Extraction of Peppermint Essential Oils and Lipophilic Compounds: Assessment of Process Kinetics and Environmental Impacts with Multiple Techniques
Abstract
:1. Introduction
2. Results and Discussion
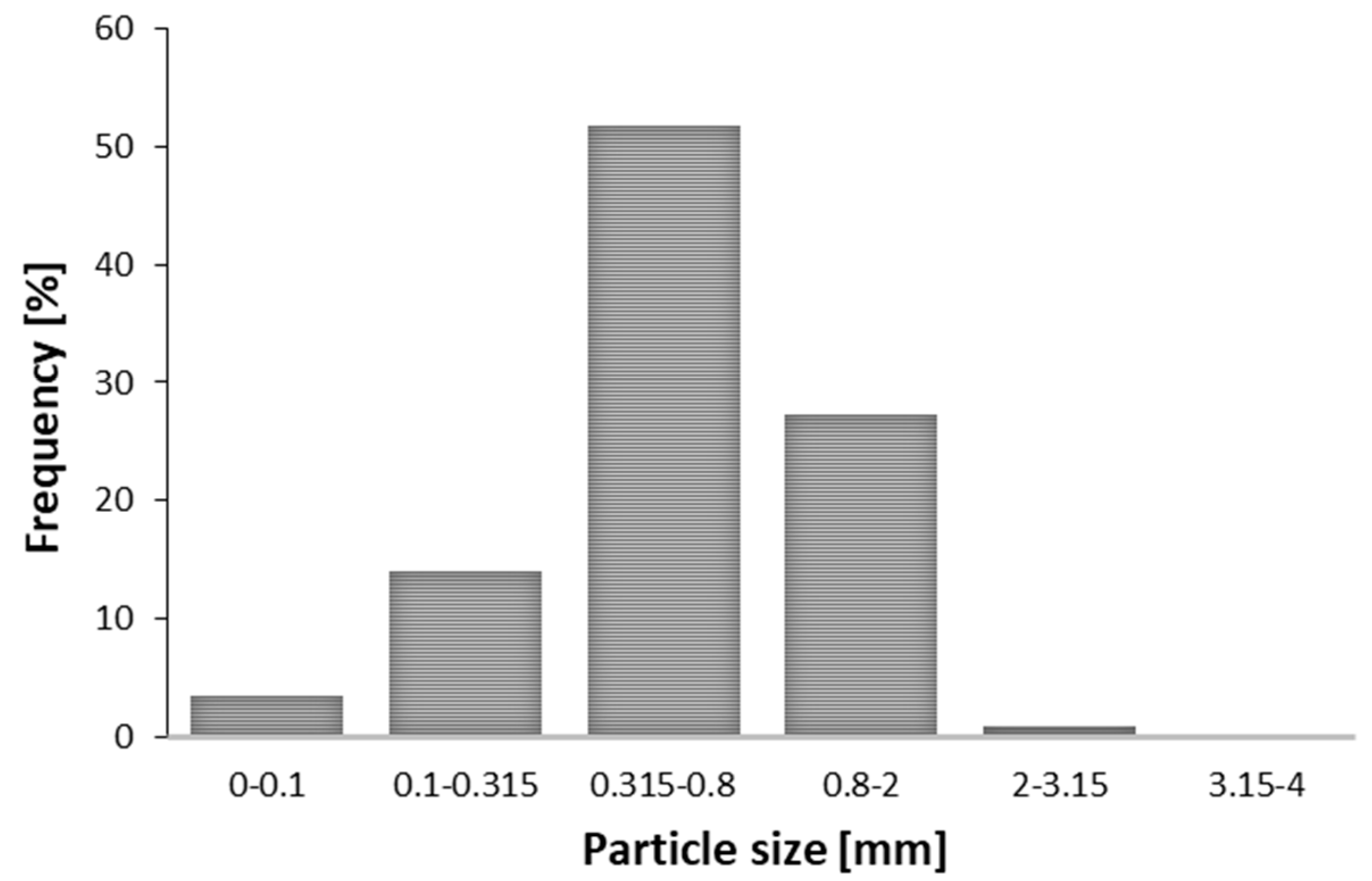
2.1. Particle Size of Extraction Materials
2.2. Separation of EO
2.3. Separation of Lipid Extract
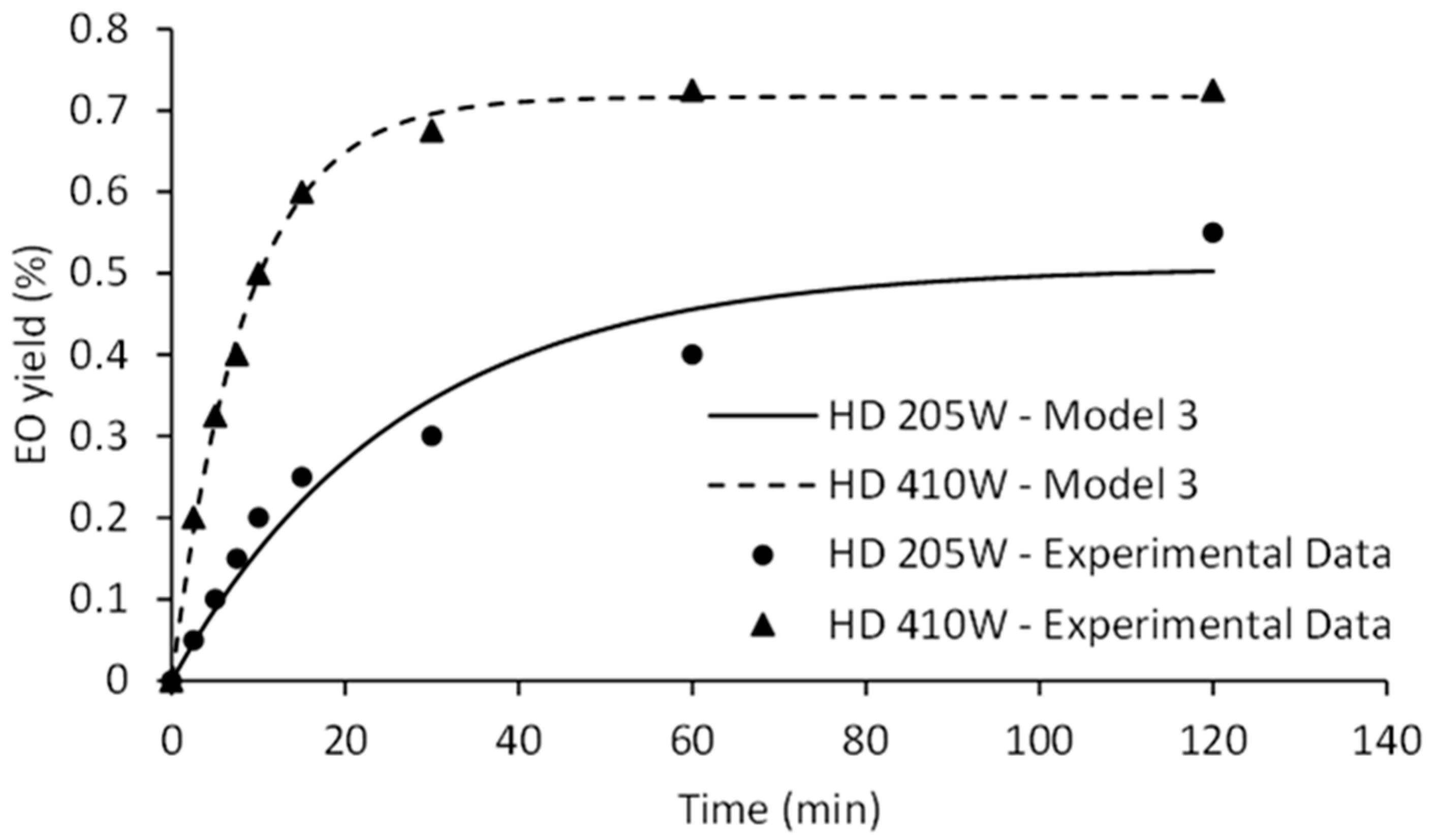
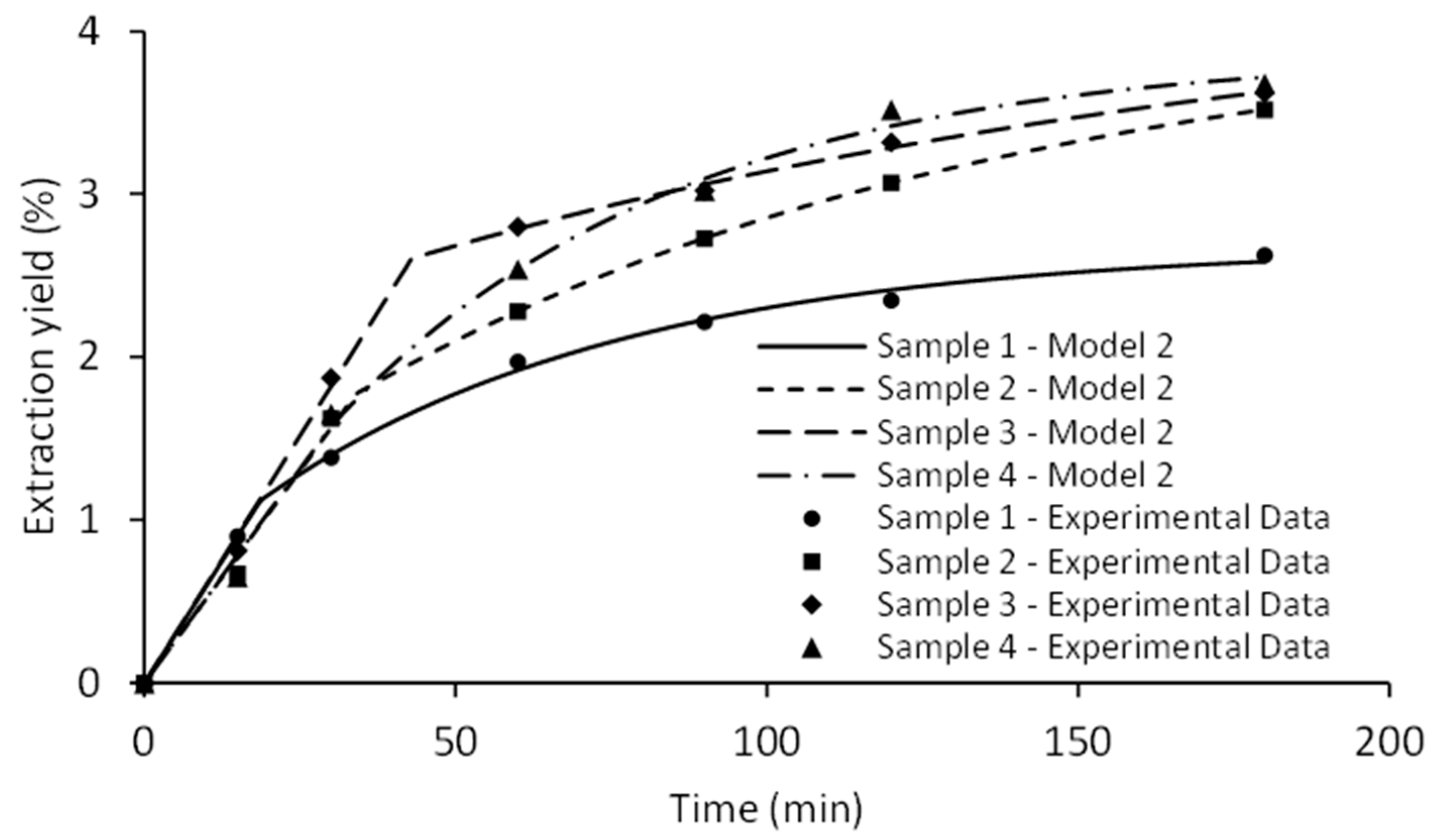
2.4. Kinetics Modeling of HD and MWHD
2.5. Kinetics Modeling of SFE
3. Materials and Methods
3.1. Plant Material and Chemicals
3.2. Isolation of Essential Oil
3.2.1. Conventional Hydrodistillation
3.2.2. Microwave-Assisted Hydrodistillation
3.3. Isolation of Lipophilic Compounds
3.3.1. Soxhlet Extraction
3.3.2. Ultrasound-Assisted Extraction
3.3.3. Microwave-Assisted Extraction
3.3.4. Supercritical Fluid Extraction
3.3.5. EO and Total Extraction Yield
3.4. Kinetics Modeling
3.4.1. Distillation Kinetics
- -
- water–plant material mixture in the distillation flask is perfectly mixed;
- -
- the EO is considered as a single component;
- -
- plant material particles are considered as isotropic, equal in size, shape, and initial EO content;
- -
- the effective coefficient of diffusion through plant particles is constant;
- -
- resistance of the EO mass transfer from the external surfaces of the plant particles could be neglected;
- -
- the EO and hydrolate are completely immiscible;
- -
- a fraction of the EO is located at the external surfaces of the plant particles (ƒ), and the rest is uniformly distributed in the plant particles (1 − ƒ);
- -
- the isolation of EO occurs via two simultaneous mechanisms: (a) “washing” of the EO from the external surfaces of the plant particles and (b) the diffusion of EO from the interior of the plant particles towards their external surfaces.
3.4.2. SFE Kinetics Modeling
3.5. Environmental Impact of EO Distillation
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pushpangadan, P.; Tewari, S. Peppermint. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2006; pp. 460–481. [Google Scholar]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Mint Essential Oil Market Size Worth $330.02 Million by 2025|CAGR: 9.2%: Grand View Research, Inc. Available online: https://www.prnewswire.com/news-releases/mint-essential-oil-market-size-worth-330-02-million-by-2025--cagr-9-2-grand-view-research-inc-300971922.html#:~:text=SAN%20FRANCISCO%2C%20Dec.,reach%2066.38%20kilotons%20by%202025 (accessed on 20 February 2021).
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction-An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- García-Ayuso, L.E.; Velasco, J.; Dobarganes, M.C.; De Castro, M.D.L. Determination of the oil content of seeds by focused microwave-assisted soxhlet extraction. Chromatographia 2000, 52, 103–108. [Google Scholar] [CrossRef]
- Mohamed, R.S.; Mansoori, G.A. The Use of Supercritical Fluid Extraction Technology in Food Processing. Food Technol. Mag. 2004, 20, 134–139. [Google Scholar]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Eur. Pharmacopoeia 2014.
- Radivojac, A.; Bera, O.; Micić, D.; Đurović, S.; Zeković, Z.; Blagojević, S.; Pavlić, B. Conventional versus microwave-assisted hydrodistillation of sage herbal dust: Kinetics modeling and physico-chemical properties of essential oil. Food Bioprod. Process. 2020, 123, 90–101. [Google Scholar] [CrossRef]
- Zeković, Z.; Vladić, J.; Vidović, S.; Adamović, D.; Pavlić, B. Optimization of microwave-assisted extraction (MAE) of coriander phenolic antioxidants—Response surface methodology approach. J. Sci. Food Agric. 2016, 96, 4613–4622. [Google Scholar] [CrossRef] [PubMed]
- Pekić, B.; Zeković, Z.; Petrović, L.; Tolić, A. Behavior of (–)-α-Bisabolol and (–)-α-Bisabololoxides A and B in Camomile Flower Extraction with Supercritical Carbon Dioxide. Sep. Sci. Technol. 1995, 30, 3567–3576. [Google Scholar] [CrossRef]
- Milojević, S.Ž.; Radosavljević, D.B.; Pavićević, V.P.; Pejanović, S.; Veljković, V.B. Modeling the kinetics of essential oil hydro-distillation from plant materials [Modelovanje kinetike hidrodestilacije etarskog ulja iz biljnih materijala]. Hem. Ind. 2013, 67, 843–859. [Google Scholar] [CrossRef] [Green Version]
- Brunner, G. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Springer: Berlin, Germany, 2013. [Google Scholar]
- Sovová, H. Steps of supercritical fluid extraction of natural products and their characteristic times. J. Supercrit. Fluids 2012, 66, 73–79. [Google Scholar] [CrossRef]
- Drinić, Z.; Pljevljakušić, D.; Živković, J.; Bigović, D.; Šavikin, K. Microwave-assisted extraction of O. vulgare L. spp. hirtum essential oil: Comparison with conventional hydro-distillation. Food Bioprod. Process. 2020, 120, 158–165. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Smadja, J.; Chemat, F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J. Chromatogr. A 2006, 1112, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Zhao, S.; Kwok, K.-C.; Liang, H. Investigation on ultrasound assisted extraction of saikosaponins from Radix Bupleuri. Sep. Purif. Technol. 2007, 55, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Simándi, B. Effects of particle size distribution, moisture content, and initial oil content on the supercritical fluid extraction of paprika. J. Supercrit. Fluids 2008, 46, 293–298. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Rezvanpanah, S.; Rezaei, K.; Razavi, S.H.; Moini, S. Use of Microwave-assisted Hydrodistillation to Extract the Essential Oils from Satureja hortensis and Satureja montana. Food Sci. Technol. Res. 2008, 14, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing; Wiley-Vch: Weinheim, Germany, 2002. [Google Scholar]
- Flannigan, D.J.; Suslick, K.S. Inertially confined plasma in an imploding bubble. Nat. Phys. 2010, 6, 598–601. [Google Scholar] [CrossRef] [Green Version]
- Zaidul, I.; Norulaini, N.N.; Omar, A.M.; Sato, Y.; Smith, R. Separation of palm kernel oil from palm kernel with supercritical carbon dioxide using pressure swing technique. J. Food Eng. 2007, 81, 419–428. [Google Scholar] [CrossRef]
- Cassel, E.; Vargas, R.; Martinez, N.; Lorenzo, D.; Dellacassa, E. Steam distillation modeling for essential oil extraction process. Ind. Crop. Prod. 2009, 29, 171–176. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Mahfud, M. Response surface methodology for optimization studies of microwave-assisted extraction of san-dalwood oil. J. Mater. Environ. Sci. 2016, 7, 1958–1971. [Google Scholar]
- Sodeifian, G.; Sajadian, S.A.; Ardestani, N.S. Optimization of essential oil extraction from Launaea acanthodes Boiss: Utilization of supercritical carbon dioxide and cosolvent. J. Supercrit. Fluids 2016, 116, 46–56. [Google Scholar] [CrossRef]
- Pavlić, B.; Bera, O.; Vidović, S.; Ilić, L.; Zeković, Z. Extraction kinetics and ANN simulation of supercritical fluid extraction of sage herbal dust. J. Supercrit. Fluids 2017, 130, 327–336. [Google Scholar] [CrossRef]
- Bojanić, N.; Teslić, N.; Rakić, D.; Brdar, M.; Fišteš, A.; Zeković, Z.; Bodroža-Solarov, M.; Pavlić, B. Extraction kinetics modeling of wheat germ oil supercritical fluid extraction. J. Food Process. Preserv. 2019, 43, e14098. [Google Scholar] [CrossRef]
- Pavlić, B.; Pezo, L.; Marić, B.; Tukuljac, L.P.; Zeković, Z.; Solarov, M.B.; Teslić, N. Supercritical fluid extraction of raspberry seed oil: Experiments and modelling. J. Supercrit. Fluids 2020, 157, 104687. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical Fluid Extraction of Bioactive Compounds: Fundamentals, Applications and Economic Perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Albuquerque, C.L.; Meireles, M.A.A. Supercritical CO2 extraction of cupuassu butter from defatted seed residue: Experimental data, mathematical modeling and cost of manufacturing. Food Bioprod. Process. 2016, 97, 48–62. [Google Scholar] [CrossRef]



| Run | Model I | Model II | Model III | Model IV | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | AARD (%) | R2 | AARD (%) | R2 | AARD (%) | R2 | AARD (%) | |
| HD | ||||||||
| 205 W | 0.996 | 7.71 | 0.964 | 13.82 | 0.961 | 13.21 | 0.980 | 8.23 |
| 410 W | 0.997 | 2.88 | 0.997 | 2.64 | 0.997 | 2.88 | 0.990 | 5.14 |
| MWHD | ||||||||
| 90 W | 0.932 | 20.15 | 0.928 | 23.10 | 0.928 | 22.41 | 0.929 | 21.57 |
| 180 W | 0.975 | 6.81 | 0.969 | 7.03 | 0.969 | 7.55 | 0.937 | 12.72 |
| 360 W | 0.994 | 3.69 | 0.994 | 3.58 | 0.994 | 3.69 | 0.986 | 5.48 |
| 600 W | 0.995 | 3.96 | 0.995 | 3.75 | 0.995 | 3.96 | 0.981 | 7.62 |
| 800 W | 0.980 | 5.68 | 0.980 | 5.21 | 0.980 | 5.68 | 0.998 | 1.75 |
| Mean | 0.981 | 7.27 | 0.975 | 8.45 | 0.975 | 8.48 | 0.971 | 8.93 |
| Run | Experiment | Model I | Model II | Model III | Model IV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| q∞ | q∞ | kd1 | k1 | ƒ | q∞ | kd1 | ƒ | q∞ | kd1 | q∞ | k2 | |
| HD | ||||||||||||
| 205 W | 0.55 | 1.99 | 0.0016 | 0.1086 | 0.880 | 0.53 | 0.0297 | 0.060 | 0.51 | 0.0379 | 0.63 | 0.0603 |
| 410 W | 0.73 | 0.72 | 0.1175 | 0.1175 | −0.275 | 0.72 | 0.1156 | 0.011 | 0.72 | 0.1175 | 0.80 | 0.1884 |
| MWHD | ||||||||||||
| 90 W | 0.15 | 0.33 | 0.0043 | 0.0574 | 0.906 | 0.18 | 0.0140 | −0.008 | 0.19 | 0.0132 | 0.28 | 0.0332 |
| 180 W | 0.20 | 0.20 | 0.0664 | 0.0741 | −6.033 | 0.20 | 0.1286 | −0.022 | 0.21 | 0.1252 | 0.23 | 0.7161 |
| 360 W | 0.65 | 0.64 | 0.1366 | 0.1366 | −0.001 | 0.64 | 0.1355 | 0.006 | 0.64 | 0.1366 | 0.71 | 0.2558 |
| 600 W | 0.75 | 0.74 | 0.1480 | 0.1499 | −0.378 | 0.74 | 0.1533 | −0.014 | 0.74 | 0.1505 | 0.82 | 0.2465 |
| 800 W | 0.80 | 0.76 | 0.2229 | 0.2229 | −1.002 | 0.77 | 0.2119 | 0.037 | 0.76 | 0.2229 | 0.83 | 0.4213 |
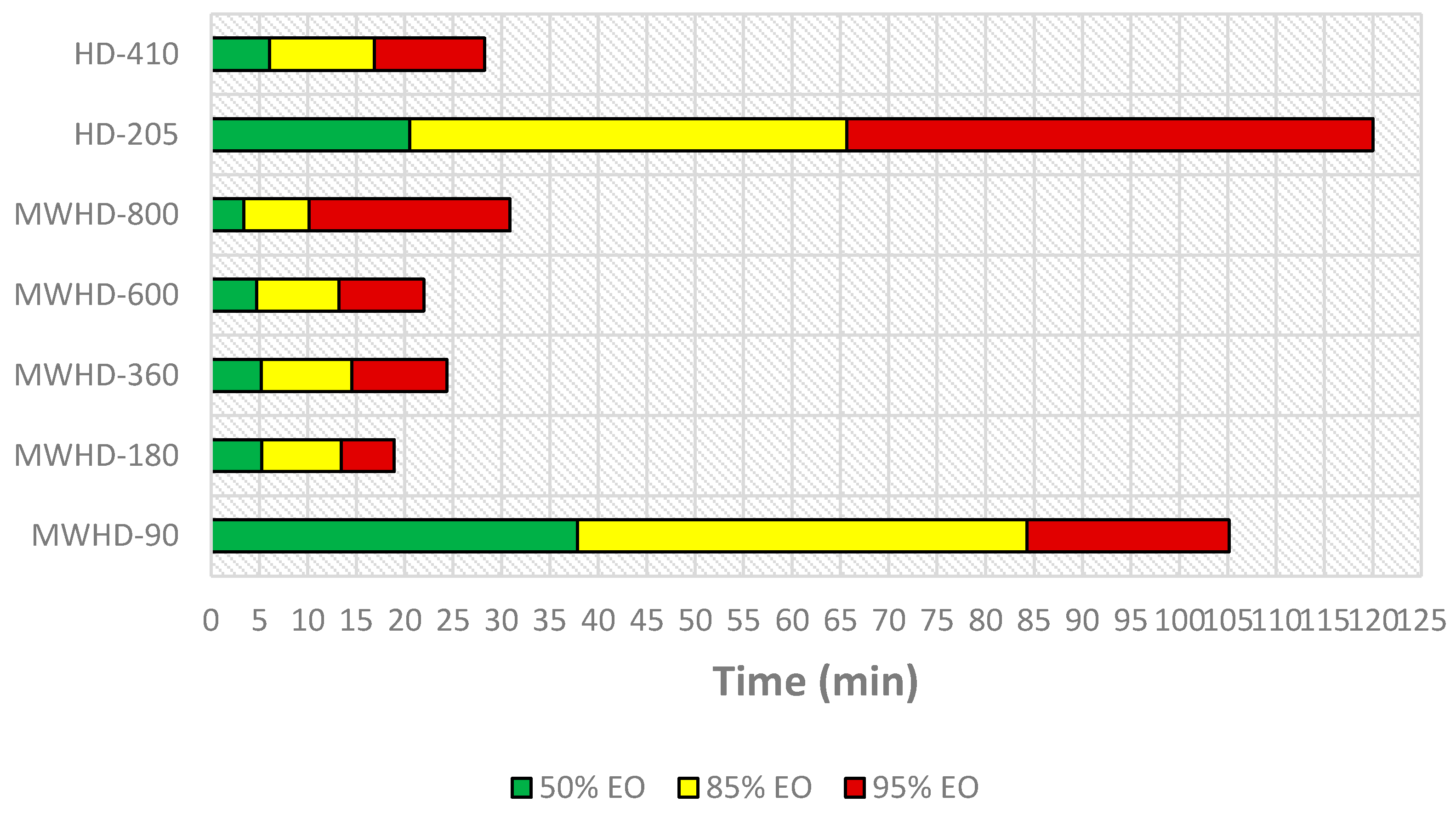
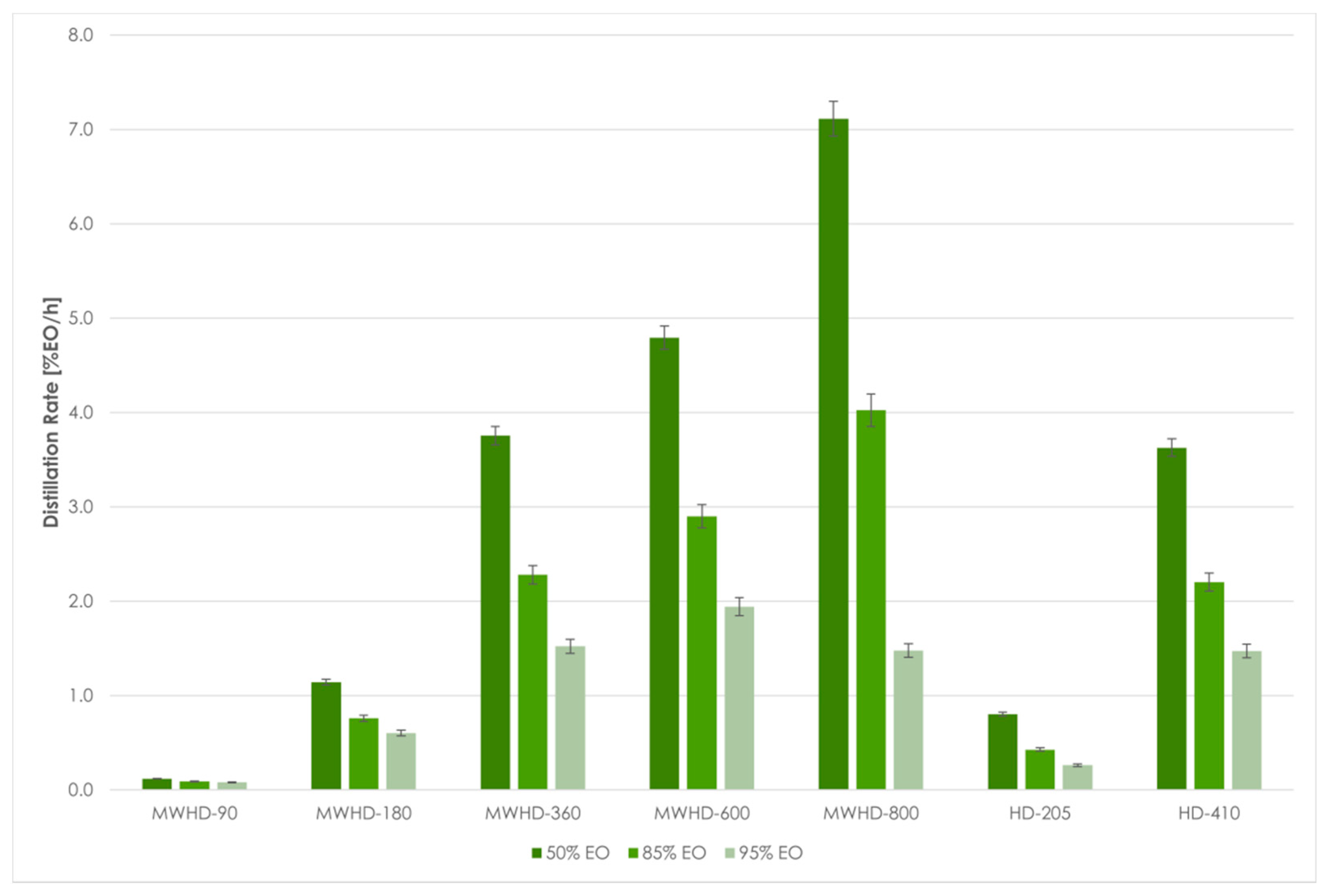
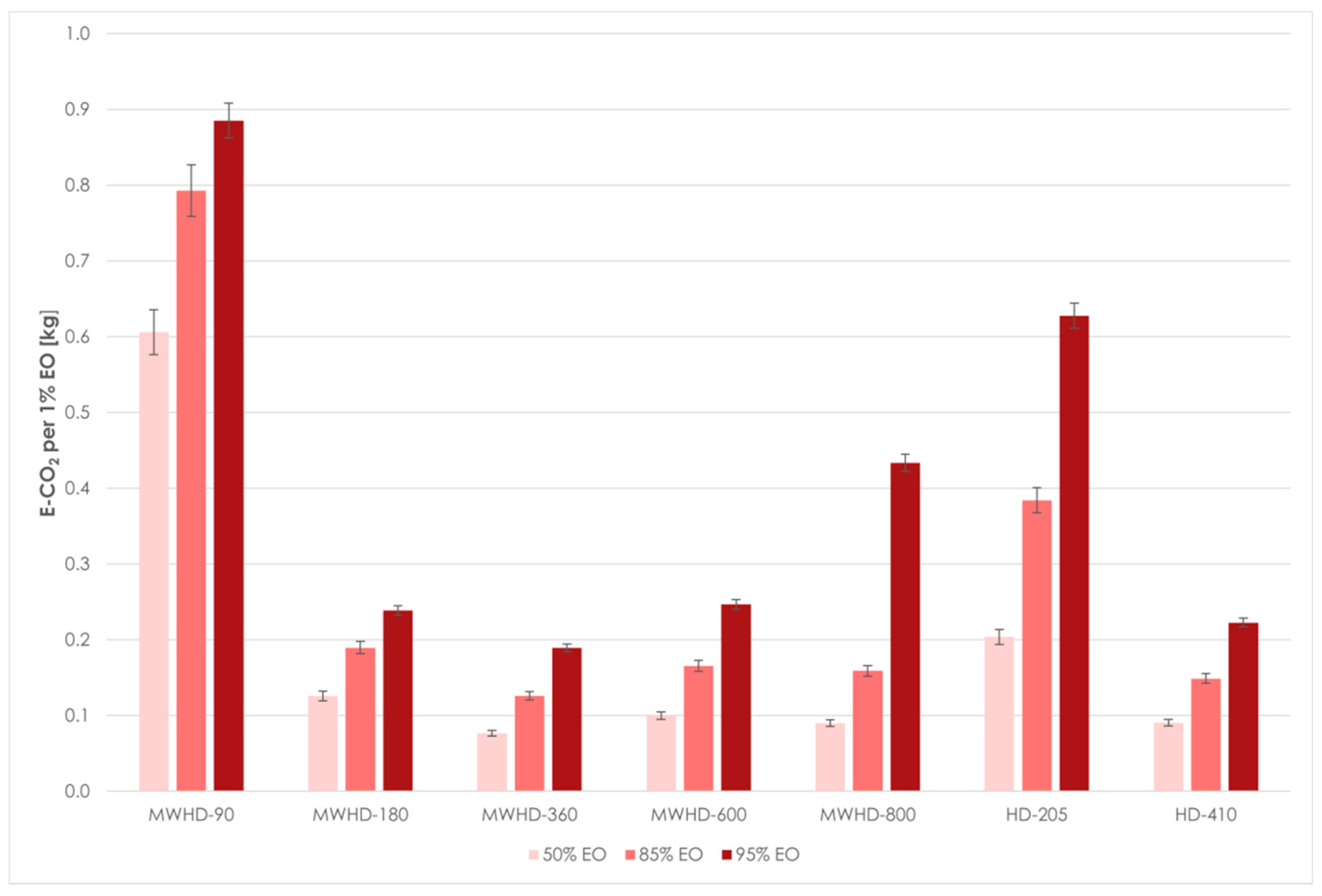
| Distillation Threshold | Run | Experiment q∞ | %EO 1 | t (min) 2 | t (h) 2 | Distillation Rate (%EO/h) | A 3 per 1% EO (kWh) | E-CO2 4 per 1% EO (kg) |
|---|---|---|---|---|---|---|---|---|
| 50% | MWHD-90 | 0.15 | 0.08 | 37.87 | 0.63 | 0.12 | 0.76 | 0.61 |
| MWHD-180 | 0.20 | 0.10 | 5.24 | 0.09 | 1.14 | 0.16 | 0.13 | |
| MWHD-360 | 0.65 | 0.33 | 5.19 | 0.09 | 3.76 | 0.10 | 0.08 | |
| MWHD-600 | 0.75 | 0.38 | 4.69 | 0.08 | 4.79 | 0.13 | 0.10 | |
| MWHD-800 | 0.80 | 0.40 | 3.37 | 0.06 | 7.11 | 0.11 | 0.09 | |
| HD-205 | 0.55 | 0.28 | 20.50 | 0.34 | 0.80 | 0.25 | 0.20 | |
| HD-410 | 0.73 | 0.37 | 6.04 | 0.10 | 3.63 | 0.11 | 0.09 | |
| 85% | MWHD-90 | 0.15 | 0.13 | 84.24 | 1.40 | 0.09 | 0.99 | 0.79 |
| MWHD-180 | 0.20 | 0.17 | 13.43 | 0.22 | 0.76 | 0.24 | 0.19 | |
| MWHD-360 | 0.65 | 0.55 | 14.53 | 0.24 | 2.28 | 0.16 | 0.13 | |
| MWHD-600 | 0.75 | 0.64 | 13.19 | 0.22 | 2.90 | 0.21 | 0.17 | |
| MWHD-800 | 0.80 | 0.68 | 10.14 | 0.17 | 4.02 | 0.20 | 0.16 | |
| HD-205 | 0.55 | 0.47 | 65.68 | 1.09 | 0.43 | 0.48 | 0.38 | |
| HD-410 | 0.73 | 0.62 | 16.89 | 0.28 | 2.20 | 0.19 | 0.15 | |
| 95% | MWHD-90 | 0.15 | 0.14 | 105.15 | 1.75 | 0.08 | 1.11 | 0.89 |
| MWHD-180 | 0.20 | 0.19 | 18.92 | 0.32 | 0.60 | 0.30 | 0.24 | |
| MWHD-360 | 0.65 | 0.62 | 24.34 | 0.41 | 1.52 | 0.24 | 0.19 | |
| MWHD-600 | 0.75 | 0.71 | 21.99 | 0.37 | 1.94 | 0.31 | 0.25 | |
| MWHD-800 | 0.80 | 0.76 | 30.88 | 0.51 | 1.48 | 0.54 | 0.43 | |
| HD-205 | 0.55 | 0.52 | 120.00 | 2.00 | 0.26 | 0.78 | 0.63 | |
| HD-410 | 0.73 | 0.69 | 28.26 | 0.47 | 1.47 | 0.28 | 0.22 |
| Sample | Model I | Model II | ||
|---|---|---|---|---|
| R2 | AARD (%) | R2 | AARD (%) | |
| SFE-100 | 0.995 | 3.68 | 0.998 | 1.36 |
| SFE-200 | 0.993 | 6.47 | 0.999 | 3.36 |
| SFE-300 | 0.992 | 7.33 | 0.999 | 3.01 |
| SFE-400 | 0.994 | 8.08 | 0.997 | 5.37 |
| Mean | 0.993 | 6.39 | 0.998 | 3.28 |
| Sample | Experiment | Model I | Model II | |||||
|---|---|---|---|---|---|---|---|---|
| Y∞ (%) | Y∞ (%) | k (min−1) | Y∞ (%) | G | Km | t1 (min) | ti (min) | |
| SFE-100 | 2.62 | 2.53 | 0.0258 | 2.68 | 0.4176 | 0.2696 | 18.66 | 57.71 |
| SFE-200 | 3.52 | 3.63 | 0.0166 | 4.12 | 0.4309 | 0.1526 | 34.02 | 106.68 |
| SFE-300 | 3.62 | 3.64 | 0.0220 | 4.37 | 0.5971 | 0.1674 | 42.97 | 157.96 |
| SFE-400 | 3.68 | 3.94 | 0.0168 | 3.87 | 0.4073 | 0.1636 | 30.00 | 55.31 |
| Mathematical Model | Equation | Reference |
|---|---|---|
| Model I | [33] | |
| Model II | [34] | |
| Model III | [34] | |
| Model IV | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radivojac, A.; Bera, O.; Zeković, Z.; Teslić, N.; Mrkonjić, Ž.; Bursać Kovačević, D.; Putnik, P.; Pavlić, B. Extraction of Peppermint Essential Oils and Lipophilic Compounds: Assessment of Process Kinetics and Environmental Impacts with Multiple Techniques. Molecules 2021, 26, 2879. https://doi.org/10.3390/molecules26102879
Radivojac A, Bera O, Zeković Z, Teslić N, Mrkonjić Ž, Bursać Kovačević D, Putnik P, Pavlić B. Extraction of Peppermint Essential Oils and Lipophilic Compounds: Assessment of Process Kinetics and Environmental Impacts with Multiple Techniques. Molecules. 2021; 26(10):2879. https://doi.org/10.3390/molecules26102879
Chicago/Turabian StyleRadivojac, Aleksandar, Oskar Bera, Zoran Zeković, Nemanja Teslić, Živan Mrkonjić, Danijela Bursać Kovačević, Predrag Putnik, and Branimir Pavlić. 2021. "Extraction of Peppermint Essential Oils and Lipophilic Compounds: Assessment of Process Kinetics and Environmental Impacts with Multiple Techniques" Molecules 26, no. 10: 2879. https://doi.org/10.3390/molecules26102879
APA StyleRadivojac, A., Bera, O., Zeković, Z., Teslić, N., Mrkonjić, Ž., Bursać Kovačević, D., Putnik, P., & Pavlić, B. (2021). Extraction of Peppermint Essential Oils and Lipophilic Compounds: Assessment of Process Kinetics and Environmental Impacts with Multiple Techniques. Molecules, 26(10), 2879. https://doi.org/10.3390/molecules26102879









