A Multimethodological Characterization of Cannabis sativa L. Inflorescences from Seven Dioecious Cultivars Grown in Italy: The Effect of Different Harvesting Stages
Abstract
:1. Introduction
2. Results
2.1. Targeted Analyses: Cannabinoids, Chlorophylls, Total Carotenoids, and Phenolics Content, Antioxidant Activity
2.2. Untargeted Analysis of Metabolite Profile by NMR and PCA
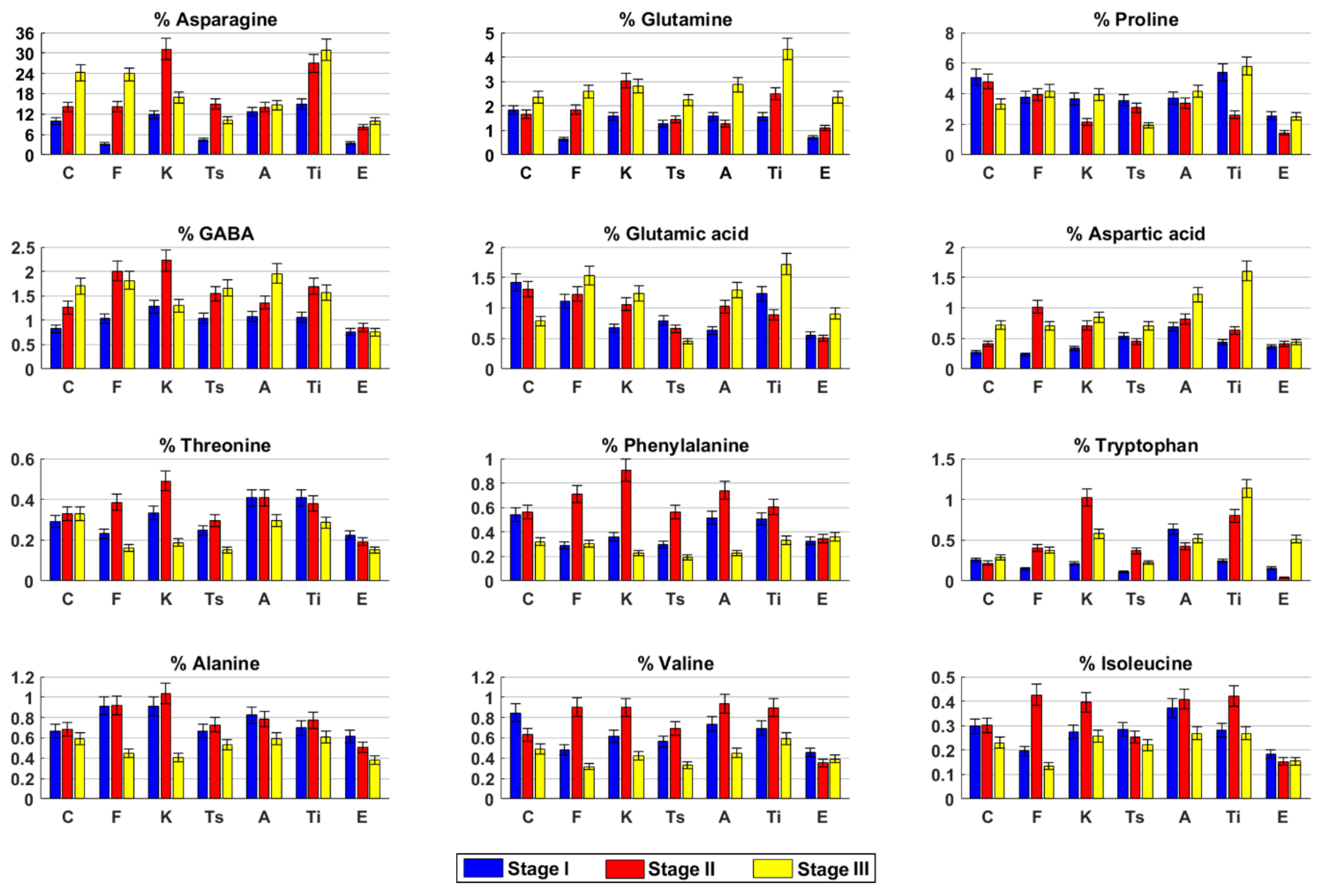
2.3. Metabolite Profile of the Inflorescences at Three Harvesting Stage
2.3.1. Carmagnola
2.3.2. Fibranova
2.3.3. Kompolti
2.3.4. Tisza
2.3.5. Antal
2.3.6. Tiborszallasi
2.3.7. Eletta Campana
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Hemp Plant Material
4.3. Cannabinoids Content by UHPLC Analyses
4.4. Untargeted NMR Analysis and Multivariate Statistical Analysis
4.5. Spectrophotometric Analysis of Chlorophylls and Total Carotenoids
4.6. Spectrophotometric Analysis of Total Phenolics Content and Antioxidant Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Piluzza, G.; Delogu, G.; Cabras, A.; Marceddu, S.; Bullitta, S. Differentiation between fiber and drug types of hemp (Cannabis sativa L.) from a collection of wild and domesticated accessions. Genet. Resour. Crop. Evol. 2013, 60, 2331–2342. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ash, A.L. Hemp-production and utilization. Econ. Bot. 1948, 2, 158–169. [Google Scholar] [CrossRef]
- EUR-Lex. Access to European Union Law. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:01999R1251-20040701 (accessed on 3 March 2020).
- EU. Plant Variety Database. Available online: https://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchVariety&ctl_type=A&species_id=240&variety_name=antal&listed_in=0&show_current=on&show_deleted=on (accessed on 20 March 2020).
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Ind. Crop. Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Verma, S.K.; Chauhan, A.; Darokar, M.P. The essential oil of “bhang” (Cannabis sativa L.) for non-narcotic applications. Curr. Sci. 2014, 107, 645–650. [Google Scholar]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crop. Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Ceccarini, L.; Tavarini, S.; Flamini, G.; Angelini, L.G. Valorisation of hemp inflorescence after seed harvest: Cultivation site and harvest time influence agronomic characteristics and essential oil yield and composition. Ind. Crop. Prod. 2019, 139, 139. [Google Scholar] [CrossRef]
- Amaducci, S.; Zatta, A.; Pelatti, F.; Venturi, G. Influence of agronomic factors on yield and quality of hemp (Cannabis sativa L.) fibre and implication for an innovative production system. Field Crop. Res. 2008, 107, 161–169. [Google Scholar] [CrossRef]
- Mediavilla, V.; Meier, C. Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil. J. Int. Hemp. Assoc. 1998, 5, 16–20. [Google Scholar]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Di Sotto, A.; Di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. Inflorescences from Monoecious Cultivars Grown in Central Italy: An Untargeted Chemical Characterization from Early Flowering to Ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef]
- Ranalli, P. Current status and future scenarios of hemp breeding. Euphytica 2004, 140, 121–131. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L.; Springer: New York, NY, USA, 2017; Volume 103, ISBN 9783319455419. [Google Scholar]
- Ingallina, C.; Capitani, D.; Mannina, L.; Carradori, S.; Locatelli, M.; Di Sotto, A.; Di Giacomo, S.; Toniolo, C.; Pasqua, G.; Valletta, A.; et al. Phytochemical and biological characterization of Italian “sedano bianco di Sperlonga” Protected Geographical Indication celery ecotype: A multimethodological approach. Food Chem. 2020, 309, 125649. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Mannina, L.; Capitani, D.; Sanzò, G.; Ingallina, C.; Botta, B.; Fornarini, S.; Crestoni, M.E.; Chiavarino, B.; Carradori, S.; et al. A multi-methodological approach in the study of Italian PDO “Cornetto di Pontecorvo” red sweet pepper. Food Chem. 2018, 255, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Marincola, F.C.; Capitani, D.; Mannina, L. Use of NMR applications to tackle future food fraud issues. Trends Food Sci. Technol. 2019, 91, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Sobolev, A.P.; Circi, S.; Capitani, D.; Ingallina, C.; Mannina, L. Molecular fingerprinting of food authenticity. Curr. Opin. Food Sci. 2017, 16, 59–66. [Google Scholar] [CrossRef]
- Mazzoccanti, G.; Ismail, O.H.; D’Acquarica, I.; Villani, C.; Manzo, C.; Wilcox, M.; Cavazzini, A.; Gasparrini, F. Cannabis through the looking glass: Chemo- and enantio-selective separation of phytocannabinoids by enantioselective ultra high performance supercritical fluid chromatography. Chem. Commun. 2017, 53, 12262–12265. [Google Scholar] [CrossRef] [Green Version]
- Hillig, K.W.; Mahlberg, P.G. A chemotaxonomic analysis of cannabinoid variation inCannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef] [Green Version]
- Glivar, T.; Eržen, J.; Kreft, S.; Zagožen, M.; Čerenak, A.; Čeh, B.; Benković, E.T. Cannabinoid content in industrial hemp (Cannabis sativa L.) varieties grown in Slovenia. Ind. Crop. Prod. 2020, 145, 112082. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- Rapa, M.; Ciano, S.; Ruggieri, R.; Vinci, G. Bioactive compounds in cherry tomatoes (Solanum Lycopersicum var. Cerasiforme): Cultivation techniques classification by multivariate analysis. Food Chem. 2021, 355, 129630. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A. Rapid Assays to Evaluate the Antioxidant Capacity of Phenols in Virgin Olive Oil. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2010; pp. 625–635. [Google Scholar]
- Di Pierro, F. A nutraceutical role for cannabidiol. Why not? Nutrafoods 2015, 14, 111–117. [Google Scholar] [CrossRef]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 2009, 159, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Delong, G.T.; Wolf, C.E.; Poklis, A.; Lichtman, A.H. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ9-tetrahydrocannabinol. Drug Alcohol Depend. 2010, 112, 126–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechtler, K.; Bailer, J.; De Hueber, K. Variations of Δ9-THC content in single plants of hemp varieties. Ind. Crop. Prod. 2004, 19, 19–24. [Google Scholar] [CrossRef]
- Stanley, L.; Yuan, Y. Transcriptional Regulation of Carotenoid Biosynthesis in Plants: So Many Regulators, So Little Consensus. Front. Plant Sci. 2019, 10, 1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolken, J.J.; Mellon, A.D. The Relationship between Chlorophyll and the Carotenoids in the Algal Flagellate, Euglena. J. Gen. Physiol. 1956, 39, 675–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as Natural Antioxidants: Sources, Extraction and Applications in Food, Cosmetics and Drugs. Green Chem. Sustain. Technol. 2019, 197–235. [Google Scholar] [CrossRef]
- Mkpenie, V.N.; Essien, E.E.; Udoh, I.I. Effect of extraction conditions on total polyphenol contents, antioxidant and antimicrobial activities of Cannabis sativa L. Electron. J. Environ. Agric. Food Chem. 2012, 11, 300–307. [Google Scholar]
- Hacke, A.C.M.; Lima, D.; De Costa, F.; Deshmukh, K.; Li, N.; Chow, A.M.; Marques, J.A.; Pereira, R.P.; Kerman, K. Probing the antioxidant activity of Δ9-tetrahydrocannabinol and cannabidiol in Cannabis sativa extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef]
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Cannabinoids: Extraction Methods, Analysis, and Physicochemical Characterization. In Studies in Natural Products Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 61, pp. 143–173. [Google Scholar]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Supriatno; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Nahar, L.; Uddin, S.J.; Alam, A.; Sarker, S.D. Extraction of naturally occurring cannabinoids: An update. Phytochem. Anal. 2021, 32, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Guo, M.; Sarker, S.D. Gas chromatographic analysis of naturally occurring cannabinoids: A review of literature published during the past decade. Phytochem. Anal. 2020, 31, 135–146. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Giusti, A.M.; Mannina, L. New Hybrid Tomato Cultivars: An NMR-Based Chemical Characterization. Appl. Sci. 2020, 10, 1887. [Google Scholar] [CrossRef] [Green Version]
- Solovchenko, A.E.; Chivkunova, O.B.; Merzlyak, M.N.; Reshetnikova, I.V. A Spectrophotometric Analysis of Pigments in Apples. Russ. J. Plant Physiol. 2001, 48, 693–700. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Preti, R.; Rapa, M.; Vinci, G. Effect of Steaming and Boiling on the Antioxidant Properties and Biogenic Amines Content in Green Bean (Phaseolus vulgaris) Varieties of Different Colours. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]


| Cultivar | Harvesting Stage | (–)-Δ9-THC | CBD | (–)-Δ9-THCA | CBDA | CBG | CBDV | CBC | CBN |
|---|---|---|---|---|---|---|---|---|---|
| Carmagnola | stage I | 0.24 ± 0.02 | 3.10 ± 0.40 | - | 2.02 ± 0.30 | 0.13 ± 0.01 | - | 0.19 ± 0.02 | - |
| stage II | 0.23 ± 0.01 | 3.12 ± 0.21 | - | 1.52 ± 0.02 | 0.17 ± 0.01 | - | 0.18 ± 0.01 | - | |
| stage III | 0.16 ± 0.01 | 3.82 ± 0.82 | - | - | 0.16 ± 0.01 | - | 0.13 ± 0.01 | - | |
| Fibranova | stage I | 0.19 ± 0.01 | 2.05 ± 0.03 | - | 0.88 ± 0.04 | 0.18 ± 0.01 | - | 0.21 ± 0.01 | - |
| stage II | 0.26 ± 0.01 | 2.97 ± 0.13 | - | 1.57 ± 0.07 | 0.18 ± 0.01 | 0.41 ± 0.01 | 0.33 ± 0.01 | - | |
| stage III | 0.11 ± 0.01 | 2.31 ± 0.30 | - | - | - | - | - | 0.11 ± 0.03 | |
| Kompolti | stage I | 0.28 ± 0.01 | 2.90 ± 0.20 | - | 1.40 ± 0.10 | 0.15 ± 0.01 | - | 0.18 ± 0.01 | - |
| stage II | 0.30 ± 0.01 | 5.09 ± 0.06 | - | 1.55 ± 0.02 | 0.18 ± 0.02 | - | 0.31 ± 0.01 | - | |
| stage III | 0.14 ± 0.01 | 4.64 ± 0.65 | - | - | 0.23 ± 0.01 | - | 0.15 ± 0.04 | - | |
| Tisza | stage I | 0.193 ± 0.005 | 2.400 ± 0.100 | - | 1.280 ± 0.040 | 0.210 ± 0.010 | - | 0.17 ± 0.01 | - |
| stage II | 0.250 ± 0.010 | 4.160 ± 0.220 | - | 1.910 ± 0.010 | 0.160 ± 0.010 | - | 0.21 ± 0.01 | - | |
| stage III | 0.061 ± 0.001 | 4.600 ± 0.090 | - | - | 0.091 ± 0.002 | - | - | - | |
| Antal | stage I | 0.25 ± 0.01 | 4.30 ± 0.40 | - | 1.60 ± 0.30 | 0.22 ± 0.01 | - | - | - |
| stage II | 0.14 ± 0.01 | 1.55 ± 0.15 | - | 1.11 ± 0.10 | 0.12 ± 0.01 | - | - | - | |
| stage III | 0.12 ± 0.01 | 1.96 ± 0.15 | - | - | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.03 ± 0.01 | - | |
| Tiborszallasi | stage I | 0.21 ± 0.01 | 2.76 ± 0.04 | - | 1.24 ± 0.03 | 0.24 ± 0.01 | - | 0.18 ± 0.01 | - |
| stage II | 0.51 ± 0.07 | 4.54 ± 0.22 | 0.09 ± 0.01 | 1.87 ± 0.06 | 0.21 ± 0.01 | - | 0.22 ± 0.02 | - | |
| stage III | 0.13 ± 0.01 | 3.79 ± 0.41 | - | - | 0.12 ± 0.02 | - | 0.15 ± 0.02 | - | |
| Eletta Campana | stage I | 0.17 ± 0.01 | 1.50 ± 0.10 | - | 1.00 ± 0.10 | 0.13 ± 0.01 | - | - | - |
| stage II | 0.190 ± 0.01 | 2.04 ± 0.01 | - | 1.12 ± 0.02 | 0.12 ± 0.01 | - | 0.11 ± 0.01 | - | |
| stage III | 0.06 ± 0.01 | 1.37 ± 0.12 | - | - | 0.04 ± 0.01 | - | 0.05 ± 0.01 | 0.04 ± 0.01 |
| Cultivar | Harvesting Stage | Chlorophyll a | Chlorophyll b | Chl. a/b | Total Caroteoids | Chl. (a+b)/Car |
|---|---|---|---|---|---|---|
| Carmagnola | stage I | 0.527 ± 0.050 §,e,c | 0.337 ± 0.071 §,b,d,f | 1.83 §,f | 0.210 ± 0.023 | 4.03 |
| stage II | 0.519 ± 0.004 | 0.272 ± 0.002 | 1.93 | 0.176 ± 0.002 §,f | 4.49 §,c,d,f,g | |
| stage III | 0.425 ± 0.008 * | 0.235 ± 0.002 | 1.81 | 0.158 ± 0.002 | 4.16 §,c,d,e,f,g | |
| Fibranova | stage I | 0.540 ± 0.011 §,c,e | 0.275 ± 0.004 | 2.27 §,f,g | 0.201 ± 0.004 | 3.79 |
| stage II | 0.683 ± 0.011 §,a,c,d,e,f,g | 0.362 ± 0.004 §,a,c,d,e,f | 1.89 | 0.223 ± 0.003 §,a,c,e,f | 4.68 *,§,a,c,d,f,g | |
| stage III | 0.271 ± 0.006 | 0.154 ± 0.001 | 1.76 | 0.106 ± 0.002 | 4.03 #,§,f | |
| Kompolti | stage I | 0.618 ± 0.002 §,a,b,d,e,f | 0.330 ± 0.009 §,a,b,d,e,f | 1.87 | 0.220 ± 0.001 | 4.32 |
| stage II | 0.525 ± 0.004 §,f | 0.290 ± 0.001 | 1.79 | 0.196 ± 0.002 §,a,f | 4.15 | |
| stage III | 0.394 ± 0.002 | 0.219 ± 0.007 | 1.80 | 0.161 ± 0.001 | 3.81 *,#,§,f | |
| Tisza | stage I | 0.484 ± 0.003 | 0.241 ± 0.005 | 2.01 §,c,f,g | 0.176 ± 0.001 | 4.13 |
| stage II | 0.631 ± 0.006 *,§,a,c,e,f | 0.324 ± 0.004 *,§,a,e,f | 1.97 * | 0.223 ± 0.003 *,§,a,c,e,f | 4.29 | |
| stage III | 0.261 ± 0.004 *,# | 0.161 ± 0.001 *,# | 1.62 * | 0.111 ± 0.002 *,# | 3.79 *,#,§,f | |
| Antal | stage I | 0.565 ± 0.008 §,c,e | 0.303 ± 0.008 §,d,f | 1.86 §,f,g | 0.218 ± 0.001 §,e,c | 3.98 |
| stage II | 0.580 ± 0.001 §,a,c,f | 0.304 ± 0.003 §,a,f | 1.93 | 0.188 ± 0.001 | 4.70 §,a,c,d,f,g | |
| stage III | 0.354 ± 0.002 *,# | 0.192 ± 0.003 *,# | 1.84 | 0.142 ± 0.001 | 3.85 §,f | |
| Tiborszallasi | stage I | 0.335 ± 0.002 | 0.175 ± 0.003 | 1.91 | 0.129 ± 0.001 | 3.97 |
| stage II | 0.449 ± 0.010 * | 0.256 ± 0.002 * | 1.73 | 0.162 ± 0.003 * | 4.34 *,§,c | |
| stage III | 0.218 ± 0.001 *,# | 0.122 ± 0.001 *,# | 1.79 | 0.106 ± 0.000 *,# | 3.20 *,# | |
| Eletta Campana | stage I | 0.861 ± 0.010 §,a,b,c,d,e,f | 0.432 ± 0.004 §,a,b,c,d,e,f | 1.99 | 0.317 ± 0.003 §,d,e,f | 4.08 |
| stage II | 0.637 ± 0.001 *,§,a,c,e,f | 0.366 ± 0.002 *,§,a,c,d,e,f | 1.73 | 0.232 ± 0.001 *,§,a,c,e,f | 4.32 * | |
| stage III | 0.516 ± 0.004 *,#,§,d,f | 0.275 ± 0.004*,#,§,d,f | 1.88 | 0.205 ± 0.001 *,#,§,f | 3.86 * |
| Cultivar | Harvesting Time | TPC (mg GAE/Kg FW) | DPPH (I%) | ABTS (I%) |
|---|---|---|---|---|
| Carmagnola | stage I | 1.95 ± 0.06 | 72.87 ± 0.17 | 88.03 ± 0.39 |
| stage II | 2.00 ± 0.06 | 78.74 ± 0.07 | 90.63 ± 0.74 §,c,f | |
| stage III | 1.88 ± 0.08 | 75.38 ± 0.37 | 86.54 ± 0.89 | |
| Fibranova | stage I | 1.65 ± 0.06 | 72.27 ± 0.17 | 85.56 ± 0.75 §,e |
| stage II | 2.38 ± 0.12 | 78.68 ± 0.15 | 92.68 ± 0.82 §,c,f | |
| stage III | 2.01 ± 0.10 | 71.67 ± 0.43 | 86.66 ± 0.21 | |
| Kompolti | stage I | 2.55 ± 0.08 | 75.42 ± 3.52 | 86.91 ± 0.39 |
| stage II | 2.57 ± 0.10 §,e | 75.57 ± 0.62 | 87.89 ± 0.69 * | |
| stage III | 2.11 ± 0.06 | 72.63 ± 0.76 | 81.88 ± 0.33 # | |
| Tisza | stage I | 2.16 ± 0.09 | 73.17 ± 1.16 | 85.98 ± 0.29 §,e,c |
| stage II | 2.30 ± 0.10 | 75.29 ± 0.15 | 95.24 ± 0.16 *,§,c,d | |
| stage III | 2.02 ± 0.06 | 71.97 ± 0.57 | 84.36 ± 0.42 * | |
| Antal | stage I | 1.84 ± 0.04 | 76.03 ± 1.34 | 95.35 ± 0.42 |
| stage II | 1.67 ± 0.07 | 75.08 ± 0.81 | 84.84 ± 0.90 §,c,d,f | |
| stage III | 1.54 ± 0.08 | 74.56 ± 0.52 | 84.56 ± 0.17 §,a,b,c,d,f | |
| Tiborszallasi | stage I | 2.41 ± 0.08 | 75.39 ± 0.86 | 87.32 ± 0.31 |
| stage II | 2.68 ± 0.13 §,e | 80.68 ± 1.07 §,d | 88.03 ± 1.05 * | |
| stage III | 2.42 ± 0.09 §,a,e | 79.86 ± 0.79 | 84.18 ± 0.74 # | |
| Eletta Campana | stage I | 1.80 ± 0.0985.63 | 74.40 ± 0.51 | 91.67 ± 1.19 §,e |
| stage II | 2.10 ± 0.07 | 77.32 ± 0.44 | 93.05 ± 1.12 §,c,f | |
| stage III | 2.05 ± 0.10 | 70.44 ± 3.66 | 92.40 ± 0.46 §,a,b,c,d,f |
| Compound | ppm | Compound | ppm |
|---|---|---|---|
| Isoleucine | 1.02 | Choline | 3.21 |
| Valine | 1.05 | Myo-inositol | 3.30 |
| Threonine | 1.34 | Fructose | 4.04 |
| Alanine | 1.49 | Malic acid | 4.30 |
| Proline | 2.00 | β-Glucose | 4.66 |
| Glutamic acid | 2.07 | α-Glucose | 5.25 |
| GABA | 2.30 | Sucrose | 5.42 |
| Succinic acid | 2.41 | Phenylalanine | 7.43 |
| Glutamine | 2.46 | Tryptophan | 7.53 |
| Aspartic acid | 2.83 | Formic acid | 8.47 |
| Asparagine | 2.89 | Trigonelline | 9.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spano, M.; Di Matteo, G.; Ingallina, C.; Botta, B.; Quaglio, D.; Ghirga, F.; Balducci, S.; Cammarone, S.; Campiglia, E.; Giusti, A.M.; et al. A Multimethodological Characterization of Cannabis sativa L. Inflorescences from Seven Dioecious Cultivars Grown in Italy: The Effect of Different Harvesting Stages. Molecules 2021, 26, 2912. https://doi.org/10.3390/molecules26102912
Spano M, Di Matteo G, Ingallina C, Botta B, Quaglio D, Ghirga F, Balducci S, Cammarone S, Campiglia E, Giusti AM, et al. A Multimethodological Characterization of Cannabis sativa L. Inflorescences from Seven Dioecious Cultivars Grown in Italy: The Effect of Different Harvesting Stages. Molecules. 2021; 26(10):2912. https://doi.org/10.3390/molecules26102912
Chicago/Turabian StyleSpano, Mattia, Giacomo Di Matteo, Cinzia Ingallina, Bruno Botta, Deborah Quaglio, Francesca Ghirga, Silvia Balducci, Silvia Cammarone, Enio Campiglia, Anna Maria Giusti, and et al. 2021. "A Multimethodological Characterization of Cannabis sativa L. Inflorescences from Seven Dioecious Cultivars Grown in Italy: The Effect of Different Harvesting Stages" Molecules 26, no. 10: 2912. https://doi.org/10.3390/molecules26102912
APA StyleSpano, M., Di Matteo, G., Ingallina, C., Botta, B., Quaglio, D., Ghirga, F., Balducci, S., Cammarone, S., Campiglia, E., Giusti, A. M., Vinci, G., Rapa, M., Ciano, S., Mannina, L., & Sobolev, A. P. (2021). A Multimethodological Characterization of Cannabis sativa L. Inflorescences from Seven Dioecious Cultivars Grown in Italy: The Effect of Different Harvesting Stages. Molecules, 26(10), 2912. https://doi.org/10.3390/molecules26102912