016, 618, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heverin, M.; Bogdanovic, N.; Lütjohann, D.; Bayer, T.; Pikuleva, I.; Bretillon, L.; Diczfalusy, U.; Winblad, B.; Björkhem, I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J. Lipid Res. 2004, 45, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Lütjohann, D.; Von Bergmann, K. 24S-Hydroxycholesterol: A Marker of Brain Cholesterol Metabolism. Pharmacopsychiatry 2003, 36, 102–106. [Google Scholar] [CrossRef]
- Sokratian, A.; Ziaee, J.; Kelly, K.; Chang, A.; Bryant, N.; Wang, S.; Xu, E.; Li, J.Y.; Wang, S.-H.; Ervin, J.; et al. Heterogeneity in α-synuclein fibril activity correlates to disease phenotypes in Lewy body dementia. Acta Neuropathol. 2021, 141, 547–564. [Google Scholar] [CrossRef]
- Sierra, S.; Ramos, M.C.; Molina, P.; Esteo, C.; Vázquez, J.A.; Burgos, J.S. Statins as Neuroprotectants: A Comparative In Vitro Study of Lipophilicity, Blood-Brain-Barrier Penetration, Lowering of Brain Cholesterol, and Decrease of Neuron Cell Death. J. Alzheimer’s Dis. 2011, 23, 307–318. [Google Scholar] [CrossRef]
- Yan, J.; Xu, Y.; Zhu, C.; Zhang, L.; Wu, A.; Yang, Y.; Xiong, Z.; Deng, C.; Huang, X.-F.; Yenari, M.A.; et al. Simvastatin Prevents Dopaminergic Neurodegeneration in Experimental Parkinsonian Models: The Association with Anti-Inflammatory Responses. PLoS ONE 2011, 6, e20945. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sun, J.; Huang, L.; Fu, Q.; Du, G. Simvastatin prevents neuroinflammation by inhibiting N-methyl-D-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J. Neurosci. Res. 2014, 92, 634–640. [Google Scholar] [CrossRef]
- Fassbender, K.; Simons, M.; Bergmann, C.; Stroick, M.; Lutjohann, D.; Keller, P.; Runz, H.; Kuhl, S.; Bertsch, T.; von Bergmann, K.; et al. Simvastatin strongly reduces levels of Alzheimer’s disease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 5856–5861. [Google Scholar] [CrossRef] [Green Version]
- Kojro, E.; Gimpl, G.; Lammich, S.; Marz, W.; Fahrenholz, F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the -secretase ADAM 10. Proc. Natl. Acad. Sci. USA 2001, 98, 5815–5820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolozin, B.; Kellman, W.; Ruosseau, P.; Celesia, G.G.; Siegel, G. Decreased Prevalence of Alzheimer Disease Associated With 3-Hydroxy-3-Methyglutaryl Coenzyme A Reductase Inhibitors. Arch. Neurol. 2000, 57, 1439–1443. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, K.; Kirkland, S.; Hogan, D.B.; Macknight, C.; Merry, H.; Verreault, R.; Wolfson, C.; McDowell, I. Use of Lipid-Lowering Agents, Indication Bias, and the Risk of Dementia in Community-Dwelling Elderly People. Arch. Neurol. 2002, 59, 223–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, T.D.; Breitner, J.C.; Psaty, B.M.; Fitzpatrick, A.L.; Lopez, O.L.; Newman, A.B.; Hazzard, W.R.; Zandi, P.P.; Burke, G.L.; Lyketsos, C.G.; et al. Statin use and the risk of incident dementia: The Cardiovascular Health Study. Arch. Neurol. 2005, 62, 1047–1051. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.C.; Chuang, Y.S.; Hsieh, H.M.; Lee, T.C.; Chiu, K.F.; Liu, C.K.; Wu, M.T. Early Statin Use and the Progression of Alzheimer Disease: A Total Population-Based Case-Control Study. Medicine 2015, 94, e2143. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Jia, X.; Kang, M. Statin use and risk of Parkinson’s disease: A meta-analysis. Behav. Brain Res. 2016, 309, 29–34. [Google Scholar] [CrossRef]
- Haag, M.D.M.; Hofman, A.; Koudstaal, P.J.; Stricker, B.H.C.; Breteler, M.M.B. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J. Neurol. Neurosurg. Psychiatry 2008, 80, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Alonso, A.; Guo, X.; Umbach, D.M.; Lichtenstein, M.L.; Ballantyne, C.M.; Mailman, R.B.; Mosley, T.H.; Chen, H. Statins, plasma cholesterol, and risk of Parkinson’s disease: A prospective study. Mov. Disord. 2015, 30, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sterling, N.W.; Kong, L.; Lewis, M.M.; Mailman, R.B.; Chen, H.; Leslie, D.; Huang, X. Statins may facilitate Parkinson’s disease: Insight gained from a large, national claims database. Mov. Disord. 2017, 32, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Rozani, V.; Giladi, N.; El-Ad, B.; Gurevich, T.; Tsamir, J.; Hemo, B.; Peretz, C. Statin adherence and the risk of Parkinson’s disease: A population-based cohort study. PLoS ONE 2017, 12, e0175054. [Google Scholar] [CrossRef]
- Yan, J.; Qiao, L.; Tian, J.; Liu, A.; Wu, J.; Huang, J.; Shen, M.; Lai, X. Effect of statins on Parkinson’s disease: A systematic review and meta-analysis. Medicine 2019, 98, e14852. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Song, Y.; Huang, X.; Peng, L.; Jia, J.; Liu, Y.; Lu, H. Statin Use and the Risk of Parkinson’s Disease: An Updated Meta-Analysis. PLoS ONE 2016, 11, e0152564. [Google Scholar] [CrossRef] [Green Version]
- Feldman, H.; Doody, R.S.; Kivipelto, M.; Sparks, D.L.; Waters, D.D.; Jones, R.W.; Schwam, E.; Schindler, R.; Hey-Hadavi, J.; Demicco, D.A.; et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 2010, 74, 956–964. [Google Scholar] [CrossRef]
- Sparks, D.L.; Sabbagh, M.N.; Connor, D.J.; Lopez, J.; Launer, L.J.; Browne, P.; Wasser, D.; Johnson-Traver, S.; Lochhead, J.; Ziol-wolski, C. Atorvastatin for the treatment of mild to moderate Alzheimer disease: Preliminary results. Arch. Neurol. 2005, 62, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Friedhoff, L.T.; Cullen, E.I.; Geoghagen, N.S.; Buxbaum, J.D. Treatment with controlled-release lovastatin decreases serum con-centrations of human beta-amyloid (A beta) peptide. Int. J. Neuropsychopharmacol. 2001, 4, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Appleton, J.P.; Scutt, P.; Sprigg, N.; Bath, P.M. Hypercholesterolaemia and vascular dementia. Clin. Sci. 2017, 131, 1561–1578. [Google Scholar] [CrossRef] [Green Version]
- Du, S.-Q.; Wang, X.-R.; Xiao, L.-Y.; Tu, J.-F.; Zhu, W.; He, T.; Liu, C.-Z. Molecular Mechanisms of Vascular Dementia: What Can Be Learned from Animal Models of Chronic Cerebral Hypoperfusion? Mol. Neurobiol. 2016, 54, 3670–3682. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Zhao, Z.; Li, Q.; Wang, C.; Ge, X.; Wang, X.; Wang, G.; Qin, Y. Dl-3-n-butylphthalide regulates cholinergic dysfunction in chronic cerebral hypoperfusion rats. J. Int. Med. Res. 2020, 48, 300060520936177. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Wess, J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol. Sci. 2003, 24, 414–420. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Caldarone, B.J.; King, S.L.; Zachariou, V. Nicotinic Receptors in the Brain Links between Molecular Biology and Behavior. Neuropsychopharmacolohy 2000, 22, 451–465. [Google Scholar] [CrossRef] [Green Version]
- Everitt, B.J.; Robbins, T.W. Central Cholinergic Systems and Cognition. Annu. Rev. Psychol. 1997, 48, 649–684. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Sato, Y.; Uchida, S. Activation of the intracerebral cholinergic nerve fibers originating in the basal forebrain increases regional cerebral blood flow in the rat’s cortex and hippocampus. Neurosci. Lett. 2004, 361, 90–93. [Google Scholar] [CrossRef]
- Sato, A.; Sato, Y.; Uchida, S. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Int. J. Dev. Neurosci. 2001, 19, 327–337. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. Controlling inflammation: The cholinergic anti-inflammatory pathway. Biochem. Soc. Trans. 2006, 34, 1037–1040. [Google Scholar] [CrossRef]
- Perry, E.K.; Gibson, P.H.; Blessed, G.; Perry, R.H.; Tomlinson, B.E. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J. Neurol. Sci. 1977, 34, 247–265. [Google Scholar] [CrossRef]
- Sharp, S.I.; Francis, P.T.; Elliott, M.S.; Kalaria, R.N.; Bajic, N.; Hortobágyi, T.; Ballard, C.G. Choline Acetyltransferase Activity in Vascular Dementia and Stroke. Dement. Geriatr. Cogn. Disord. 2009, 28, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Tohgi, H.; Abe, T.; Kimura, M.; Saheki, M.; Takahashi, S. Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarct types as compared with Alzheimer-type dementia. J. Neural. Transm. 1996, 103, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.P.; Jia, J.M.; Zhou, W.D.; Xu, M.; Chu, C.B.; Yan, X.; Sun, Y.X. Differential acetylcholine and choline concentrations in the cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Chin. Med. J. 2004, 117, 1161–1164. [Google Scholar]
- Sakurada, T.; Alufuzoff, I.; Winblad, B.; Nordberg, A. Substance P-like immunoreactivity, choline acetyltransferase activity and cholinergic muscarinic receptors in Alzheimer’s disease and multi-infarct dementia. Brain Res. 1990, 521, 329–332. [Google Scholar] [CrossRef]
- Waller, S.B.; Ball, M.J.; Reynolds, M.A.; London, E.D. Muscarinic Binding and Choline Acetyltransferase in Postmortem Brains of Demented Patients. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 1986, 13, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Martin-Ruiz, C.; Court, J.; Lee, M.; Piggott, M.; Johnson, M.; Ballard, C.; Kalaria, R.; Perry, R.; Perry, E. Nicotinic receptors in de-mentia of Alzheimer, Lewy body and vascular types. Acta Neurol. Scand. Suppl. 2000, 176, 34–41. [Google Scholar] [CrossRef]
- Damodaran, T.; Müller, C.P.; Hassan, Z. Chronic cerebral hypoperfusion-induced memory impairment and hippocampal long-term potentiation deficits are improved by cholinergic stimulation in rats. Pharmacol. Rep. 2019, 71, 443–448. [Google Scholar] [CrossRef]
- Sodero, A.O.; Barrantes, F.J. Pleiotropic effects of statins on brain cells. Biochim. Biophys. Acta (BBA) Biomembr. 2020, 1862, 183340. [Google Scholar] [CrossRef]
- El-Dessouki, A.M.; Galal, M.A.; Awad, A.S.; Zaki, H.F. Neuroprotective Effects of Simvastatin and Cilostazol in l-Methionine-Induced Vascular Dementia in Rats. Mol. Neurobiol. 2016, 54, 5074–5084. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Barger, S.D.; Ryan, C.M.; Flory, J.D.; Lehoczky, J.P.; Matthews, K.A.; Manuck, S.B. Effects of lovastatin on cognitive function and psychological well-being. Am. J. Med. 2000, 108, 538–546. [Google Scholar] [CrossRef]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef] [Green Version]
- Sinha, K.; Sun, C.; Kamari, R.; Bettermann, K. Current status and future prospects of pathophysiology-based neuroprotective drugs for the treatment of vascular dementia. Drug Discov. Today 2020, 25, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Davoudian, P.A.; Wilkinson, S.T. Clinical Overview of NMDA-R Antagonists and Clinical Practice; Elsevier BV: Amsterdam, The Netherlands, 2020; Volume 89, pp. 103–129. [Google Scholar]
- Meldrum, B.S. Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez-Vargas, J.A.; Muñoz-Manco, J.I.; Garcia-Segura, L.M.; Cardona-Gómez, G.P. GluN2B N-methyl-D-aspartic acid receptor subunit mediates atorvastatin-Induced neuroprotection after focal cerebral ischemia. J. Neurosci. Res. 2014, 92, 1529–1548. [Google Scholar] [CrossRef]
- Kristiansen, L.V.; Huerta, I.; Beneyto, M.; Meador-Woodruff, J.H. NMDA receptors and schizophrenia. Curr. Opin. Pharmacol. 2007, 7, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Salussolia, C.L.; Prodromou, M.L.; Borker, P.; Wollmuth, L.P. Arrangement of Subunits in Functional NMDA Receptors. J. Neurosci. 2011, 31, 11295–11304. [Google Scholar] [CrossRef]
- Groc, L.; Bard, L.; Choquet, D. Surface trafficking of N-methyl-d-aspartate receptors: Physiological and pathological perspectives. Neuroscience 2009, 158, 4–18. [Google Scholar] [CrossRef]
- Loftis, J.M.; Janowsky, A. The N-methyl-d-aspartate receptor subunit NR2B: Localization, functional properties, regulation, and clinical implications. Pharmacol. Ther. 2003, 97, 55–85. [Google Scholar] [CrossRef]
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nat. Cell Biol. 2005, 438, 185–192. [Google Scholar] [CrossRef]
- Kalia, L.V.; Kalia, S.K.; Salter, M.W. NMDA receptors in clinical neurology: Excitatory times ahead. Lancet Neurol. 2008, 7, 742–755. [Google Scholar] [CrossRef] [Green Version]
- Dobrek, Ł.; Thor, P. Glutamate NMDA Receptors in Pathophysiology and Pharmacotherapy of Selected Nervous System Diseases. PHMD 2011, 65, 338–346. [Google Scholar]
- Lau, C.G.; Zukin, R.S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 2007, 8, 413–426. [Google Scholar] [CrossRef]
- Dong, X.-X.; Wang, Y.; Qin, Z.-H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, I.J.; Hastings, T.G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 1995, 15, 3318–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnan, G.A.; Fisher, M.; Malcolm Macleod, S.M.D. Emergency and Comprehensive Care for Stroke Needed. Lancet 2008, 373, 1612–1623. [Google Scholar] [CrossRef]
- Lo, E.H.; Moskowitz, M.A.; Jacobs, T.P. Exciting, Radical, Suicidal: How Brain Cells Die after Stroke. Stroke 2005, 36, 189–192. [Google Scholar] [CrossRef] [Green Version]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [Green Version]
- Hoskison, M.; Yanagawa, Y.; Obata, K.; Shuttleworth, C. Calcium-dependent NMDA-induced dendritic injury and MAP2 loss in acute hippocampal slices. Neuroscience 2007, 145, 66–79. [Google Scholar] [CrossRef] [Green Version]
- Jurado, F.W.; Cardona-go, G.P. P120 Catenin/a N-Catenin Are Molecular Targets in the Neuroprotection and Neuronal Plasticity Mediated by Atorvastatin after Focal Cerebral Ischemia. J. Neurosci. Res. 2010, 88, 3621–3634. [Google Scholar]
- Valerio, A.; Bertolotti, P.; Delbarba, A.; Perego, C.; Dossena, M.; Ragni, M.; Spano, P.; Carruba, M.O.; De Simoni, M.G.; Nisoli, E. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J. Neurochem. 2011, 116, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Tramacere, I.; Boncoraglio, G.B.; Banzi, R.; Del Giovane, C.; Kwag, K.H.; Squizzato, A.; Moja, L. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: A systematic review and network meta-analysis. BMC Med. 2019, 17, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yebyo, H.G.; Aschmann, H.E.; Kaufmann, M.; Puhan, M.A. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: A systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am. Hear. J. 2019, 210, 18–28. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Liu, P.-Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Vargas, J.A.; Cespedes-Rubio, A.; Cardona-Gómez, G.P. Perspective of synaptic protection after post-infarction treatment with statins. J. Transl. Med. 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Maida, C.; Arnao, V.; Corte, V.D.; Simonetta, I.; Corpora, F.; Di Bona, D.; Maugeri, R.; et al. Early High-Dosage Atorvastatin Treatment Improved Serum Immune-Inflammatory Markers and Functional Outcome in Acute Ischemic Strokes Classified as Large Artery Atherosclerotic Stroke: A Randomized Trial. Medicine 2016, 95, e3186. [Google Scholar] [CrossRef] [PubMed]
- Campos-Martorell, M.; Salvador, N.; Monge, M.; Canals, F.; García-Bonilla, L.; Hernández-Guillamon, M.; Ayuso, M.I.; Chacon, P.; Rosell, A.; Alcázar, A.; et al. Brain proteomics identifies potential simvastatin targets in acute phase of stroke in a rat embolic model. J. Neurochem. 2014, 130, 301–312. [Google Scholar] [CrossRef]
- Ploughman, M.; Windle, V.; MacLellan, C.L.; White, N.; Doré, J.J.; Corbett, D. Brain-Derived Neurotrophic Factor Contributes to Recovery of Skilled Reaching after Focal Ischemia in Rats. Stroke 2009, 40, 1490–1495. [Google Scholar] [CrossRef] [Green Version]
- Hannon, J.; Hoyer, D. Molecularbiology of 5-HT receptors. Behav. Brain Res. 2008, 195, 198–213. [Google Scholar] [CrossRef]
- Abela, A.R.; Browne, C.J.; Sargin, D.; Prevot, T.D.; Ji, X.D.; Li, Z.; Lambe, E.K.; Fletcher, P.J. Median raphe serotonin neurons promote anxiety-like behavior via inputs to the dorsal hippocampus. Neuropharmacology 2020, 168, 107985. [Google Scholar] [CrossRef]
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Flower, R.J.; Henderson, G. Rang & Dale’s pharmacology, 7th ed.; Elsevier Churchill Livingstone: Edinburgh, UK, 2012; pp. 199–200. [Google Scholar]
- Huang, K.W.; Ochandarena, N.E.; Philson, A.C.; Hyun, M.; Birnbaum, J.E.; Cicconet, M.; Sabatini, B.L. Molecular and anatomicalorganization of the dorsalraphenucleus. Elife 2019, 8, e46464. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Ambrogini, P.; Chruścicka, B.; Lindskog, M.; Crespo-Ramirez, M.; Hernández-Mondragón, J.C.; Perez de la Mora, M.; Schellekens, H.; Fuxe, K. The Role of Central Serotonin Neurons and 5-HT Heteroreceptor Complexes in the Pathophysiology of Depression: A HistoricalPerspective and Future Prospects. Int. J. Mol. Sci. 2021, 22, 1927. [Google Scholar] [CrossRef]
- He, J.; Hommen, F.; Lauer, N.; Balmert, S.; Scholz, H. Serotonin transporter dependent modulation of food-seeking behavior. PLoS ONE 2020, 15, e0227554. [Google Scholar] [CrossRef] [PubMed]
- Paulus, E.V.; Mintz, E.M. Circadianrhythms of clockgeneexpression in the cerebellum of serotonin-deficient Pet-1 knockout mice. Brain Res. 2016, 1630, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell. Mol. Neurobiol. 2021, 1–22. [Google Scholar] [CrossRef]
- Müller, H.K.; Wiborg, O.; Haase, J. Subcellular Redistribution of the Serotonin Transporter by Secretory Carrier Membrane Protein 2. J. Biol. Chem. 2006, 281, 28901–28909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, K.J.; Walline, C.C.; Barker, E.L. Serotonin transporters: Implications for antidepressantdrug development. AAPS J. 2005, 7, e421–433. [Google Scholar] [CrossRef]
- Deveau, C.M.; Rodriguez, E.; Schroering, A.; Yamamoto, B.K. Serotonin transporter regulation by cholesterol-independent lipid signaling. Biochem. Pharmacol. 2021, 183, 114349. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Anuna, L.N.; Eckert, G.P.; Keller, J.H.; Igbavboa, U.; Franke, C.; Fechner, T.; Schubert-Zsilavecz, M.; Karas, M.; Müller, W.E.; Wood, W.G. Chronic administration of statins altersmultiplegene expression patterns in mouse cerebral cortex. J. Pharmacol. Exp. Ther. 2005, 312, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Molero, Y.; Cipriani, A.; Larsson, H.; Lichtenstein, P.; D’Onofrio, B.M.; Fazel, S. Associations between statin use and suicidality, depression, anxiety, and seizures: A Swedish total-population cohort study. Lancet Psychiatry 2020, 7, 982–990. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Otte, C.; Gold, S.M.; Østergaard, S.D. Statins in the treatment of depression: Hype or hope? Pharmacol. Ther. 2020, 215, 107625. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Stein, D.; Balis, D.; Grundy, S.M.; Adams-Huet, B.; Devaraj, S. Effect of hydroxymethylglutarylcoenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation 2001, 103, 1933–1935. [Google Scholar] [CrossRef] [Green Version]
- Shishehbor, M.H.; Aviles, R.J.; Brennan, M.L.; Fu, X.; Goormastic, M.; Pearce, G.L.; Gokce, N.; Keaney, J.F.; Penn, M.S.; Sprecher, D.L.; et al. Association of nitrotyrosinelevels with cardiovasculardisease and modulation by statintherapy. JAMA 2003, 289, 1675–1680. [Google Scholar] [CrossRef] [Green Version]
- Ferro, D.; Parrotto, S.; Basili, S.; Alessandri, C.; Violi, F. Simvastatininhibits the monocyteexpression of proinflammatorycytokines in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 2000, 36, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Weitz-Schmidt, G.; Welzenbach, K.; Brinkmann, V.; Kamata, T.; Kallen, J.; Bruns, C.; Cottens, S.; Takada, Y.; Hommel, U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrinsite. Nat. Med. 2001, 6, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.X.; Tarrio, M.; Grabie, N.; Zhang, Y.; Yamazaki, H.; Stavrakis, G.; Maganto-Garcia, E.; Pepper-Cunningham, Z.; Jarolim, P.; Aikawa, M.; et al. Statin-induced Krüppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J. Clin. Investig. 2010, 120, 1961–1970. [Google Scholar] [CrossRef] [Green Version]
- van Agtmaal, M.J.M.; Houben, A.J.H.M.; Pouwer, F.; Stehouwer, C.D.A.; Schram, M.T. Association of Microvascular Dysfunction With Late-Life Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2017, 74, 729–739. [Google Scholar] [CrossRef]


| Statins | Model | Group Size | Effects | References |
|---|---|---|---|---|
| All types | Rotterdam study | 6992 | Reduced risk of late-onset AD | Haag et al. [71] |
| Prospective study | 15,291 | Incerased risk of PD | Huang et al. [72] | |
| Retrospective case–control analysis | 2322 | Lipophilic statins increased risk of PD and hydrophilic statins did not affect incidence of PD | Liu et al. [73] | |
| Population-based cohort study | 232,877 | Statins did not affect incidence of PD | Rozani et al. [74] | |
| Meta-analysis | 3,845,303 | Statins, especially atorvastatin, reduced risk of PD | Yan et al. [75] | |
| Meta-analysis | 3,513,209 | Decreased risk of PD | Bai et al. [76] | |
| Meta-analysis | 2,787,249 | Statins reduced risk of PD | Sheng et al. [70] | |
| Atorvastatin | Randomized controlled trial | 640 | No therapeutic effect in AD | Feldman et al. [77] |
| Randomized controlled trial | 63 | AD progressed slowly | Sparks et al. [78] | |
| Lovastatin | Randomized controlled trial | 160 | Decreased serum Aβ | Friedhoff et al. [79] |
| Receptor | Location | Mechanism of Action | Functions |
|---|---|---|---|
| 5-HT1A | CNS | Decreased cAMP concentration by inhibition of AC | Learning and memory, depression, anxiety-like behaviors |
| 5-HT1B | CNS, vascular smooth muscle | Decreased cAMP concentration by inhibition of AC | Aggression, antimigraine effects and vasoconstriction, depression and anxiety-like behaviors |
| 5-HT1C | CNS, limfocytes | Not completely understood | Not completely understood |
| 5-HT1D | CNS, vascular smooth muscle | Decreased cAMP concentration by inhibition of AC | Pain perception, antimigraine effects, and vasoconstriction |
| 5-HT1E | CNS | Decreased cAMP concentration by inhibition of AC | Not completely understood |
| 5-HT1F | CNS, uterus, heart, GIT | Decreased cAMP concentration by inhibition of AC | Pain perception, antimigraine effects, andanxiety-like behaviors |
| 5-HT2A | CNS, PNS, thrombocytes, smooth muscles | Enhanced AC activity and IP3 | Pain perception, sensorimotor, motivation, emotionalregulation, vasoconstriction, smooth muscles cell constriction, thrombocyte aggregation |
| 5-HT2B | CNS, stomach | Enhanced PKC activity and IP3 | Anxiety-like behaviors, smooth muscle cell constriction |
| 5-HT2C | CNS, limfocytes | Enhanced PKC activity and IP3 | Anxiogenesis, sexual behavior, pain perception, regulation of serotonergic neuron activity |
| 5-HT3 | CNS, PNS | Opening of Na+, Ca2+, and K+ channels, depolarization of plasma membrane | Vomiting reflex, anxiety-like behaviors |
| 5-HT4 | CNS, PNS | Increased cAMP concentration by activation of AC | Anxiety-like behaviors, learning and memory |
| 5-HT5A | CNS | Decreased cAMP concentration by inhibition of AC | Learning and memory, emotional behaviors, acquisition of adaptive behavior, circadian rhythm |
| 5-HT6 | CNS, leukocytes | Increased cAMP concentration by activation of AC | Anxiety-like behaviors, learning and memory, cognition |
| 5-HT7 | CNS, GIT, vascular smooth muscles | Increased cAMP concentration by activation of AC | Regulation of sleep and circadian rhythm, thermoregulation, learning and memory, regulation of 5-HT release |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosowski, M.; Smolarczyk-Kosowska, J.; Hachuła, M.; Maligłówka, M.; Basiak, M.; Machnik, G.; Pudlo, R.; Okopień, B. The Effects of Statins on Neurotransmission and Their Neuroprotective Role in Neurological and Psychiatric Disorders. Molecules 2021, 26, 2838. https://doi.org/10.3390/molecules26102838
Kosowski M, Smolarczyk-Kosowska J, Hachuła M, Maligłówka M, Basiak M, Machnik G, Pudlo R, Okopień B. The Effects of Statins on Neurotransmission and Their Neuroprotective Role in Neurological and Psychiatric Disorders. Molecules. 2021; 26(10):2838. https://doi.org/10.3390/molecules26102838
Chicago/Turabian StyleKosowski, Michał, Joanna Smolarczyk-Kosowska, Marcin Hachuła, Mateusz Maligłówka, Marcin Basiak, Grzegorz Machnik, Robert Pudlo, and Bogusław Okopień. 2021. "The Effects of Statins on Neurotransmission and Their Neuroprotective Role in Neurological and Psychiatric Disorders" Molecules 26, no. 10: 2838. https://doi.org/10.3390/molecules26102838
APA StyleKosowski, M., Smolarczyk-Kosowska, J., Hachuła, M., Maligłówka, M., Basiak, M., Machnik, G., Pudlo, R., & Okopień, B. (2021). The Effects of Statins on Neurotransmission and Their Neuroprotective Role in Neurological and Psychiatric Disorders. Molecules, 26(10), 2838. https://doi.org/10.3390/molecules26102838






