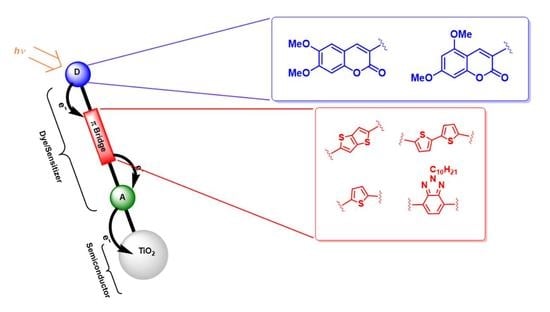
New 3-Ethynylaryl Coumarin-Based Dyes for DSSC Applications: Synthesis, Spectroscopic Properties, and Theoretical Calculations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Absorption and Fluorescence
2.3. Theoretical Calculations
2.4. Electrochemical Characterization
2.5. Photovoltaic Performance
3. Materials and Methods
3.1. General Information and Instruments
3.2. Synthesis
3.2.1. Synthesis of 6,7-Dimethoxy-3-((Trimethylsilyl)Ethynyl)Coumarin (2a) and 5,7-Dimethoxy-3-((Trimethylsilyl)Ethynyl)Coumarin (2b)
3.2.2. General Method for the Synthesis of Coupled Aldehydes (4–7)
3.2.3. General Method for the Synthesis of Final Chromophores (8–11)
3.3. Theoretical Calculations
3.4. DSSCs Fabrication and Photovoltaic Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- O’Regan, B.; Gratzel, M. A Low-Cost, High-Efficiency Solar-Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Selvaraj, P.; Ghosh, A.; Mallick, T.K.; Sundaram, S. Investigation of semi-transparent dye-sensitized solar cells for fenestration integration. Renew. Energy 2019, 141, 516–525. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, S.H. Energy Generation Performance of Window-Type Dye-Sensitized Solar Cells by Color and Transmittance. Sustainability 2020, 12, 8961. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. Dye-Sensitized Solar Cell for Indoor Applications: A Mini-Review. J. Electron. Mater. 2021. [Google Scholar] [CrossRef]
- Ghosh, A.; Selvaraj, P.; Sundaram, S.; Mallick, T.K. The colour rendering index and correlated colour temperature of dye-sensitized solar cell for adaptive glazing application. Sol. Energy 2018, 163, 537–544. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphrybaker, R.; Muller, E.; Liska, P.; Vlachopoulos, N.; Gratzel, M. Conversion of light to eletricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on nanocrystalline TiO2 electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Zakeeruddin, S.M.; Humphry-Baker, R.; Jirousek, M.; Liska, P.; Vlachopoulos, N.; Shklover, V.; Fischer, C.H.; Gratzel, M. Acid-base equilibria of (2,2′-bipyridyl-4,4′-dicarboxylic acid)ruthenium(II) complexes and the effect of protonation on charge-transfer sensitization of nanocrystalline titania. Inorg. Chem. 1999, 38, 6298–6305. [Google Scholar] [CrossRef]
- Aghazada, S.; Gao, P.; Yella, A.; Marotta, G.; Moehl, T.; Teuscher, J.; Moser, J.-E.; De Angelis, F.; Grätzel, M.; Nazeeruddin, M.K. Ligand Engineering for the Efficient Dye-Sensitized Solar Cells with Ruthenium Sensitizers and Cobalt Electrolytes. Inorg. Chem. 2016, 55, 6653–6659. [Google Scholar] [CrossRef]
- Punitharasu, V.; Kavungathodi, M.F.M.; Singh, A.K.; Nithyanandhan, J. pi-Extended cis-Configured Unsynimetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Panchromatic Response. ACS Appl. Energ. Mater. 2019, 2, 8464–8472. [Google Scholar] [CrossRef]
- Dai, P.P.; Zhu, Y.Z.; Liu, Q.L.; Yan, Y.Q.; Zheng, J.Y. Novel indeno 1,2-b indole-spirofluorene donor block for efficient sensitizers in dye-sensitized solar cells. Dyes Pigment. 2020, 175, 7. [Google Scholar] [CrossRef]
- Li, S.Z.; He, J.W.; Jiang, H.Y.; Mei, S.; Hu, Z.G.; Kong, X.F.; Yang, M.; Wu, Y.Z.; Zhang, S.H.; Tan, H.J. Comparative Studies on the Structure-Performance Relationships of Phenothiazine-Based Organic Dyes for Dye-Sensitized Solar Cells. ACS Omega 2021, 6, 6817–6823. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.B.; Ganesan, P.; Teuscher, J.; Moehl, T.; Kim, Y.J.; Yi, C.Y.; Comte, P.; Pei, K.; Holcombe, T.W.; Nazeeruddin, M.K.; et al. Influence of the Donor Size in D-pi-A Organic Dyes for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2014, 136, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.J.; Prabakaran, K.; Lee, B.; Chang, S.H.; Harutyunyan, B.; Guo, P.J.; Butler, M.R.; Timalsina, A.; Bedzyk, M.J.; Ratner, M.A.; et al. Metal-Free Tetrathienoacene Sensitizers for High-Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2015, 137, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.M.; Sun, D.Y.; Cao, Y.M.; Tsao, H.N.; Yuan, Y.; Zakeeruddin, S.M.; Wang, P.; Gratzel, M. A Stable Blue Photosensitizer for Color Palette of Dye-Sensitized Solar Cells Reaching 12.6% Efficiency. J. Am. Chem. Soc. 2018, 140, 2405–2408. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.M.; Zhou, H.R.; Eom, Y.K.; Kim, C.H.; Kim, H.K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-sensitizing Novel Thieno 3,2-b indole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10, 12. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Zehra, S.; Khan, R.A.; Alsalme, A.; Tabassum, S. Coumarin Derived “Turn on” Fluorescent Sensor for Selective Detection of Cadmium (II) Ion: Spectroscopic Studies and Validation of Sensing Mechanism by DFT Calculations. J. Fluoresc. 2019, 29, 1029–1037. [Google Scholar] [CrossRef]
- Li, H.Q.; Sun, X.Q.; Zheng, T.; Xu, Z.X.; Song, Y.X.; Gu, X.H. Coumarin-based multifunctional chemosensor for arginine/lysine and Cu2+/Al3+ ions and its Cu2+ complex as colorimetric and fluorescent sensor for biothiols. Sens. Actuator B-Chem. 2019, 279, 400–409. [Google Scholar] [CrossRef]
- Li, X.Q.; Huo, F.J.; Yue, Y.K.; Zhang, Y.B.; Yin, C.X. A coumarin-based “off-on” sensor for fluorescence selectivily discriminating GSH from Cys/Hcy and its bioimaging in living cells. Sens. Actuator B-Chem. 2017, 253, 42–49. [Google Scholar] [CrossRef]
- Peng, S.; Zhao, Y.H.; Fu, C.X.; Pu, X.M.; Zhou, L.; Huang, Y.; Lu, Z.Y. Acquiring High-Performance Deep-Blue OLED Emitters through an Unexpected Blueshift Color-Tuning Effect Induced by Electron-Donating-OMe Substituents. Chem-Eur. J. 2018, 24, 8056–8060. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, K.J.; Kim, B.; Lee, J.; Kay, K.Y.; Park, J. New Ambipolar Blue Emitting Materials Based on Amino Coumarin Derivatives with High Efficiency for Organic Lightemitting Diodes. J. Nanosci. Nanotechnol. 2013, 13, 8020–8024. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.C.; Gong, Y.X.; Yu, T.Z.; Zhao, Y.L. Synthesis and characterization of SFX-based coumarin derivatives for OLEDs. Dyes Pigment. 2021, 185, 6. [Google Scholar] [CrossRef]
- Esnal, I.; Duran-Sampedro, G.; Agarrabeitia, A.R.; Banuelos, J.; Garcia-Moreno, I.; Macias, M.A.; Pena-Cabrera, E.; Lopez-Arbeloa, I.; de la Moya, S.; Ortiz, M.J. Coumarin-BODIPY hybrids by heteroatom linkage: Versatile, tunable and photostable dye lasers for UV irradiation. Phys. Chem. Chem. Phys. 2015, 17, 8239–8247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Feng, C.; Lu, Z.Z. Tunable co-doped dye laser of coumarin 440 and coumarin 460. Laser Phys. 2021, 31, 3. [Google Scholar] [CrossRef]
- Liu, X.G.; Cole, J.M.; Waddell, P.G.; Lin, T.C.; Radia, J.; Zeidler, A. Molecular Origins of Optoelectronic Properties in Coumarin Dyes: Toward Designer Solar Cell and Laser Applications. J. Phys. Chem. A 2012, 116, 727–737. [Google Scholar] [CrossRef]
- Hara, K.; Wang, Z.S.; Sato, T.; Furube, A.; Katoh, R.; Sugihara, H.; Dan-Oh, Y.; Kasada, C.; Shinpo, A.; Suga, S. Oligothiophene-containing coumarin dyes for efficient dye-sensitized solar cells. J. Phys. Chem. B 2005, 109, 15476–15482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Cui, Y.; Hara, K.; Dan-Oh, Y.; Kasada, C.; Shinpo, A. A high-light-harvesting-efficiency coumarin dye for stable dye-sensitized solar cells. Adv. Mater. 2007, 19, 1138–1141. [Google Scholar] [CrossRef]
- Wang, Z.S.; Cui, Y.; Dan-Oh, Y.; Kasada, C.; Shinpo, A.; Hara, K. Molecular Design of Coumarin Dyes for Stable and Efficient Organic Dye-Sensitized Solar Cells. J. Phys. Chem. C 2008, 112, 17011–17017. [Google Scholar] [CrossRef]
- Wang, Z.S.; Cui, Y.; Dan-Oh, Y.; Kasada, C.; Shinpo, A.; Hara, K. Thiophene-functionalized coumarin dye for efficient dye-sensitized solar cells: Electron lifetime improved by coadsorption of deoxycholic acid. J. Phys. Chem. C 2007, 111, 7224–7230. [Google Scholar] [CrossRef]
- Jiang, S.L.; Chen, Y.Q.; Li, Y.J.; Han, L. Novel D-D-pi-A indoline-linked coumarin sensitizers for dye-sensitized solar cells. J. Photochem. Photobiol. A-Chem. 2019, 384, 7. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Gao, J.R.; Han, L. New D-D-pi-A triphenylamine-coumarin sensitizers for dye-sensitized solar cells. Photochem. Photobiol. Sci. 2017, 16, 1049–1056. [Google Scholar] [CrossRef]
- Vekariya, R.L.; Vaghasiya, J.V.; Dhar, A. Coumarin based sensitizers with ortho-halides substituted phenylene spacer for dye sensitized solar cells. Org. Electron. 2017, 48, 291–297. [Google Scholar] [CrossRef]
- Martins, S.; Avo, J.; Lima, J.; Nogueira, J.; Andrade, L.; Mendes, A.; Pereira, A.; Branco, P.S. Styryl and phenylethynyl based coumarin chromophores for dye sensitized solar cells. J. Photochem. Photobiol. A 2018, 353, 564–569. [Google Scholar] [CrossRef]
- Teng, C.; Yang, X.C.; Yang, C.; Tian, H.N.; Li, S.F.; Wang, X.N.; Hagfeldt, A.; Sun, L.C. Influence of Triple Bonds as pi-Spacer Units in Metal-Free Organic Dyes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 11305–11313. [Google Scholar] [CrossRef]
- Gordo, J.; Avo, J.; Parola, A.J.; Lima, J.C.; Pereira, A.; Branco, P.S. Convenient Synthesis of 3-Vinyl and 3-Styryl Coumarins. Org. Lett. 2011, 13, 5112–5115. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.M.A.; Branco, P.C.S.; Pereira, A. An Efficient Methodology for the Synthesis of 3-Styryl Coumarins. J. Braz. Chem. Soc. 2012, 23, 688–693. [Google Scholar] [CrossRef]
- Leao, R.A.C.; de Moraes, P.D.; Pedro, M.; Costa, P.R.R. Synthesis of Coumarins and Neoflavones through Zinc Chloride Catalyzed Hydroarylation of Acetylenic Esters with Phenols. Synthesis 2011, 2011, 3692–3696. [Google Scholar] [CrossRef]
- Su, J.L.; Zhang, Y.; Chen, M.R.; Li, W.M.; Qin, X.W.; Xie, Y.P.; Qin, L.X.; Huang, S.H.; Zhang, M. A Copper Halide Promoted Regioselective Halogenation of Coumarins Using N-Halosuccinimide as Halide Source. Synlett 2019, 30, 630–634. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhao, K.; Yao, F.; Xiong, J.; Tian, H.; Han, J. Fluorescent Nucleoside and Preparation Method Thereof. 2017. Available online: https://patents.google.com/patent/CN107325141A/en (accessed on 2 May 2019).
- Icli, M.; Pamuk, M.; Algi, F.; Onal, A.M.; Cihaner, A. Donor-Acceptor Polymer Electrochromes with Tunable Colors and Performance. Chem. Mat. 2010, 22, 4034–4044. [Google Scholar] [CrossRef]
- Pina, J.; de Castro, C.S.; Delgado-Pinar, E.; Sérgio Seixas de Melo, J. Characterization of 4-methylesculetin and of its mono- and di-methoxylated derivatives in water and organic solvents in its ground, singlet and triplet excited states. J. Mol. Liq. 2019, 278, 616–626. [Google Scholar] [CrossRef]
- Donovalová, J.; Cigáň, M.; Stankovičová, H.; Gašpar, J.; Danko, M.; Gáplovský, A.; Hrdlovič, P. Spectral Properties of Substituted Coumarins in Solution and Polymer Matrices. Molecules 2012, 17, 3259–3276. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Holze, R. Optical and Electrochemical Band Gaps in Mono-, Oligo-, and Polymeric Systems: A Critical Reassessment. Organometallics 2014, 33, 5033–5042. [Google Scholar] [CrossRef]
- Moreira, L.M.; de Melo, M.M.; Martins, P.A.; Lyon, J.P.; Romani, A.P.; Codognoto, L.; dos Santos, S.C.; de Oliveira, H.P.M. Photophysical Properties of Coumarin Compounds in Neat and Binary Solvent Mixtures: Evaluation and Correlation Between Solvatochromism and Solvent Polarity Parameters. J. Braz. Chem. Soc. 2014, 25, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Bhagwat, A.A.; Sekar, N. Fluorescent 7-Substituted Coumarin Dyes: Solvatochromism and NLO Studies. J. Fluoresc. 2019, 29, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Almenningen, A.; Bastiansen, O.; Svendsas, P. Electron Diffraction Studies of 2,2′-Dithienyl Vapour. Acta Chem. Scand. 1958, 12, 1671–1674. [Google Scholar] [CrossRef]
- Koumura, N.; Wang, Z.S.; Mori, S.; Miyashita, M.; Suzuki, E.; Hara, K. Alkyl-functionalized organic dyes for efficient molecular photovoltaics. J. Am. Chem. Soc. 2006, 128, 14256–14257. [Google Scholar] [CrossRef] [PubMed]
- Forneli, A.; Planells, M.; Sarmentero, M.A.; Martinez-Ferrero, E.; O’Regan, B.C.; Ballester, P.; Palomares, E. The role of para-alkyl substituents on meso-phenyl porphyrin sensitised TiO2 solar cells: Control of the e(TiO2)/electrolyte(+) recombination reaction. J. Mater. Chem. 2008, 18, 1652–1658. [Google Scholar] [CrossRef]
- Kim, S.; Choi, H.; Baik, C.; Song, K.; Kang, S.O.; Ko, J. Synthesis of conjugated organic dyes containing alkyl substituted thiophene for solar cell. Tetrahedron 2007, 63, 11436–11443. [Google Scholar] [CrossRef]
- Bradley, D.; Williams, G.; Lawton, M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef]
- Still, W.C.; Kahn, M.; Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. A New Mixing of Hartree-Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; Defrees, D.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. 23. A Polarization-Type Basis Set for 2nd-Row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Cammi, R.; Corni, S.; Mennucci, B.; Tomasi, J. Electronic excitation energies of molecules in solution: State specific and linear response methods for nonequilibrium continuum solvation models. J. Chem. Phys. 2005, 122, 104513. [Google Scholar] [CrossRef]
- O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Pinto, A.L.; Oliveira, J.; Araujo, P.; Calogero, G.; de Freitas, V.; Pina, F.; Parola, A.J.; Lima, J.C. Study of the multi-equilibria of red wine colorants pyranoanthocyanins and evaluation of their potential in dye-sensitized solar cells. Sol. Energy 2019, 191, 100–108. [Google Scholar] [CrossRef]





| Dye | (mm) | Solid (nm) | ε (cm−1M−1) | (nm) | Solid (nm) | ΔSS (nm) | ΔSS (cm−1) |
|---|---|---|---|---|---|---|---|
| 8 | 417 | 386 | 64,470 | 489 | 476 | 72 | 3589 |
| 9a | 420 | 380 | 30,190 | 464 | 510 | 44 | 2201 |
| 9b | 418 | 380 | 45,480 | 499 | 500 | 81 | 3998 |
| 10 | 414 | 375 | 15,300 | 486 | 488 | 72 | 3578 |
| 11 | 416 | 375 | 12,060 | 462 | 490 | 46 | 2451 |
| Dye | (nm) | Dipole Moment (D) | Transition and Orbitals Major Contributions | Oscillator Strength, F | Eg (eV) a | |
|---|---|---|---|---|---|---|
| 8 | 416 | 415 | 14.560 | S0→S1, HOMO→LUMO (83%) | 2.146 | 2.85 [2.70] |
| 9a | 421 | 424 | 19.139 | S0→S1, HOMO→LUMO (79%) | 2.156 | 2.77 [2.75] |
| 9b | 416 | 428 | 22.394 | S0→S1, HOMO→LUMO (78%); | 2.147 | 2.72 [2.73] |
| 10 | 414 | 404 | 24.230 | S0→S1, HOMO→LUMO (86%) | 1.878 | 2.97 [2.78] |
| 11 | 415 | 407 | 15.56 | S0→S1, HOMO→LUMO (84%) | 1.764 | 2.75 [2.76] |
| Dye | HOMO Energy (eV) | LUMO Energy (eV) | Eg (eV) |
|---|---|---|---|
| 8 | −5.70 | −3.57 | 2.13 |
| 9a | −5.64 | −3.58 | 2.06 |
| 9b | −5.60 | −3.53 | 2.07 |
| 10 | −5.74 | −3.62 | 2.12 |
| 11 | −5.65 | −3.60 | 2.05 |
| Dye | Voc (mV) | Jsc (mA/cm2) | Jmax (mA/cm2) | Vmax (mV) | FF | η (%) |
|---|---|---|---|---|---|---|
| 8 | 367 ± 5 | 9.3 ± 0.1 | 7.5 ± 0.2 | 256 ± 3 | 0.56 ± 0.01 | 2.00 ± 0.06 |
| 9a | 289 ± 6 | 6.7 ± 0.3 | 4.9 ± 0.3 | 193 ± 4 | 0.49 ± 0.02 | 0.95 ± 0.07 |
| 9b | 339 ± 3 | 10.2 ± 0.1 | 7.8 ± 0.2 | 227 ± 2 | 0.51 ± 0.01 | 1.78 ± 0.06 |
| 10 | 311 ± 5 | 6.4 ± 0.1 | 4.9 ± 0.3 | 214 ± 5 | 0.54 ± 0.02 | 1.07 ± 0.05 |
| 11 | 359 ± 2 | 5.4 ± 0.1 | 4.3 ± 0.2 | 258 ± 1 | 0.58 ± 0.02 | 1.13 ± 0.04 |
| N719 | 440 ± 6 | 15.5 ± 0.4 | 13.1 ± 0.2 | 305 ± 4 | 0.59 ± 0.02 | 4.06 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarrato, J.; Pinto, A.L.; Malta, G.; Röck, E.G.; Pina, J.; Lima, J.C.; Parola, A.J.; Branco, P.S. New 3-Ethynylaryl Coumarin-Based Dyes for DSSC Applications: Synthesis, Spectroscopic Properties, and Theoretical Calculations. Molecules 2021, 26, 2934. https://doi.org/10.3390/molecules26102934
Sarrato J, Pinto AL, Malta G, Röck EG, Pina J, Lima JC, Parola AJ, Branco PS. New 3-Ethynylaryl Coumarin-Based Dyes for DSSC Applications: Synthesis, Spectroscopic Properties, and Theoretical Calculations. Molecules. 2021; 26(10):2934. https://doi.org/10.3390/molecules26102934
Chicago/Turabian StyleSarrato, João, Ana Lucia Pinto, Gabriela Malta, Eva G. Röck, João Pina, João Carlos Lima, A. Jorge Parola, and Paula S. Branco. 2021. "New 3-Ethynylaryl Coumarin-Based Dyes for DSSC Applications: Synthesis, Spectroscopic Properties, and Theoretical Calculations" Molecules 26, no. 10: 2934. https://doi.org/10.3390/molecules26102934
APA StyleSarrato, J., Pinto, A. L., Malta, G., Röck, E. G., Pina, J., Lima, J. C., Parola, A. J., & Branco, P. S. (2021). New 3-Ethynylaryl Coumarin-Based Dyes for DSSC Applications: Synthesis, Spectroscopic Properties, and Theoretical Calculations. Molecules, 26(10), 2934. https://doi.org/10.3390/molecules26102934