Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments
Abstract
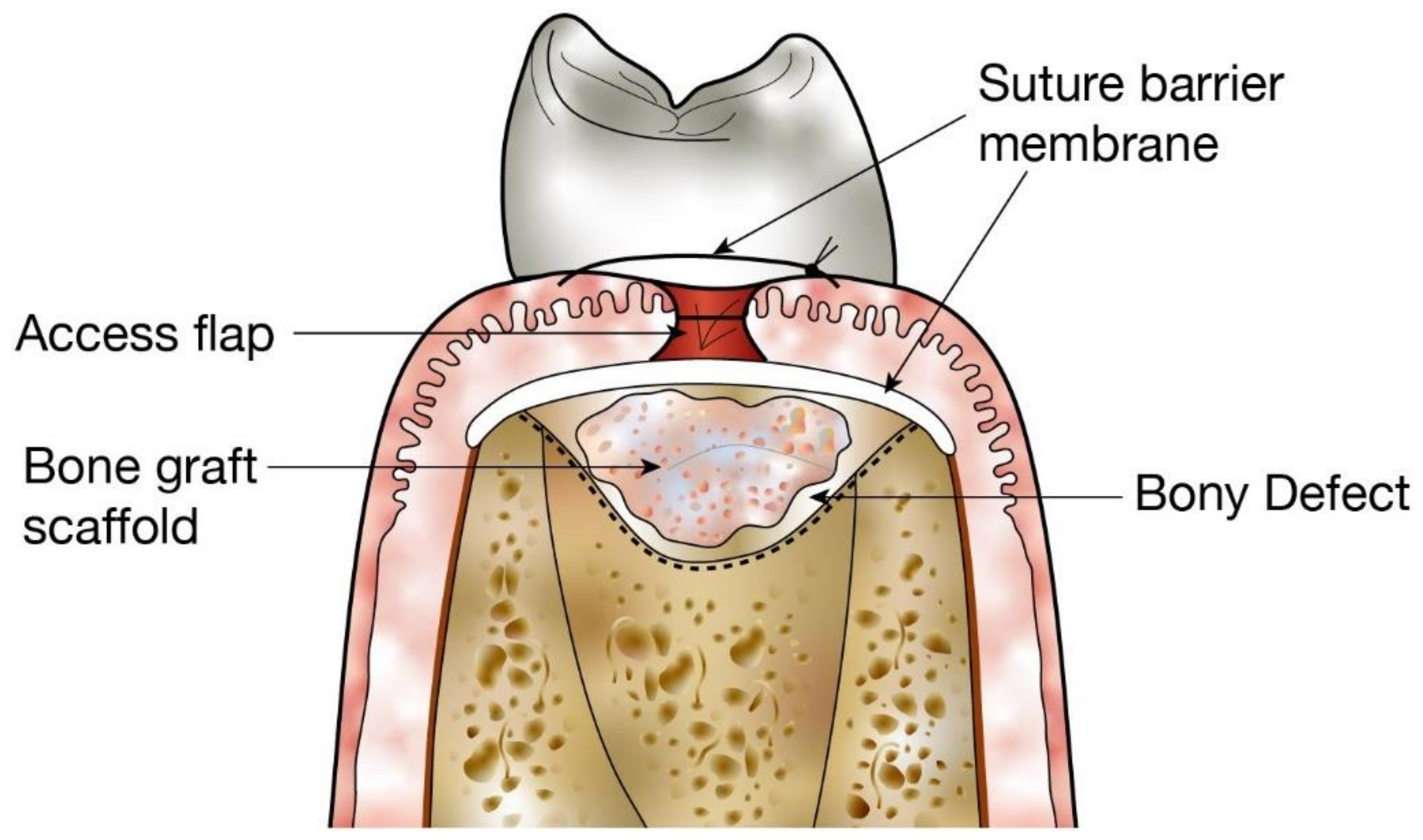
:1. Introduction
2. Characteristics of an Ideal Bone Grafting Material
3. Classification of Dental Bone Graft and Substitute Materials
3.1. Natural Bone Graft and Substitute Materials
3.1.1. Autografts
3.1.2. Allografts
3.1.3. Xenografts
3.1.4. Phytogenic Material
3.2. Synthetic Bone Substitute Materials
3.2.1. Hydroxyapatite (HA)
3.2.2. Tricalcium Phosphate Ceramics (β-TCP)
3.2.3. Biphasic Calcium Phosphate Ceramics (HA and β-TCP Ceramics)
3.2.4. Bioactive Glass
3.2.5. Calcium Phosphate Cements (CPCs)
3.2.6. Calcium Sulfates
3.2.7. Polymers
3.2.8. Metals
3.3. Composite Bone Substitute Materials
3.4. Growth Factor-Based Bone Substitutes (GFBSs)
3.5. Bone Substitutes with Infused Living Osteogenic Cells
4. Future of Bone Substitute Materials in Dentistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cypher, T.J.; Grossman, J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- Elsalanty, M.E.; Genecov, D.G. Bone Grafts in Craniofacial Surgery. Craniomaxillofacial Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [Green Version]
- Abhay, S.; Haines, S.J. Repairing holes in the head: A history of cranioplasty. Neurosurgery 1997, 40, 588–603. [Google Scholar]
- Kumar, P.; Fathima, G.; Vinitha, B. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Pommer, B.; Zechner, W.; Watzek, G.; Palmer, G.W.A.R. To Graft or Not to Graft? Evidence-Based Guide to Decision Making in Oral Bone Graft Surgery. Bone Grafting 2012, 2012, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Cha, H.-S.; Kim, J.-W.; Hwang, J.-H.; Ahn, K.-M. Frequency of bone graft in implant surgery. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef]
- Dental Bone Graft Substitute Market by Type (Synthetic Bone Grafts, Xenograft, Allograft, Alloplast), Application (Sinus Lift, Ridge Augmentation, Socket Preservation), Product (Bio-OSS, OsteoGraf, Grafton), End User (Hospital)-Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/dental-bone-graft-substitutes-market-159678690.html (accessed on 15 March 2021).
- Bhatt, R.A.; Rozental, T.D. Bone Graft Substitutes. Hand Clin. 2012, 28, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Ratnayake, J.T.; Ross, E.D.; Dias, G.J.; Shanafelt, K.M.; Taylor, S.S.; Gould, M.L.; Guan, G.; Cathro, P.R. Preparation, characterisation and in-vitro biocompatibility study of a bone graft developed from waste bovine teeth for bone regeneration. Mater. Today Commun. 2020, 22, 100732. [Google Scholar] [CrossRef]
- Moore, W.R.; E Graves, S.; I Bain, G. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef]
- Kao, S.T.; Scott, D.D. A Review of Bone Substitutes. Oral Maxillofac. Surg. Clin. North Am. 2007, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Dietsh, F. Bone-grafting materials in implant dentistry. Implant Dent. 1993, 2, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Horch, H.-H.; Sader, R.; Pautke, C.; Neff, A.; Deppe, H.; Kolk, A. Synthetic, pure-phase β-tricalcium phosphate ceramic granules (Cerasorb®) for bone regeneration in the reconstructive surgery of the jaws. Int. J. Oral Maxillofac. Surg. 2006, 35, 708–713. [Google Scholar] [CrossRef]
- Buser, D.; Hoffmann, B.; Bernard, J.-P.; Lussi, A.; Mettler, D.; Schenk, R.K. Evaluation of filling materials in membrane-protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin. Oral Implant. Res. 1998, 9, 137–150. [Google Scholar] [CrossRef]
- Nevins, M.; Parma-Benfenati, S.; Janke, U.W.; Kleyer, A.; Rasperini, G.; Tinti, C.; Schupbach, P.; Kim, D.M. The efficacy of mineralized allograft cortical and cancellous chips in maxillary sinus augmentations. Int. J. Periodontics Restor. Dent. 2014, 34, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Toscano, N.; Holtzclaw, D.; Mazor, Z.; Rosen, P.; Horowitz, R.; Toffler, M. Horizontal Ridge Augmentation Utilizing a Composite Graft of Demineralized Freeze-Dried Allograft, Mineralized Cortical Cancellous Chips, and a Biologically Degradable Thermoplastic Carrier Combined With a Resorbable Membrane: A Retrospective Evaluation of 73 Consecutively Treated Cases From Private Practices. J. Oral Implant. 2010, 36, 467–474. [Google Scholar] [CrossRef]
- Fuentes, R.; Issa, J.P.M.; Iyomasa, M.M.; Oporto, G.; Prieto, R.; Borie, E. The Behavior of Demineralized Bone Matrix (DBM) in Post-Extraction Sockets. Int. J. Morphol. 2012, 30, 394–398. [Google Scholar] [CrossRef]
- Hanes, P.J. Bone Replacement Grafts for the Treatment of Periodontal Intrabony Defects. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 499–512. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Schärer, P.; Marinello, C.P.; Schüpbach, P.; Berglundh, T. Alveolar ridge augmentation with Bio-Oss: A histologic study in humans. Int. J. Periodontics Restor. Dent. 2001, 21, 288–295. [Google Scholar]
- Ibrahim, A.M.; Khalil, M.M.; El Halawani, G.N. Evaluation Of Anterior Maxillary Horizontal Ridge Augmentation With Simultanous Implant Placement Using Cerabone® Versus Cerabone® Combined With Platelet Rich Plasma (Randomized Clinical Trial). Alex. Dent. J. 2020, 10. [Google Scholar] [CrossRef]
- Ewers, R. Maxilla Sinus Grafting With Marine Algae Derived Bone Forming Material: A Clinical Report of Long-Term Results. J. Oral Maxillofac. Surg. 2005, 63, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Christian, S.; Doris, M.; Alexis, S.; Georgios, L.; Else, S.; Franz, K.; Karl, D.; Rolf, E. The fluorohydroxyapatite (FHA) FRIOS® Algipore® is a suitable biomaterial for the reconstruction of severely atrophic human maxillae. Clin. Oral Implant. Res. 2003, 14, 743–749. [Google Scholar] [CrossRef]
- Yukna, R.A.; Yukna, C.N. A 5-year follow-up of 16 patients treated with coralline calcium carbonate (Biocoral™) bone replacement grafts in infrabony defects. J. Clin. Periodontol. 1998, 25, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Kenney, E.; Lekovic, V.; Sa Ferreira, J.; Han, T.; Dimitrijevic, B.; Carranza, F., Jr. Bone formation within porous hydroxylapatite implants in human periodontal defects. J. Periodontol. 1986, 57, 76–83. [Google Scholar] [CrossRef]
- Misch, C.M. Use of the Mandibular Ramus as a Donor Site for Onlay Bone Grafting. J. Oral Implant. 2000, 26, 42–49. [Google Scholar] [CrossRef]
- Smith, B.; Rajchel, J., II. Anatomic Considerations in Mandibular Ramus Osteotomies. Modern Practice in Orthognathic and Reconstructive Surgery; WB Saunders: Philadelphia, PA, USA, 1992; pp. 2347–2360. [Google Scholar]
- Misch, C.M. Autogenous Bone: Is It Still the Gold Standard? Implant. Dent. 2010, 19, 361. [Google Scholar] [CrossRef] [PubMed]
- Pikos, M.A. Block autografts for localized ridge augmentation: Part II. The posterior mandible. Implant Dent. 2000, 9, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Deshmukh, J.; Deshpande, S.; Khatri, R.; Deshpande, S. Vertical and horizontal ridge augmentation in anterior maxilla using autograft, xenograft and titanium mesh with simultaneous placement of endosseous implants. J. Indian Soc. Periodontol. 2014, 18, 661. [Google Scholar]
- Bostrom, M.P.G.; Seigerman, D.A. The Clinical Use of Allografts, Demineralized Bone Matrices, Synthetic Bone Graft Substitutes and Osteoinductive Growth Factors: A Survey Study. HSS J. 2005, 1, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.H.; Gibbons, C.L.M.H.; Athanasou, N.A. The pathology of bone allograft. J. Bone Jt. Surgery. Br. Vol. 1999, 81, 333–335. [Google Scholar] [CrossRef]
- Malinin, T.; Temple, H.; Garg, A. Bone allografts in dentistry: A review. Dentistry 2014, 4, 1. [Google Scholar]
- Amorfini, L.; Migliorati, M.; Signori, A.; Silvestrini-Biavati, A.; Benedicenti, S. Block Allograft Technique versus Standard Guided Bone Regeneration: A Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2013, 16, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Pendarvis, W.T.; Sandifer, J.B. Localized ridge augmentation using a block allograft with subsequent implant placement: A case series. Int. J. Periodontics Restor. Dent. 2008, 28, 509–515. [Google Scholar]
- Winkler, T.; Sass, F.; Duda, G.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Joint Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- El-Chaar, E.S. Demineralized bone matrix in extraction sockets: A clinical and histologic case series. Implant Dent. 2013, 22, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Uwagie, E.A.; Awasum, A.C.; Kene, R.C.; Chilaka, F.C. Use of native bovine bone morphogenetic protein extract in healing segmental tibial bone defects in goats. J. Vet. Sci. Technol. 2016, 7, 329. [Google Scholar]
- Wong, R.W.; Rabie, A.B.M. Effect of Gusuibu Graft on Bone Formation. J. Oral Maxillofac. Surg. 2006, 64, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.; Saravia, D.; Arias, A.; Prieto, R.; Dias, F. Mandibular dental implant placement using demineralized bone matrix (DBM). Biomed. Res. 2017, 28, 2656–2660. [Google Scholar]
- Zhang, M.; Powers, R.M., Jr.; Wolfinbarger, L., Jr. Effect (s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J. Periodontol. 1997, 68, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Kozusko, S.D.; Riccio, C.; Goulart, M.; Bumgardner, J.; Jing, X.L.; Konofaos, P. Chitosan as a Bone Scaffold Biomaterial. J. Craniofacial Surg. 2018, 29, 1788–1793. [Google Scholar] [CrossRef]
- Oliveira, G.; Pignaton, T.B.; de Almeida Ferreira, C.E.; Peruzzo, L.C.; Marcantonio, E., Jr. New bone formation comparison in sinuses grafted with anorganic bovine bone and β-TCP. Clin. Oral Implants Res. 2019, 30, 483. [Google Scholar] [CrossRef]
- Scarano, A.; Degidi, M.; Iezzi, G.; Pecora, G.; Piattelli, M.; Orsini, G.; Caputi, S.; Perrotti, V.; Mangano, C.; Piattelli, A. Maxillary Sinus Augmentation With Different Biomaterials: A Comparative Histologic and Histomorphometric Study in Man. Implant. Dent. 2006, 15, 197–207. [Google Scholar] [CrossRef]
- Özkan, Y.; Akoğlu, B.; Kulak-Özkan, Y. Maxillary Sinus Floor Augmentation Using Bovine Bone Grafts With Simultaneous Implant Placement: A 5-Year Prospective Follow-Up Study. Implant. Dent. 2011, 20, 455–459. [Google Scholar] [CrossRef]
- Uzbek, U.H.; Rahman, S.A.; Alam, M.K.; Gillani, S.W. Bone Forming Potential of An-Organic Bovine Bone Graft: A Cone Beam CT study. J. Clin. Diagn. Res. 2014, 8, ZC73–ZC76. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.H.; Simon, C.G., Jr. Fast setting calcium phosphate–chitosan scaffold: Mechanical properties and biocompatibility. Biomaterials 2005, 26, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Jebahi, S.; Oudadesse, H.; Ben Saleh, G.; Saoudi, M.; Mesadhi, S.; Rebai, T.; Keskes, H.; el Feki, A.; el Feki, H. Chitosan-based bioglass composite for bone tissue healing: Oxidative stress status and antiosteoporotic performance in a ovariectomized rat model. Korean J. Chem. Eng. 2014, 31, 1616–1623. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Sun, Z.; Ali, M.A. Injectable gel from squid pen chitosan for bone tissue engineering applications. J. Sol Gel Sci. Technol. 2015, 77, 675–687. [Google Scholar] [CrossRef]
- Nie, L.; Deng, Y.; Li, P.; Hou, R.; Shavandi, A.; Yang, S. Hydroxyethyl Chitosan-Reinforced Polyvinyl Alcohol/Biphasic Calcium Phosphate Hydrogels for Bone Regeneration. ACS Omega 2020, 5, 10948–10957. [Google Scholar] [CrossRef] [PubMed]
- Wattanutchariya, W.; Changkowchai, W. Characterization of porous scaffold from chitosan-gelatin/hydroxyapatite for bone grafting. In Proceedings of the International Multiconference of Engineers and Computer Scientists, Hong Kong, China, 12–14 March 2014. [Google Scholar]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, K.-J.; Seok, H. Silk Protein-Based Membrane for Guided Bone Regeneration. Appl. Sci. 2018, 8, 1214. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.R.; Tsukada, M.; Gotoh, Y.; Morikawa, H.; Freddi, G.; Shiozaki, H. Physical properties and dyeability of silk fibers degummed with citric acid. Bioresour. Technol. 2010, 101, 8439–8445. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, J.; Chen, C.; Yao, C.; Chung, S.-M.; Yao, J.; Lee, I.-S.; Kong, X. Silk fibroin membrane used for guided bone tissue regeneration. Mater. Sci. Eng. C 2017, 70, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Jo, Y.-Y.; Seok, H.; Kim, S.-G.; Chae, W.-S.; Sapru, S.; Kundu, S.C.; Park, N.-R.; Che, X.; Choi, J.-Y. In vivo bone regeneration ability of different layers of natural silk cocoon processed using an eco-friendly method. Macromol. Res. 2017, 25, 806–816. [Google Scholar] [CrossRef]
- Zafar, M.S.; Al-Samadani, K.H. Potential use of natural silk for bio-dental applications. J. Taibah Univ. Med. Sci. 2014, 9, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Ha, Y.-Y.; Park, Y.-W.; Kweon, H.; Jo, Y.-Y.; Kim, S.-G. Comparison of the physical properties and in vivo bioactivities of silkworm-cocoon-derived silk membrane, collagen membrane, and polytetrafluoroethylene membrane for guided bone regeneration. Macromol. Res. 2014, 22, 1018–1023. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Abdallah, M.-N.; Glogauer, M.; Grynpas, M. Natural and synthetic bone replacement graft materials for dental and maxillofacial applications. Adv. Dent. Biomater. 2019, 347–376. [Google Scholar] [CrossRef]
- Li, Y. Local use of iontophoresis with traditional Chinese herbal medicine, e.g., Gu-Sui-Bu (Rhizoma Drynariae) may accelerate orthodontic tooth movement. Dent. Hypotheses 2013, 4, 50. [Google Scholar] [CrossRef]
- Sun, J.-S.; Lin, C.-Y.; Dong, G.-C.; Sheu, S.-Y.; Lin, F.-H.; Chen, L.-T.; Wang, Y.-J. The effect of Gu-Sui-Bu (Drynaria fortunei J. Sm) on bone cell activities. Biomaterials 2002, 23, 3377–3385. [Google Scholar] [CrossRef]
- McPherson, R. Bone Grafting with Coralline Hydroxyapatite. EC Dent. Sci. 2019, 18, 2413–2423. [Google Scholar]
- Shavandi, A.; Wilton, V.; Bekhit, A.E.-D.A. Synthesis of macro and micro porous hydroxyapatite (HA) structure from waste kina (Evechinus chloroticus) shells. J. Taiwan Inst. Chem. Eng. 2016, 65, 437–443. [Google Scholar] [CrossRef]
- Giuliani, A.; Manescu, A.; Larsson, E.; Tromba, G.; Luongo, G.; Piattelli, A.; Mangano, F.; Iezzi, G.; Mangano, C. In Vivo Regenerative Properties of Coralline-Derived (Biocoral) Scaffold Grafts in Human Maxillary Defects: Demonstrative and Comparative Study with Beta-Tricalcium Phosphate and Biphasic Calcium Phosphate by Synchrotron Radiation X-Ray Microtomography. Clin. Implant. Dent. Relat. Res. 2013, 16, 736–750. [Google Scholar] [CrossRef]
- Karaismailoğlu, T.N.; Tomak, Y.; Andaç, A.; Ergün, E. Comparison of autograft, coralline graft, and xenograft in promoting posterior spinal fusion. Acta Orthop. Traumatol. Turc. 2002, 36, 147–154. [Google Scholar] [PubMed]
- Sivakumar, M.; Manjubala, I. Preparation of hydroxyapatite/fluoroapatite-zirconia composites using Indian corals for biomedical applications. Mater. Lett. 2001, 50, 199–205. [Google Scholar] [CrossRef]
- Sethmann, I.; Luft, C.; Kleebe, H.-J. Development of phosphatized calcium carbonate biominerals as bioactive bone graft substitute materials, part I: Incorporation of magnesium and strontium ions. J. Funct. Biomater. 2018, 9, 69. [Google Scholar] [CrossRef]
- Du, B.; Gao, Y.; Deng, Y.; Zhao, Y.; Lai, C.; Guo, Z.; Rong, M.; Zhou, L. Local delivery of rhVEGF165 through biocoated nHA/coral block grafts in critical-sized dog mandible defects: A histological study at the early stages of bone healing. Int. J. Clin. Exp. Med. 2015, 8, 4940–4953. [Google Scholar]
- Diaz-Rodriguez, P.; López-Álvarez, M.; Serra, J.; González, P.; Landín, M. Current stage of marine ceramic grafts for 3D bone tissue regeneration. Mar. Drugs 2019, 17, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damien, E.; Revell, P. Coralline hydroxyapatite bone graft substitute: A review of experimental studies and biomedical applications. J. Appl. Biomater. Biomech. 2004, 2, 65–73. [Google Scholar]
- Quiñones, C.R.; Hürzeler, M.B.; Schüpbachs, P.; Kirsch, A.; Blum, P.; Caffesse, R.G.; Strub, J.R. Maxillary sinus augmentation using different grafting materials and osseointegrated dental implants in monkeys. Part II. Evaluation of porous hydroxyapatite as a grafting material. Clin. Oral Implant. Res. 1997, 8, 487–496. [Google Scholar] [CrossRef]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Padial-Molina, M.; Lopez-Chaichio, L.; Gutiérrez-Garrido, L.; Martín-Morales, N.; O’Valle, F. Algae-derived hydroxyapatite behavior as bone biomaterial in comparison with anorganic bovine bone: A split-mouth clinical, radiological, and histologic randomized study in humans. Clin. Oral Implant Res. 2020, 31, 536–548. [Google Scholar] [CrossRef]
- Turhani, D.; Weissenböck, M.; Watzinger, E.; Yerit, K.; Cvikl, B.; Ewers, R.; Thurnher, D. In vitro study of adherent mandibular osteoblast-like cells on carrier materials. Int. J. Oral Maxillofac. Surg. 2005, 34, 543–550. [Google Scholar] [CrossRef]
- Smiler, D.; Soltan, M.; Lee, J.W. A histomorphogenic analysis of bone grafts augmented with adult stem cells. Implant Dent. 2007, 16, 42–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanschitz, F.; Nell, A.; Patruta, S.; Wagner, A.; Ewers, R. Influence of three currently used bone replacing materials on the in vitro proliferation of human peripheral blood mononuclear cells. Clin. Oral Implants Res. 2005, 16, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Kübler, A.; Neugebauer, J.; Oh, J.-H.; Scheer, M.; Zöller, J.E. Growth and Proliferation of Human Osteoblasts on Different Bone Graft Substitutes An In Vitro Study. Implant. Dent. 2004, 13, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.; Degidi, M.; Piattelli, A.; Mangano, C.; Scarano, A.; Shibli, J.A.; Perrotti, V. Comparative histological results of different biomaterials used in sinus augmentation procedures: A human study at 6 months. Clin. Oral Implant. Res. 2011, 23, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Thorwarth, M.; Wehrhan, F.; Srour, S.; Schultze-Mosgau, S.; Felszeghy, E.; Bader, R.; Schlegel, K. Evaluation of substitutes for bone: Comparison of microradiographic and histological assessments. Br. J. Oral Maxillofac. Surg. 2007, 45, 41–47. [Google Scholar] [CrossRef]
- Poeschl, P.W.; Ziya-Ghazvini, F.; Schicho, K.; Buchta, C.; Moser, D.; Seemann, R.; Ewers, R.; Schopper, C. Application of Platelet-Rich Plasma for Enhanced Bone Regeneration in Grafted Sinus. J. Oral Maxillofac. Surg. 2012, 70, 657–664. [Google Scholar] [CrossRef]
- Zhou, A.J.-J.; Clokie, C.M.L.; Peel, S.A.F. Bone Formation in Algae-Derived and Synthetic Calcium Phosphates With or Without Poloxamer. J. Craniofacial Surg. 2013, 24, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Herr, G.; Wahl, D.; Küsswetter, W. Osteogenic activity of bone morphogenetic protein and hydroxyapatite composite implants. Ann. Chir. Gynaecol. Suppl. 1993, 207, 99–107. [Google Scholar]
- Luczyszyn, S.M.; Papalexiou, V.; Novaes, A.B., Jr.; Grisi, M.F.; Souza, S.L.; Taba, M., Jr. Acellular dermal matrix and hydroxyapatite in prevention of ridge deformities after tooth extraction. Implant Dent. 2005, 14, 176–184. [Google Scholar] [CrossRef]
- Chitsazi, M.; Shirmohammadi, A.; Faramarzie, M.; Pourabbas, R.; Rostamzadeh, A. A clinical comparison of nano-crystalline hydroxyapatite (Ostim) and autogenous bone graft in the treatment of periodontal intrabony defects. Med. Oral Patol. Oral Cirugia Bucal 2011, 16, e448–e453. [Google Scholar] [CrossRef]
- Kamboj, M.; Arora, R.; Gupta, H. Comparative evaluation of the efficacy of synthetic nanocrystalline hydroxyapatite bone graft (Ostim®) and synthetic microcrystalline hydroxyapatite bone graft (Osteogen®) in the treatment of human periodontal intrabony defects: A clinical and denta scan study. J. Indian Soc. Periodontol. 2016, 20, 423. [Google Scholar] [PubMed]
- Ad De, R.; Meijer, G.; Dormaar, T.; Janssen, N.; Van Der Bilt, A.; Slootweg, P.; De Bruijn, J.; Van Rijn, L.; Koole, R. β-TCP versus autologous bone for repair of alveolar clefts in a goat model. Cleft Palate Craniofacial J. 2011, 48, 654–662. [Google Scholar] [CrossRef]
- Ogose, A.; Kondo, N.; Umezu, H.; Hotta, T.; Kawashima, H.; Tokunaga, K.; Ito, T.; Kudo, N.; Hoshino, M.; Gu, W. Histological assessment in grafts of highly purified β-tricalcium phosphate (OSferion®) in human bones. Biomaterials 2006, 27, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, M.; Ueno, T.; Hirata, A.; Iida, S.; Aghaloo, T.; Moy, P.K. Histologic Evaluation of Human Alveolar Sockets Treated With an Artificial Bone Substitute Material. J. Craniofacial Surg. 2011, 22, 490–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakar, A.; Rao, B.H.S.; Hegde, S.; Deshpande, N.; Lindner, A.; Nagursky, H.; Patney, A.; Mahajan, H. Ridge preservation using an in situ hardening biphasic calcium phosphate (β-TCP/HA) bone graft substitute-a clinical, radiological, and histological study. Int. J. Implant. Dent. 2017, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Norton, M.R.; Wilson, J. Dental implants placed in extraction sites implanted with bioactive glass: Human histology and clinical outcome. Int. J. Oral Maxillofac. Implant. 2002, 17, 249–257. [Google Scholar]
- Wadhawan, A.; Gowda, T.M.; Mehta, D.S. Gore-tex® versus resolut adapt® GTR membranes with perioglas® in periodontal regeneration. Contemp. Clin. Dent. 2012, 3, 406. [Google Scholar] [CrossRef] [PubMed]
- Chacko, N.L.; Abraham, S.; Rao, H.N.S.; Sridhar, N.; Moon, N.; Barde, D.H. A Clinical and Radiographic Evaluation of Periodontal Regenerative Potential of PerioGlas®: A Synthetic, Resorbable Material in Treating Periodontal Infrabony Defects. J. Int. Oral Health 2014, 6, 20–26. [Google Scholar]
- Sugawara, A.; Fujikawa, K.; Kusama, K.; Nishiyama, M.; Murai, S.; Takagi, S.; Chow, L.C. Histopathologic reaction of a calcium phosphate cement for alveolar ridge augmentation. J. Biomed. Mater. Res. 2002, 61, 47–52. [Google Scholar] [CrossRef]
- Stanton, D.C.; Chou, J.C.; Carrasco, L.R. Injectable calcium-phosphate bone cement (Norian) for reconstruction of a large mandibular defect: A case report. J. Oral Maxillofac. Surg. 2004, 62, 235–240. [Google Scholar] [CrossRef]
- Maragos, P.; Bissada, N.F.; Wang, R.; Cole, B.P. Comparison of three methods using calcium sulfate as a graft/barrier material for the treatment of Class II mandibular molar furcation defects. Int. J. Periodontics Restor. Dent. 2002, 22, 493–501. [Google Scholar]
- Petruskevicius, J.; Nielsen, M.S.; Kaalund, S.; Knudsen, P.R.; Overgaard, S. No effect of Osteoset ®, a bone graft substitute, on bone healing in humans: A prospective randomized double-blind study. Acta Orthop. Scand. 2002, 73, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Sunitha, J.; Abid, S. Evaluation of HTR polymer (Bioplant® HTR®) as a bone graft material in the treatment of interproximal vertical bony defects: A clinical and radiological study. Indian J. Dent. Res. 2010, 21, 179. [Google Scholar] [CrossRef]
- Yukna, R.A.; Yukna, C.N. Six-year clinical evaluation of HTR synthetic bone grafts in human grade II molar furcations. J. Periodontal Res. 1997, 32, 627–633. [Google Scholar] [CrossRef]
- Jeng, M.-D.; Chiang, C.-P. Autogenous bone grafts and titanium mesh-guided alveolar ridge augmentation for dental implantation. J. Dent. Sci. 2020, 15, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Greco, G.; Gherlone, E. A Preshaped Titanium Mesh for Guided Bone Regeneration with an Equine-Derived Bone Graft in a Posterior Mandibular Bone Defect: A Case Report. Dent. J. 2019, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Abshagen, K.; Schrodi, I.; Gerber, T.; Vollmar, B. In vivo analysis of biocompatibility and vascularization of the synthetic bone grafting substitute NanoBone®. J. Biomed. Mater. Res. Part A 2009, 91, 557–566. [Google Scholar] [CrossRef]
- Ghanem, W.A.; Ahmed, I.H.; Kilany, O.E.; Mortada, I.M. Effect of nanobone graft on socket healing after teeth extraction. Dent. J. 2016, 62, 3553. [Google Scholar]
- Ucer, C.; Mangham, D.; Coulton, L. Clinical and histological results of socket preservation using Fortoss Vital: 554 Posters-Tissue Augmentation and Engineering. Clin. Oral Implant Res. 2012, 23, 256–257. [Google Scholar]
- Sukumar, S.; Drízhal, I.; Paulusová, V.; Bukac, J. Surgical Treatment of Periodontal Intrabony Defects with Calcium Sulphate in Combination with Beta-Tricalcium Phosphate: Clinical Observations Two Years Post-Surgery. Acta Med. 2011, 54, 13–20. [Google Scholar] [CrossRef]
- Abuelnaga, M.; Elbokle, N.; Khashaba, M. Evaluation of custom made xenogenic bone grafts in mandibular alveolar ridge augmentation versus particulate bone graft with titanium mesh. Egypt. J. Oral Maxillofac. Surg. 2018, 9, 62–73. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, D.; Perale, G.; Milazzo, M.; Moscato, S.; Stefanini, C.; Pertici, G.; Danti, S. Bovine bone matrix/poly( l -lactic- co -ε-caprolactone)/gelatin hybrid scaffold (SmartBone®) for maxillary sinus augmentation: A histologic study on bone regeneration. Int. J. Pharm. 2017, 523, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–-Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, 36138. [Google Scholar] [CrossRef] [Green Version]
- Dewi, A.H.; Ana, I.D. The use of hydroxyapatite bone substitute grafting for alveolar ridge preservation, sinus augmentation, and periodontal bone defect: A systematic review. Heliyon 2018, 4, e00884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Leeuwenburgh, S.C.; Li, Y.; Jansen, J.A. The Use of Micro- and Nanospheres as Functional Components for Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2012, 18, 24–39. [Google Scholar] [CrossRef]
- Sakamoto, M. Development and evaluation of superporous hydroxyapatite ceramics with triple pore structure as bone tissue scaffold. J. Ceram. Soc. Jpn. 2010, 118, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bünger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Arcos, D.; Vallet-Regí, M. Upgrading Calcium Phosphate Scaffolds for Tissue Engineering Applications. Key Eng. Mater. 2008, 377, 19–42. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef]
- Horowitz, R.A.; Leventis, M.D.; Rohrer, M.D.; Prasad, H.S. Bone grafting: History, rationale, and selection of materials and techniques. Compend. Contin. Educ. Dent. 2014, 35, 1–6. [Google Scholar] [PubMed]
- Suneelkumar, C.; Datta, K.; Srinivasan, M.R.; Kumar, S.T. Biphasic calcium phosphate in periapical surgery. J. Conserv. Dent. 2008, 11, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavropoulos, A.; Windisch, P.; Szendröi-Kiss, D.; Peter, R.; Gera, I.; Sculean, A. Clinical and Histologic Evaluation of Granular Beta-Tricalcium Phosphate for the Treatment of Human Intrabony Periodontal Defects: A Report on Five Cases. J. Periodontol. 2010, 81, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Fiorellini, J.P.; Kim, D.M.; Weber, H.P. Regeneration of standardized mandibular bone defects using expanded polytetrafluoroethylene membrane and various bone fillers. Int. J. Periodontics Restor. Dent. 2007, 27, 151–159. [Google Scholar]
- Gallinetti, S.; Canal, C.; Ginebra, M.P. Development and characterization of biphasic hydroxyapatite/β-TCP cements. J. Am. Ceram. Soc. 2014, 97, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Saikia, K.C.; Bhattacharya, T.D.; Bhuyan, S.K.; Talukdar, D.J.; Saikia, S.P.; Jitesh, P. Calcium phosphate ceramics as bone graft substitutes in filling bone tumor defects. Indian J. Orthop. 2008, 42, 169–172. [Google Scholar] [CrossRef]
- Spivak, J.M.; Hasharoni, A. Use of hydroxyapatite in spine surgery. Eur. Spine J. 2001, 10, S197–S204. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Chang, J.; Kim, J.; You, C.; Kwon, H.; Lee, D. The role of osteoclast in resorption of hydroxyapatite and β-tricalcium phosphate coating layer. Key Eng. Mater. 2009, 396, 81–84. [Google Scholar]
- Campana, V.; Milano, G.; Pagano, E.D.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [Green Version]
- Esfahanizadeh, N.; Nourani, M.R.; Bahador, A.; Akhondi, N.; Montazeri, M. The Anti-biofilm Activity of Nanometric Zinc doped Bioactive Glass against Putative Periodontal Pathogens: An in vitro Study. Biomed. Glas. 2018, 4, 95–107. [Google Scholar] [CrossRef]
- Lovelace, T.B.; Mellonig, J.T.; Meffert, R.M.; Jones, A.A.; Nummikoski, P.V.; Cochran, D.L. Clinical Evaluation of Bioactive Glass in the Treatment of Periodontal Osseous Defects in Humans. J. Periodontol. 1998, 69, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Yang, S.S. Clinical Application of Unigraft® in the Treatment of Human Periodontal Defects. 2005. Available online: https://www.unicarebiomedical.com/pdf/UnigraftClinicalStudy.pdf (accessed on 15 March 2021).
- Krishnan, V.; Lakshmi, T. Bioglass: A novel biocompatible innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78. [Google Scholar] [CrossRef]
- Hench, L.; Hench, J.W.; Greenspan, D. Bioglass: A short history and bibliography. J. Australas. Ceram. Soc. 2004, 40, 1–42. [Google Scholar]
- El Shazley, N.; Hamdy, A.; El-Eneen, H.A.; El Backly, R.M.; Saad, M.M.; Essam, W.; Moussa, H.; El Tantawi, M.; Jain, H.; Marei, M.K. Bioglass in alveolar bone regeneration in orthodontic patients: Randomized controlled clinical trial. JDR Clin. Transl. Res. 2016, 1, 244–255. [Google Scholar] [CrossRef]
- Ezzat, A.E.M.; El-Shenawy, H.M. Repair of cleft alveolar bone with bioactive glass material using Z-plasty flap. Int. J. Appl. Basic Med. Res. 2015, 5, 211–213. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Lu, H.; Li, W.; Chen, F.-M.; Zhao, Y.-M. The use of calcium phosphate-based biomaterials in implant dentistry. J. Mater. Sci. Mater. Electron. 2012, 23, 853–862. [Google Scholar] [CrossRef]
- Burguera, E.F.; Xu, H.H.K.; Sun, L. Injectable calcium phosphate cement: Effects of powder-to-liquid ratio and needle size. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 84, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Khairoun, I.; Boltong, M.G.; Driessens, F.C.M.; A Planell, J. Some factors controlling the injectability of calcium phosphate bone cements. J. Mater. Sci. Mater. Electron. 1998, 9, 425–428. [Google Scholar] [CrossRef]
- O’Hara, R.M.; Dunne, N.J.; Orr, J.F.; Buchanan, F.J.; Wilcox, R.; Barton, D.C. Optimisation of the mechanical and handling properties of an injectable calcium phosphate cement. J. Mater. Sci. Mater. Electron. 2010, 21, 2299–2305. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; A Reynolds, M. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, C.; Shao, Z.; Zou, D.; Lu, J. Ridge Alterations following Socket Preservation Using a Collagen Membrane in Dogs. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, W.S.; Ronk, R. Calcium Sulfate Bone Void Filler: A Review and a Look Ahead. J. Craniofacial Surg. 2000, 11, 327–333. [Google Scholar] [CrossRef]
- Kutkut, A.; Andreana, S. Medical-grade calcium sulfate hemihydrate in clinical implant dentistry: A review. J. Long Term Eff. Med Implant 2010, 20, 295–301. [Google Scholar] [CrossRef]
- Kumar, Y.; Nalini, K.; Menon, J.; Patro, D.K.; Banerji, B. Calcium sulfate as bone graft substitute in the treatment of osseous bone defects, a prospective study. J. Clin. Diagn. Res. JCDR 2013, 7, 2926. [Google Scholar] [CrossRef] [PubMed]
- Evaniew, N.; Tan, V.; Parasu, N.; Jurriaans, E.; Finlay, K.; Deheshi, B.; Ghert, M. Use of a Calcium Sulfate–Calcium Phosphate Synthetic Bone Graft Composite in the Surgical Management of Primary Bone Tumors. Orthopedics 2013, 36, e216–e222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hak, D.J. The Use of Osteoconductive Bone Graft Substitutes in Orthopaedic Trauma. J. Am. Acad. Orthop. Surg. 2007, 15, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Baranes, D.; Kurtzman, G.M. Biphasic calcium sulfate as an alternative grafting material in various dental applications. J. Oral Implantol. 2019, 45, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherji, A.; Rath, S.K. Calcium sulfate in periodontics: A time tested versatile alloplast. J. Sci. Soc. 2016, 43, 18. [Google Scholar] [CrossRef]
- Fuchs, J.R.; Nasseri, B.A.; Vacanti, J.P. Tissue engineering: A 21st century solution to surgical reconstruction. Ann. Thorac. Surg. 2001, 72, 577–591. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Runge, M.B.; Dadsetan, M.; Chen, Q.; Lu, L.; Yaszemski, M.J. Cross-linking Characteristics and Mechanical Properties of an Injectable Biomaterial Composed of Polypropylene Fumarate and Polycaprolactone Co-polymer. J. Biomater. Sci. Polym. Ed. 2011, 22, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairag, R.; Rosenzweig, D.H.; Garcialuna, J.L.R.; Weber, M.H.; Haglund, L. Three-Dimensional Printed Polylactic Acid Scaffolds Promote Bone-like Matrix Deposition in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 15306–15315. [Google Scholar] [CrossRef]
- Danoux, C.B.; Barbieri, D.; Yuan, H.; De Bruijn, J.D.; A Van Blitterswijk, C.; Habibovic, P. In vitro and in vivo bioactivity assessment of a polylactic acid/hydroxyapatite composite for bone regeneration. Biomatter 2014, 4, e27664. [Google Scholar] [CrossRef] [Green Version]
- Ai, C.; Sheng, D.; Chen, J.; Cai, J.; Wang, S.; Jiang, J.; Chen, S. Surface modification of vascular endothelial growth factor-loaded silk fibroin to improve biological performance of ultra-high-molecular-weight polyethylene via promoting angiogenesis. Int. J. Nanomed. 2017, 12, 7737–7750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as bone substitutes: A review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef] [PubMed]
- Guduric, V.; Metz, C.; Siadous, R.; Bareille, R.; Levato, R.; Engel, E.; Fricain, J.-C.; Devillard, R.; Luzanin, O.; Catros, S. Layer-by-layer bioassembly of cellularized polylactic acid porous membranes for bone tissue engineering. J. Mater. Sci. Mater. Electron. 2017, 28, 78. [Google Scholar] [CrossRef] [Green Version]
- Parani, M.; Lokhande, G.; Singh, A.; Gaharwar, A.K. Engineered Nanomaterials for Infection Control and Healing Acute and Chronic Wounds. ACS Appl. Mater. Interfaces 2016, 8, 10049–10069. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral Sci. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Briguglio, F.; Falcomatà, D.; Marconcini, S.; Fiorillo, L.; Farronato, D. The Use of Titanium Mesh in Guided Bone Regeneration: A Systematic Review. Int. J. Dent. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nabiyouni, M.; Brückner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Zhao, D.; Zhang, N.; Li, H.; Li, J.; Zhao, Y.; Yan, J.; Zhou, Y. In vivo study of microarc oxidation coated Mg alloy as a substitute for bone defect repairing: Degradation behavior, mechanical properties, and bone response. Colloids Surf. B Biointerfaces 2019, 181, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wan, P.; Tan, L.L.; Wang, K.; Yang, K. Preclinical investigation of an innovative magnesium-based bone graft substitute for potential orthopaedic applications. J. Orthop. Transl. 2014, 2, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Slutsky, D.J.; Osterman, A.L. Fractures and Injuries of the Distal Radius and Carpus E-Book: The Cutting Edge-Expert Consult: Online and Print; Elsevier Health Sciences: Philadelphia, PA, USA, 2008. [Google Scholar]
- Bienengräber, V.; Gerber, T.; Henkel, K.-O.; Bayerlein, T.; Proff, P.; Gedrange, T. The clinical application of a new synthetic bone grafting material in oral and maxillofacial surgery. Folia Morphol. 2006, 65, 84–88. [Google Scholar]
- Seifi, M.; Arayesh, A.; Shamloo, N.; Hamedi, R. Effect of Nanocrystalline Hydroxyapatite Socket Preservation on Orthodontically Induced Inflammatory Root Resorption. Cell J. 2015, 16, 514–527. [Google Scholar] [PubMed]
- Eldibany, R.; Shokry, M. The effect of Nanobone® in combination with platelet rich fibrin on bone regeneration following enucleation of large mandibular cysts. Tanta Dent. J. 2014, 11, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Fairbairn, P.; Leventis, M. Protocol for bone augmentation with simultaneous early implant placement: A retrospective multicenter clinical study. Int. J. Dent. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.-K.; Choi, E.-J.; Cho, K.-S.; Chai, J.-K.; Wikesjö, U.M. Periodontal Repair in Intrabony Defects Treated With a Calcium Carbonate Implant and Guided Tissue Regeneration. J. Periodontol. 1996, 67, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Squier, C.; Collins, P. The relationship between soft tissue attachment, epithelial downgrowth and surface porosity. J. Periodontal Res. 1981, 16, 434–440. [Google Scholar] [CrossRef]
- Coetzee, A.S. Regeneration of Bone in the Presence of Calcium Sulfate. Arch. Otolaryngol. Head Neck Surg. 1980, 106, 405–409. [Google Scholar] [CrossRef]
- Oliveira, É.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.; Shavandi, A. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Licata, M.E.; Polizzi, B.; Campisi, G. Platelet-rich plasma (PRP) in dental and oral surgery: From the wound healing to bone regeneration. Immun. Ageing 2013, 10, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicciù, M. Growth Factor Applied to Oral and Regenerative Surgery. Int. J. Mol. Sci. 2020, 21, 7752. [Google Scholar] [CrossRef] [PubMed]
- Cabbar, F.; Güler, N.; Kürkçü, M.; Işeri, U.; Şençift, K. The Effect of Bovine Bone Graft With or Without Platelet-Rich Plasma on Maxillary Sinus Floor Augmentation. J. Oral Maxillofac. Surg. 2011, 69, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Bottini, D.J.; Spallone, D.; Curcio, B.C.; Cervelli, V. Application of platelet-rich plasma in maxillofacial surgery: Clinical evaluation. J. Craniofacial Surg. 2010, 21, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Mehta, D. Evaluation of immediately loaded dental implants bioactivated with platelet-rich plasma placed in the mandibular posterior region: A clinico-radiographic study. J. Indian Soc. Periodontol. 2012, 16, 89. [Google Scholar]
- Sallent, I.; Capella-Monsonís, H.; Procter, P.; Bozo, I.Y.; Deev, R.V.; Zubov, D.; Vasyliev, R.; Perale, G.; Pertici, G.; Baker, J.; et al. The Few Who Made It: Commercially and Clinically Successful Innovative Bone Grafts. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.-S.; Huang, B.; Kim, J.; Park, W.E.; Park, C.C. Utilization of autologous concentrated growth factors (CGF) enriched bone graft matrix (Sticky bone) and CGF-enriched fibrin membrane in Implant Dentistry. J. Implant Adv. Clin. Dent. 2015, 7, 11–18. [Google Scholar]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Bruder, S.P.; Kraus, K.H.; Goldberg, V.M.; Kadiyala, S. The Effect of Implants Loaded with Autologous Mesenchymal Stem Cells on the Healing of Canine Segmental Bone Defects*. J. Bone Jt. Surg. Am. Vol. 1998, 80, 985–996. [Google Scholar] [CrossRef]
- Kok, I.J.D.; Drapeau, S.J.; Young, R.; Cooper, L.F. Evaluation of mesenchymal stem cells following implantation in alveolar sockets: A canine safety study. Int. J. Oral Maxillofac. Implant 2005, 20, 511–518. [Google Scholar]
- Dallari, D.; Fini, M.; Stagni, C.; Torricelli, P.; Nicolialdini, N.; Giavaresi, G.; Cenni, E.; Baldini, N.; Cenacchi, A.; Bassi, A. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet rich plasma and freeze-dried bone allografts, alone and in combination. J. Orthop. Res. 2005, 24, 877–888. [Google Scholar] [CrossRef]
- Bertolai, R.; Catelani, C.; Aversa, A.; Rossi, A.; Giannini, D.; Bani, D. Bone graft and mesenchimal stem cells: Clinical observations and histological analysis. Clin. Cases Miner. Bone Metab. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Wiesmann, H.-P.; Berr, K.; Depprich, R.A.; Kübler, N.R.; Naujoks, C.; Cohnen, M.; Ommerborn, M.A.; Meyer, U.; Handschel, J. Embryonic stem cells induce ectopic bone formation in rats. Bio Med Mater. Eng. 2010, 20, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, Z.; Xie, Y.; Hu, J.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.-T.; Wang, S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res. Ther. 2015, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wu, C.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Amghar-Maach, S.; Gay-Escoda, C.; Sánchez-Garcés, M. Ángeles Regeneration of periodontal bone defects with dental pulp stem cells grafting: Systematic Review. J. Clin. Exp. Dent. 2019, 11, e373–e381. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jeon, S.H.; Choung, P.-H. Efficacy of Periodontal Stem Cell Transplantation in the Treatment of Advanced Periodontitis. Cell Transplant. 2011, 20, 271–286. [Google Scholar] [CrossRef] [Green Version]
- Deev, R.V.; Drobyshev, A.Y.; Bozo, I.Y.; Isaev, A. Ordinary and Activated Bone Grafts: Applied Classification and the Main Features. BioMed Res. Int. 2015, 2015, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ducheyne, P. Comprehensive Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]



| Material Type | Product Name | Material Source | Forms Available | Clinical Applications | Advantages | Limitations | Type of Study and Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| Cortical Allograft | MinerOss CorticalTM | Mineralized cortical allograft | Fresh, frozen, freeze-dried Whole bone segments, block, pieces |
|
|
| Clinical trial Bone formation 6 months following sinus augmentation procedures. Average of 3.5 mm horizontal ridge width gain, 4 months following placement of FDBA | [21,22] |
| Cancellous Allograft | MinerOss CancellousTM | Mineralized cancellous allograft | Fresh, frozen, freeze-dried Chips, wedges, pegs, powder |
|
|
| ||
| Demineralised Bone Matrix | Dynagraft D PuttyTM OpteformTM Grafton DBMTM | Human DBM | Putty, moldable pastes, blocks, particulates, powder |
|
|
| Clinical trial 50–60% resolution of periodontal intrabony defects Remineralization and new bone formation following sinus augmentation with DBM | [23,24] |
| Deproteinised bovine bone | BioOssTM OsteoGrafTM CeraboneTM | Bovine | Block, granules, particulates |
|
|
| Clinical Trial New bone formation, intermingled with BioOssTM particles 6-7 months following graft placement 14/14 implants placed in patients with insufficient alveolar ridge width in the maxillary lateral incisor region successfully osseo integrated and were functionally stable | [25,26] |
| Algae-based | AlgiporeTM | Red algae | Granules |
|
|
| Clinical trial 95% implant survival rate in atrophic maxilla grafted with Algipore 14 years following graft placement New bone formation around and within the pores of implanted AlgiporeTM particles, 7 months following graft placement | [27,28] |
| Coral-based | ProOsteonTM BioCoralTM InterPoreTM | Marine coral | Block, Granules |
|
|
| Clinical trial Decrease in periodontal probing depths and gingival recession 5 years following grafting with BioCoral Bone formation within, and along the walls of the pores of grafted Interpore 200TM, starting 3 months and continuing beyond 6 months following graft placement in periodontal osseous defects of three recipients | [29,30] |
| Material Type | Product Name | Forms Available | Indications | Advantages | Limitations | Type of Study and Outcome | Reference |
|---|---|---|---|---|---|---|---|
| Hydroxyapatite | OstimTM EndobonTM | Blocks, wedges and granules |
|
|
| Clinical trial Significant bone regeneration in 2 and 3-wall intrabony periodontal defects 6 months following placement of OstimTM graft Decreased periodontal pocket depth, decreased clinical attachment loss, decreased intrabony defect depth, 6 months following placement of OstimTM graft | [93,94] |
| Tricalcium phosphate ceramics | CerasorbTM OSferionTM OrthograftTM | Blocks, cylinders, wedges, granules |
|
|
| In vivo (goat) Bone regeneration comparable to that of autografts in alveolar clefts, 6 months following placement of β-TCP Clinical trial Successful osseointegration and prominent bone formation along graft surface evident 28 days after placement of OSferionTM | [95,96] |
| Biphasic calcium phosphate ceramics | MASTERGRAFTTM | Moldable putty, granules |
|
|
| Clinical trial New bone formation with histological observation of osteogenic activity surrounding MASTERGRAFT granules, 4-5 months following graft placement New bone formation and minimal ridge width reduction observed in post-extraction alveolar ridges of 15 patients | [97,98] |
| Bioglasses | PerioglasTM BiogranTM | Particulates |
|
|
| Clinical trial 88.6% success rates of implants placed in sites grafted with bioactive glasses, 29 months following bioglass material Decreases in periodontal pocketing depth, clinical attachment loss, gingival recession, depth of bony defect observed, 9 months after placement of PerioglasTM either alone, or in combination with a non-resorbable membrane GoreTexTM or bioresorbable membrane Resolut AdaptTM | [99,100,101] |
| Calcium phosphate cements | NorianTM ChronOS injectTM HydrosetTM BoneSourceTM | Injectable paste, moldable putty |
|
|
| Clinical Trial Nearly complete bone regeneration in alveolar ridge defects, 6 months following placement of CPC material Case Report Complete replacement by newly formed bone of NorianTM graft placed in a large 3-wall mandibular defect, one year following graft placement | [102,103] |
| Calcium sulfates | OsteoSetTM | Various sizes pellets |
|
|
| Clinical trial When used in combination with FDBA, resulted in the reduction of periodontal probing depths, gains in clinical attachment, defect fill and resolution, 12 months following placement of calcium sulfate graft material Double-blind randomized trial 42% of bony defect filled with new bone, 6 weeks after placement of OsteoSetTM graft. No statistically significant additional bone formation observed during 3-6 months period. | [104,105] |
| Polymers | Bioplant HTR Synthetic BoneTM | Particulates, granules, ready to use in syringe |
|
|
| Clinical trial Reduction in periodontal probing depths, clinical attachment gain and significant resolution of defects in alveolar crest bone, 6 months following placement of Bioplant HTR Synthetic BoneTM Decreased periodontal probing depths, mean horizontal and vertical furcation probing attachment levels, six years after placement of Bioplant HTR Synthetic BoneTM | [106,107] |
| Metals | OSS BuilderTM | Mesh/membrane available in lateral and papilla design forms |
|
|
| Clinical trial Significant bone formation in alveolar ridge, 4 months following placement of autograft with titanium mesh Case Report Increase in alveolar crestal bone width and height observed, 5 months after placement of autograft mixed with equine-derived xenograft and a titanium mesh | [108,109] |
| Composites | NanoBoneTM (nanocrystalline HA/silicon dioxide) | Putty, granulate, block, ready to use “QD” |
|
|
| In vivo (mouse) New trabecular bone formation, followed by resorption of graft material, 8 months following placement of NanoBoneTMIn vivo (dog) Significantly greater amount of new bone formed in extraction sockets observed at 45 and 90 days after placement of NanoBoneTM with PRF than NanoBoneTM alone or in the control group | [110,111] |
| Fortoss VitalTM (β-TCP/calcium sulphate) | Paste |
|
|
| Clinical trial Formation of new viable bone, 12 weeks after placement of Fortoss VitalTM Reduction in periodontal pocketing depth, clinical attachment loss, but increases in gingival recession observed 2 years after placement of Fortoss VitalTM | [112,113] | |
| SmartBoneTM (DBM/polymer/collagen) | Blocks, microchips, plate, granules, wedge, cylinder, rod |
|
|
| Clinical trial Formation of new bone, and increases in alveolar bone dimension, 4 months following placement of SmartBoneTM Successful osseointegration and new bone formation observed surrounded by vascular connective tissue, 4 months following placement of SmartBoneTM graft. | [114,115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. https://doi.org/10.3390/molecules26103007
Zhao R, Yang R, Cooper PR, Khurshid Z, Shavandi A, Ratnayake J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules. 2021; 26(10):3007. https://doi.org/10.3390/molecules26103007
Chicago/Turabian StyleZhao, Rusin, Ruijia Yang, Paul R. Cooper, Zohaib Khurshid, Amin Shavandi, and Jithendra Ratnayake. 2021. "Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments" Molecules 26, no. 10: 3007. https://doi.org/10.3390/molecules26103007
APA StyleZhao, R., Yang, R., Cooper, P. R., Khurshid, Z., Shavandi, A., & Ratnayake, J. (2021). Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules, 26(10), 3007. https://doi.org/10.3390/molecules26103007









