Mechanism of Producing Metallic Nanoparticles, with an Emphasis on Silver and Gold Nanoparticles, Using Bottom-Up Methods
Abstract
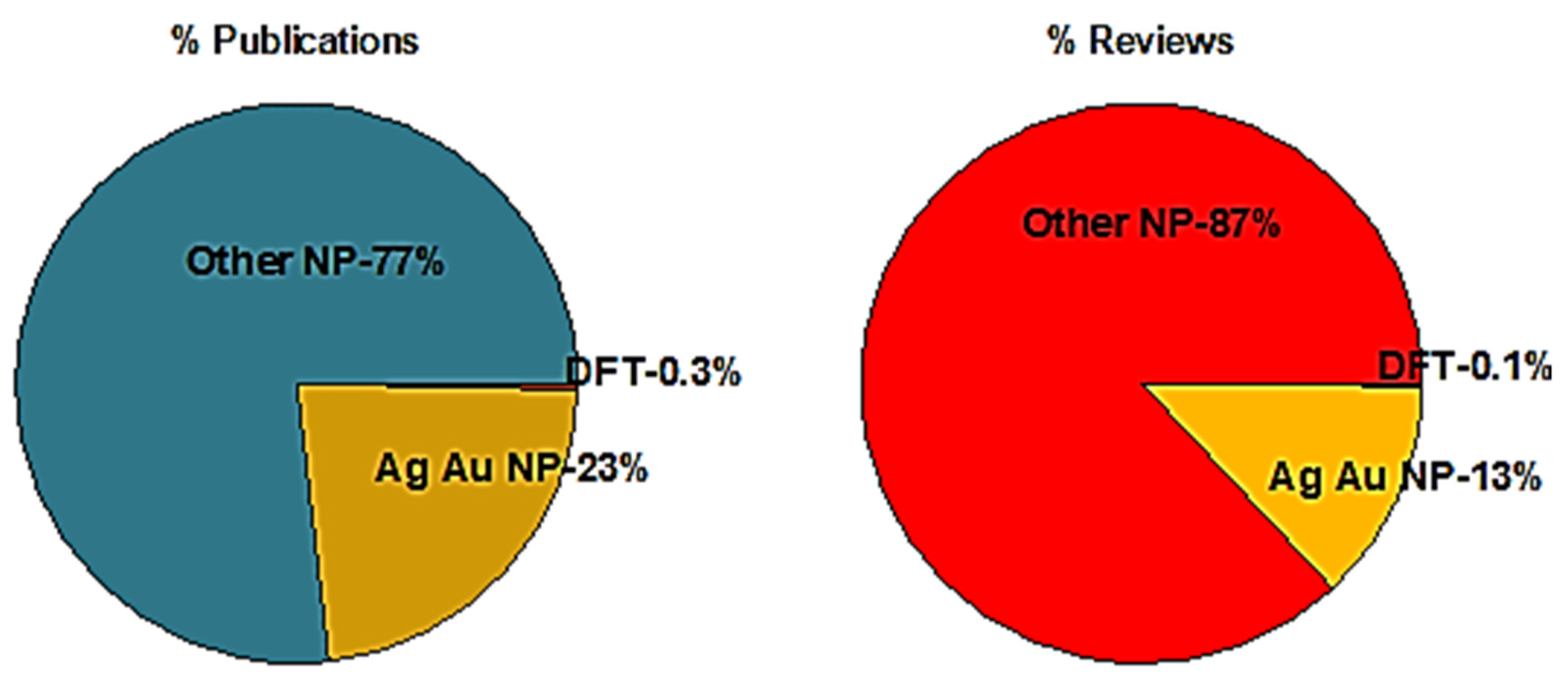
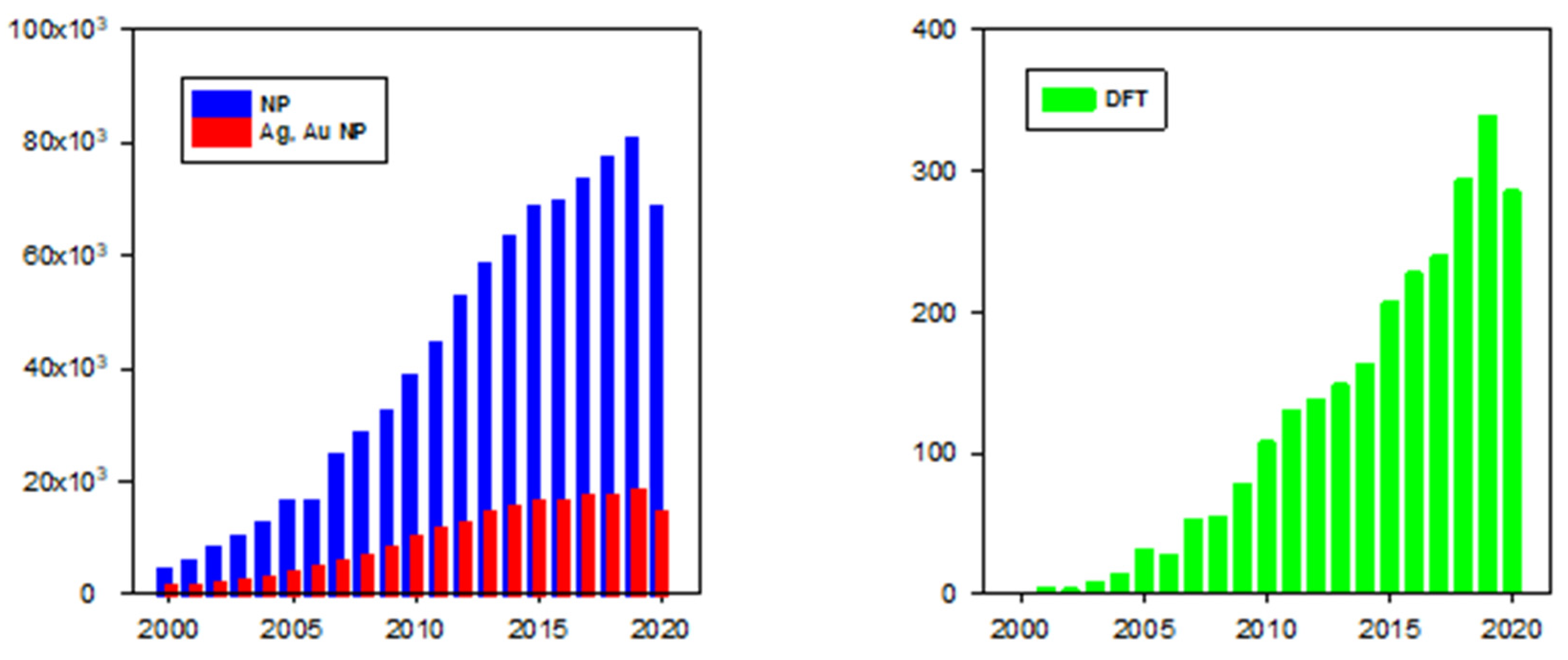
1. Introduction
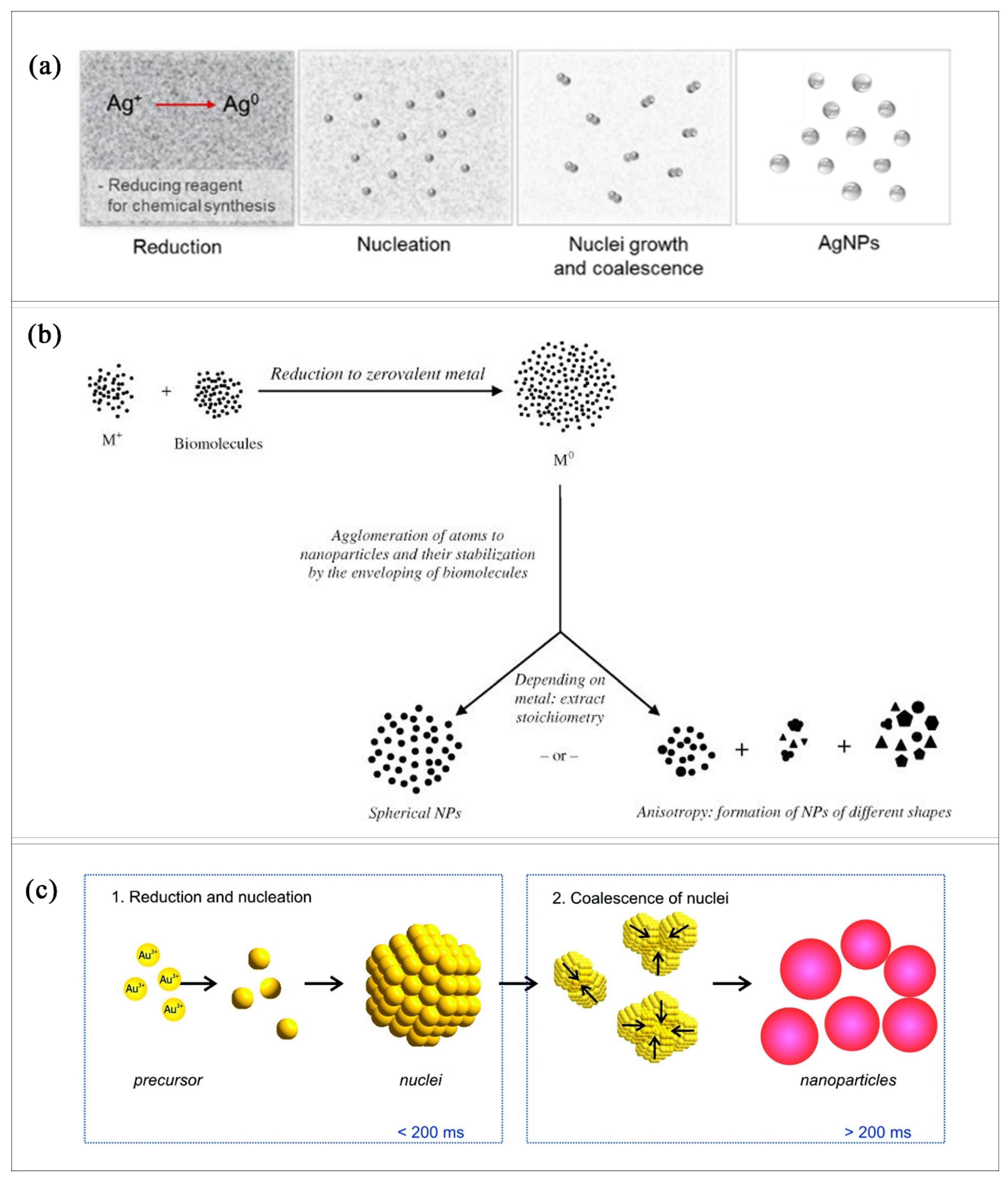
2. Production of AgNPs and AuNPs—Assumed Mechanism
3. Standard Reduction Potentials
4. Formation of M-M M-MI and MI-MI Bonds
5. Formation of AgNPs and AuNPs—A New Approach
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiss, F.D.; Miotto, R.; Ferraz, A.C. Size effects on silver nanoparticles’ properties. Nanotechnology 2011, 22, 275708. [Google Scholar] [CrossRef]
- González, A.L.; Noguez, C.; Beránek, J.; Barnard, A.S. Size, shape, stability, and color of plasmonic silver nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y. Ben Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M.; Stener, M.; Guo, Y.; Chen, S.; Crespo, P.; García, M.A.; Hernando, A.; Pengo, P.; Pasquato, L. Physico-Chemical Characteristics of Gold Nanoparticles. In Comprehensive Analytical Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 66, pp. 81–152. [Google Scholar]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Moyano, D.F.; Rotello, V.M. Nano meets biology: Structure and function at the nanoparticle interface. Langmuir 2011, 27, 10376–10385. [Google Scholar] [CrossRef]
- Mokammel, M.A.; Islam, M.J.; Hasanuzzaman, M.; Hashmi, S. Nanoscale Materials for Self-Cleaning and Antibacterial Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. Gold Nanoparticles as a Potent Radiosensitizer: A Transdisciplinary Approach from Physics to Patient. Cancers 2020, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular Transport of Silver and Gold Nanoparticles and Biological Responses: An Update. Int. J. Mol. Sci. 2018, 19, 1305. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, D.H.; Lee, H.S.; Jang, C.W.; Kim, J.M.; Seo, S.W.; Kim, S.; Choi, S.H. Enhancement of efficiency in graphene/porous silicon solar cells by co-doping graphene with gold nanoparticles and bis(trifluoromethanesulfonyl)-amide. J. Mater. Chem. C 2017, 5, 9005–9011. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1–8. [Google Scholar] [CrossRef]
- Singh, P.; Singh, H.; Kim, Y.J.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Extracellular synthesis of silver and gold nanoparticles by Sporosarcina koreensis DC4 and their biological applications. Enzym. Microb. Technol. 2016, 86, 75–83. [Google Scholar] [CrossRef]
- Soshnikova, V.; Kim, Y.J.; Singh, P.; Huo, Y.; Markus, J.; Ahn, S.; Castro-Aceituno, V.; Kang, J.; Chokkalingam, M.; Mathiyalagan, R.; et al. Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Xu, S.; Ouyang, W.; Xie, P.; Lin, Y.; Qiu, B.; Lin, Z.; Chen, G.; Guo, L. Highly Uniform Gold Nanobipyramids for Ultrasensitive Colorimetric Detection of Influenza Virus. Anal. Chem. 2017, 89, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Koduru, J.R.; Desai, M.L.; Park, T.J.; Singhal, R.K.; Basu, H. Recent progress on surface chemistry of plasmonic metal nanoparticles for colorimetric assay of drugs in pharmaceutical and biological samples. TrAC Trends Anal. Chem. 2018, 105, 106–120. [Google Scholar] [CrossRef]
- Reznickova, A.; Novotna, Z.; Kvitek, O.; Kolska, Z.; Svorcik, V. Gold, silver and carbon nanoparticles grafted on activated polymers for biomedical applications. J. Nanosci. Nanotechnol. 2015, 15, 10053–10073. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ardjmand, M.; Baei, M.S.; Rad, A.S.; Akbarzadeh, A. Conjugation of pectin biopolymer with Au-nanoparticles as a drug delivery system: Experimental and DFT studies. Appl. Organomet. Chem. 2020, 34. [Google Scholar] [CrossRef]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef]
- Panda, M.K.; Singh, Y.D.; Behera, R.K.; Dhal, N.K. Biosynthesis of Nanoparticles and Their Potential Application in Food and Agricultural Sector. In Green Nanoparticles. Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020; pp. 213–225. [Google Scholar]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Kumar, S.; Srivastava, A.K.; Sharma, A.K. Myconanotechnology in agriculture: A perspective. World J. Microbiol. Biotechnol. 2013, 29, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, N.R.; Wilson, K.S.J. Optical properties of silver nanocomposites and photonic band gap—Pressure dependence. Opt. Commun. 2016, 368, 174–179. [Google Scholar] [CrossRef]
- Venditti, I. Gold Nanoparticles in Photonic Crystals Applications: A Review. Materials 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, S.; Liu, B.; Verzhbitskiy, I.; Li, S.; Giustiniano, F.; Kozawa, D.; Loh, K.P.; Matsuda, K.; Okamoto, K.; et al. Exciton-Plasmon Coupling and Electromagnetically Induced Transparency in Monolayer Semiconductors Hybridized with Ag Nanoparticles. Adv. Mater. 2016, 28, 2709–2715. [Google Scholar] [CrossRef]
- Feng, L.; Gao, G.; Huang, P.; Wang, K.; Wang, X.; Luo, T.; Zhang, C. Optical properties and catalytic activity of bimetallic gold-silver nanoparticles. Nano Biomed. Eng. 2010, 2, 258–267. [Google Scholar] [CrossRef]
- Teranishi, T. Fabrication and electronic properties of gold nanoparticle superlattices. Comptes Rendus Chim. 2003, 6, 979–987. [Google Scholar] [CrossRef]
- Peng, P.; Hu, A.; Gerlich, A.P.; Zou, G.; Liu, L.; Zhou, Y.N. Joining of Silver Nanomaterials at Low Temperatures: Processes, Properties, and Applications. ACS Appl. Mater. Interfaces 2015, 7, 12597–12618. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative plasmonic materials: Beyond gold and silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Daneshafruz, H.; Sheibani, H. Gold nanoparticles on cyanuric citric acid functionalized magnetic SBA-16 as an effective catalyst for dye reduction. Phys. E Low-Dimens. Syst. Nanostructures 2021, 126, 114392. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cha, S.-H.; Kim, H.; Choi, S.E.; Cho, S.; Park, Y. Diallyl disulphide-loaded spherical gold nanoparticles and acorn-like silver nanoparticles synthesised using onion extract: Catalytic activity and cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2020, 48, 948–960. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, H.; Zhang, H.; Jiang, J. A promising Ag2CrO4/LaFeO3 heterojunction photocatalyst applied to photo-Fenton degradation of RhB. Environ. Technol. 2020, 41, 1486–1503. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dhaliwal, A.S. Plasmon-induced photocatalytic degradation of methylene blue dye using biosynthesized silver nanoparticles as photocatalyst. Environ. Technol. 2020, 41, 1520–1534. [Google Scholar] [CrossRef]
- Sudheeshkumar, V.; Sulaiman, K.O.; Scott, R.W.J. Activation of atom-precise clusters for catalysis. Nanoscale Adv. 2020, 2, 55–69. [Google Scholar] [CrossRef]
- Khurana, K.; Jaggi, N. Localized Surface Plasmonic Properties of Au and Ag Nanoparticles for Sensors: A Review. Plasmonics 2021, 1, 3. [Google Scholar]
- Freitas de Freitas, L.; Varca, G.; dos Santos Batista, J.; Benévolo Lugão, A. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Daruich De Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. In Supramolecular Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S.; Tsunoji, N.; Henzie, J.; Takei, T.; Yamauchi, Y.; Ide, Y. Templated synthesis of atomically-thin Ag nanocrystal catalysts in the interstitial space of a layered silicate. Chem. Commun. 2018, 54, 4402–4405. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.E.; Liu, X.; Assadi, M.H.N.; Younis, A.; Yasukawa, Y.; Karan, S.K.; Zhang, J.; Kim, J.; Wang, D.; Morisako, A.; et al. Au quantum dots engineered room temperature crystallization and magnetic anisotropy in CoFe2O4 thin films. Nanoscale Horiz. 2019, 4, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wu, X.; Wang, H.; Wang, H.; Zhou, J. Photo-reduction of Ag nanoparticles by using cellulose-based micelles as soft templates: Catalytic and antimicrobial activities. Carbohydr. Polym. 2019, 213, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Ahn, H.Y.; Mun, J.; Lee, Y.Y.; Kim, M.; Cho, N.H.; Chang, K.; Kim, W.S.; Rho, J.; Nam, K.T. Amino-acid- A nd peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 2018, 556, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Werner, D.; Uwada, T. Studies on the interaction of pulsed lasers with plasmonic gold nanoparticles toward light manipulation, heat management, and nanofabrication. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 28–54. [Google Scholar] [CrossRef]
- Gutenberg Open Science: Understanding Shape Control in Gold Nanoparticles from Molecular Dynamics Simulations. Available online: https://openscience.ub.uni-mainz.de/handle/20.500.12030/4558 (accessed on 15 March 2021).
- Jiang, S.; Zhang, Y.; Gan, Y.; Chen, Z.; Peng, H. Molecular dynamics study of neck growth in laser sintering of hollow silver nanoparticles with different heating rates. J. Phys. D Appl. Phys. 2013, 46, 335302. [Google Scholar] [CrossRef]
- Zeng, Q.; Jiang, X.; Yu, A.; Lu, G. Growth mechanisms of silver nanoparticles: A molecular dynamics study. Nanotechnology 2007, 18, 035708. [Google Scholar] [CrossRef]
- Meena, S.K.; Sulpizi, M. Understanding the microscopic origin of gold nanoparticle anisotropic growth from molecular dynamics simulations. Langmuir 2013, 29, 14954–14961. [Google Scholar] [CrossRef]
- Yang, P.; Zeng, Q.; Dong, K.; Zhu, H. A quick method for developing interparticle force models of spherical gold nanoparticles from molecular dynamics simulation. Powder Technol. 2020, 362, 501–506. [Google Scholar] [CrossRef]
- Kaminski, M.; Jurkiewicz, K.; Burian, A.; Brodka, A. The structure of gold nanoparticles: Molecular dynamics modeling and its verification by X-ray diffraction. J. Appl. Crystallogr. 2020, 53, 1–8. [Google Scholar] [CrossRef]
- Pan, H.; Ko, S.H.; Grigoropoulos, C.P. The solid-state neck growth mechanisms in low energy laser sintering of gold nanoparticles: A Molecular dynamics simulation study. J. Heat Transf. 2008, 130. [Google Scholar] [CrossRef]
- Barngrover, B.M.; Aikens, C.M. The golden pathway to thiolate-stabilized nanoparticles: Following the formation of gold(I) thiolate from gold(III) chloride. J. Am. Chem. Soc. 2012, 134, 12590–12595. [Google Scholar] [CrossRef][Green Version]
- Toroz, D.; Corni, S. Peptide synthesis of gold nanoparticles: The early steps of gold reduction investigated by density functional theory. Nano Lett. 2011, 11, 1313–1318. [Google Scholar] [CrossRef]
- Barngrover, B.M.; Manges, T.J.; Aikens, C.M. Prediction of nonradical Au(0)-containing precursors in nanoparticle growth processes. J. Phys. Chem. A 2015, 119, 889–895. [Google Scholar] [CrossRef]
- Yoneya, M.; Sugisawa, S.Y. Simulation of Colloidal Silver Nanoparticle Formation from a Precursor Complex. J. Phys. Chem. C 2019, 123, 11257–11263. [Google Scholar] [CrossRef]
- Li, Y.; Zaluzhna, O.; Zangmeister, C.D.; Allison, T.C.; Tong, Y.J. Different mechanisms govern the two-phase Brust-Schiffrin dialkylditelluride syntheses of Ag and Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 1990–1992. [Google Scholar] [CrossRef] [PubMed]
- Saldías, C.; Leiva, Á.; Bonardd, S.; Quezada, C.; Saldías, S.; Pino, M.; Radic’, D. A facile one-step synthesis of noble metal nanoparticles in DMSO using poly(ethylene glycol)-poly(ε-caprolactone) block copolymers. React. Funct. Polym. 2015, 96, 78–88. [Google Scholar] [CrossRef]
- Zehlike, L.; Peters, A.; Ellerbrock, R.H.; Degenkolb, L.; Klitzke, S. Aggregation of TiO2 and Ag nanoparticles in soil solution—Effects of primary nanoparticle size and dissolved organic matter characteristics. Sci. Total Environ. 2019, 688, 288–298. [Google Scholar] [CrossRef]
- Singh, D.K.; Jagannathan, R.; Khandelwal, P.; Abraham, P.M.; Poddar, P. In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: An experimental and density functional theory investigation. Nanoscale 2013, 5, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.E.; Giacomelli, C.E. Stability of silver nanoparticles: Agglomeration and oxidation in biological relevant conditions. J. Nanoparticle Res. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Pillay, D.; Wang, Y.; Hwang, G.S. Nucleation and growth of 1B metal clusters on rutile TiO2(1 1 0): Atomic level understanding from first principles studies. Catal. Today 2005, 105, 78–84. [Google Scholar] [CrossRef]
- Tamuly, C.; Hazarika, M.; Bordoloi, M.; Bhattacharyya, P.K.; Kar, R. Biosynthesis of Ag nanoparticles using pedicellamide and its photocatalytic activity: An eco-friendly approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Ojea-Jiménez, I.; Campanera, J.M. Molecular modeling of the reduction mechanism in the citrate-mediated synthesis of gold nanoparticles. J. Phys. Chem. C 2012, 116, 23682–23691. [Google Scholar] [CrossRef]
- Graham, T.R.; Renslow, R.; Govind, N.; Saunders, S.R. Precursor Ion-Ion Aggregation in the Brust-Schiffrin Synthesis of Alkanethiol Nanoparticles. J. Phys. Chem. C 2016, 120, 19837–19847. [Google Scholar] [CrossRef]
- Hudgens, J.W.; Pettibone, J.M.; Senftle, T.P.; Bratton, R.N. Reaction mechanism governing formation of 1,3-bis(diphenylphosphino) propane-protected gold nanoclusters. Inorg. Chem. 2011, 50, 10178–10189. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Schaaff, T.G.; Shafigullin, M.N.; Khoury, J.T.; Vezmar, I.; Whetten, R.L.; Cullen, W.G.; First, P.N.; Gutiérrez-Wing, C.; Ascensio, J.; Jose-Yacamán, M.J. Isolation of smaller nanocrystal Au molecules: Robust quantum effects in optical spectra. J. Phys. Chem. B 1997, 101, 7885–7891. [Google Scholar] [CrossRef]
- Briñas, R.P.; Hu, M.; Qian, L.; Lymar, E.S.; Hainfeld, J.F. Gold nanoparticle size controlled by polymeric Au(I) thiolate precursor size. J. Am. Chem. Soc. 2008, 130, 975–982. [Google Scholar] [CrossRef]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-protected gold clusters revisited: Bridging the gap between gold(I)-thiolate complexes and thiolate-protected gold nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lanni, E.; Garg, N.; Bier, M.E.; Jin, R. Kinetically controlled, high-yield synthesis of Au25 clusters. J. Am. Chem. Soc. 2008, 130, 1138–1139. [Google Scholar] [CrossRef]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, C.; Guo, C.; Wang, X.; Sun, P.; Zhou, D.; Chen, W.; Xue, G. New insight into intermediate precursors of Brust-Schiffrin gold nanoparticles synthesis. J. Phys. Chem. C 2013, 117, 11399–11404. [Google Scholar] [CrossRef]
- Anuradha, J.; Abbasi, T.; Abbasi, S.A. An eco-friendly method of synthesizing gold nanoparticles using an otherwise worthless weed pistia (Pistia stratiotes L.). J. Adv. Res. 2015, 6, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Erler, R.; Thünemann, A.F.; Sokolov, S.; Ahner, T.T.; Rademann, K.; Emmerling, F.; Kraehnert, R. Nucleation and growth of gold nanoparticles studied via in situ small angle X-ray scattering at millisecond time resolution. ACS Nano 2010, 4, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Liz-Marzán, M. Formation and Stabilization of Silver Nanoparticles through Reduction by N,N-Dimethylformamide. Langmuir 1999, 15, 948–951. [Google Scholar] [CrossRef]
- Onesto, V.; Gentile, F.; Russo, M.; Villani, M.; Candeloro, P.; Perozziello, G.; Malara, N.; Fabrizio, E.D.; Coluccio, M.L. Kinetic Rate Constants of Gold Nanoparticle Deposition on Silicon. Langmuir 2019, 35, 14258–14265. [Google Scholar] [CrossRef]
- Zavras, A.; Ariafard, A.; Khairallah, G.N.; White, J.M.; Mulder, R.J.; Canty, A.J.; O’Hair, R.A.J. Synthesis, structure and gas-phase reactivity of the mixed silver hydride borohydride nanocluster [Ag3(μ3-H)(μ3-BH4)LPh3]BF4 (LPh = bis(diphenylphosphino)methane). Nanoscale 2015, 7, 18129–18137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, J.Y.; Wu, W.W. In Situ Observation of Au Nanostructure Evolution in Liquid Cell TEM. J. Phys. Chem. C 2017, 121, 26069–26075. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 85th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bratsch, S.G. Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef]
- Mondal, T.; Sermiagin, A.; Meyerstein, D.; Zidki, T.; Kornweitz, H. On the mechanism of reduction of M(H2O)mn+ by borohydride: The case of Ag(H2O)2+. Nanoscale 2020, 12, 1657–1672. [Google Scholar] [CrossRef]
- Linnert, T.; Mulvaney, P.; Henglein, A.; Weller, H. Long-Lived Nonmetallic Silver Clusters in Aqueous Solution: Preparation and Photolysis. J. Am. Chem. Soc. 1990, 112, 4657–4664. [Google Scholar] [CrossRef]
- Henglein, A. Colloidal Silver Nanoparticles: Photochemical Preparation and Interaction with O2, CCl4, and Some Metal Ions. Chem. Mater. 1998, 10, 444–450. [Google Scholar] [CrossRef]
- Halpern, J. Homogeneous catalytic activation of molecular hydrogen by metal ions and complexes. J. Phys. Chem. 1959, 63, 398–403. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, A.Q.; Li, H.J.; Qian, D.J.; Chen, M. Synthesis, Study, and Discrete Dipole Approximation Simulation of Ag-Au Bimetallic Nanostructures. Nanoscale Res. Lett. 2016, 11, 209. [Google Scholar] [CrossRef]
- Merga, G.; Wilson, R.; Lynn, G.; Milosavljevic, B.H.; Meisel, D. Redox catalysis on “naked” silver nanoparticles. J. Phys. Chem. C 2007, 111, 12220–12226. [Google Scholar] [CrossRef]
- Kopple, K.; Meyerstein, D.; Meisel, D. Mechanism of the catalytic hydrogen production by gold sols. H/D isotope effect studies. J. Phys. Chem. 1980, 84, 870–875. [Google Scholar] [CrossRef]
- Huang, H.; Du Toit, H.; Ben-Jaber, S.; Wu, G.; Panariello, L.; Thanh, N.T.K.; Parkin, I.P.; Gavriilidis, A. Rapid synthesis of gold nanoparticles with carbon monoxide in a microfluidic segmented flow system. React. Chem. Eng. 2019, 4, 884–890. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Jia, C.J.; Schüth, F. Colloidal metal nanoparticles as a component of designed catalyst. Phys. Chem. Chem. Phys. 2011, 13, 2457–2487. [Google Scholar] [CrossRef]
- Evanoff, D.D.; Chumanov, G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lub, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef] [PubMed]
- Roucoux, A.; Schulz, J.; Patin, H. Reduced transition metal colloids: A novel family of reusable catalysts? Chem. Rev. 2002, 102, 3757–3778. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.E. Iron nanoparticles as potential magnetic carriers. J. Magn. Magn. Mater. 2001, 225, 17–20. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.N. Chemical Catalysis by Colloids and Clusters. Chem. Rev. 1993, 93, 2693–2730. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Tatykhanova, G.S.; Selenova, B.S. Polymer Protected and Gel Immobilized Gold and Silver Nanoparticles in Catalysis. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1198–1211. [Google Scholar] [CrossRef]
- Adhikary, J.; Meyerstein, D.; Marks, V.; Meistelman, M.; Gershinsky, G.; Burg, A.; Shamir, D.; Kornweitz, H.; Albo, Y. Sol-gel entrapped Au0- and Ag0-nanoparticles catalyze reductive de-halogenation of halo-organic compounds by BH4−. Appl. Catal. B Environ. 2018, 239, 450–462. [Google Scholar] [CrossRef]
- Mondal, J.; Kundu, S.K.; Hung Ng, W.K.; Singuru, R.; Borah, P.; Hirao, H.; Zhao, Y.; Bhaumik, A. Fabrication of Ruthenium Nanoparticles in Porous Organic Polymers: Towards Advanced Heterogeneous Catalytic Nanoreactors. Chem. Eur. J. 2015, 21, 19016–19027. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jeong, Y.; Yu, J. Spontaneous hydrolysis of borohydride required before its catalytic activation by metal nanoparticles. Catal. Commun. 2016, 84, 80–84. [Google Scholar] [CrossRef]
- Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C.; Mulfinger, L. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar] [CrossRef]
- Shahwan, T.; Abu Sirriah, S.; Nairat, M.; Boyaci, E.; Eroĝlu, A.E.; Scott, T.B.; Hallam, K.R. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Hafez, M.E.; Ma, H.; Peng, Y.Y.; Ma, W.; Long, Y.T. Correlated Anodic-Cathodic Nanocollision Events Reveal Redox Behaviors of Single Silver Nanoparticles. J. Phys. Chem. Lett. 2019, 10, 3276–3281. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis; W. H. Freeman: New York, NY, USA, 2007; ISBN 9781319164300. [Google Scholar]
- Povar, I.; Spinu, O. Ruthenium redox equilibria: 3. Pourbaix diagrams for the systems Ru-H2O and Ru-Cl--H2O. J. Electrochem. Sci. Eng. 2016, 6, 145–153. [Google Scholar] [CrossRef]
- Kornweitz, H.; Meyerstein, D. Mechanisms of Reduction of M(H2O)kn+ to Form M°-Nano-Particles in Aqueous Solutions Differs from That Commonly Assumed: The Reduction of Ag(H2O)2+ by H2. J. Phys. Chem. C 2018, 122, 25043–25050. [Google Scholar] [CrossRef]
- Mondal, T.; Sermiagin, A.; Zidki, T.; Bogot, A.; Meyerstein, D.; Kornweitz, H. On the Differences in the Mechanisms of Reduction of AuCl2− and Ag(H2O)2+ with BH4−. J. Phys. Chem. A 2021. [Google Scholar] [CrossRef]
- Baetzold, R.C. Silver-Water Clusters: A Computation of Agn(H2O)m for n = 1–6; M = 1–8. J. Phys. Chem. C 2017, 121, 11811–11823. [Google Scholar] [CrossRef]
- Henglein, A.; Mulvaney, P.; Linnert, T. Chemistry of Agn aggregates in aqueous solution: Non-metallic oligomeric clusters and metallic particles. Faraday Discuss. 1991, 92, 31–44. [Google Scholar] [CrossRef]
- Jin, R.; Zhu, Y.; Qian, H. Quantum-sized gold nanoclusters: Bridging the gap between organometallics and nanocrystals. Chem. Eur. J. 2011, 17, 6584–6593. [Google Scholar] [CrossRef] [PubMed]
- Azubel, M.; Koivisto, J.; Malola, S.; Bushnell, D.; Hura, G.L.; Koh, A.L.; Tsunoyama, H.; Tsukuda, T.; Pettersson, M.; Häkkinen, H.; et al. Electron microscopy of gold nanoparticles at atomic resolution. Science 2014, 345, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Nhat, P.V.; Si, N.T.; Nguyen, M.T. Structural Evolution and Stability Trend of Small-Sized Gold Clusters Aun (n = 20–30). J. Phys. Chem. A 2020, 124, 1289–1299. [Google Scholar] [CrossRef]
- Reilly, S.M.; Krick, T.; Dass, A. Surfactant-free synthesis of ultrasmall gold nanoclusters. J. Phys. Chem. C 2010, 114, 741–745. [Google Scholar] [CrossRef]
- Donkers, R.L.; Lee, D.; Murray, R.W. Synthesis and isolation of the molecule-like cluster Au38(PhCH2CH2S)24. Langmuir 2004, 20, 1945–1952. [Google Scholar] [CrossRef]
- Barngrover, B.M.; Aikens, C.M. Electron and hydride addition to gold(I) thiolate oligomers: Implications for gold-thiolate nanoparticle growth mechanisms. J. Phys. Chem. Lett. 2011, 2, 990–994. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of Colloidal Silver Nanoparticles: Capping Action of Citrate Arnim Henglein. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Ershov, B.G.; Janata, E.; Henglein, A.; Fojtik, A. Silver atoms and clusters in aqueous solution: Absorption spectra and the particle growth in the absence of stabilizing Ag+ ions. J. Phys. Chem. 1993, 97, 4589–4594. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, H. Strong Silver-Silver Interactions in Three Silver(I) Carboxylate Complexes with High Cytotoxicity Properties. Synth. React. Inorg. Met. Nano-Metal Chem. 2005, 35, 325–332. [Google Scholar] [CrossRef]
- Bazhanova, Z.G.; Tarasov, Y.I.; Kovtun, D.M.; Boltalin, A.I.; Novosadov, B.K.; Kochikov, I.V. A quantum chemical study of the structure of fluorinated silver acetate(I) monomers and dimers. J. Struct. Chem. 2010, 51, 409–418. [Google Scholar] [CrossRef]
- Jordan, A.J.; Lalic, G.; Sadighi, J.P. Coinage Metal Hydrides: Synthesis, Characterization, and Reactivity. Chem. Rev. 2016, 116, 8318–8372. [Google Scholar] [CrossRef]
- Dubiel, M.; Brunsch, S.; Kolb, U.; Gutwerk, D.; Bertagnolli, H. Experimental studies investigating the structure of soda-lime glasses after silver-sodium ion exchange. J. Non-Cryst. Solids 1997, 220, 30–44. [Google Scholar] [CrossRef]
- Loh, N.D.; Sen, S.; Bosman, M.; Tan, S.F.; Zhong, J.; Nijhuis, C.A.; Král, P.; Matsudaira, P.; Mirsaidov, U. Multistep nucleation of nanocrystals in aqueous solution. Nat. Chem. 2017. [Google Scholar] [CrossRef]
- Wu, Z.; Suhan, J.; Jin, R. One-pot synthesis of atomically monodisperse, thiol-functionalized Au 25 nanoclusters. J. Mater. Chem. 2009, 19, 622–626. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1979, 75, 790–798. [Google Scholar] [CrossRef]
| Metal | Redox Couple | E0(Mn+/) (M0solid) V vs. SHE [89] | E0(Mn+/) (M0aq) V vs. SHE [91] |
|---|---|---|---|
| Zinc | Zn2+(aq)/Zn0 | −0.76 | −1.25 |
| Cadmium | Cd2+(aq)/Cd0 | −0.4 | −0.8 |
| Mercury | Hg2+(aq)/Hg0 | +0.85a | 0.68 |
| Copper | Cu+(aq)/Cu0 | 0.52 | −2.57 |
| Silver | Ag+(aq)/Ag0 | 0.8 | −1.74 |
| Gold | AuCl2−(aq)/Au0 +2Cl−(aq) | 1.15 [114] | −2.23 |
| Nickel | Ni2+(aq)/Ni0 | −0.25 | −2.24 |
| Palladium | Pd2+(aq)/Pd0 | 0.95 | −0.81 |
| Platinum | Pt2+(aq)/Pt0 | 1.18 | −1.51 |
| Cobalt | Co2+(aq)/Co0 | −0.28 | −2.25 |
| Rhodium | RhCl63−(aq)/(Rh0+6Cl−(aq)) | +0.43 | −1.33 |
| Iridium | IrCl62−(aq)/(Ir0 + 6Cl−(aq)) | +0.77 | −0.83 |
| Iron | Fe2+(aq)/Fe0 | −0.44 | −2.36 |
| Ruthenium | RuCl3(aq)/(Ru0 + 3Cl−(aq)) | 0.51 [115] | −1.55 |
| Osmium | (OsO4(aq) + 8H+(aq))/(Os0 + 4H2O) | +0.84 | −0.12 |
| Manganese | Mn2+(aq)/Mn0 | −1.19 | −2.43 |
| Chromium | Cr3+(aq)/Cr0 | −0.74 | −1.95 |
| Vanadium | V2+(aq)/V0 | −1.17 | −5.08 |
| Titanium | Ti3+(aq)/Ti0 | −1.37 | −3.17 |
| Reaction | ΔG0 (kcal/mol) | ΔG# (kcal/mol) | ||
|---|---|---|---|---|
| M = Ag+ [91] | M = Au+ [117] | M = Ag+ [91] | M = Au+ [117] | |
| ML(BH4)n + H2O → ML(BH3OH)n + H2 a | 0.38 | −3.61 | 20.09 | 23.73 |
| M(BH4)2− + H2O → M(BH4)(BH3OH)− + H2 | −0.05 | −3.01 | 19.88 | 22.16 |
| M(BH4)(BH3OH)− + H2O → M(BH4)2− + H2 | 0.09 | −3.00 | 19.72 | 20.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimadom, B.R.; Kornweitz, H. Mechanism of Producing Metallic Nanoparticles, with an Emphasis on Silver and Gold Nanoparticles, Using Bottom-Up Methods. Molecules 2021, 26, 2968. https://doi.org/10.3390/molecules26102968
Karimadom BR, Kornweitz H. Mechanism of Producing Metallic Nanoparticles, with an Emphasis on Silver and Gold Nanoparticles, Using Bottom-Up Methods. Molecules. 2021; 26(10):2968. https://doi.org/10.3390/molecules26102968
Chicago/Turabian StyleKarimadom, Basil Raju, and Haya Kornweitz. 2021. "Mechanism of Producing Metallic Nanoparticles, with an Emphasis on Silver and Gold Nanoparticles, Using Bottom-Up Methods" Molecules 26, no. 10: 2968. https://doi.org/10.3390/molecules26102968
APA StyleKarimadom, B. R., & Kornweitz, H. (2021). Mechanism of Producing Metallic Nanoparticles, with an Emphasis on Silver and Gold Nanoparticles, Using Bottom-Up Methods. Molecules, 26(10), 2968. https://doi.org/10.3390/molecules26102968








