Chiral Capillary Electrokinetic Chromatography: Principle and Applications, Detection and Identification, Design of Experiment, and Exploration of Chiral Recognition Using Molecular Modeling
Abstract
1. Introduction
2. Chiral Capillary Electrokinetic Chromatography Principle and Selectors
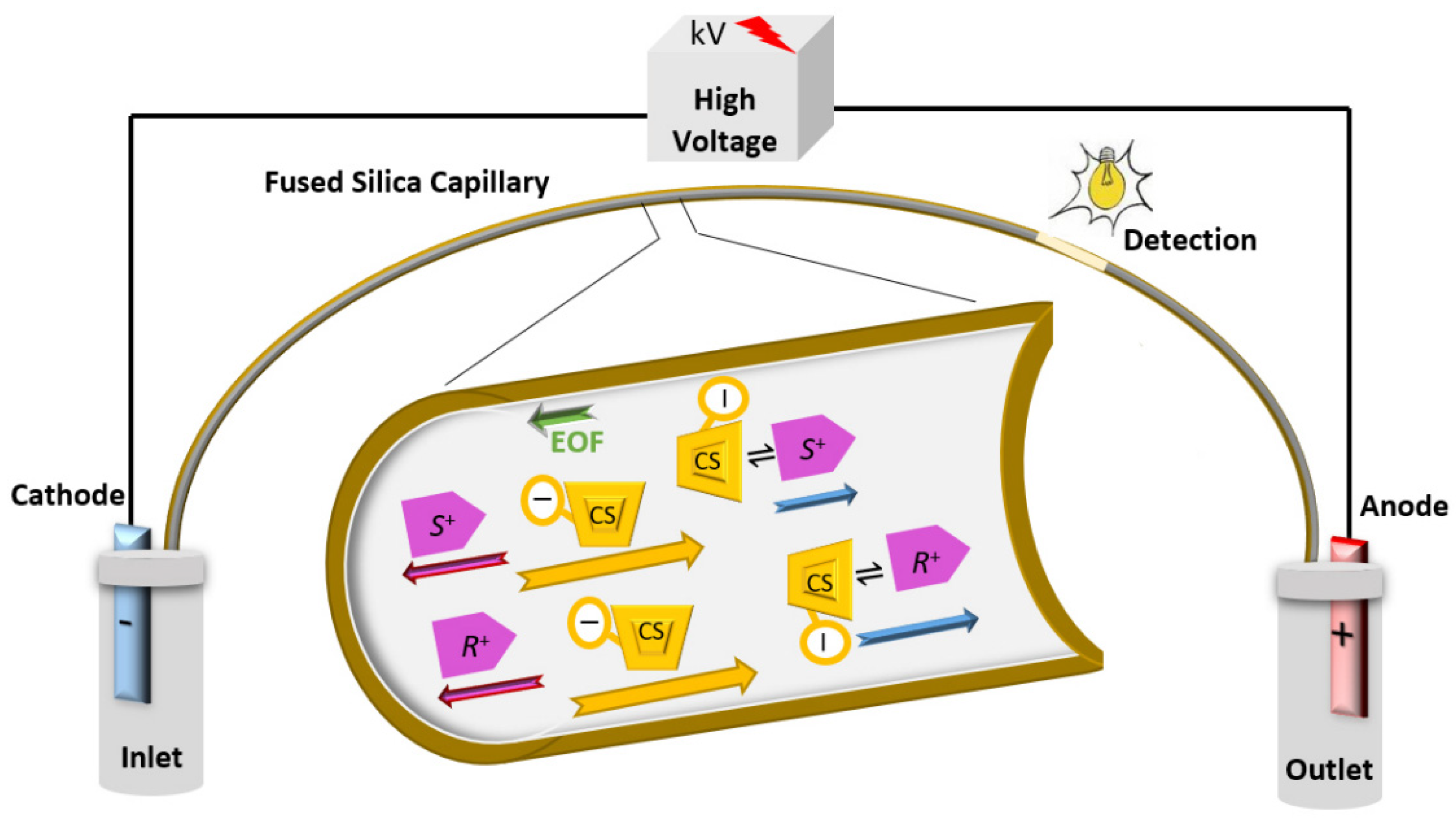
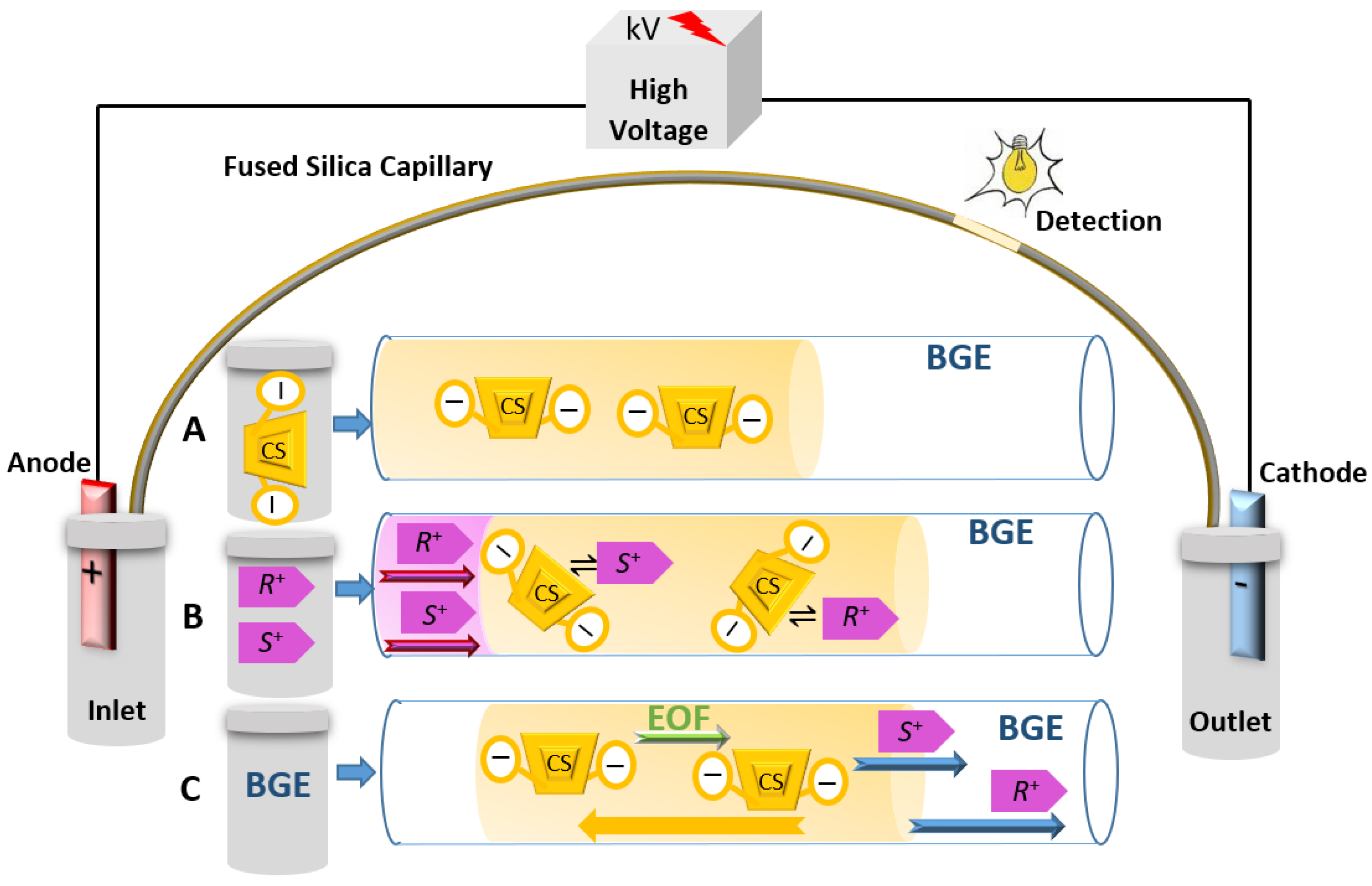
2.1. Principle of CEKC
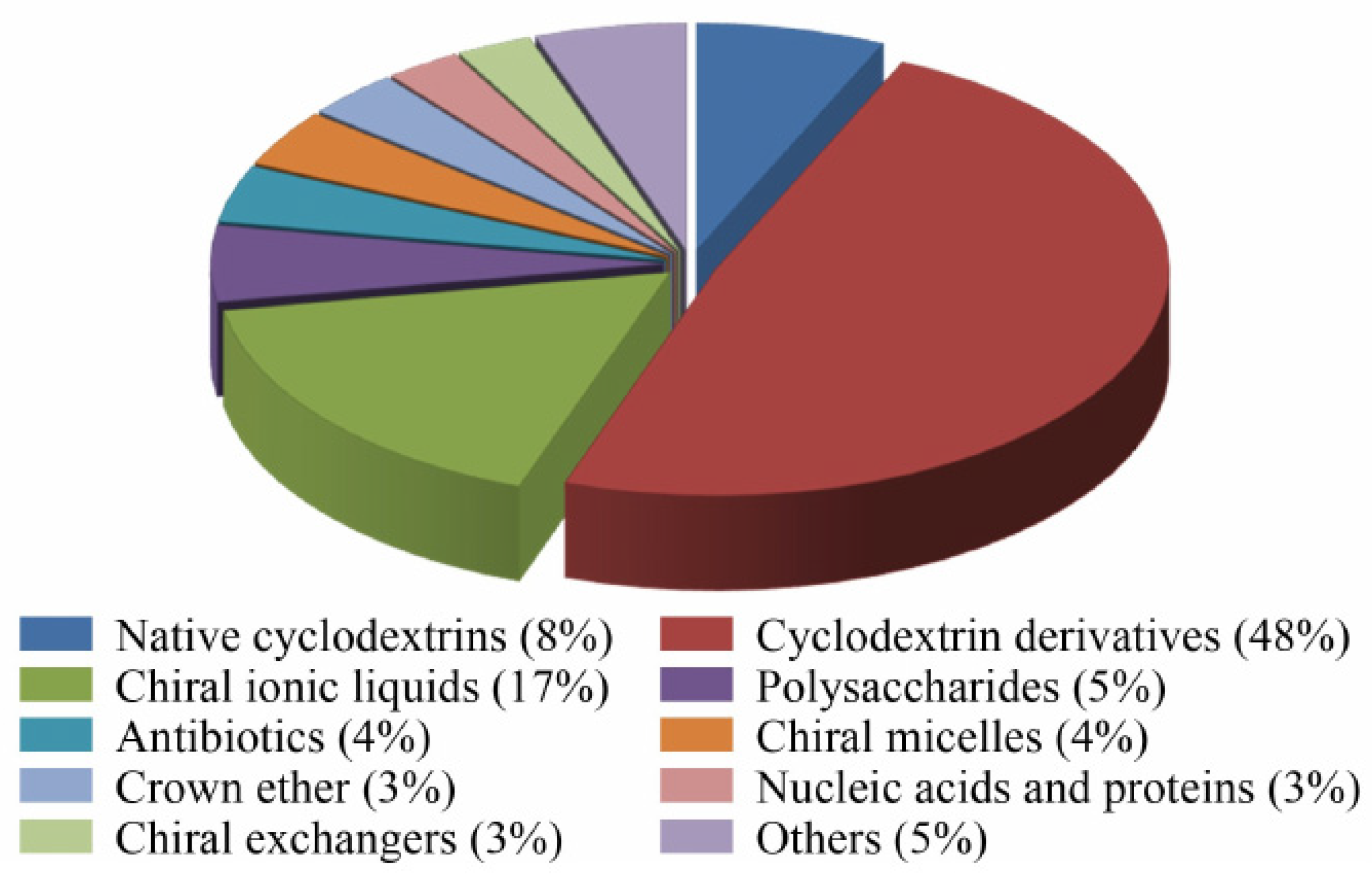
2.2. Types of Chiral Selectors in CEKC
3. Methodological and Instrumental Aspects
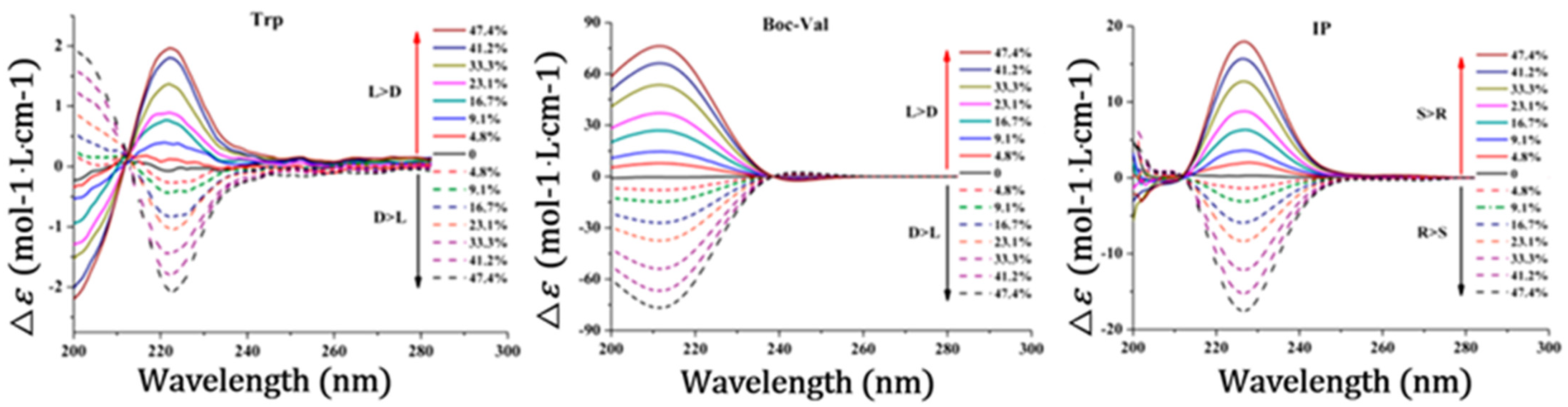
3.1. Detection Sensitivity
3.2. Identification of Individual Enantiomers
3.3. Design of Experiment
| Application | Screening Design | Screened Factors | Optimization Design | CMPs | CQAs and ATP | Optimum Conditions | Software | Ref. |
|---|---|---|---|---|---|---|---|---|
| Enantioseparation of donepezil, rivastigmine, ketoconazole, itraconazole, fluconazole, sertaconazole | None | Type of CD (highly sulfated α, γ-CDs, hydroxyl propyl-β-CD, and sulfobutyl-ether-β-CD) | FFD (32) | pH, % CD | Rs; t | BGE: 50 mM phosphate-triethanolamine, 15 kV and 25 °C, pH 2.5 at low % highly sulfated-γ-CD or high % sulfobutyl-ether-β-CD | Minitab17 (USA) | [141] |
| Chiral purity of pregabalin as dansyl derivative | D-optimal | BGE, pH, concentration of chiral selector, voltage, temperature. | Face-centered CCD and MCS | All screened factors | Rs; t Determine impurities at ≤0.015% S-eutomer | BGE: 100 mM PhB, pH 2.5, 40 mg/mL heptakis (2,3,6-tri-O-methyl)-β-CDat 25 °C and 15 kV. | MODDE 11 (Sweden) | [143] |
| Chiral purity of cinacalcet | Ishikawa fishbone diagram and CNX tool | Type of CD: (2-carboxyethyl)-β-CD and (2-hydroxypropyl)-γ-CD | BBD and MCS | Voltage, buffer pH, % methanol, CD concentration | Rs; t Determine impurities at ≤0.1% R-eutomer | BGE: 150 mM PhB pH 2.70, 3.1 mM HPγCyD; 2.00% v/v methanol at 18 °C and 26 kV. | MODDE 10 (Sweden) | [144] |
| Chiral purity of dexmedetomidine | FFD | Type of CD (native α-CD, β-CD, γ-CD, neutral and charged derivatives), buffer type, addition of triethanolamine | Face-centered CCD | Voltage, temperature, buffer pH and concentration, CD concentration | Rs; t Determine impurities at 0.1% eutomer | BGE: 50 mM PhB pH 6.5, 40 mg/mL sulfated β-CD at 17 °C and 10 kV | MODDE 11 (Sweden) | [145] |
| Chiral purity of levomepromazine | fFD | Type of CD (charged α-CD, β-CD; γ-CD, and its neutral derivative hydroxypropyl-γ-CD). Type of buffer. | Face-centered CCD, MCS | CD concentration, buffer pH and concentration temperature, voltage | Rs; t; absence of separation of sulfoxide diastereomers. Quantify 0.1% of dextromepromazine and levomepromazine sulfoxide impurities | 100 mM citric acid buffer pH 2.85, 3.6 mg/mL hydroxypropyl-γ-CD, at 15 °C and 25 kV. | MODDE 11 (Sweden) | [146] |
| Chiral purity of levodropropizine | OFAT | Type of CD: sulfated-α-CD; carboxymethyl-α-CD; succinyl-β-CD; Sulfated-β-CD | Face-centered CCD; MCS | CD concentration, % propan-2-ol; temperature; voltage | Rs; t; max analyses time 20 min. Quantify 0.5% of dextrodropropizine and 1-phenylpiperazine maximum analysis time of 20 min | 25 mM PhB pH 7.0, 23.5 mg/mL sulfated-β-CD and 10% propan-2-ol at 16.3 °C and 16.5 kV | MODDE 12 (Sweden) | [147] |
| Enantiosepartion of venlafaxine | OFAT | Type of CD (neutral α-CD, β-CD, γ-CD, hydroxypropyl-β-CD, randomly methylated-β-CD, heptakis(2,6-di-o-methyl)-β-CD, heptakis(2,4,6-tri-o-methyl)-β-CD, carboxymethyl-β-CD, sulfobuthyl ether β-CD), pH | Face-centered CCD | CD concentration, BGE concentration, temperature, voltage, injection pressure | Rs; t | 10 mM carboxymethyl-β-CD; pH 2.5; at 15 °C and 25 kV | Design Expert 7.0 | [110] |
3.4. Molecular Modeling Applied to Enantioseparations
| Analyte | CDs | Separation Conditions | Theoretical Methodology | Evidence of Interactions | Ref. |
|---|---|---|---|---|---|
| Tramadol | Sulfated-β-CD, carboxymethyl-β-CD | Phosphate buffer 125 mM (pH 10), 20 kV, 30 mbar/4 s, 10 °C, detection at 195 nm | Semiempirical PM3/DFT (B3LYP/6-31G+(d,p))/water solvent (PCM) | Hydrogen bonds | [44] |
| Bupropion and hydroxybupropion | sulfated-β-CD | BGE phosphate 75 mM pH 7.0, 15 kV, 30 mbar/4 s, 15 °C, detection at 210 nm | Molecular dynamics simulations with AMBER/DFT force field (B97D(6-31G(d,p))/water solvent (SMD) | Hydrogen bonds | [155] |
| Clenpenterol | β-CD/heptakis(2,3-di-O-acetyl)-β-CD | Phosphate buffer 50 mM pH 2.0 | Molecular dynamics simulations (100 ns trajectories) using amber force field | Van der Waals interactions, hydrogen bonds, and desolvation energy | [154] |
| Quinurenine | α-CD, mono-6A-deoxy-6-(1-allylimidazolium)-β-CD chloride, and their mixture | Borax buffer 50 mM pH 9.0, 15 kV, 50 mbar/5 s, 25 °C, detection at 226 nm | Molecular docking/molecular mechanics MM2 | Hydrogen bonds | [156] |
| Medetomidine | β-CD, γ-CD, Sulfated-β-CD and highly sulfated-β-CD | Phosphate buffer 50 mM pH 2.5, 20/−20 kV | Molecular dynamics simulations in water (TIP3P/12 Å, 100 ns trajectories) and use of amber force field | Hydrogen bonds | [157] |
| Citalopram | Carboxymethyl-β-CD, | Phosphate buffer 25 mM pH 7.0, 15 kV, 50 mbar/1 s, 17.5 °C, detection at 240 nm | Semiempirical RM1/DFT M06-2X-D3(6-31G**)/water solvent by SM8 method | Details of structural analysis were not provided | [158] |
| Asenapine | β-CD | TRIS-acetate buffer 160 mM pH 3.5, 15 kV, 50 mbar/4 s, 20 °C | Semiempirical PM3/DFT PBE def2-SVP/water solvent by COSMO method | Hydrogen bonds | [159] |
| Sutezolid | heptakis-(2,3-diacetyl-6-sulfo)-β-CD heptakis-(2,3-dimethyl-6-sulfo)-β-cyclodextrin | NACE buffer (methanol: acetonitrile: trifluoroacetic acid), 25 kV, 0.5 psi/5 s, 22 °C, detection at 200 and 258 nm | DFT/B3LYP(6-31G*)/water solvent (TIP3/14 Å) (Amber 14 molecular dynamics simulations (MM-GBSA/MMPBSA, trajectories up to 500 ns) | Hydrogen bonds | [160] |
| Oxybutynin, clenbuterol, salbutamol and peneiclidine | heptakis-(2,3-diacetyl-6-sulfo)-β-CD | BGE TRIS-H3PO4 50 mM pH 2.5, 10 kV, 10 mbar/3 s, detection at 210 nm | Molecular Mechanics Powell Method (Strength Field Tripos)/Molecular Dynamics Simulations with LGA Algorithm | Hydrogen bonding, nonclassical hydrogen bonding and π-S | [161] |
| Rasagiline | Sulfobutyl-ether-β-CD | Glycine-HCl buffer 50 mM pH 2.0, 12 kV, 25 mbar/2 s, 35 °C, detection at 200 nm | Molecular docking simulations/implicit solvency of Generalized Born | Van der Waals interactions, hydrogen bonds, and type-π | [162] |
| Ofloxacin | Methylated-β-CD | Phosphate buffer 50 mM pH 3.1, 20 kV, 50 mbar/5 s, 20 °C, detection at 276 nm | Molecular Mechanics MM2 | Van der Waals interactions and electrostatic interactions (interactions of loads, dipoles, and quadrupoles) | [163] |
| Bronpheniramine, chlorpheniramine and pheniramine | heptakis {2,6-di-O-[3-(1,3-dicarboxylpropylamino)-2-hydroxypropyl]}-β-CD | Phosphate buffer 120 mM pH 2.5–4.0, 20 kV, 20 psi, 20 °C, detection at 210 nm | Hybrid method ONIOM2: Semiempirical PM3/DFT (B3LYP/6-31G(d,p)) | Van der Waals interactions and electrostatic interactions | [164] |
| Acebutelol | heptakis-(2,3-diacetyl-6-sulfo)-β-CD/heptakis(2,3-di-O-methyl-6-O-sulfo)-β-CD | Phosphate buffer 100 mM pH 3.0 and ammonium format 0.75 mM, 25 kV, 50 mbar/3 s, 15 °C, detection at 230 nm. | Molecular dynamics simulations with general amber force field with AM1-BCC load. For purpose solvent model IEFPCM (aqueous solution and methanol solution) | Hydrophobic effect and hydrogen bonds | [165] |
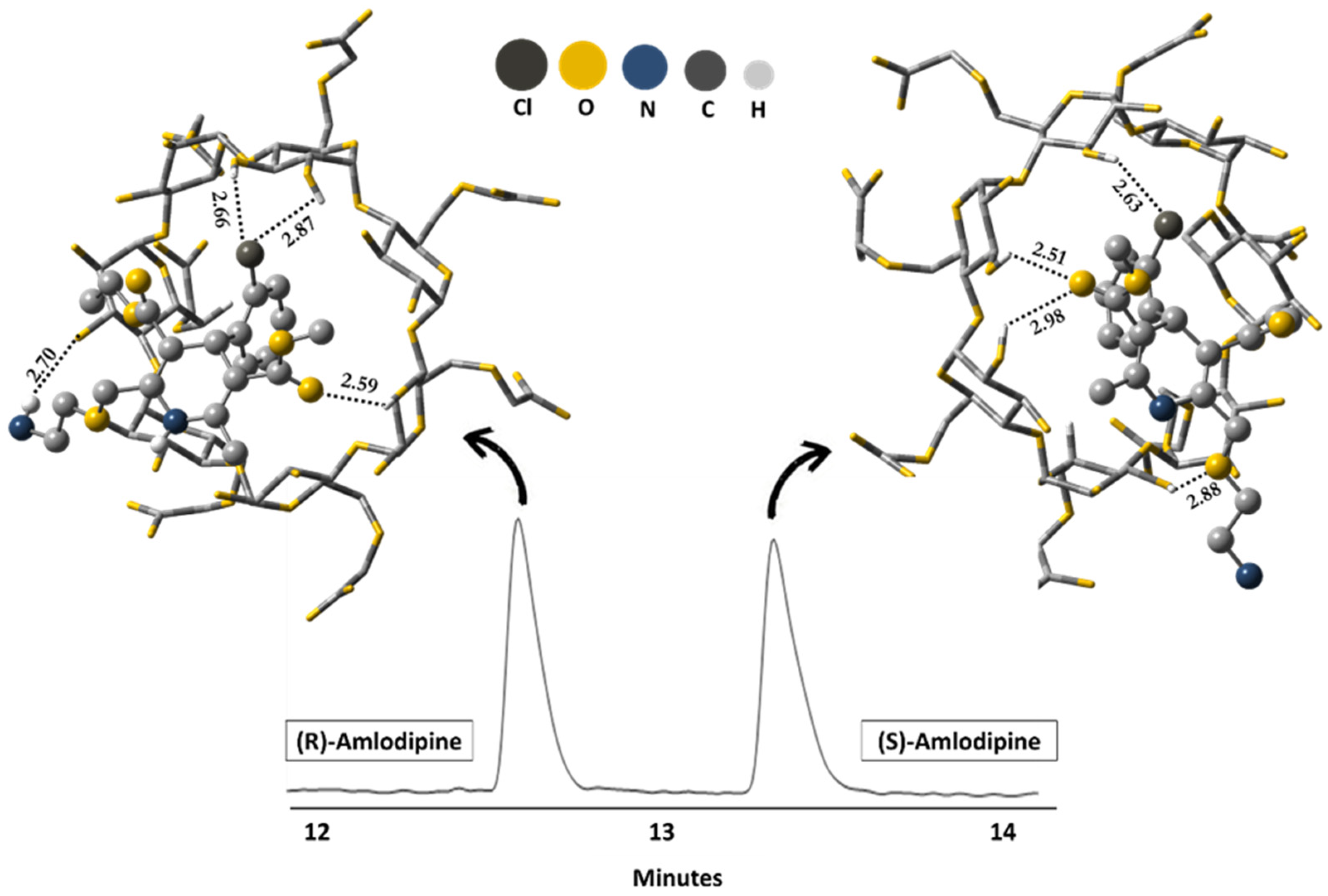
| Amlodipine | carboxymethyl-β-CD | BGE TRIS-H3PO4 20 mM pH 3.5, 20 kV, 50 mbar/5 s, 20 °C, detection at 237 nm. | Molecular mechanics (MM2) and molecular dynamics simulations/Semiempirical method PM3 | Hydrogen bond, dipole-dipole, and π-π. | [90] |
| Terbutalin | heptakis {2,6-di-O-[2-hydroxy-3-(sulfoamino)propoxy]}-β-CD | Phosphate buffer 60 mM pH 2.5, 10 kV, 0.5 psi/5 s, detection at 210 nm. | Hybrid method ONIOM2: Semiempirical PM3/DFT (B3LYP/6-31G(d,p)) | Electrostatic interactions and hydrogen bonding | [166] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, D.R.; Comeskey, D.; Hunt, M.B.; Rasmussen, L.E.L. Chirality in Elephant Pheromones. Nature 2005, 438, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.; Patel, N.H. Nodal Signalling Is Involved in Left–Right Asymmetry in Snails. Nature 2009, 457, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Espada, A.; Molina-Martin, M. Capillary Electrophoresis and Small Molecule Drug Discovery: A Perfect Match? Drug Discov. Today 2012, 17, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Tiritan, M.E.; Pinto, M.; Fernandes, C. Enantioselective Synthesis, Enantiomeric Separations and Chiral Recognition. Molecules 2020, 25, 1713. [Google Scholar] [CrossRef]
- Bonner, W.A. Parity Violation and the Evolution of Biomolecular Homochirality. Chirality 2000, 12, 114–126. [Google Scholar] [CrossRef]
- Casado, N.; Valimaña-Traverso, J.; García, M.Á.; Marina, M.L. Enantiomeric Determination of Drugs in Pharmaceutical Formulations and Biological Samples by Electrokinetic Chromatography. Crit. Rev. Anal. Chem. 2020, 50, 554–584. [Google Scholar] [CrossRef]
- Ratih, R.; Wätzig, H.; Stein, M.; el Deeb, S. Investigation of the Enantioselective Interaction between Selected Drug Enantiomers and Human Serum Albumin by Mobility Shift-affinity Capillary Electrophoresis. J. Sep. Sci. 2020, 43, 3960–3968. [Google Scholar] [CrossRef]
- Mozafari, M.; el Deeb, S.; Krull, F.; Wildgruber, R.; Weber, G.; Reiter, C.G.; Wätzig, H. Interaction of Albumins and Heparinoids Investigated by Affinity Capillary Electrophoresis and Free Flow Electrophoresis. Electrophoresis 2018, 39, 569–580. [Google Scholar] [CrossRef]
- Albishri, H.M.; Deeb, S.E.; AlGarabli, N.; AlAstal, R.; Alhazmi, H.A.; Nachbar, M.; El-Hady, D.A.; Wätzig, H. Recent Advances in Affinity Capillary Electrophoresis for Binding Studies. Bioanalysis 2014, 6, 3369–3392. [Google Scholar] [CrossRef]
- Deeb, S.E.; Wätzig, H.; El-Hady, D.A.; Albishri, H.M.; de Griend, C.S.; Scriba, G.K.E. Recent Advances in Capillary Electrophoretic Migration Techniques for Pharmaceutical Analysis. Electrophoresis 2014, 35, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Deeb, S.E.; Wätzig, H.; El-Hady, D.A. Capillary Electrophoresis to Investigate Biopharmaceuticals and Pharmaceutically-Relevant Binding Properties. Trac Trends Anal. Chem. 2013, 48, 112–131. [Google Scholar] [CrossRef]
- Fortuna, A.; Alves, G.; Falcão, A. Chiral Chromatographic Resolution of Antiepileptic Drugs and Their Metabolites: A Challenge from the Optimization to the Application. Biomed. Chromatogr. 2014, 28, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Girón, A.B.; Marina, M.L.; Crego, A.L. Chiral Separation of a Basic Drug with Two Chiral Centers by Electrokinetic Chromatography for Its Pharmaceutical Development. J. Chromatogr. A 2016, 1467, 427–435. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, Y.; Lin, B.; Yu, J. Enantioseparation and Determination of Alminoprofen in Rat Plasma and Its Application to a Stereoselective Pharmacokinetic Study. J. Pharm. Biomed. Anal. 2020, 191, 113552. [Google Scholar] [CrossRef] [PubMed]
- Simó, C.; Barbas, C.; Cifuentes, A. Chiral Electromigration Methods in Food Analysis. Electrophoresis 2003, 24, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Montero, L.; Llorens, L.; Castro-Puyana, M.; Cifuentes, A. Recent Advances in the Application of Capillary Electromigration Methods for Food Analysis and Foodomics. Electrophoresis 2018, 39, 136–159. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; García, M.Á.; Marina, M.L. Chiral Separation of Metalaxyl and Benalaxyl Fungicides by Electrokinetic Chromatography and Determination of Enantiomeric Impurities. J. Chromatogr. A 2011, 1218, 4877–4885. [Google Scholar] [CrossRef]
- Carrão, D.B.; Perovani, I.S.; de Albuquerque, N.C.P.; de Oliveira, A.R.M. Enantioseparation of Pesticides: A Critical Review. Trac Trends Anal. Chem. 2020, 122, 115719. [Google Scholar] [CrossRef]
- Caslavska, J.; Thormann, W. Bioanalysis of Drugs and Their Metabolites by Chiral Electromigration Techniques (2010–2020). Electrophoresis 2021, elps.202000383. [Google Scholar] [CrossRef]
- di Venere, M.; Viglio, S.; Sassera, D.; Fumagalli, M.; Bardoni, A.; Salvini, R.; Cagnone, M.; Iadarola, P. Do the Complementarities of Electrokinetic and Chromatographic Procedures Represent the “Swiss Knife” in Proteomic Investigation? An Overview of the Literature in the Past Decade. Electrophoresis 2017, 38, 1538–1550. [Google Scholar] [CrossRef]
- Bernardo-Bermejo, S.; Sánchez-López, E.; Castro-Puyana, M.; Marina, M.L. Chiral Capillary Electrophoresis. Trac Trends Anal. Chem. 2020, 124, 115807. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, Z. Advances in Capillary Electro-Chromatography. J. Pharm. Anal. 2019, 9, 227–237. [Google Scholar] [CrossRef]
- Dubey, R.; Bhushan, R. Enantioseparation by Thin-Layer Chromatography. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1985, pp. 35–44. [Google Scholar]
- Scherz, H.; Huck, C.W.; Bonn, G.K. CEC and EKC of Natural Compounds. Electrophoresis 2007, 28, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Declerck, S.; vander Heyden, Y.; Mangelings, D. Enantioseparations of Pharmaceuticals with Capillary Electrochromatography: A Review. J. Pharm. Biomed. Anal. 2016, 130, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Kutter, J.P. Recent Advances in Microchip Enantioseparation and Analysis. Electrophoresis 2020, 41, 2122–2135. [Google Scholar] [CrossRef]
- Fanali, S.; Chankvetadze, B. Some Thoughts about Enantioseparations in Capillary Electrophoresis. Electrophoresis 2019, elps.201900144. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.B.; Quirino, J.P. Chiral Selectors in Capillary Electrophoresis: Trends During 2017–2018. Molecules 2019, 24, 1135. [Google Scholar] [CrossRef]
- Koster, N.; Clark, C.P.; Kohler, I. Past, Present, and Future Developments in Enantioselective Analysis Using Capillary Electromigration Techniques. Electrophoresis 2021, 42, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Salido-Fortuna, S.; Castro-Puyana, M.; Marina, M.L. Chiral Micellar Electrokinetic Chromatography. J. Chromatogr. A 2020, 1626, 461383. [Google Scholar] [CrossRef]
- Orlandini, S.; Pasquini, B.; Caprini, C.; del Bubba, M.; Squarcialupi, L.; Colotta, V.; Furlanetto, S. A Comprehensive Strategy in the Development of a Cyclodextrin-Modified Microemulsion Electrokinetic Chromatographic Method for the Assay of Diclofenac and Its Impurities: Mixture-Process Variable Experiments and Quality by Design. J. Chromatogr. A 2016, 1466, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Deeb, S.; Iriban, M.A.; Gust, R. MEKC as a Powerful Growing Analytical Technique. Electrophoresis 2011, 32, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Deeb, S.; Dawwas, H.A.; Gust, R. Recent Methodological and Instrumental Development in MEKC. Electrophoresis 2013, 34, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Altria, K.; McEvoy, E.; Donegan, S.; Power, J. A Review of Developments in the Methodology and Application of Microemulsion Electrokinetic Chromatography. Electrophoresis 2013, 34, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, J.; Yu, H.; Li, Z.; Gao, X.; Chang, Y. The Microemulsion Electrokinetic Capillary Chromatography Combined with Reversed-Electrode Polarity Stacking Mode for Enriching and Quantifying Lignanoids and Ginsenosides in TCMs Preparation Shengmai Injection. Electrophoresis 2018, 39, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Scriba, G.K.E. Differentiation of Enantiomers by Capillary Electrophoresis. In Differentiation of Enantiomers I; Springer: Berlin/Heidelberg, Germany, 2013; Volume 340, pp. 209–275. [Google Scholar]
- Terabe, S. Capillary Separation: Micellar Electrokinetic Chromatography. Annu. Rev. Anal. Chem. 2009, 2, 99–120. [Google Scholar] [CrossRef]
- Lu, H.; Chen, G. Recent Advances of Enantioseparations in Capillary Electrophoresis and Capillary Electrochromatography. Anal. Methods 2011, 3, 488. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral Recognition Mechanisms in Analytical Separation Sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral Recognition in Separation Science—An Update. J. Chromatogr. A 2016, 1467, 56–78. [Google Scholar] [CrossRef]
- Alvira, E. Theoretical Study of the β-Cyclodextrin Inclusion Complex Formation of Eugenol in Water. Molecules 2018, 23, 928. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B.; Lindner, W.; Scriba, G.K.E. Enantiomer Separations in Capillary Electrophoresis in the Case of Equal Binding Constants of the Enantiomers with a Chiral Selector: Commentary on the Feasibility of the Concept. Anal. Chem. 2004, 76, 4256–4260. [Google Scholar] [CrossRef]
- Liu, Y.; Shamsi, S.A. Chiral Capillary Electrophoresis–Mass Spectrometry: Developments and Applications in the Period 2010–2015: A Review. J. Chromatogr. Sci. 2016, bmw100. [Google Scholar] [CrossRef][Green Version]
- Cecilio Fonseca, M.; Santos da Silva, R.C.; Nascimento, C.S.; Bastos Borges, K. Computational Contribution to the Electrophoretic Enantiomer Separation Mechanism and Migration Order Using Modified β-Cyclodextrins. Electrophoresis 2017, 38, 1860–1868. [Google Scholar] [CrossRef]
- Peng, Z.-L.; Yi, F.; Guo, B.; Lin, J.-M. Temperature Effects on the Enantioselectivity of Basic Analytes in Capillary EKC Using Sulfated β-CDs as Chiral Selectors. Electrophoresis 2007, 28, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, X.-B.; Jiang, R.; Sun, X.-L.; Li, X.-Y.; Liu, W.-M.; Zhang, S.-Y. Analysis of Optical Purity and Impurity of Syntheticd-Phenylalanine Products Using Sulfated β-Cyclodextrin as Chiral Selector by Reversed-Polarity Capillary Electrophoresis. Chirality 2006, 18, 84–90. [Google Scholar] [CrossRef]
- Chu, B.-L.; Feng, Q.; Wang, Z.; Lin, J.-M. Enantiomeric Separation of Two Antiparkinsonian Drugs by Electrokinetic Chromatography Using Dextran Sulfate. Chromatographia 2009, 70, 817–824. [Google Scholar] [CrossRef]
- el Deeb, S.; Hasemann, P.; Wätzig, H. Strategies in Method Development to Quantify Enantiomeric Impurities Using CE. Electrophoresis 2008, 29, 3552–3562. [Google Scholar] [CrossRef]
- Růžička, M.; Koval, D.; Vávra, J.; Reyes-Gutiérrez, P.E.; Teplý, F.; Kašička, V. Interactions of Helquats with Chiral Acidic Aromatic Analytes Investigated by Partial-Filling Affinity Capillary Electrophoresis. J. Chromatogr. A 2016, 1467, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Talele, H.R.; Koval, D.; Severa, L.; Reyes-Gutiérrez, P.E.; Císařová, I.; Sázelová, P.; Šaman, D.; Bednárová, L.; Kašička, V.; Teplý, F. Diquats with Robust Chirality: Facile Resolution, Synthesis of Chiral Dyes, and Application as Selectors in Chiral Analysis. Chem. A Eur. J. 2018, 24, 7601–7604. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z. Recent Advance of Novel Chiral Separation Systems in Capillary Electrophoresis. Chin. J. Chromatogr. 2020, 38, 1028–1037. [Google Scholar]
- Modified Cyclodextrins for Chiral Separation; Tang, W., Ng, S.-C., Sun, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-37647-4. [Google Scholar]
- Fejős, I.; Kalydi, E.; Malanga, M.; Benkovics, G.; Béni, S. Single Isomer Cyclodextrins as Chiral Selectors in Capillary Electrophoresis. J. Chromatogr. A 2020, 1627, 461375. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xu, S.; Guo, X.; Wei, L.; Yu, J.; Wang, T. Use of Various β-Cyclodextrin Derivatives as Chiral Selectors for the Enantiomeric Separation of Ofloxacin and Its Five Related Substances by Capillary Electrophoresis. J. Sep. Sci. 2017, 40, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Saz, J.M.; Marina, M.L. Recent Advances on the Use of Cyclodextrins in the Chiral Analysis of Drugs by Capillary Electrophoresis. J. Chromatogr. A 2016, 1467, 79–94. [Google Scholar] [CrossRef]
- Guo, C.; Xiao, Y. Negatively Charged Cyclodextrins: Synthesis and Applications in Chiral Analysis-A Review. Carbohydr. Polym. 2021, 256, 117517. [Google Scholar] [CrossRef]
- Cucinotta, V.; Messina, M.; Contino, A.; Maccarrone, G.; Orlandini, S.; Giuffrida, A. Chiral Separation of Terbutaline and Non-Steroidal Anti-Inflammatory Drugs by Using a New Lysine–Bridged Hemispherodextrin in Capillary Electrophoresis. J. Pharm. Biomed. Anal. 2017, 145, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Salido-Fortuna, S.; Casado, N.; Castro-Puyana, M.; Marina, M.L. Use of Choline Chloride-D-Sorbitol Deep Eutectic Solvent as Additive in Cyclodextrin-Electrokinetic Chromatography for the Enantiomeric Separation of Lacosamide. Microchem. J. 2021, 160, 105669. [Google Scholar] [CrossRef]
- Wiedmer, S.K.; King, A.W.T.; Riekkola, M.-L. Phosphonium-Based Ionic Liquids in Electrokinetic Capillary Chromatography for the Separation of Neutral Analytes. J. Chromatogr. A 2012, 1253, 171–176. [Google Scholar] [CrossRef]
- Nie, L.; Yohannes, A.; Yao, S. Recent Advances in the Enantioseparation Promoted by Ionic Liquids and Their Resolution Mechanisms. J. Chromatogr. A 2020, 1626, 461384. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Use of Chiral Amino Acid Ester-Based Ionic Liquids as Chiral Selectors in CE. Electrophoresis 2013, 34, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ren, S.; Xue, S.; Li, A.; Liu, S.; Sun, X. Tetraalkylammonium-l-Tartrate Ionic Liquids as Sole Chiral Selectors in Capillary Electrophoresis. Sep. Purif. Technol. 2021, 256, 117842. [Google Scholar] [CrossRef]
- Greño, M.; Marina, M.L.; Castro-Puyana, M. Enantioseparation by Capillary Electrophoresis Using Ionic Liquids as Chiral Selectors. Crit. Rev. Anal. Chem. 2018, 48, 429–446. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, S.; Feng, Z.; Du, Y.; Yan, Z. Evaluation of Synergistic Enantioseparation Systems with Chiral Spirocyclic Ionic Liquids as Additives by Capillary Electrophoresis. Anal. Bioanal. Chem. 2016, 408, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Shamsi, S.A. Synthesis, Characterization, and Application of Chiral Ionic Liquids and Their Polymers in Micellar Electrokinetic Chromatography. Anal. Chem. 2006, 78, 7061–7069. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.B.; Quirino, J.P. Ionic Liquids in Electrokinetic Chromatography. J. Chromatogr. A 2021, 1637, 461801. [Google Scholar] [CrossRef]
- Salido-Fortuna, S.; Greño, M.; Castro-Puyana, M.; Marina, M.L. Amino Acid Chiral Ionic Liquids Combined with Hydroxypropyl-β-Cyclodextrin for Drug Enantioseparation by Capillary Electrophoresis. J. Chromatogr. A 2019, 1607, 460375. [Google Scholar] [CrossRef] [PubMed]
- Wahl, J.; Holzgrabe, U. Capillary Electrophoresis Separation of Phenethylamine Enantiomers Using Amino Acid Based Ionic Liquids. J. Pharm. Biomed. Anal. 2018, 148, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, Y.; Feng, Z.; Liu, Z.; Li, J. Establishment and Molecular Modeling Study of Maltodextrin-Based Synergistic Enantioseparation Systems with Two New Hydroxy Acid Chiral Ionic Liquids as Additives in Capillary Electrophoresis. J. Chromatogr. A 2018, 1559, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Tabani, H.; Fakhari, A.R.; Nojavan, S. Maltodextrins as Chiral Selectors in CE: Molecular Structure Effect of Basic Chiral Compounds on the Enantioseparation. Chirality 2014, 26, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.A.; Woods, C.D.; Stanley, F.E.; Stalcup, A.M. Chiral separations using heparin and polyelectrolyte multilayers in capillary electrophoresis. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2218–2231. [Google Scholar] [CrossRef]
- Sun, X.; Yu, T.; Xu, G.; Du, Y.; Chen, J.; Li, X. Evaluation of the Enantioselectivity of Capillary Electrokinetic Chromatography Using Ethanediamine-Bonded Poly (Glycidyl Methacrylate) Microspheres as the Pseudostationary Phases. Chirality 2019, 31, 118–126. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Gasper, M.P.; Rundlett, K.L. Highly Enantioselective Capillary Electrophoretic Separations with Dilute Solutions of the Macrocyclic Antibiotic Ristocetin A. J. Chromatogr. A 1995, 689, 285–304. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Ali, I. Macrocyclic Antibiotics as Effective Chiral Selectors for Enantiomeric Resolution by Liquid Chromatography and Capillary Electrophoresis. Chromatographia 2000, 52, 679–691. [Google Scholar] [CrossRef]
- Prokhorova, A.F.; Shapovalova, E.N.; Shpak, A.; Staroverov, S.M.; Shpigun, O.A. Enantiorecognition of Profens by Capillary Electrophoresis Using a Novel Chiral Selector Eremomycin. J. Chromatogr. A 2009, 1216, 3674–3677. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Nachbar, M.; el Deeb, S. Precise Small Volume Sample Handling for Capillary Electrophoresis. Electrophoresis 2015, 36, 2665–2669. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhang, Q.; Xue, S.; Liu, S.; Rui, M. Use of Gamithromycin as a Chiral Selector in Capillary Electrophoresis. J. Chromatogr. A 2020, 1624, 461099. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, S.; Xue, S. Investigation of Fusidic Acid as a Chiral Selector in Capillary Electrophoresis. Sep. Purif. Technol. 2020, 242, 116768. [Google Scholar] [CrossRef]
- Schmid, M.G. Chiral Metal-Ion Complexes for Enantioseparation by Capillary Electrophoresis and Capillary Electrochromatography: A Selective Review. J. Chromatogr. A 2012, 1267, 10–16. [Google Scholar] [CrossRef]
- Zheng, Z.-X.; Wei, Y.; Lin, J.-M. Chiral Separation Based on Ligand-Exchange Capillary Electrophoresis Using a Copper(II)-L-Ornithine Ternary Complex as Selector. Electrophoresis 2005, 26, 1007–1012. [Google Scholar] [CrossRef]
- Barker, G.E.; Russo, P.; Hartwick, R.A. Chiral Separation of Leucovorin with Bovine Serum Albumin Using Affinity Capillary Electrophoresis. Anal. Chem. 1992, 64, 3024–3028. [Google Scholar] [CrossRef]
- Asmari, M.; Ratih, R.; Alhazmi, H.A.; el Deeb, S. Thermophoresis for Characterizing Biomolecular Interaction. Methods 2018, 146, 107–119. [Google Scholar] [CrossRef]
- Rogez-Florent, T.; Foulon, C.; Drucbert, A.-S.; Schifano, N.; Six, P.; Devassine, S.; Depreux, P.; Danzé, P.-M.; Goossens, L.; Danel, C.; et al. Chiral Separation of New Sulfonamide Derivatives and Evaluation of Their Enantioselective Affinity for Human Carbonic Anhydrase II by Microscale Thermophoresis and Surface Plasmon Resonance. J. Pharm. Biomed. Anal. 2017, 137, 113–122. [Google Scholar] [CrossRef]
- Quintana, S.; García, M.Á.; Marina, M.L.; Gómez, R.; de la Mata, F.J.; Ortega, P. Synthesis of Chiral Carbosilane Dendrimers with l -Cysteine and N -Acetyl- l -Cysteine on Their Surface and Their Application as Chiral Selectors for Enantiomer Separation by Capillary Electrophoresis. Tetrahedron Asymmetry 2017, 28, 1797–1802. [Google Scholar] [CrossRef]
- Müllerová, L.; Dubský, P.; Gaš, B. Twenty Years of Development of Dual and Multi-Selector Models in Capillary Electrophoresis: A Review. Electrophoresis 2014, 35, 2688–2700. [Google Scholar] [CrossRef]
- La, Z.; Danel, C.; Grolaux, G.; Charton, J.; Furman, C.; Lipka, E. Electrophoretic Separation of Multiple Chiral Center Analyte with a Three Cyclodextrins Mixture. Electrophoresis 2021, elps.202000342. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Chen, M.; Zhang, G.; Chang, R.; Chen, A. Separation and Determination of Corynoxine and Corynoxine B Using Chiral Ionic Liquid and Hydroxypropyl-β-Cyclodextrin as Additives by Field-Amplified Sample Stacking in Capillary Electrophoresis. Electrophoresis 2018, 39, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.A.; Hancu, G.; Gyéresi, Á.; Kelemen, H.; Szabó, Z.; Noszál, B.; Dubský, P.; Tóth, G. Chiral Separation of Lansoprazole and Rabeprazole by Capillary Electrophoresis Using Dual Cyclodextrin Systems. Electrophoresis 2019, 40, 2799–2805. [Google Scholar] [CrossRef]
- Nicolaou, A.G.; Mavroudi, M.C.; Stavrou, I.J.; Weatherly, C.A.; Kapnissi-Christodoulou, C.P. Synergistic Enantioseparation Systems with Either Cyclodextrins or Cyclofructans and L-Alanine Tert Butyl Ester Lactate. Electrophoresis 2019, 40, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, Z.; Yu, T.; Du, Y.; Chen, J.; Liu, Z.; Xi, Y. Study of the Enantioselectivity and Recognition Mechanism of Chiral Dual System Based on Chondroitin Sulfate D in Capillary Electrophoresis. Anal. Bioanal. Chem. 2018, 410, 5889–5898. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Sun, X. Investigation of Maltodextrin-Based Synergistic System with Amino Acid Chiral Ionic Liquid as Additive for Enantioseparation in Capillary Electrophoresis. Chirality 2017, 29, 824–835. [Google Scholar] [CrossRef] [PubMed]
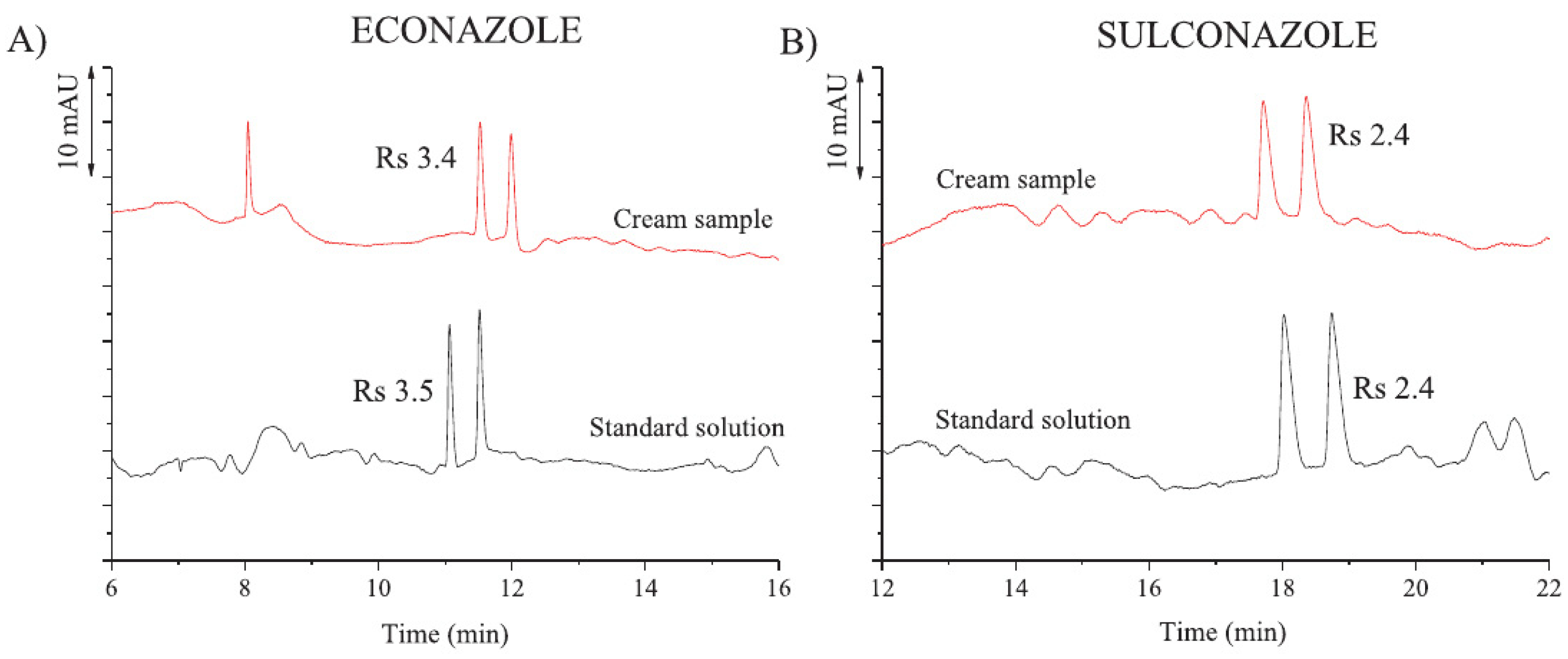
- Salido-Fortuna, S.; Marina, M.L.; Castro-Puyana, M. Enantiomeric Determination of Econazole and Sulconazole by Electrokinetic Chromatography Using Hydroxypropyl-β-Cyclodextrin Combined with Ionic Liquids Based on L-Lysine and L-Glutamic Acid. J. Chromatogr. A 2020, 1621, 461085. [Google Scholar] [CrossRef]
- Chalavi, S.; Fakhari, A.R.; Nojavan, S. Development of a Modified Partial Filling Method in Capillary Electrophoresis Using Two Chiral Plugs for the Simultaneous Enantioseparation of Chiral Drugs: Comparison with Mixed Chiral Selector Capillary Electrophoresis. J. Chromatogr. A 2018, 1567, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Baciu, T.; Borrull, F.; Calull, M.; Aguilar, C. Enantioselective Determination of Cathinone Derivatives in Human Hair by Capillary Electrophoresis Combined In-Line with Solid-Phase Extraction. Electrophoresis 2016, 37, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Prior, A.; Sánchez-Hernández, L.; Sastre-Toraño, J.; Marina, M.L.; de Jong, G.J.; Somsen, G.W. Enantioselective Analysis of Proteinogenic Amino Acids in Cerebrospinal Fluid by Capillary Electrophoresis-Mass Spectrometry. Electrophoresis 2016, 37, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Gruba, E.; Cielecka-Piontek, J.; Bednarek, E. Chiral Separation of Tedizolid Using Charge Single Isomer Derivatives of Cyclodextrins by Capillary Electrokinetic Chromatography. J. Pharm. Biomed. Anal. 2016, 120, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Gruba, E.; Bocian, W.; Cielecka-Piontek, J. Enantioselective Recognition of Radezolid by Cyclodextrin Modified Capillary Electrokinetic Chromatography and Electronic Circular Dichroism. J. Pharm. Biomed. Anal. 2017, 139, 98–108. [Google Scholar] [CrossRef]
- Hamidi, S.; Khoubnasabjafari, M.; Ansarin, K.; Jouyban-Gharamaleki, V.; Jouyban, A. Chiral Separation of Methadone in Exhaled Breath Condensate Using Capillary Electrophoresis. Anal. Methods 2017, 9, 2342–2350. [Google Scholar] [CrossRef]
- Menéndez-López, N.; Valimaña-Traverso, J.; Castro-Puyana, M.; Salgado, A.; García, M.Á.; Marina, M.L. Enantiomeric Separation of the Antiuremic Drug Colchicine by Electrokinetic Chromatography. Method Development and Quantitative Analysis. J. Pharm. Biomed. Anal. 2017, 138, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Z.-I.; Gál, R.; Szőcs, L.; Ludmerczki, R.; Muntean, D.-L.; Noszál, B.; Tóth, G. Validated Capillary Electrophoretic Method for the Enantiomeric Quality Control of R -Praziquantel. Electrophoresis 2017, 38, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, L.; Zhang, H.; Chen, D. Dispersive Micro-Solid-Phase Extraction Combined with Online Preconcentration by Capillary Electrophoresis for the Determination of Glycopyrrolate Stereoisomers in Rat Plasma. J. Sep. Sci. 2018, 41, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhao, Y.; Zhang, X.; Yu, J.; Guo, X. Determination of Brompheniramine Enantiomers in Rat Plasma by Cation-Selective Exhaustive Injection and Sweeping Cyclodextrin Modified Electrokinetic Chromatography Method. Electrophoresis 2018, 39, 2099–2106. [Google Scholar] [CrossRef]
- Casado, N.; Salgado, A.; Castro-Puyana, M.; García, M.Á.; Marina, M.L. Enantiomeric Separation of Ivabradine by Cyclodextrin-Electrokinetic Chromatography. Effect of Amino Acid Chiral Ionic Liquids. J. Chromatogr. A 2019, 1608, 460407. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, B.; Li, S.; Yu, J.; Guo, X. Enantioselective Analysis of Pheniramine in Rat Using Large Volume Sample Stacking or Cation-Selective Exhaustive Injection and Sweeping Coupled with Cyclodextrin Modified Electrokinetic Chromatography. Talanta 2019, 192, 226–232. [Google Scholar] [CrossRef]
- Tseng, W.-B.; Hsieh, M.-M.; Chiu, T.-C.; Yu, P.-L.; Chen, S.-H. Enantioseparation of Phenothiazines through Capillary Electrophoresis with Solid Phase Extraction and Polymer Based Stacking. J. Food Drug Anal. 2018, 26, 1171–1179. [Google Scholar] [CrossRef]
- Casado, N.; Saz, J.M.; García, M.Á.; Marina, M.L. Modeling-Based Optimization of the Simultaneous Enantiomeric Separation of Multicomponent Mixtures of Phenoxy Acid Herbicides Using Dual Cyclodextrin Systems by Capillary Electrophoresis. J. Chromatogr. A 2020, 1610, 460552. [Google Scholar] [CrossRef] [PubMed]
- Greño, M.; Castro-Puyana, M.; Marina, M.L. Enantiomeric Separation of Homocysteine and Cysteine by Electrokinetic Chromatography Using Mixtures of γ-Cyclodextrin and Carnitine-Based Ionic Liquids. Microchem. J. 2020, 157, 105070. [Google Scholar] [CrossRef]
- Cârcu-Dobrin, M.; Sabău, A.G.; Hancu, G.; Árpád, G.; Rusu, A.; Kelemen, H.; Papp, L.A.; Cârje, A. Chiral Discrimination of Amlodipine from Pharmaceutical Products Using Capillary Electrophoresis. Braz. J. Pharm. Sci. 2020, 56, 1–9. [Google Scholar] [CrossRef]
- Lorenzo, M.P.; Valiente, L.; Buendia, I.; Gortázar, A.R.; Garcia, A. Optimization and Validation of a Chiral CE-LIF Method for Quantitation of Aspartate, Glutamate and Serine in Murine Osteocytic and Osteoblastic Cells. J. Chromatogr. B 2020, 1152, 122259. [Google Scholar] [CrossRef]
- Milan, A.; Hancu, G.; Lupu, D.; Budău, M.; Garaj, V.; Kelemen, H. Venlafaxine Chiral Separation by Capillary Electrophoresis Using Cyclodextrin Derivatives as Chiral Selector and Experimental Design Method Optimization. Symmetry 2020, 12, 849. [Google Scholar] [CrossRef]
- Pieckowski, M.; Kowalski, P.; Bączek, T. Combination of Large Volume Sample Stacking with Polarity Switching and Cyclodextrin Electrokinetic Chromatography (LVSS-PS-CDEKC) for the Determination of Selected Preservatives in Pharmaceuticals. Talanta 2020, 211, 120673. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, S.; Amariei, G.; Boltes, K.; García, M.Á.; Marina, M.L. Enantiomeric Separation of Panthenol by Capillary Electrophoresis. Analysis of Commercial Formulations and Toxicity Evaluation on Non-Target Organisms. J. Chromatogr. A 2021, 1639, 461919. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, W. Recent (2018–2020) Development in Capillary Electrophoresis. Anal. Bioanal. Chem. 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Breadmore, M.C.; Grochocki, W.; Kalsoom, U.; Alves, M.N.; Phung, S.C.; Rokh, M.T.; Cabot, J.M.; Ghiasvand, A.; Li, F.; Shallan, A.I.; et al. Recent Advances in Enhancing the Sensitivity of Electrophoresis and Electrochromatography in Capillaries and Microchips (2016–2018). Electrophoresis 2019, 40, 17–39. [Google Scholar] [CrossRef]
- Šlampová, A.; Malá, Z.; Gebauer, P. Recent Progress of Sample Stacking in Capillary Electrophoresis (2016–2018). Electrophoresis 2019, 40, 40–54. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Zhu, P.; Duan, G.; Li, Y.; Song, F. Chiral Separation of Bupivacaine Hydrochloride by Capillary Electrophoresis with High Frequency Conductivity Detection and Its Application to Rabbit Serum and Pharmaceutical Injection. Pharmazie 2012, 67, 25–30. [Google Scholar]
- Wuethrich, A.; Quirino, J.P. Derivatisation for Separation and Detection in Capillary Electrophoresis (2015–2017). Electrophoresis 2018, 39, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Míguez, R.; Marina, M.L.; Castro-Puyana, M. Enantiomeric Separation of Non-Protein Amino Acids by Electrokinetic Chromatography. J. Chromatogr. A 2016, 1467, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, L.; Castro-Puyana, M.; Marina, M.L.; Crego, A.L. Recent Approaches in Sensitive Enantioseparations by CE. Electrophoresis 2012, 33, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Crego, A.L.; Mateos, M.; Nozal, L. Recent Contributions for Improving Sensitivity in Chiral CE. Electrophoresis 2018, 39, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, D.L.; Jaramillo, M.; Green, T.K. Enantioseparation and Stacking of Cyanobenz[f]Isoindole-Amino Acids by Reverse Polarity Capillary Electrophoresis and Sulfated β-Cyclodextrin. Anal. Chem. 2007, 79, 736–743. [Google Scholar] [CrossRef]
- Aranas, A.T.; Guidote, A.M.; Quirino, J.P. Sweeping and New On-Line Sample Preconcentration Techniques in Capillary Electrophoresis. Anal. Bioanal. Chem. 2009, 394, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wuethrich, A.; Haddad, P.R.; Quirino, J.P. Chiral Capillary Electromigration Techniques-Mass Spectrometry-Hope and Promise. Electrophoresis 2014, 35, 2–11. [Google Scholar] [CrossRef]
- Wang, X.; Hou, J.; Jann, M.; Hon, Y.Y.; Shamsi, S.A. Development of a Chiral Micellar Electrokinetic Chromatography–Tandem Mass Spectrometry Assay for Simultaneous Analysis of Warfarin and Hydroxywarfarin Metabolites: Application to the Analysis of Patients Serum Samples. J. Chromatogr. A 2013, 1271, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Otsuka, K.; Terabe, S. Separation of Enantiomers by Capillary Electrophoresis–Mass Spectrometry Employing a Partial Filling Technique with a Chiral Crown Ether. J. Chromatogr. A 2000, 875, 323–330. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Martín-Biosca, Y.; Medina-Hernández, M.J.; Sagrado, S. Enantioresolution in Electrokinetic Chromatography-Complete Filling Technique Using Sulfated Gamma-Cyclodextrin. Software-Free Topological Anticipation. J. Chromatogr. A 2016, 1467, 391–399. [Google Scholar] [CrossRef]
- Nishi, H. Enantiomer Separation of Drugs by Electrokinetic Chromatography. J. Chromatogr. A 1996, 735, 57–76. [Google Scholar] [CrossRef]
- Wuethrich, A.; Haddad, P.R.; Quirino, J.P. Online Sample Concentration in Partial-Filling Chiral Electrokinetic Chromatography—Mass Spectrometry. Chirality 2014, 26, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Castro-Puyana, M.; Marina, M.L. Chiral Capillary Electrophoresis-Mass Spectrometry. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1985, pp. 391–405. [Google Scholar]
- Lee, S.; Kim, S.-J.; Bang, E.; Na, Y.-C. Chiral Separation of Intact Amino Acids by Capillary Electrophoresis-Mass Spectrometry Employing a Partial Filling Technique with a Crown Ether Carboxylic Acid. J. Chromatogr. A 2019, 1586, 128–138. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Endresz, G.; Blaschke, G. About Some Aspects of the Use of Charged Cyclodextrins for Capillary Electrophoresis Enantioseparation. Electrophoresis 1994, 15, 804–807. [Google Scholar] [CrossRef]
- Cui, X.; Liang, C.; Gong, F.; Wang, R.; Ni, C.; Wu, Y.; Chen, G.; Zhang, Y. Simultaneous Chiral Analysis of Amphetamine-Type Stimulants and Ephedrine by Capillary Electrophoresis Coupled to Time-of-Flight Mass Spectrometry. Chirality 2018, 30, 1079–1087. [Google Scholar] [CrossRef]
- Šebestová, A.; Baron, D.; Pechancová, R.; Pluháček, T.; Petr, J. Determination of Oxaliplatin Enantiomers at Attomolar Levels by Capillary Electrophoresis Connected with Inductively Coupled Plasma Mass Spectrometry. Talanta 2019, 205, 120151. [Google Scholar] [CrossRef] [PubMed]
- Desiderio, C.; Rossetti, D.; Perri, F.; Giardina, B.; Messana, I.; Castagnola, M. Enantiomeric Separation of Baclofen by Capillary Electrophoresis Tandem Mass Spectrometry with Sulfobutylether-β-Cyclodextrin as Chiral Selector in Partial Filling Mode. J. Chromatogr. B 2008, 875, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Švidrnoch, M.; Přibylka, A.; Bekárek, V.; Ševčík, J.; Smolka, V.; Maier, V. Enantioseparation of d, l-2-Hydroxyglutaric Acid by Capillary Electrophoresis with Tandem Mass Spectrometry—Fast and Efficient Tool for d-and l -2-Hydroxyglutaracidurias Diagnosis. J. Chromatogr. A 2016, 1467, 383–390. [Google Scholar] [CrossRef]
- Machida, Y.; Nishi, H. Chiral Purity in Drug Analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Hashim, P.K.; Tamaoki, N. Enantioselective Photochromism under Circularly Polarized Light. ChemPhotoChem 2019, 3, 347–355. [Google Scholar] [CrossRef]
- Liu, M.; Chen, L.; Tian, T.; Zhang, Z.; Li, X. Identification and Quantitation of Enantiomers by Capillary Electrophoresis and Circular Dichroism Independent of Single Enantiomer Standard. Anal. Chem. 2019, 91, 13803–13809. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B. Enantiomer Migration Order in Chiral Capillary Electrophoresis. Electrophoresis 2002, 23, 4022–4035. [Google Scholar] [CrossRef]
- el Deeb, S. Evaluation of a Vancomycin-Based LC Column in Enantiomeric Separation of Atenolol: Method Development, Repeatability Study and Enantiomeric Impurity Determination. Chromatographia 2010, 71, 783–787. [Google Scholar] [CrossRef]
- Abdel-Megied, A.M.; Hanafi, R.S.; Aboul-Enein, H.Y. A Chiral Enantioseparation Generic Strategy for Anti-Alzheimer and Antifungal Drugs by Short End Injection Capillary Electrophoresis Using an Experimental Design Approach. Chirality 2018, 30, 165–176. [Google Scholar] [CrossRef]
- Zhu, Q.; Scriba, G.K.E. Analysis of Small Molecule Drugs, Excipients and Counter Ions in Pharmaceuticals by Capillary Electromigration Methods—Recent Developments. J. Pharm. Biomed. Anal. 2018, 147, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Harnisch, H.; Chien, Y.; Scriba, G.K.E. Capillary Electrophoresis Method for the Chiral Purity Determination of Pregabalin Derivatized with Dansyl Chloride. Chromatographia 2018, 81, 719–725. [Google Scholar] [CrossRef]
- Pasquini, B.; Orlandini, S.; Villar-Navarro, M.; Caprini, C.; del Bubba, M.; Douša, M.; Giuffrida, A.; Gotti, R.; Furlanetto, S. Chiral Capillary Zone Electrophoresis in Enantioseparation and Analysis of Cinacalcet Impurities: Use of Quality by Design Principles in Method Development. J. Chromatogr. A 2018, 1568, 205–213. [Google Scholar] [CrossRef]
- Krait, S.; Scriba, G.K.E. Quality by Design-Assisted Development of a Capillary Electrophoresis Method for the Chiral Purity Determination of Dexmedetomidine. Electrophoresis 2018, 39, 2575–2580. [Google Scholar] [CrossRef]
- Niedermeier, S.; Scriba, G.K.E. A Quality by Design-Based Approach to a Capillary Electrokinetic Assay for the Determination of Dextromepromazine and Levomepromazine Sulfoxide as Impurities of Levomepromazine. J. Pharm. Biomed. Anal. 2017, 146, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Niedermeier, S.; Scriba, G.K.E.E.; Gerhard, K.E. Scriba Quality by Design-Based Development of a Chiral Capillary Electrophoresis Method for the Determination of Dextrodropropizine and 1-Phenylpiperazine as Impurities of Levodropropizine. Chromatographia 2020, 83, 123–129. [Google Scholar] [CrossRef]
- Elbashir, A.A.; Aboul-Enein, H.Y. Capillary Electrophoresis and Molecular Modeling as a Complementary Technique for Chiral Recognition Mechanism. Crit. Rev. Anal. Chem. 2013, 43, 131–137. [Google Scholar] [CrossRef]
- Chankvetadze, B. Enantioseparations by Capillary Electromigration Techniques. In Chiral Analysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 565–605. [Google Scholar]
- Scriba, G.K.E. Chiral Recognition in Separation Sciences. Part I: Polysaccharide and Cyclodextrin Selectors. Trac Trends Anal. Chem. 2019, 120, 115639. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral Recognition in Separation Sciences. Part II: Macrocyclic Glycopeptide, Donor-Acceptor, Ion-Exchange, Ligand-Exchange and Micellar Selectors. Trac Trends Anal. Chem. 2019, 119, 115628. [Google Scholar] [CrossRef]
- Peluso, P.; Dessì, A.; Dallocchio, R.; Mamane, V.; Cossu, S. Recent Studies of Docking and Molecular Dynamics Simulation for Liquid-Phase Enantioseparations. Electrophoresis 2019, 40, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, A. Fundamental Aspects of Chiral Separations by Capillary Electrophoresis. Electrophoresis 2001, 22, 3079–3106. [Google Scholar] [CrossRef]
- Salgado, A.; Tatunashvili, E.; Gogolashvili, A.; Chankvetadze, B.; Gago, F. Structural Rationale for the Chiral Separation and Migration Order Reversal of Clenpenterol Enantiomers in Capillary Electrophoresis Using Two Different β-Cyclodextrins. Phys. Chem. Chem. Phys. 2017, 19, 27935–27939. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.C.; Coelho, J.F.; Silva, C.F.; Dutra, F.V.A.; Guimarães, L.; Nascimento, C.S.; Borges, K.B. Enantioselective Separation of Bupropion and Its Major Metabolite Hydroxybupropion: An Experimental and Theoretical Study. Chem. Phys. Lett. 2019, 730, 1–7. [Google Scholar] [CrossRef]
- Rizvi, A.S.; Murtaza, G.; Irfan, M.; Xiao, Y.; Qu, F. Determination of Kynurenine Enantiomers by Alpha-Cyclodextrin, Cationic-Βeta-Cyclodextrin and Their Synergy Complemented with Stacking Enrichment in Capillary Electrophoresis. J. Chromatogr. A 2020, 1622, 461128. [Google Scholar] [CrossRef]
- Krait, S.; Salgado, A.; Chankvetadze, B.; Gago, F.; Scriba, G.K.E. Investigation of the Complexation between Cyclodextrins and Medetomidine Enantiomers by Capillary Electrophoresis, NMR Spectroscopy and Molecular Modeling. J. Chromatogr. A 2018, 1567, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Budău, M.; Hancu, G.; Muntean, D.L.; Papp, L.A.; Cârje, A.G.; Garaj, V. Enantioseparation of Citalopram Enantiomers by Capillary Electrophoresis: Method Development through Experimental Design and Computational Modeling. Chirality 2020, 32, 1119–1128. [Google Scholar] [CrossRef]
- Szabó, Z.-I.; Tóth, G.; Völgyi, G.; Komjáti, B.; Hancu, G.; Szente, L.; Sohajda, T.; Béni, S.; Muntean, D.-L.; Noszál, B. Chiral Separation of Asenapine Enantiomers by Capillary Electrophoresis and Characterization of Cyclodextrin Complexes by NMR Spectroscopy, Mass Spectrometry and Molecular Modeling. J. Pharm. Biomed. Anal. 2016, 117, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Bocian, W.; Bednarek, E.; Pałys, B.; Cielecka-Piontek, J. Enantioselective Recognition of Sutezolid by Cyclodextrin Modified Non-Aqueous Capillary Electrophoresis and Explanation of Complex Formation by Means of Infrared Spectroscopy, NMR and Molecular Modelling. J. Pharm. Biomed. Anal. 2019, 169, 49–59. [Google Scholar] [CrossRef]
- Yao, Y.; Song, P.; Wen, X.; Deng, M.; Wang, J.; Guo, X. Chiral Separation of 12 Pairs of Enantiomers by Capillary Electrophoresis Using Heptakis-(2,3-Diacetyl-6-Sulfato)-β-Cyclodextrin as the Chiral Selector and the Elucidation of the Chiral Recognition Mechanism by Computational Methods. J. Sep. Sci. 2017, 40, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Z.-I.; Ludmerczki, R.; Fiser, B.; Noszál, B.; Tóth, G. Chiral Separation of Rasagiline Using Sulfobutylether-β-Cyclodextrin: Capillary Electrophoresis, NMR and Molecular Modeling Study. Electrophoresis 2019, 40, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Székely-Szentmiklósi, B. Béla Tőkés study of ofloxacin—Random by methylated–β–cyclodextrin inclusion complex. Farmacia 2016, 64, 147–151. [Google Scholar]
- Liu, Y.; Deng, M.; Yu, J.; Jiang, Z.; Guo, X. Capillary Electrophoretic Enantioseparation of Basic Drugs Using a New Single-Isomer Cyclodextrin Derivative and Theoretical Study of the Chiral Recognition Mechanism. J. Sep. Sci. 2016, 39, 1766–1775. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Lin, H.; Feng, Y.; Shen, H.; Huang, R.; Liu, L.; Zhao, Z. Combination of Capillary Electrophoresis and Molecular Modeling to Study the Enantiomer Affinity Pattern between Β-blockers and Anionic Cyclodextrin Derivatives in a Methanolic and Water Background Electrolyte. J. Sep. Sci. 2019, jssc.201800884. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, C.; Ma, Y.; Zhou, S.; Li, Z.; Sun, T. Effectively Enhancing the Enantioseparation Ability of β-Cyclodextrin Derivatives by de Novo Design and Molecular Modeling. Analyst 2017, 142, 3699–3706. [Google Scholar] [CrossRef]
- Loukas, Y.L.; Sabbah, S.; Scriba, G.K.E. Method Development and Validation for the Chiral Separation of Peptides in the Presence of Cyclodextrins Using Capillary Electrophoresis and Experimental Design. J. Chromatogr. A 2001, 931, 141–152. [Google Scholar] [CrossRef]
- Scriba, G.K.E.V. Cyclodextrins in Capillary Electrophoresis Enantioseparations—Recent Developments and Applications. J. Sep. Sci. 2008, 31, 1991–2011. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Budău, M.; Kántor, L.K.; Cârje, A. Cyclodextrine Screening for the Chiral Separation of Amlodipine Enantiomers by Capillary Electrophoresis. Adv. Pharm. Bull. 2015, 5, 35–40. [Google Scholar] [PubMed]
- Li, W.; Tan, G.; Zhao, L.; Chen, X.; Zhang, X.; Zhu, Z.; Chai, Y. Computer-Aided Molecular Modeling Study of Enantioseparation of Iodiconazole and Structurally Related Triadimenol Analogues by Capillary Electrophoresis: Chiral Recognition Mechanism and Mathematical Model for Predicting Chiral Separation. Anal. Chim. Acta 2012, 718, 138–147. [Google Scholar] [CrossRef] [PubMed]



| Analyte | Matrix | Separation Conditions | Detection | LOD/LOQ | Ref. |
|---|---|---|---|---|---|
| Cathinone derivatives | Human hair | Preconcentration by solid phase extraction, fused silica capillary of 50 µm i.d. and 80 cm total length, separation voltage of 35 kV, BGE of 80 mM disodium phosphate at pH 2.5, β-CD as CS | DAD at 200 nm | 0.02 ng/mg 0.2 ng/mg | [94] |
| Proteinogenic amino acids | Cerebrospinal fluid | Derivatization by 9-fluorenylmethyl chloroformate fused silica capillary of 50 µm i.d. with a length of 70 cm to the detector and 80 cm of total length, separation voltage of 35 kV, BGE of 50 mM ammonium bicarbonate at pH 8 containing 15% (v/v) isopropanol and 10 mM β-CD, Sheath liquid interfacing of propan-2-ol:water: 1 M ammonium bicarbonate (50:50:1, v/v/v) at a flow rate of 3 L/min, and a nebulizer gas pressure of 2 psi | ESI-MS | 0.9 µM - | [95] |
| Tedizolid | Pharmaceutical formulation | Fused silica capillary of 45 cm (effective length 35 cm) × 25 µm i.d., separation voltage of 12 kV, BGE of 37.5 mM heptakis-(2,3-diacetyl6-sulfo)-β-CD dissolved in 50 mM formic buffer pH 4.0 with the addition of acetonitrile (81.4:18.6, v/v) | DAD at 200 nm | - - | [96] |
| Radezolid | Pharmaceutical preparation | Fused silica capillary of 45 total length and 35 cm effective length and 25 µm i.d., separation voltage −28 kV, BGE of 40 mM heptakis(2,3-di-O-methyl-6-sulfo)-β-CD dissolved in 50 mM phosphate buffer pH 2.5 | DAD at 265 nm | - - | [97] |
| Methadone | Exhaled breath condensate | Fused silica capillary of 50 cm length, 41.5 cm effective length, and 50 µm i.d., separation voltage of 25 kV, BGE of 150 mM phosphoric acid-tetraethylammonium at pH 2.5 containing 30% (v/v) methanol and 0.8% (w/v) carboxymethyl-β-CD | DAD at 200 nm | - 0.15 µg/mL | [98] |
| Colchicine | Pharmaceutical preparation | Fused silica capillary 58.5 cm (50 cm effective length) × 50 μm i.d., separation voltage of 20 kV, 50 mM or 25 mM borate buffer pH 9.0 using succinyl-γ-cyclodextrin or sulfated-γ-CD | UV at 243 nm | 0.3 mg/mL 1 mg/mL | [99] |
| Praziquantel | Pharmaceutical preparation | Fused silica capillary of 50 µm i.d., 48.5 cm total and 40 cm effective length, separation voltage of 15 kV, BGE of 50 mM phosphate buffer pH 2.0, supplied with 15 mM sulfated-β-CD | DAD at 210 nm | 0.75 µg/mL 2.0 µg/mL | [100] |
| Glycopyrrolate | Rat plasma | Online preconcentration by cation-selective exhaustive injection-sweeping, fused silica capillary (40.2 cm × 75 μm), separation voltage of −20 kV, BGE 30 mM phosphate solution at pH 2.0 containing 20 mg/mL sulfated-β-CD and 5% acetonitrile | DAD at 200 nm | 2.0 ng/mL 0.0625 µg/mL | [101] |
| Brompheniramine | Rat plasma | Online preconcentration by cation-selective exhaustive injection and sweeping, fused silica capillary of the total length of 50 cm (effective length 40 cm) × 50 µm i.d., separation voltage of −20 kV, BGE in 50 mM phosphate buffer pH 3.5, containing 10% (v/v) acetonitrile and 30 mg/mL sulfated-β-CD | UV at 210 nm | - 0.01 µg/mL | [102] |
| Ivabradine | Pharmaceutical formulation | Fused silica capillary of 58.5 cm (50 cm to the detector window) × 50 µm i.d., separation voltage of −30 kV, BGE of 5 mM tetrabutylammonium-aspartic acid in 50 mM formate buffer pH 2.0 containing 4 mM sulfated-γ-CD | UV at 200 nm | 0.22 and 0.28 µg/mL 0.73 and 0.93 µg/mL | [103] |
| Lansoprazole and rabeprazole | Pharmaceutical preparations | Fused silica capillary of 48 cm total, and 40 cm effective length and 50 μm i.d., separation voltage of +20 kV, BGE for lansoprazole: 25 mM phosphate buffer pH 7, 10 mM sulfobutyl-ether-β-CD/20mM γ-CD, +20 kV voltage; BGE for rabeprazole: 25 mM phosphate buffer pH 7, 15 mM sulfobutyl-ether-β-CD/30 mM γ-CD | UV at 210 nm | 2 and 2 µg/mL 6 and 6 µg/mL | [88] |
| Pheniramine | Rat plasma | Online preconcentration by large volume sample stacking and sweeping, fussed silica capillary of a total length of 50 cm (effective length 40 cm) × 50 μm i.d., separation voltage of −20 kV, BGE of 30 mM phosphate buffer at pH 3.0 with 30 mg/mL sulfated-β-CD | UV at 262 nm | - 10 ng/mL | [104] |
| Phenothiazines | Urine sample | Preconcentration by solid phase extraction, fused silica capillary of 75 µm i.d. and 365 µm o.d., separation voltage from 10 to 12 kV, BGE of 75 mM phosphate buffer pH 3.0 and 0.9% poly (diallyldimethylammonium chloride), hydroxypropyl-γ-CD as a CS. | UV at 254 nm | 2.1 to 6.3 nM- | [105] |
| Six phenoxy acid herbicides (Fenoprop 1, Fenoprop 2, Mecoprop 1, Mecoprop 2, Dichlorprop 1, Dichlorprop 2 | Mixture of herbicides | Fused silica capillary of 58.5 cm total length and 50 cm length to the detector and 50 µm i.d., separation voltage of 25 kV, BGE of 50 mM phosphate buffer pH 7.0, dual CD (4 mM hydroxyl--β-CD and 16 mM heptakis(2,3,6-tri-O-methyl)-β-CD. | DAD at 200 nm for mecoprop, chlorprop, and 210 nm for fenoprop | - - | [106] |
| Homocysteine and cysteine | Stock standard solutions in borate buffer | Derivatization by 9-fluorenylmethyl chloroformate, fused silica capillary of 58.5 cm total length and 50 cm effective length and 50 µm i.d., separation voltage 20 kV, BGE for homocysteine: 2 mM γ-CD +5 mM L-Carnitine C1NTf2 in borate buffer pH 9.0, BGE for Cysteine: 2 mM γ-CD +5 mM L-CarnitineC1Lac in phosphate buffer pH 7.0 | DAD at 210 nm | - - | [107] |
| Amlodipine | Pharmaceutical formulation | Fused silica capillary of 48 cm length (40 cm effective length) ×50 μm i.d., separation voltage of 25 kV, BGE of 25 mM phosphate buffer pH 9.0, 15 mM carboxymethyl-β-CD | UV at 230 nm | S 0.27 and R 0.32 µg/mL S 0.8 and R 0.96 µg/mL | [108] |
| L/D-Asp, L/D-Glu, and L/D-Ser | Bone cell lines (murine osteocytes and osteoblast | Derivatization by 4-fluoro-7-nitro-2,1,3-benzoxadiazole, capillary fused silica with 75 μm i.d. and a total length of 60 cm, separation voltage of 30 kV, BGE 137.5 mM borate buffer pH 10.25 and 12.5 mM β-CDs | LIF at λex = 488 nm and λem = 522 nm | 0.25 µmol/L | [109] |
| Venlafaxine | Pharmaceutical preparations | Fused silica capillary of 30 cm length (effective length 22 cm) × 50 µm, separation voltage of 25 kV, BGE of 25 mM phosphate buffer pH 2.5, 10 mM carboxymethyl-β-CD | UV at 230 nm | 0.07 and 0.06 mg/mL 0.21 and 0.18 mg/mL | [110] |
| Methylparaben, ethylparaben, propylparaben, butylparaben, isobutylparaben, sorbic acid, benzoic acid, p-hydroxybenzoic acid | Pharmaceutical preparations | Online preconcentration by large volume sample stacking, fused silica capillary of 75 µm i.d. × 50 cm length, separation voltage 25 kV, BGE of 25 mM tetraborate pH 9.3 and α-CD | UV at 195 nm for ethylparaben, benzoic acid, and p-hydroxybenzoic acid, at 296 nm for methylparaben, propylparaben, butylparaben, and isobutylparaben, at 254 nm for sorbic acid at 254 nm. | 0.8 to 5 ng/mL 3 to 16 ng/mL | [111] |
| L-Panthenol dexapanthenol | Pharmaceutical and cosmetic formulations | Fused silica capillary of a total length of 58.5 cm (50 cm effective length) and 50 µm i.d., separation voltage of 30 kV, BGE of 25 mM (2-carboxyethyl)-β-CD in 100 mM borate buffer pH 9.0 | UV at 205 nm | 1.0 and 4.0 mg/L 3.3 and 13.3 mg/L | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Deeb, S.; Silva, C.F.; Junior, C.S.N.; Hanafi, R.S.; Borges, K.B. Chiral Capillary Electrokinetic Chromatography: Principle and Applications, Detection and Identification, Design of Experiment, and Exploration of Chiral Recognition Using Molecular Modeling. Molecules 2021, 26, 2841. https://doi.org/10.3390/molecules26102841
El Deeb S, Silva CF, Junior CSN, Hanafi RS, Borges KB. Chiral Capillary Electrokinetic Chromatography: Principle and Applications, Detection and Identification, Design of Experiment, and Exploration of Chiral Recognition Using Molecular Modeling. Molecules. 2021; 26(10):2841. https://doi.org/10.3390/molecules26102841
Chicago/Turabian StyleEl Deeb, Sami, Camilla Fonseca Silva, Clebio Soares Nascimento Junior, Rasha Sayed Hanafi, and Keyller Bastos Borges. 2021. "Chiral Capillary Electrokinetic Chromatography: Principle and Applications, Detection and Identification, Design of Experiment, and Exploration of Chiral Recognition Using Molecular Modeling" Molecules 26, no. 10: 2841. https://doi.org/10.3390/molecules26102841
APA StyleEl Deeb, S., Silva, C. F., Junior, C. S. N., Hanafi, R. S., & Borges, K. B. (2021). Chiral Capillary Electrokinetic Chromatography: Principle and Applications, Detection and Identification, Design of Experiment, and Exploration of Chiral Recognition Using Molecular Modeling. Molecules, 26(10), 2841. https://doi.org/10.3390/molecules26102841









