Profiling of Volatile Compounds and Associated Gene Expression in Two Anthurium Cultivars and Their F1 Hybrid Progenies
Abstract
1. Introduction
2. Results
2.1. Floral Scent Compounds in the Spadix of A. ‘Mystral’ and A. ‘Alabama’
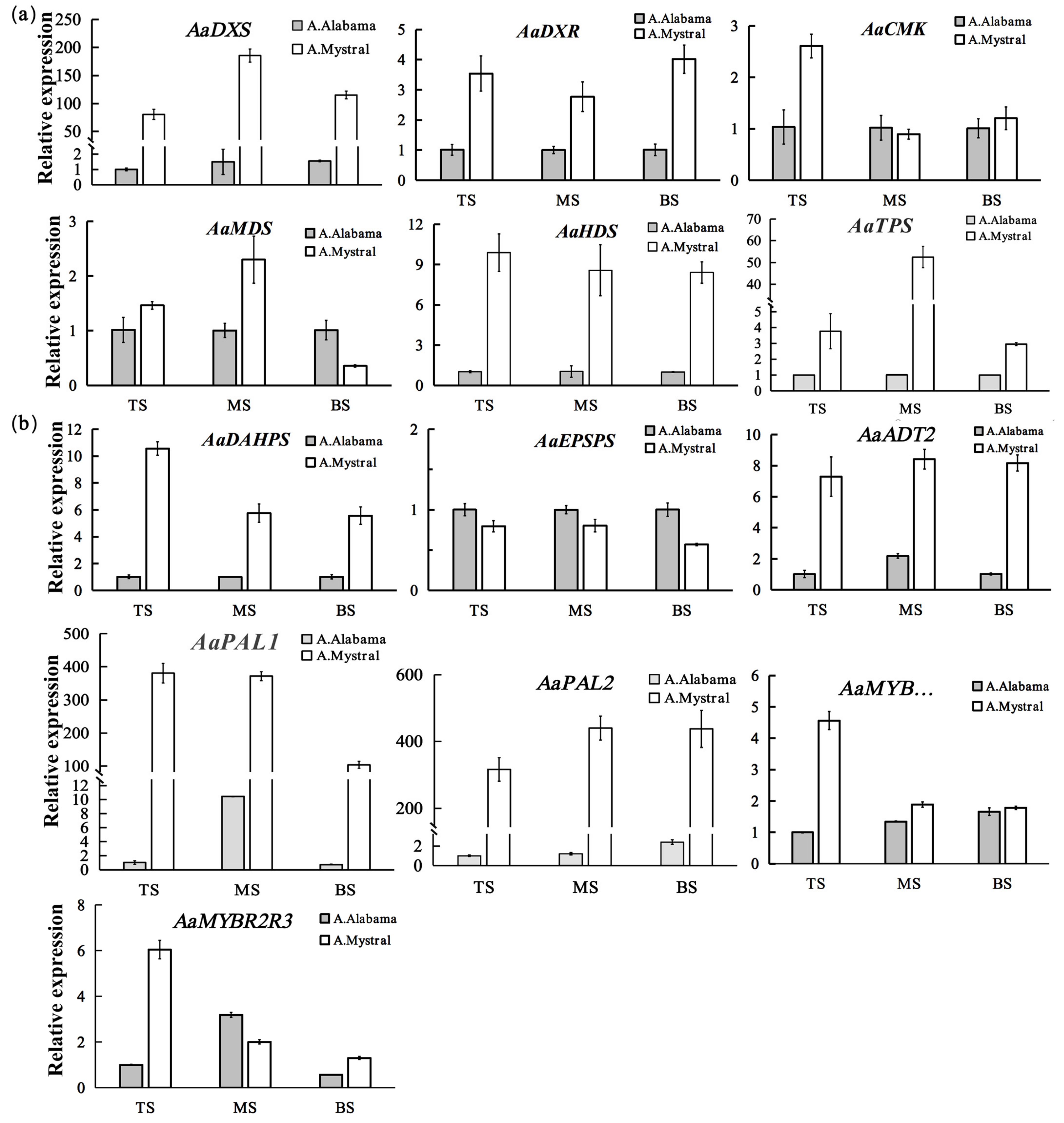
2.2. Analysis of the Key Genes Involved in Volatile Organic Compound (VOC) Biosynthetic Pathways
2.3. Segregation of Floral Scent Traits in Hybrid Progenies of A. ‘Mystral’ × A. ‘Alabama’
2.4. The Compounds of Floral Scent in Hybrid Progenies of A. ‘Mystral’ × A. ‘Alabama’
2.5. Analysis of Volatile Organic Compound Biosynthetic Pathway-Related Genes in Hybrid Progenies
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Extraction and Determination of Volatile Compounds
4.3. Analysis of Quantitative Real-Time PCR (qRT-PCR)
4.4. The Olfactory Tests of the Hybrid Progenies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Filella, I.; Primante, C.; Llusià, J.; Martín González, A.M.; Seco, R.; Farré-Armengol, G.; Rodrigo, A.; Bosch, J.; Peñuelas, J. Floral advertisement scent in a changing plant-pollinators market. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Byers, K.J.R.P.; Vela, J.P.; Peng, F.; Riffell, J.A.; Bradshaw, H.D. Floral volatile alleles can contribute to pollinator-mediated reproductive isolation in monkeyflowers (Mimulus). Plant J. 2014, 80, 1031–1042. [Google Scholar] [CrossRef]
- Zhang, T.; Bao, F.; Yang, Y.; Hu, L.; Ding, A.; Ding, A.; Wang, J.; Cheng, T.; Zhang, Q. A comparative analysis of floral scent compounds in intraspecific cultivars of Prunus mume with different corolla colours. Molecules 2020, 25, 145. [Google Scholar] [CrossRef]
- Kishimoto, K.; Nakayama, M.; Yagi, M.; Onozaki, T.; Oyama-Okubo, N. Evaluation of wild Dianthus species as genetic resources for fragrant carnation breeding based on their floral scent composition. J. Jpn. Soc. Hortic. Sci. 2011, 80, 175–181. [Google Scholar] [CrossRef]
- Magnard, J.L.; Roccia, A.; Caissard, J.C.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Oyant, L.H.S.; Jullien, F.; Nicolè, F.; et al. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Zheng, R.; Cai, X.; Luo, J.; Zou, J.; Wang, C. Emission and accumulation of monoterpene and the key terpene synthase(TPS)associated with monoterpene biosynthesis in osmanthus fragrans lour. Front. Plant Sci. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Gao, F.; Liu, B.; Li, M.; Gao, X.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identification and characterization of terpene synthase genes accounting for volatile terpene emissions in flowers of Freesia x hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Yue, Y.; Shi, M.; Chen, M.; Yang, X.; Wang, L. Exploration of floral volatile organic compounds in six typical lycoris taxa by GC-MS. Plants 2019, 8, 422. [Google Scholar] [CrossRef]
- Obi Johnson, B.; Golonka, A.M.; Blackwell, A.; Vazquez, I.; Wolfram, N. Floral Scent Variation in the Heterostylous Species Gelsemium sempervirens. Molecules 2019, 24, 2818. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Wang, T.; Fan, J.; Liu, Z.; Zong, J.; Fan, W.; Han, Y.; Grierson, D. Volatile composition and classification of Lilium flower aroma types and identification, polymorphisms, and alternative splicing of their monoterpene synthase genes. Hortic. Res. 2019, 6. [Google Scholar] [CrossRef]
- Li, Z.G.; Cao, H.; Lee, M.R.; Shen, D.L. Analysis of volatile compounds emitted from Chimonanthus praecox (L.) Link in different florescence and QSRR study of GC retention indices. Chromatographia 2009, 70, 1153–1162. [Google Scholar] [CrossRef]
- Kutty, N.N.; Mitra, A. Profiling of volatile and non-volatile metabolites in Polianthes tuberosa L. flowers reveals intraspecific variation among cultivars. Phytochemistry 2019, 162, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Ahn, M.S.; Jo, G.S.; Suh, J.N.; Seo, K.H.; Kim, W.H.; Kang, Y.I.; Lee, Y.R.; Choi, Y.J. Volatile Compounds and Gene Expression. Plants 2020, 9, 1597. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Kitaoka, N.; Lu, X.; Yang, B.; Peters, R.J. The application of synthetic biology to elucidation of plant mono-, sesqui-, and diterpenoid metabolism. Mol. Plant. 2015, 8, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yu, R.; Fan, Y. Characterization of two monoterpene synthases involved in floral scent formation in Hedychium coronarium. Planta 2014, 240, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Utsumi, R. Diversity, regulation, and genetic manipulation of plant mono-and sesquiterpenoid biosynthesis. Cell. Mol. Life Sci. 2009, 66, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Amrhein, N. Molecular organization of the shikimate pathway in higher plants. Phytochemistry 1995, 39, 737–749. [Google Scholar] [CrossRef]
- Yoo, H.; Widhalm, J.R.; Qian, Y.; Maeda, H.; Cooper, B.R.; Jannasch, A.S.; Gonda, I.; Lewinsohn, E.; Rhodes, D.; Dudareva, N. An alternative pathway contributes to phenylalanine biosynthesis in plants via a cytosolic tyrosine:phenylpyruvate aminotransferase. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Guy, A.; Galili, G.; Dor, E.; Schweitzer, R.; Amir, R.; Hacham, Y. Enhanced Production of Aromatic Amino Acids in Tobacco Plants Leads to Increased Phenylpropanoid Metabolites and Tolerance to Stresses. Front. Plant Sci. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Picazo-Aragonés, J.; Terrab, A.; Balao, F. Plant volatile organic compounds evolution: Transcriptional regulation, epigenetics and polyploidy. Int. J. Mol. Sci. 2020, 21, 956. [Google Scholar] [CrossRef] [PubMed]
- Gigot, C.; Ongena, M.; Fauconnier, M.L.; Wathelet, J.P.; du Jardin, P.; Thonart, P. The lipoxygenase metabolic pathway in plants: Potential for industrial production of natural green leaf volatiles. Biotechnol. Agron. Soc. Environ. 2010, 14, 451–460. [Google Scholar] [CrossRef]
- Hong, G.J.; Xue, X.Y.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell. 2012, 24, 2635–2648. [Google Scholar] [CrossRef]
- Reeves, P.H.; Ellis, C.M.; Ploense, S.E.; Wu, M.F.; Yadav, V.; Tholl, D.; Chételat, A.; Haupt, I.; Kennerley, B.J.; Hodgens, C.; et al. A regulatory network for coordinated flower maturation. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Gao, F.; Jin, W.; Li, S.; Kimani, S.; Yang, S.; Bao, T.; Gao, X.; Wang, L. MYB21 interacts with MYC2 to control the expression of terpene synthase genes in flowers of Freesia hybrida and Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 4140–4158. [Google Scholar] [CrossRef]
- Qi, T.; Huang, H.; Song, S.; Xie, D. Regulation of jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in arabidopsis. Plant Cell. 2015, 27, 1620–1633. [Google Scholar] [CrossRef]
- Skibbe, M.; Qu, N.; Galis, I.; Baldwin, I.T. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell. 2008, 20, 1984–2000. [Google Scholar] [CrossRef]
- Spitzer-rimon, B.; Farhi, M.; Albo, B.; Cna, A.; Moyal, M.; Zvi, B.; Masci, T.; Edelbaum, O.; Yu, Y.; Shklarman, E.; et al. The R2R3-MYB – Like Regulatory Factor EOBI, Acting Downstream of EOBII, Regulates Scent Production by Activating ODO1 and Structural Scent-Related Genes in Petunia. Plant Cell 2012, 24, 5089–5105. [Google Scholar] [CrossRef]
- Flowers, P.; Verdonk, J.C.; Haring, M.A.; Van Tunen, A.J.; Schuurink, R.C. ODORANT1 Regulates Fragrance Biosynthesis in Petunia Flowers. Plant Cell 2005, 17, 1612–1624. [Google Scholar] [CrossRef]
- Van Moerkercke, A.; Haring, M.A.; Schuurink, R.C. The transcription factor EMISSION OF BENZENOIDS II activates the MYB ODORANT1 promoter at a MYB binding site specific for fragrant petunias. Plant J. 2011, 67, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, T.A.; Clark, D.G. Unraveling the regulation of floral fragrance biosynthesis. Plant Signal. Behav. 2011, 6, 378–381. [Google Scholar] [CrossRef]
- Pirrello, J.; Leclercq, J.; Dessailly, F.; Rio, M.; Piyatrakul, P.; Kuswanhadi, K.; Tang, C.; Montoro, P. Transcriptional and post-transcriptional regulation of the jasmonate signalling pathway in response to abiotic and harvesting stress in Hevea brasiliensis. BMC Plant Biol. 2014, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Browse, J. The power of mutants for investigating jasmonate biosynthesis and signaling. Phytochemistry 2009, 70, 1539–1546. [Google Scholar] [CrossRef]
- Geyter, D.E.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional machineries in jamonate-elicited plant secondary metabolism. Trends Plant Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Kuanprasert, N.; Kuehnle, A.R.; Tang, C.S. Floral fragrance compounds of some Anthurium (Araceae) species and hybrids. Phytochemistry 1998, 49, 521–528. [Google Scholar] [CrossRef]
- Majetic, C.J.; Raguso, R.A.; Ashman, T.L. The impact of biochemistry vs. population membership on floral scent profiles in colour polymorphic Hesperis matronalis. Ann. Bot. 2008, 102, 911–922. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, R.; Fan, Y. Transcriptome profiling provides new insights into the formation of floral scent in Hedychium coronarium. BMC Genom. 2015, 16, 1–23. [Google Scholar] [CrossRef]
- Li, C.; Qiu, J.; Yang, G.; Huang, S.; Yin, J. Ectopic expression of the Anthurium andraeanum (Hort.) R2R3-MYB genes AaMYB4 and AaMYB5 enhance the flower color in transgenic tobacco. Plant Cell Tissue Organ Cult. 2019, 139, 105–117. [Google Scholar] [CrossRef]



| No. | Compounds | Molecular Formula | RT 1 (min) | Content (μg·gFW·h−1) 2 ± SD 3 | |
|---|---|---|---|---|---|
| A. ‘Mystral’ | A. ‘Alabama’ | ||||
| monoterpenes | |||||
| 1 | Eucalyptol | C10H18O | 10.211 | 49.2 ± 2.8 | - |
| 2 | α, α-4-trimethyl-3-Cyclohexene-1-methanol | C10H18O | 14.182 | 7.07 ± 0.6 | - |
| 3 | β-Pinene | C10H16 | 8.62 | 1.313 ± 0.4 | - |
| 4 | β-Phellandrene | C10H16 | 8.551 | 0.566 ± 0.0 | - |
| 5 | 1-methyl-4-(1-methylethylidene)-cyclohexene | C10H16 | 11.876 | 0.414 ± 0.1 | - |
| 6 | (E)-1,3,6-Octatriene, 3,7-dimethyl- | C10H16 | 10.691 | 0.232 ± 0.1 | - |
| 7 | α-Pinene | C10H16 | 7.436 | 0.212 ± 0.0 | - 4 |
| 8 | β-Myrcene | C10H16 | 9.026 | 0.202 ± 0.1 | - |
| 9 | 4-methyl-1-(1-methylethyl)-3-Cyclohexen-1-ol | C12H20O2 | 14.474 | 0.152 ± 0.2 | - |
| 10 | cis-2-Cyclohexen-1-ol,2-methyl-5-(1-methylethenyl)-, acetate | C12H18O2 | 18.874 | 0.121 ± 0.0 | - |
| 11 | Thujone | C10H16O | 12.711 | 0.061 ± 0.0 | - |
| 12 | 3-methyl-6-(1-methylethylidene)-cyclohexene | C10H16 | 13.592 | 0.051 ± 0.0 | - |
| 13 | 2,6-Octadienoic acid, 3,7-dimethyl-, methyl ester | C11H18O2 | 18.49 | 0.03 ± 0.0 | - |
| sesquiterpenes | |||||
| 14 | 1H-Cyclopropa[a]naphthalene,1a,2, 3,3a,4,5,6,7b-octahydro-1,1,3a,7-tetramethyl | C15H24 | 21.454 | 0.172 ± 0.0 | - |
| 15 | γ-Himachalene | C15H24 | 22.576 | 0.121 ± 0.0 | - |
| phenylpropanoid/benzenoids | |||||
| 16 | Acetic acid, phenylmethyl ester | C9H10O2 | 14.19 | 19.835 ± 1.5 | - |
| 17 | Benzoic acid, methyl ester | C11H12O2 | 12.059 | 3.848 ± 0.5 | - |
| 18 | Benzaldehyde | C7H6O | 8.242 | 0.212 ± 0.1 | - |
| 19 | 2-Propenoic acid, 3-phenyl-, methyl ester | C16H14O2 | 20.07 | 0.131 ± 0.0 | - |
| 20 | Indole | C8H7N | 17.672 | 0.131 ± 0.2 | - |
| 21 | Butylated Hydroxytoluene | C15H24O | 23.337 | 0.091 ± 0.0 | - |
| 22 | 1-ethyl-2,4,5-trimethyl-, Benzene | C11H16 | 18.227 | 0.081 ± 0.0 | - |
| Others | |||||
| 23 | Tetradecane | C14H30 | 20.453 | 0.242 ± 0.2 | 0.226 ± 0.2 |
| 24 | Heptadecane,2,6,10,14-tetramethyl | C21H44 | 22.015 | 0.242 ± 0.2 | 0.183± 0.2 |
| 25 | Pentadecane | C15H32 | 22.948 | 0.242 ± 0.2 | 0.279± 0.2 |
| 26 | 1,2-Benzenedicarboxylic acid, butyl 2-ethylhexyl ester | C16H22O4 | 32.011 | 0.232 ± 0.1 | - |
| 27 | 2,6,10-trimethyl-Dodecane | C15H32 | 19.852 | 0.1 ± 0.0 | 0.082 ± 0.0 |
| 28 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | 18.628 | 0.081 ± 0.0 | 0.059 ± 0.0 |
| 29 | decamethyl-cyclopentasiloxane | C10H30O5Si5 | 13.844 | 0.051 ± 0.0 | - |
| 30 | Tridecane | C13H28 | 17.832 | 0.051 ± 0.0 | - |
| 31 | 10-Methylnonadecane | C20H42 | 19.692 | 0.04 ± 0.0 | - |
| 32 | 2,6,11,15-tetramethyl-Hexadecane | C20H42 | 26.415 | 0.04 ± 0.0 | 0.054 ± 0.0 |
| 33 | 3,5-dimethyl-Undecane | C13H28 | 26.518 | - | 0.055 ± 0.0 |
| 34 | Hexadecane | C16H34 | 27.777 | - | 0.103 ± 0.1 |
| 85.567 | 1.041 | ||||
| Hybrid Combinations | No. of Plants (Strong Floral Scent) | No. of Plants (Weak Floral Scent) | No. of Plants (Fragrance Free) | Ratio (No. Strong/No. Fragrance Free) |
|---|---|---|---|---|
| A. ‘Mystral’ ♀ × A. ‘Alabama’ ♂ (08-377) | 60 | 210 | 93 | 3:1 |
| A. ‘Alabama’ ♀ × A. ‘Mystral’ ♂ (08-382) | 6 | 48 | 37 | 3:2 |
| No. | Compounds | Molecular Formula | RT 1 (min) | Relative Amount (μg·gFW·h−1) 2 ± SD 3 | |||
|---|---|---|---|---|---|---|---|
| 08-377-9 | 08-377-3 | 08-382-20 | 08-382-48 | ||||
| Strong | Weak | Strong | Weak | ||||
| Monoterpenes | |||||||
| 1 | α-Pinene | C10H16 | 7.436 | 0.342 ± 0.0 | - 4 | 1.424 ± 0.1 | 1.048 ± 0.1 |
| 2 | β-Pinene | C10H16 | 8.62 | 0.467 ± 0.1 | - | 0.937 ± 0.1 | - |
| 3 | β -Phellandrene | C10H16 | 8.694 | - | - | 2.13 ± 0.2 | 0.382 ± 0.1 |
| 4 | 4-methylene-1-(1-methylethyl)-cyclohexene | C10H16 | 8.78 | - | - | - | 1.214 ± 0.1 |
| 5 | Eucalyptol | C10H18O | 10.199 | 4.215 ± 0.3 | - | 37.497 ± 1.3 | 11.49 ± 0.6 |
| 6 | 3,7-dimethyl-1,6-Octadien-3-ol | C10H16O | 12.225 | - | - | 27.09 ± 11.0 | - |
| 7 | (E)-1,3,6-Octatriene, 3,7-dimethyl- | C10H16 | 10.691 | 0.023 ± 0.0 | - | 0.109 ± 0.1 | - |
| 8 | cis-Linalol oxide | C10H18O2 | 11.441 | - | - | 0.803 ± 0.1 | - |
| 9 | 1-methyl-4-(1-methylethylidene)-cyclohexene | C10H16 | 11.882 | 0.023 ± 0.0 | - | - | - |
| 10 | cis-Limonene oxide | C10H15O | 13.198 | 0.023 ± 0.0 | - | 0.56 ± 0.0 | 0.077 ± 0.0 |
| 11 | trans-Limonene oxide | C10H15O | 13.329 | 2.154 ± 0.2 | 0.013 ± 0.0 | 11.124 ± 0.6 | 1.265 ± 0.1 |
| 12 | 4-methyl-1-(1-methylethyl)-3-Cyclohexen-1-ol | C10H18O | 14.485 | - | - | - | - |
| 13 | α,α-4-trimethyl-3-Cyclohexene-1-methanol | C10H18O | 14.868 | 0.296 ± 0.0 | - | 5.72 ± 0.6 | - |
| 14 | 4-methyl-1-(1-methylethenyl)-Cyclohexene | C10H16 | 14.971 | 0.308 ± 0.0 | - | 1.4 ± 0.1 | - |
| 15 | trans-2-methyl-5-(1-methylethenyl)-Cyclohexanone | C10H16O | 15.057 | 2.37 ± 0.2 | - | 10.309 ± 0.9 | 0.226 ± 0.0 |
| 16 | 2-methyl-5-(1-methylethenyl)-2-Cyclohexen-1-ol | C10H18O | 15.269 | 0.057 ± 0.0 | - | 0.596 ± 0.1 | - |
| 17 | (S)-2-methyl-5-(1-methylethenyl)-2-Cyclohexen-1-one | C10H18O | 16.367 | 0.103 ± 0.0 | 0.011 ± 0.0 | 1.789 ± 0.5 | 1.2980.1 |
| 18 | trans-Carvone oxide | C10H14O | 16.934 | 1.425 ± 0.1 | - | 10.126 ± 0.6 | 0.703 ± 0.0 |
| 19 | cis-2-Cyclohexen-1-ol,2-methyl-5-(1-methylethenyl)-, acetate | C12H18O2 | 15.967 | - | - | - | 0.112 ± 0.0 |
| Sesquiterpenes | |||||||
| 20 | (S)-1-methyl-4-(5-methyl-1-methylene-4-hexenyl)-Cyclohexene | C15H24 | 23.234 | 0.011 ± 0.0 | - | - | - |
| Benzene | |||||||
| 21 | Benzyl Alcohol | C7H8O | 10.308 | - | 0.035 ± 0.0 | - | - |
| 22 | Benzoic acid, methyl ester | C8H8O2 | 12.082 | - | 0.148 ± 0.0 | - | - |
| 23 | Acetic acid, phenylmethyl ester | C9H10O2 | 14.273 | 87.442 ± 1.9 | 6.956 ± 0.5 | - | 0.294 ± 0.0 |
| 24 | Benzoic acid, ethyl ester | C9H10O2 | 14.285 | - | 0.014 ± 0.0 | - | - |
| 25 | Propanoic acid, phenylmethyl ester | C10H12O2 | 16.728 | 0.011 ± 0.0 | - | - | - |
| 26 | Butylated Hydroxytoluene | C15H24O | 23.354 | - | 0.03 ± 0.0 | - | - |
| 27 | Benzyl Benzoate | C14H12O2 | 29.133 | 4.809 ± 0.8 | - | - | 0.082 ± 0.0 |
| 28 | Benzoic acid, 2-hydroxy-, phenylmethyl ester | C14H12O3 | 32.074 | 0.547 ± 0.0 | - | - | - |
| Fatty Acid Derivatives | |||||||
| 29 | 1-Butanol, 3-methyl-, acetate | C7H14O2 | 6.028 | - | 0.2066 ± 0.0 | - | - |
| 30 | 1,2-Benzenedicarboxylic acid, butyl 2-ethylhexyl ester | C16H22O4 | 32.097 | - | - | 0.316 ± 0.0 | - |
| 31 | Phthalic acid, isobutyl octyl ester | C20H30O4 | 32.028 | - | 0.025 ± 0.0 | - | 0.233 ± 0.0 |
| 32 | Hexanedioic acid, bis(2-ethylhexyl) ester | C22H42O4 | 34.958 | 4.627 ± 0.2 | - | - | - |
| 33 | 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-Butanone | C13H22O2 | 21.569 | - | - | 0.195 ± 0.0 | - |
| Others | |||||||
| 34 | Tridecane | C13H28 | 17.832 | 0.034 ± 0.0 | 0.017 ± 0.0 | - | - |
| 35 | Tetradecane | C14H30 | 20.459 | 0.125 ± 0.0 | 0.11 ± 0.0 | 0.207 ± 0.0 | 0.265 ± 0.0 |
| 36 | Pentadecane | C15H32 | 22.942 | 0.148 ± 0.0 | 0.092 ± 0.0 | 0.256 ± 0.0 | 0.203 ± 0.1 |
| 37 | Heptacosane | C27H56 | 22.238 | - | - | - | - |
| 38 | Hexadecane | C16H34 | 25.3 | 0.034 ± 0.0 | 0.032 ± 0.0 | - | - |
| 39 | 2,6,10-trimethyl-Pentadecane | C18H38 | 27.669 | - | 0.02 ± 0.0 | - | - |
| 40 | 3-methyl-Tetradecane | C15H32 | 22.244 | - | 0.023 ± 0.0 | - | - |
| 41 | 5-methyl-3-Octyne | C9H16 | 17.346 | - | - | 0.207 ± 0.0 | - |
| 42 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | 18.628 | 0.034 ± 0.0 | 0.032 ± 0.0 | 0.11 ± 0.0 | 0.051 ± 0.0 |
| 43 | 10-Methylnonadecane | C20H42 | 19.515 | 0.023 ± 0.0 | 0.016 ± 0.0 | - | - |
| 44 | 3-methyl-Tridecane | C14H30 | 19.709 | - | 0.012 ± 0.0 | - | - |
| 45 | 2,6,10-trimethyl-Dodecane | C15H32 | 19.852 | 0.046 ± 0.0 | 0.14 ± 0.0 | 0.073 ± 0.0 | 0.056 ± 0.0 |
| 46 | 3-cyclohexyl-Decane | C16H32 | 21.683 | - | 0.03 ± 0.0 | - | - |
| 47 | Heptadecane,2,6,10,14-tetramethyl | C21H44 | 22.021 | 0.114 ± 0.0 | - | 0.158 ± 0.0 | - |
| 109.811 | 7.9626 | 113.137 | 18.999 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Xia, Q.; Wang, Y.; Chen, W.; Liu, C.; Zeng, R.; Xie, L.; Yi, M.; Guo, H. Profiling of Volatile Compounds and Associated Gene Expression in Two Anthurium Cultivars and Their F1 Hybrid Progenies. Molecules 2021, 26, 2902. https://doi.org/10.3390/molecules26102902
Wei Q, Xia Q, Wang Y, Chen W, Liu C, Zeng R, Xie L, Yi M, Guo H. Profiling of Volatile Compounds and Associated Gene Expression in Two Anthurium Cultivars and Their F1 Hybrid Progenies. Molecules. 2021; 26(10):2902. https://doi.org/10.3390/molecules26102902
Chicago/Turabian StyleWei, Qian, Qing Xia, Yue Wang, Wen Chen, Cuiling Liu, Ruizhen Zeng, Li Xie, Maosheng Yi, and Herong Guo. 2021. "Profiling of Volatile Compounds and Associated Gene Expression in Two Anthurium Cultivars and Their F1 Hybrid Progenies" Molecules 26, no. 10: 2902. https://doi.org/10.3390/molecules26102902
APA StyleWei, Q., Xia, Q., Wang, Y., Chen, W., Liu, C., Zeng, R., Xie, L., Yi, M., & Guo, H. (2021). Profiling of Volatile Compounds and Associated Gene Expression in Two Anthurium Cultivars and Their F1 Hybrid Progenies. Molecules, 26(10), 2902. https://doi.org/10.3390/molecules26102902






