Metal Doped PVA Films for Opto-Electronics-Optical and Electronic Properties, an Overview
Abstract
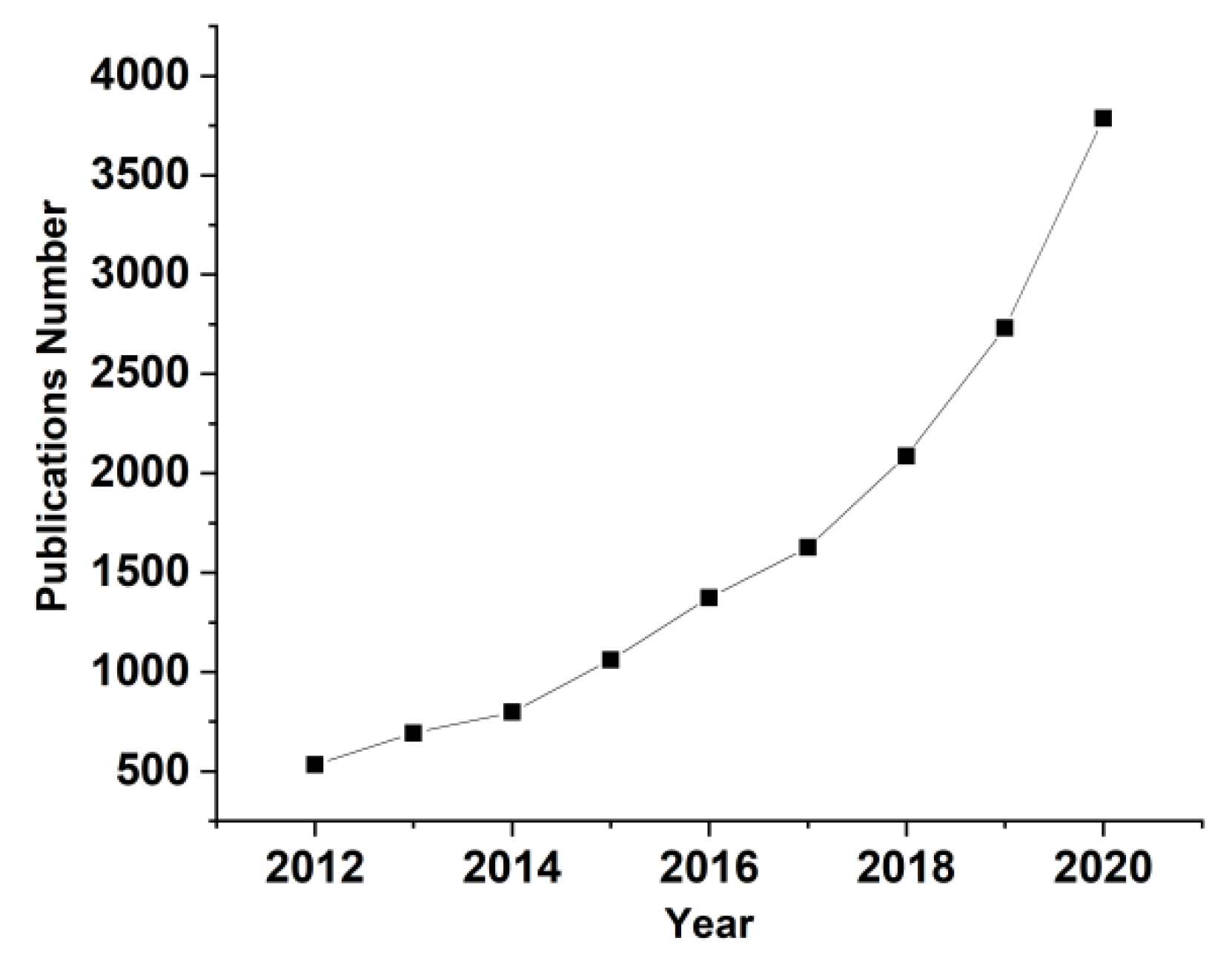
:1. Introduction
2. PVA-Synthesis and Properties
2.1. PVA-Applications
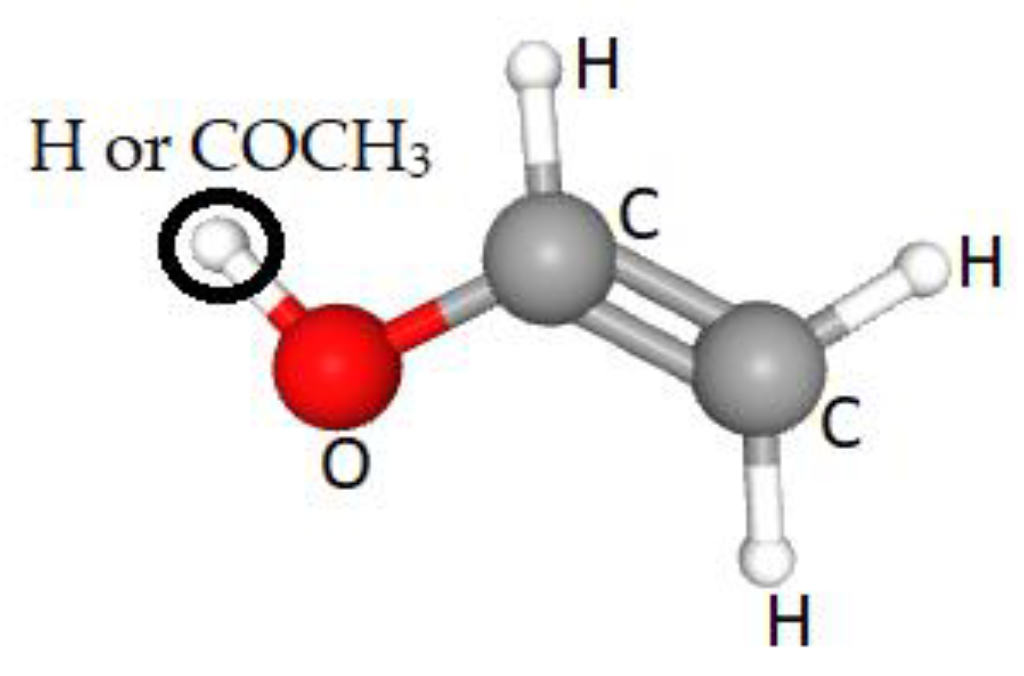
2.2. PVA Films-Synthesis and Tailoring Properties
2.3. Preparation of Doped PVA Films
3. Tuning the Optical and Electronic Properties of Doped PVA Films
4. Conclusions
Funding
Conflicts of Interest
References
- Bhagyaraj, S.; Oluwafemi, O.S.; Krupa, I. Polymers in Optics. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 423–455. [Google Scholar]
- Beckers, M.; Schlüter, T.; Vad, T.; Gries, T.; Bunge, C.A. Fabrication Techniques for Polymer Optical Fibres; Woodhead Publishing: Cambridge, UK, 2017; pp. 187–199. [Google Scholar]
- Loste, J.; Lopez-Cuesta, J.-M.; Billon, L.; Garay, H.; Save, M. Transparent Polymer Nanocomposites: An Overview on Their Synthesis and Advanced Properties. Prog. Polym. Sci. 2019, 89, 133–158. [Google Scholar] [CrossRef]
- Fréchet, J.M.J. Functional Polymers: From Plastic Electronics to Polymer-Assisted Therapeutics. Prog. Polym. Sci. 2005, 30, 844–857. [Google Scholar] [CrossRef]
- Alberto, N.; Domingues, M.; Marques, C.; André, P.; Antunes, P. Optical Fiber Magnetic Field Sensors Based on Magnetic Fluid: A Review. Sensors 2018, 18, 4325. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl Alcohol: A Review of Research Status and Use of Polyvinyl Alcohol Based Nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Akhtar, M.; Kadhum, A.; Mohamad, A.; Al-Amiery, A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. Biopolymer Composites with High Dielectric Performance: Interface Engineering. Biopolym. Compos. Electron. 2017, 27–128. [Google Scholar] [CrossRef]
- Tanio, N.; Koike, Y. What Is the Most Transparent Polymer? Polym. J. 2000, 32, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Ponassi, V.; Martorana, V. Tailoring of Physical and Chemical Properties of Macro- and Microhydrogels Based on Telechelic PVA. Biomacromolecules 2002, 3, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Plapcianu, C.; Filoti, G. Optical and electronic properties of (Fe+Sb):PVA, for real time holography. J. Optoelectron. Adv. Mater. 2006, 8, 1225–1229. [Google Scholar]
- Bulinski, M.; Kuncser, V.; Iova, I.; Bela, A.; Franke, H.; Russo, U.; Filoti, G. Mixed-Valence Ion-Doped PVA As Potential Materials for Real-Time Holography. In Proceedings of the Sixth Symposium on Optoelectronics, Bucharest, Romania, 22–24 September 1999; pp. 17–25, Publisher SPIE–4068-03 (2000). [Google Scholar]
- Ta, V.D.; Nguyen, T.; Pham, Q.; Nguyen, T. Biocompatible Microlasers Based on Polyvinyl Alcohol Microspheres. Opt. Commun. 2020, 459, 124925. [Google Scholar] [CrossRef]
- Adamow, A.; Szukalski, A.; Sznitko, L.; Persano, L.; Pisignano, D.; Camposeo, A.; Mysliwiec, J. Electrically Controlled White Laser Emission through Liquid Crystal/Polymer Multiphases. Light Sci. Appl. 2020, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Koutavarapu, R.; Manepalli, R.K.N.R.; Madhav, B.T.P.; Satyanarayana, T.; Nagarjuna, G.; Shim, J.; Rao, M.C. Optical, Electrical and Photoluminescence Studies on Al2O3 Doped PVA Capped ZnO Nanoparticles for Optoelectronic Device Application. Optik 2020, 205, 164236. [Google Scholar] [CrossRef]
- Seto, M.; Maeda, Y.; Matsuyama, T.; Yamaoka, H.; Sakai, H. Light Polarization in Iodine-Doped Polyvinyl Alcohol Films. Hyperfine Interact. 1992, 68, 221–224. [Google Scholar] [CrossRef]
- Sata, R.; Suzuki, H.; Ueno, N.; Morisawa, Y.; Hatanaka, M.; Wakabayashia, T. UV-Polarizing Linear Polyyne Molecules Aligned in PVA. Chin. J. Chem. Phys. 2019, 32, 175–181. [Google Scholar] [CrossRef]
- Ghoshal, D.; Bhattacharya, D.; Mondal, D.; Das, S.; Bose, N.; Basu, M. Methylene Blue/PVA Composite Film for Flexible, Wide-Scale UV–VIS Laser Cut-off Filter. Mater. Res. Express 2019, 6, 075332. [Google Scholar] [CrossRef]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Use of Polymers in Controlled Release of Active Agents. In Basic Fundamentals of Drug Delivery; Tekade, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 113–172. [Google Scholar]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.-X. Polymer Properties and Characterization: Developing Solid Oral Dosage Forms, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 181–223. [Google Scholar]
- Amann, M.; Minge, O. Biodegradability of Poly(Vinyl Acetate) and Related Polymers. In Synthetic Biodegradable Polymers; Rieger, B., Künkel, A., Coates, G.W., Reichard, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 137–172. [Google Scholar]
- Lewis, R.J., Sr. (Ed.) Sax’s Dangerous Properties of Industrial Materials, 12th ed.; Wiley-Interscience, Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. 3710. [Google Scholar]
- Shie, J.-L.; Chen, Y.-H.; Chang, C.-Y.; Lin, J.-P.; Lee, D.-J.; Wu, C.-H. Thermal Pyrolysis of Poly(Vinyl Alcohol) and Its Major Products. Energy Fuels 2002, 16, 109–118. [Google Scholar] [CrossRef]
- Rudnik, E. Properties and applications. In Compostable Polymer Materials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 49–98. [Google Scholar]
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.M.; Peijs, T. Transparent Semi-Crystalline Polymeric Materials and Their Nanocomposites: A Review. Polym. Eng. Sci. 2020, 60, 2351–2376. [Google Scholar] [CrossRef]
- Bodurov, I.; Vlaeva, I.; Viraneva, A.; Yovcheva, T.; Sainov, S. Modified design of a laser refractometer. Int. J. Biomed. Nanosci. Nanotechnol. 2016, 16, 31–33. [Google Scholar]
- Liu, X.; Chen, Q.; Lv, L.; Feng, X.; Meng, X. Preparation of Transparent PVA/TiO2 Nanocomposite Films with Enhanced Visible-Light Photocatalytic Activity. Catal. Commun. 2015, 58, 30–33. [Google Scholar] [CrossRef]
- Satoh, K. Poly(Vinyl Alcohol) (PVA). In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–6. [Google Scholar]
- Vandervoort, J.; Ludwig, A. Biocompatible Stabilizers in the Preparation of PLGA Nanoparticles: A Factorial Design Study. Int. J. Pharm. 2002, 238, 77–92. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S. Crosslinked Poly(Vinyl Alcohol) Hydrogels for Wound Dressing Applications: A Review of Remarkably Blended Polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hassan, C.M.; Peppas, N.A. Structure and Applications of Poly(Vinyl Alcohol) Hydrogels Produced by Conventional Crosslinking or by Freezing/Thawing Methods. Biopolymers · PVA Hydrogels, Anionic Polymerisation Nanocomposites. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar]
- Mukherjee, P.; Rani, A.; Saravanan, P. Polymeric Materials for 3D Bioprinting. 3D Print. Technol. Nanomed. 2019, 63–81. [Google Scholar] [CrossRef]
- Muppalaneni, S.; Omidian, H. Polyvinyl Alcohol in Medicine and Pharmacy: A Perspective. J. Dev. Drugs 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Uthaman, S.; Muthiah, M.; Park, I.-K.; Cho, C.-S. Fabrication and Development of Magnetic Particles for Gene Therapy. Polym. Nanomater. Gene Ther. 2016, 215–230. [Google Scholar] [CrossRef]
- Hallensleben, M.L. Polyvinyl Compounds, Others. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Ibrahim, M.S.; El-Wassefy, N.A.; Farahat, D.S. Biocompatibility of Dental Biomaterials. Biomater. Oral Dent. Tissue Eng. 2017, 117–140. [Google Scholar] [CrossRef]
- Raghul, S.S.; Bhat, S.G.; Chandrasekaran, M.; Francis, V.; Thachil, E.T. Biodegradation of Polyvinyl Alcohol-Low Linear Density Polyethylene-Blended Plastic Film by Consortium of Marine Benthic Vibrios. Int. J. Environ. Sci. Technol. 2013, 11, 1827–1834. [Google Scholar] [CrossRef] [Green Version]
- Aziz, S.B.; Hamsan, M.H.; Karim, W.O.; Marif, A.S.; Abdulwahid, R.T.; Kadir, M.F.Z.; Brza, M.A. Study of Impedance and Solid-State Double-Layer Capacitor Behavior of Proton (H+)-Conducting Polymer Blend Electrolyte-Based CS:PS Polymers. Ionics 2020, 26, 4635–4649. [Google Scholar] [CrossRef]
- Aziz, S.B. Modifying Poly(Vinyl Alcohol) (PVA) from Insulator to Small-Bandgap Polymer: A Novel Approach for Organic Solar Cells and Optoelectronic Devices. J. Electron. Mater. 2015, 45, 736–745. [Google Scholar] [CrossRef]
- Lampman, S. Characterization and Failure Analysis of Plastics; ASM International: Novelty, OH, USA, 2003; p. 29. [Google Scholar]
- Ahmed, K.; Kanwal, F.; Ramay, S.M.; Atiq, S.; Khan, A.; Mahmood, A. Study of the effect of PVA on dielectric constant and structure of TiO2/polypyrrole composites prepared by in-situ polymerization. Dig. J. Nanomater. Bios. 2017, 12, 775–783. [Google Scholar]
- Abdulwahid, R.T.; Abdullah, O.G.; Aziz, S.B.; Hussein, S.A.; Muhammad, F.F.; Yahya, M.Y. The Study of Structural and Optical Properties of PVA:PbO2 Based Solid Polymer Nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 12112–12118. [Google Scholar] [CrossRef]
- Suh, J.H.; Shin, J.W.; Kim, H.K.; Kim, H.S.; Kim, Y.W.; Kang, H.J. Effect of Poly(Vinyl Alcohol) Adhesives on the Dimensional Stability of LCD Polarizer. Polym. Korea 2010, 34, 560–564. [Google Scholar] [CrossRef]
- Jain, N.; Singh, V.K.; Chauhan, S. A Review on Mechanical and Water Absorption Properties of Polyvinyl Alcohol Based Composites/Films. J. Mech. Behav. Mater. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the Degree of Crystallinity of Poly(Vinyl Alcohol) by FTIR Spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Su, Y.; Wu, Y.; Liu, M.; Qing, Y.; Zhou, J.; Wu, Y. Ferric Ions Modified Polyvinyl Alcohol for Enhanced Molecular Structure and Mechanical Performance. Materials 2020, 13, 1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhosiny, H.; Khairy, Y. Microstructure and optical properties of Ni2+ doped PVA for optoelectronic devices. Phys. B Condens. Matter 2019, 570, 41–47. [Google Scholar] [CrossRef]
- Aziz, S.B.; Marf, A.S.; Dannoun, E.M.A.; Brza, M.A.; Abdullah, R.M. The Study of the Degree of Crystallinity, Electrical Equivalent Circuit, and Dielectric Properties of Polyvinyl Alcohol (PVA)-Based Biopolymer Electrolytes. Polymers 2020, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Premalatha, M.; Vijaya, N.; Selvasekarapandian, S.; Selvalakshmi, S. Characterization of Blend Polymer PVA-PVP Complexed with Ammonium Thiocyanate. Ionics 2016, 22, 1299–1310. [Google Scholar] [CrossRef]
- Mondal, A.; Mandal, B. CO2 Separation Using Thermally Stable Crosslinked Poly(Vinyl Alcohol) Membrane Blended with Polyvinylpyrrolidone/Polyethyleneimine/Tetraethylenepentamine. J. Membr. Sci. 2014, 460, 126–138. [Google Scholar] [CrossRef]
- Nishino, T.; Kani, S.; Gotoh, K.; Nakamae, K. Melt Processing of Poly(Vinyl Alcohol) through Blending with Sugar Pendant Polymer. Polymer 2002, 43, 2869–2873. [Google Scholar] [CrossRef]
- Deng, L.; Hägg, M.-B. Carbon Nanotube Reinforced PVAm/PVA Blend FSC Nanocomposite Membrane for CO2/CH4 Separation. Int. J. Greenh. Gas Control 2014, 26, 127–134. [Google Scholar] [CrossRef]
- Khurana, K.; Patel, A.K.; Das, K. Refractive Indices Studies on PVA and PVP Blends. Int. J. Sci. Res. Dev. 2016, 4, 984–985. [Google Scholar]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Russo, P.; Speranza, V.; Vignali, A.; Tescione, F.; Buonocore, G.G.; Lavorgna, M. Structure and Physical Properties of High Amorphous Polyvinyl Alcohol/Clay Composites. Aip Conf. Proc. 2015, 1695, 020035. [Google Scholar]
- El Salmawi, K.M. Application of Polyvinyl Alcohol (PVA)/Carboxymethyl Cellulose (CMC) Hydrogel Produced by Conventional Crosslinking or by Freezing and Thawing. J. Macromol. Sci. Part A 2007, 44, 619–624. [Google Scholar] [CrossRef]
- Laput, O.A.; Zuza, D.A.; Vasenina, I.V.; Kurzina, I.A. Chemical State and Morphology of Zn and Mg Ion-Implanted Polyvinyl Alcohol. Surf. Coat. Technol. 2020, 389, 125558. [Google Scholar] [CrossRef]
- Shanthini, G.M.; Sakthivel, N.; Menon, R.; Nabhiraj, P.Y.; Gómez-Tejedor, J.A.; Meseguer-Dueñas, J.M.; Gómez Ribelles, J.L.; Krishna, J.B.M.; Kalkura, S.N. Surface Stiffening and Enhanced Photoluminescence of Ion Implanted Cellulose–Polyvinyl Alcohol–Silica Composite. Carbohydr. Polym. 2016, 153, 619–630. [Google Scholar] [CrossRef]
- Thomas, D.; Zhuravlev, E.; Wurm, A.; Schick, C.; Cebe, P. Fundamental Thermal Properties of Polyvinyl Alcohol by Fast Scanning Calorimetry. Polymer 2018, 137, 145–155. [Google Scholar] [CrossRef]
- Swapna, V.P.; Selvin Thomas, P.; Suresh, K.I.; Saranya, V.; Rahana, M.P.; Stephen, R. Thermal properties of poly (vinyl alcohol)(PVA)/halloysite nanotubes reinforced nanocomposites. Int. J. Plast. Technol. 2015, 19, 1–13. [Google Scholar]
- Nagarkar, R.; Patel, J. Polyvinyl Alcohol: A Comprehensive Study. Acta Sci. Pharm. Sci. 2019, 3.4, 34–44. [Google Scholar]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Gohil, J.M.; Bhattacharya, A.; Ray, P. Studies On The Crosslinking Of Poly (Vinyl Alcohol). J. Polym. Res. 2005, 13, 161–169. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, T.; Peng, L.; Dan, Y. Acrylamide Modified Poly(Vinyl Alcohol): Crystalline and Enhanced Water Solubility. RSC Adv. 2015, 5, 86598–86605. [Google Scholar] [CrossRef]
- Yokoyama, F.; Masada, I.; Shimamura, K.; Ikawa, T.; Monobe, K. Morphology and Structure of Highly Elastic Poly(Vinyl Alcohol) Hydrogel Prepared by Repeated Freezing-And-Melting. Colloid Polym. Sci. 1986, 264, 595–601. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Hyon, S.-H.; Cha, W.-I.; Ikada, Y.; Kita, M.; Ogura, Y.; Honda, Y. Poly(Vinyl Alcohol) Hydrogels as Soft Contact Lens Material. J. Biomater. Sci. Polym. Ed. 1994, 5, 397–406. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Nagano, S.; Hara, M.; Hyon, S.-H.; Patel, M.; Matsumura, K. Facile Preparation of Transparent Poly(Vinyl Alcohol) Hydrogels with Uniform Microcrystalline Structure by Hot-Pressing without Using Organic Solvents. Polym. J. 2017, 49, 535–542. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in Medical and Pharmaceutical Applications: Perspectives and Challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Yi, C.; Wang, Z.; Li, M.; Wang, J.; Wang, S. Facilitated Transport of CO2 through Polyvinylamine/Polyethlene Glycol Blend Membranes. Desalination 2006, 193, 90–96. [Google Scholar] [CrossRef]
- Ghoshal, S.; Denner, P.; Stapf, S.; Mattea, C. Study of the Formation of Poly(Vinyl Alcohol) Films. Macromolecules 2012, 45, 1913–1923. [Google Scholar] [CrossRef]
- Chan, K.S.; Senin, H.B.; Naimah, I.; Rusop, M.; Soga, T. Structural and mechanical properties of polyvinyl alcohol (PVA) thin film. Aip Conf. Proc. 2009, 1136, 366. [Google Scholar] [CrossRef]
- Nguyen, N.T. Fabrication Technologies in Micro and Nano Technologies, Micromixers, 2nd ed.; William Andrew Publishing: Norwich, NY, USA, 2012; pp. 113–161. [Google Scholar]
- Chen, X.; Hu, Y.; Xie, Z.; Wang, H. Materials and Design of Photocatalytic Membranes. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 71–96. [Google Scholar]
- Scriven, L.E. Physics and Applications of DIP Coating and Spin Coating. MRS Proc. 1988, 121, 121. [Google Scholar] [CrossRef]
- Manakasettharn, S.; Taylor, J.A.; Krupenkin, T. Superhydrophobicity at Micron and Submicron Scale. In Wiederrecht, Comprehensive Nanoscience and Technology; Academic Press: Cambridge, MA, USA, 2011; pp. 383–411. [Google Scholar]
- Ciampi, E.; McDonald, P.J. Skin Formation and Water Distribution in Semicrystalline Polymer Layers Cast from Solution: A Magnetic Resonance Imaging Study. Macromolecules 2003, 36, 8398–8405. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Yan, N.; Wang, Z.; Guo, L.; Steinhart, M.; Wang, Y. Fast Evaporation Enabled Ultrathin Polymer Coatings on Nanoporous Substrates for Highly Permeable Membranes. Innovation 2021, 2, 100088. [Google Scholar] [CrossRef]
- Xiuxiu, J.; Wang, J.; Dai, L.; Liu, X.; Li, L.; Yang, Y.Y.; Cao, Y.; Wang, W.; Wu, W.; Guo, S. Flame-retardant poly(vinyl alcohol)/MXene multilayered films with outstanding electromagnetic interference shielding and thermal conductive performances. Chem. Eng. J. 2020, 380, 122475. [Google Scholar]
- Campbell, J.H.; Grens, J.Z.; Poco, J.F.; Ives, B.H. Preparation and Properties of Polyvinyl Alcohol Microspheres; Lawrence Livermore National Laboratory, University of California: Livermore, CA, USA, 1986. [Google Scholar]
- Flaim, T.D.; Wang, Y.; Mercado, R. High-Refractive-Index Polymer Coatings for Optoelectronics Applications. Proc. SPIE 2004, 5250, 423–434. [Google Scholar]
- Phan, C.M.; Nguyen, H.M. Role of Capping Agent in Wet Synthesis of Nanoparticles. J. Phys. Chem. A 2017, 121, 3213–3219. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Basak, D. Effect of Surface Capping with Poly(Vinyl Alcohol) on the Photocarrier Relaxation of ZnO Nanowires. ACS Appl. Mater. Interfaces 2009, 1, 2066–2070. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Kamari, H.M.; Saleh, M.A.; Flaifel, M.H.; Al-Ghaili, A.M.; Kasim, H.; Baqer, A.A.; Saion, E.; Jihua, W. Morphological, Structural and Optical Behaviour of PVA Capped Binary (NiO)0.5 (Cr2O3)0.5 Nanoparticles Produced via Single Step Based Thermal Technique. Results Phys. 2020, 17, 103059. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.C.A.; Zerefa, E.; Adimasu, Y. PVA Assisted ZnO Based Mesoporous Ternary Metal Oxides Nanomaterials: Synthesis, Optimization, and Evaluation of Antibacterial Activity. Mater. Res. Express 2020, 7, 045011. [Google Scholar] [CrossRef]
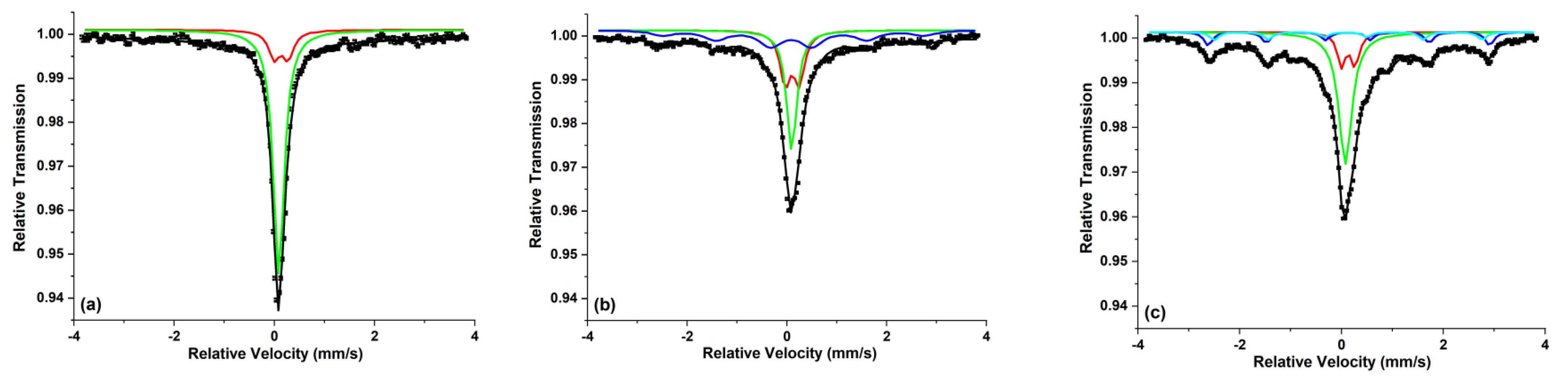
- Kuncser, V.; Filoti, G.; Podgorsek, R.; Biebricher, M.; Franke, H. The Diffraction Efficiency in Fe:PVA Explained by Mössbauer Spectroscopy. J. Phys. D Appl. Phys. 1998, 31, 2315–2318. [Google Scholar] [CrossRef]
- Bulinski, M.; Iova, I.; Belea, A.; Kuncser, V.; Filoti, G. Experimental investigation of the nonlinear optical response in Fe:PVA. J. Mater. Sci. Lett. 2000, 19, 27–28. [Google Scholar] [CrossRef]
- Hamdalla, T.A.; Hanafy, T.A.; Bekheet, A.E. Influence of Erbium Ions on the Optical and Structural Properties of Polyvinyl Alcohol. J. Spectrosc. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, O.G.; Saleem, S.A. Effect of Copper Sulfide Nanoparticles on the Optical and Electrical Behavior of Poly(Vinyl Alcohol) Films. J. Electron. Mater. 2016, 45, 5910–5920. [Google Scholar] [CrossRef]
- Kumar, D.; Jat, S.K.; Khanna, P.K.; Vijayan, N.; Banerjee, S. Synthesis, Characterization, and Studies of PVA/Co-Doped ZnO Nanocomposite Films. Int. J. Green Nanotechnol. 2012, 4, 408–416. [Google Scholar] [CrossRef]
- Hemalatha, K.S.; Sriprakash, G.; Ambika Prasad, M.V.N.; Damle, R.; Rukmani, K. Temperature Dependent Dielectric and Conductivity Studies of Polyvinyl Alcohol-ZnO Nanocomposite Films by Impedance Spectroscopy. J. Appl. Phys. 2015, 118, 154103. [Google Scholar] [CrossRef]
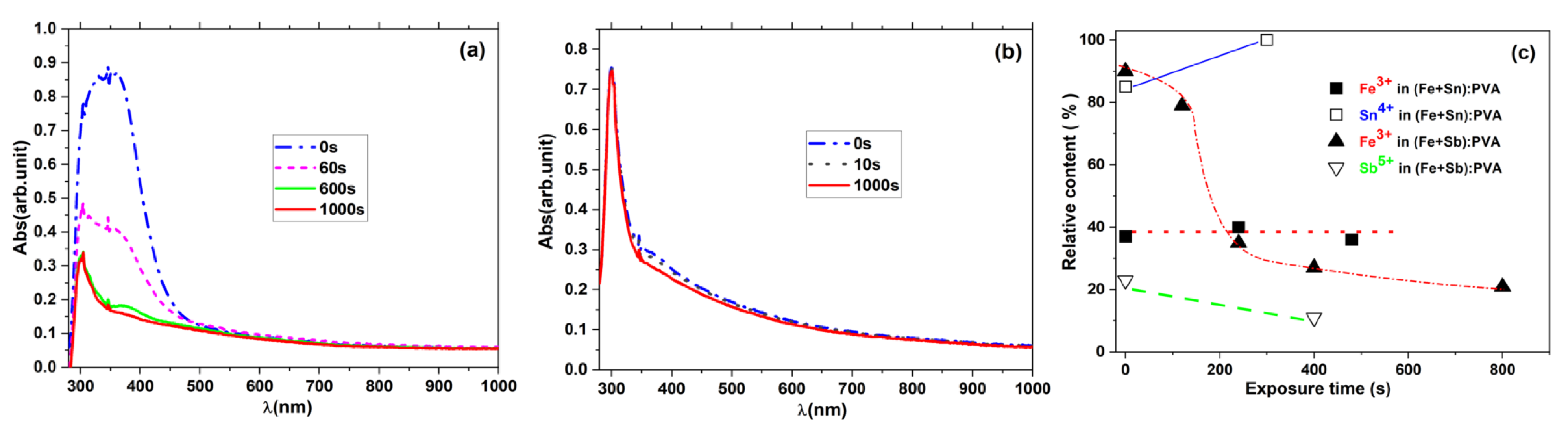
- Obreja, P.; Cristea, D.; Budianu, E.; Rebigan, R.; Kuncser, V.; Bulinski, M.; Filoti, G. Effect of Dopant on the Physical Properties of Polymer Films for Microphotonics. Prog. Solid State Chem. 2006, 34, 103–109. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Effect of Separate Zinc, Copper and Graphene Oxides Nanofillers on Electrical Properties of PVA Based Composite Strips. J. Electron. Mater. 2018, 48, 1116–1121. [Google Scholar] [CrossRef]
- Hemalatha, K.S.; Rukmani, K. Synthesis, Characterization and Optical Properties of Polyvinyl Alcohol–Cerium Oxide Nanocomposite Films. RSC Adv. 2016, 6, 74354–74366. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.E.; Algarni, H.; Khairy, Y. Influence of Cobalt-Metal Concentration on the Microstructure and Optical Limiting Properties of PVA. Opt. Mater. 2020, 108, 110212. [Google Scholar] [CrossRef]
- Rahma, M.A.; Saadon, H.L.; Mahdi, M.A. High-Performance All-Optical Limiting Based on Nonlinear Refraction of Metal-Doped PbS/PVA Freestanding Nanocomposite Films. Optik 2018, 174, 580–590. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Huang, W.; Hu, L.; Tang, Y.; Liu, J.; Fu, S.; Wang, B. MXene-PVA Thin Film for Efficient All-Optical Modulator and All-Optical Signal Processing with High Performances. J. Phys. Photonics 2020, 2, 045004. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Massaro, M.; Piscopiello, E.; Tapfer, L. Metal Ion Implantation in Inert Polymers for Strain Gauge Applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. 2010, 268, 2878–2882. [Google Scholar] [CrossRef]
- Kulish, J.R.; Franke, H.; Singh, A.; Lessard, R.A.; Knystautas, E.J. Ion Implantation, a Method for Fabricating Light Guides in Polymers. J. Appl. Phys. 1988, 63, 2517–2521. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Q.; Li, L. Morphology and Properties of Highly Talc- and CaCO3-Filled Poly(Vinyl Alcohol) Composites Prepared by Melt Processing. J. Appl. Polym. Sci. 2013, 130, 3050–3057. [Google Scholar] [CrossRef]
- Cotton, S.A. Iron(III) Chloride and Its Coordination Chemistry. J. Coord. Chem. 2018, 71, 3415–3443. [Google Scholar] [CrossRef]
- Kuncser, V.; Avramescu, A.; Filoti, G.; Rotaru, P.; Podgorsek, R.; Biebricher, M.; Franke, H. Optical and Mössbauer Study of the Real Time Holographic Organometallic Material Fe:PVA. J. Alloys Compd. 1997, 256, 269–275. [Google Scholar] [CrossRef]
- Cariati, E.; Roberto, D.; Ugo, R.; Lucenti, E. The Surface of Inorganic Oxides or Zeolites as a Nonconventional Reaction Medium for the Selective Synthesis of Metal Carbonyl Complexes and Clusters. Chem. Rev. 2003, 103, 3707–3732. [Google Scholar] [CrossRef]
- Schmid, G.; Fenske, D. Metal Clusters and Nanoparticles. Philos. Trans. R. Soc. A 2010, 368, 1207–1210. [Google Scholar] [CrossRef]
- Abdelaziz, M.; Ghannam, M.M. Influence of Titanium Chloride Addition on the Optical and Dielectric Properties of PVA Films. Phys. B Condens. Matter 2010, 405, 958–964. [Google Scholar] [CrossRef]
- Bulinski, M.; Kuncser, V.; Plapcianu, C.; Cristea, D.; Elena, M.; Obreja, P.; Krautwald, S.; Franke, H.; Wagner, F.E.; Filoti, G. Mixed valence compounds for integrated optics. In Proceedings of the International Semiconductor Conference, Sinaia, Romania, 8–12 October 2002. [Google Scholar]
- Ali, H.E.H.; Khairy, Y.; Yahia, I.S.; Nasrallah, D.A. Vanadium Chloride Impregnated Polyvinyl Alcohol Composite as Efficient Linear, Non-Linear, and Limiting Optical Applications: Microstructure, Electrical, and Optical Properties. Phys. Solid State 2021, 63, 165–182. [Google Scholar] [CrossRef]
- Bhajantri, R.F.; Ravindrachary, V.; Harisha, A.; Ranganathaiah, C.; Kumaraswamy, G.N. Effect of Barium Chloride Doping on PVA Microstructure: Positron Annihilation Study. Appl. Phys. A 2007, 87, 797–805. [Google Scholar] [CrossRef]
- Ali, H.E.; Khairy, Y. Optical and Electrical Performance of Copper Chloride Doped Polyvinyl Alcohol for Optical Limiter and Polymeric Varistor Devices. Phys. B Condens. Matter 2019, 572, 256–265. [Google Scholar] [CrossRef]
- Bulinski, M.; Kuncser, V.; Plapcianu, C.; Krautwald, S.; Franke, H.; Rotaru, P.; Filoti, G. Optical and Electronic Properties of Polyvinyl Alcohol Doped with Pairs of Mixed Valence Metal Ions. J. Phys. D Appl. Phys. 2004, 37, 2437–2441. [Google Scholar] [CrossRef]

| Treatment | Parameter Values: {up}-Increasing; {dw}-Decreasing | Refs. |
|---|---|---|
| Adding dopants (conductive ions) | {dw}: Degree of crystallinity, tensile strength | [45,46,47] |
| Blending (plasticizers, polymers) | {up}: Thermal stability, electrical, mechanical, and electrochemical properties | [48,49,50] |
| Heat treatment (freezing and thawing) | {dw}: Degree of crystallinity | [31] |
| Reinforced with poly(GEMA) | {up}: Thermal decomposition temperature | [51] |
| Reinforced with carbon nanotube | {up}: Mechanical properties | [52] |
| Blending with biodegradable PVP | {up}: Refractive index | [53] |
| Ultrasonic | {up}: Tensile strength. {dw}: Water vapor permeability, strain at break | [54] |
| Polymer additive | {up}: Glass transition temperature, mechanical properties | [55] |
| Gamma ray irradiation | {dw}: Degree of crystallinity | [56] |
| Ion beam | {up}: Reflecting coefficient, degree of crystallinity, photoluminescence | [57,58] |
| Dopant | {up}-Increasing; {dw}-Decreasing | Application Areas | Refs. |
|---|---|---|---|
| FeCl3 | {up}: VIS absorption. {dw}: Refractive index | Real time holography | [86,87] |
| ErCl3 | {up}: Refractive index. {dw}: Band gap. | Optoelectronics | [88] |
| CuS | {up}: Electrical conductivity {dw}: Band gap, dielectric constant, dielectric loss | Optoelectronics | [89] |
| Co-ZnO | {up}: Photoluminescence, Thermal stability. {dw}: Band gap. | UV-shielding, nanophotonics | [90] |
| ZnO | {up}: AC conductivity, dielectric constant, Tensile strength, elongation at break. {dw}: Band gap, dielectric loss. | Optoelectronics, EMI, and UV shielding, microwave absorption, UV luminescence | [91] |
| CrO3+CuO | {dw}: Refractive index, high transmittance | Sensor applications | [92] |
| CuO | {dw}: Band gap, dielectric constant, dielectric loss, AC conductivity | Optoelectronics | [93] |
| CeO2 | {up}: Absorption in the UV; Photoluminescence (UV, blue–green) | Filters; Solar Cells | [94] |
| Co-metal | {up}: Absorption. {dw}: Energy gap | Optical limiting in photonic devices | [95] |
| Zn-PbS | {dw}: Band gap, optical nonlinearity, dynamic range | All-optical limiting | [96] |
| MXene | {up}: Broad spectrum, optical modulation | Polarization-dependent all-optical modulator | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulinski, M. Metal Doped PVA Films for Opto-Electronics-Optical and Electronic Properties, an Overview. Molecules 2021, 26, 2886. https://doi.org/10.3390/molecules26102886
Bulinski M. Metal Doped PVA Films for Opto-Electronics-Optical and Electronic Properties, an Overview. Molecules. 2021; 26(10):2886. https://doi.org/10.3390/molecules26102886
Chicago/Turabian StyleBulinski, Mircea. 2021. "Metal Doped PVA Films for Opto-Electronics-Optical and Electronic Properties, an Overview" Molecules 26, no. 10: 2886. https://doi.org/10.3390/molecules26102886





