Curcumin Attenuated Neurotoxicity in Sporadic Animal Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
2.1. CUR Treatment Acts on Body and Organ Weights Affected by AlCl3 Administration
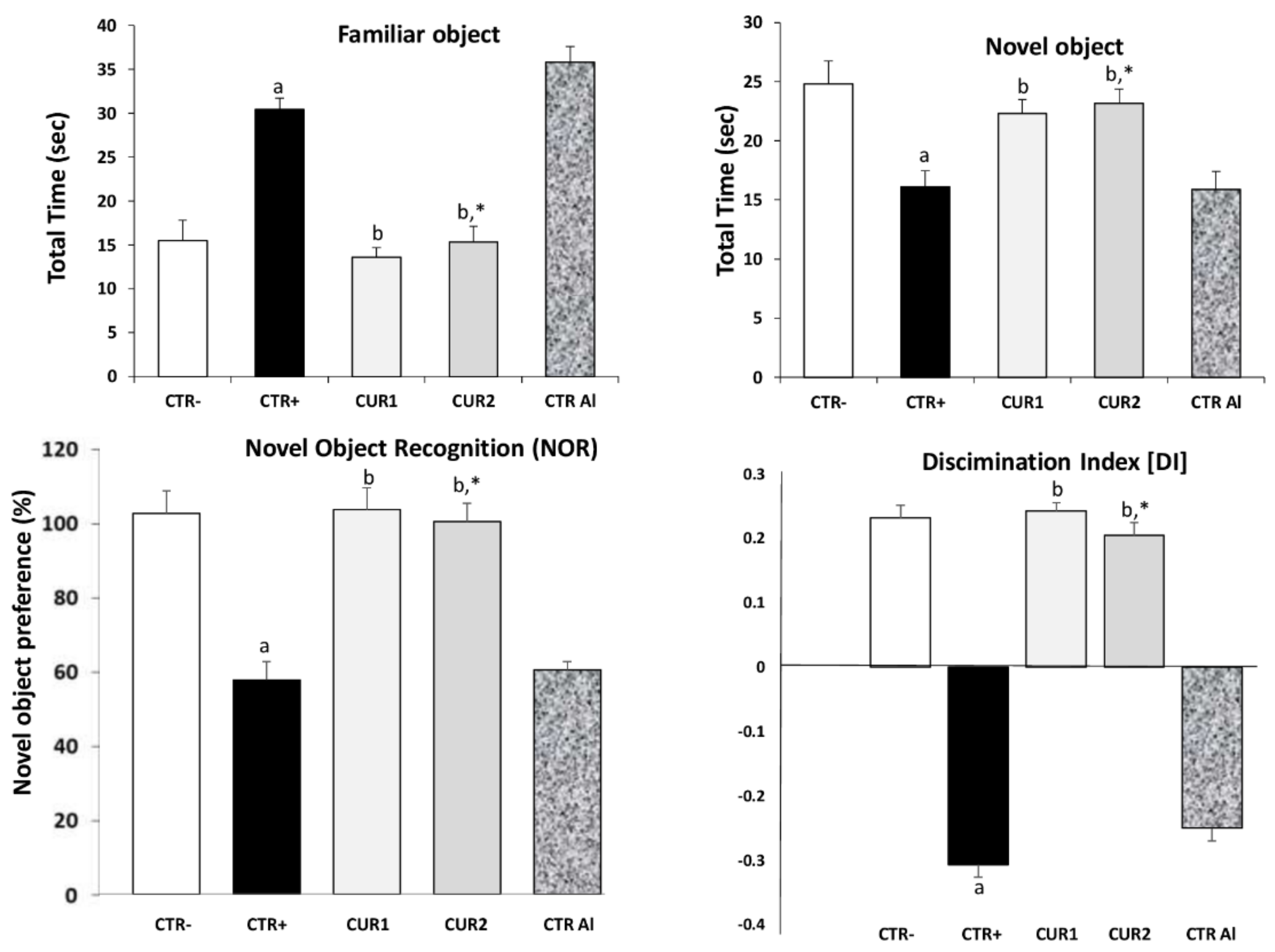
2.2. CUR Protects against AlCl3-Induced Cognitive Impairment
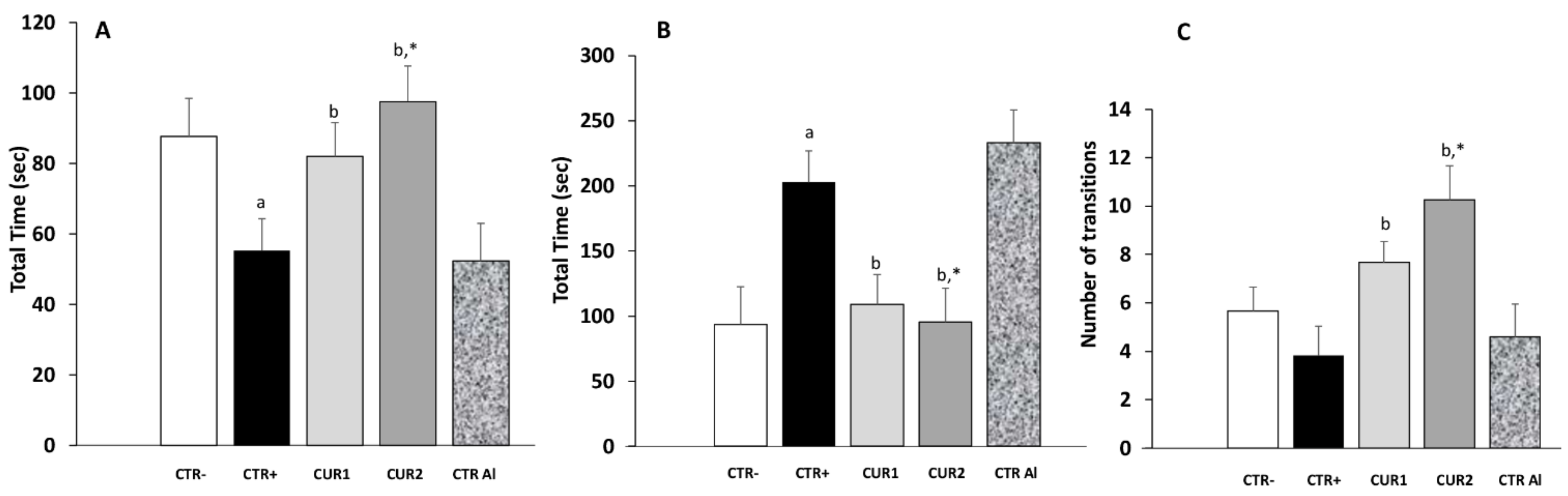
2.3. CUR Protects against AlCl3-Induced Anxiety
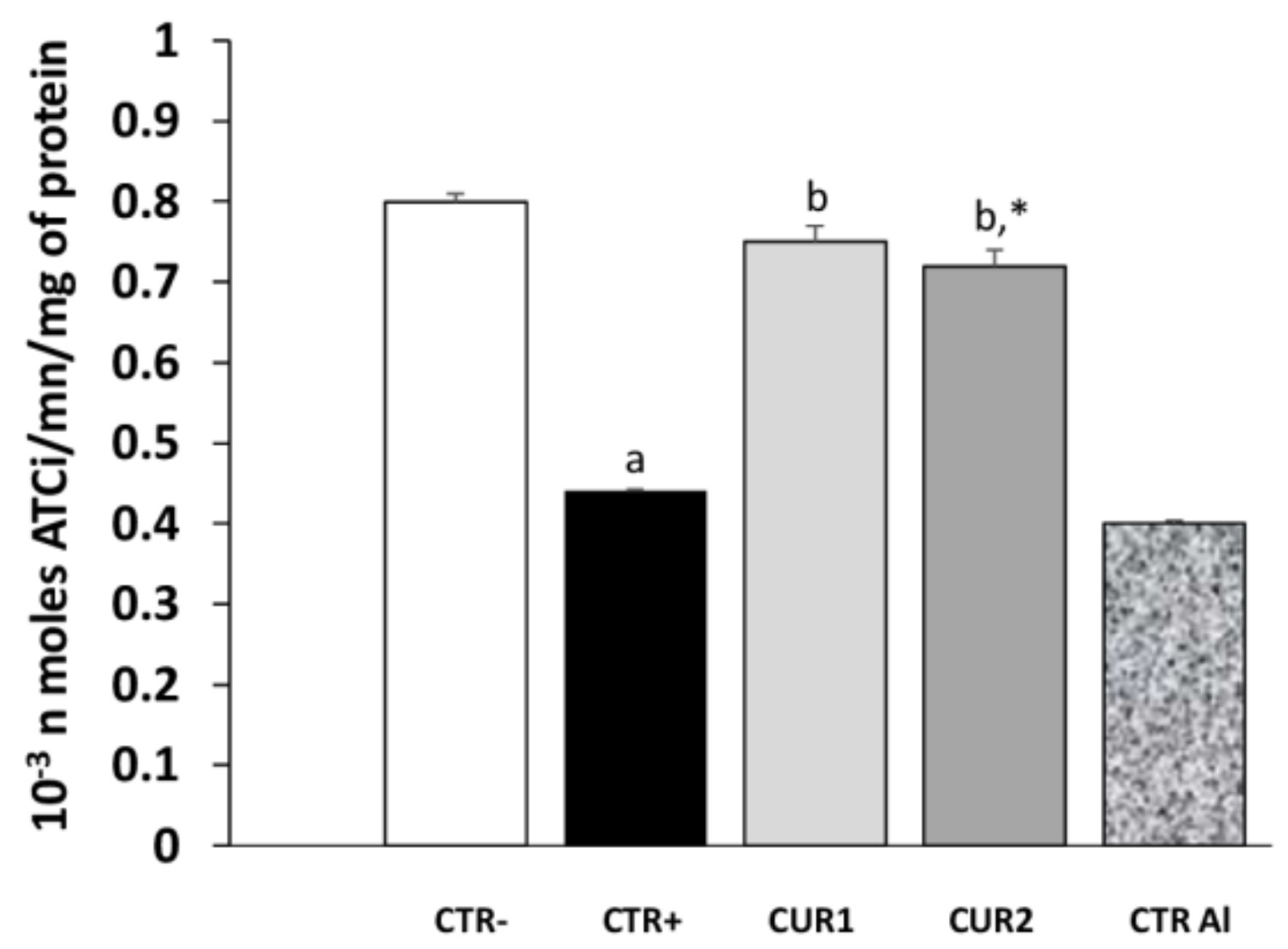
2.4. CUR Improves AlCl3-Induced AChE Alteration
2.5. CUR Nullifies AlCl3-Induced Oxidative Stress
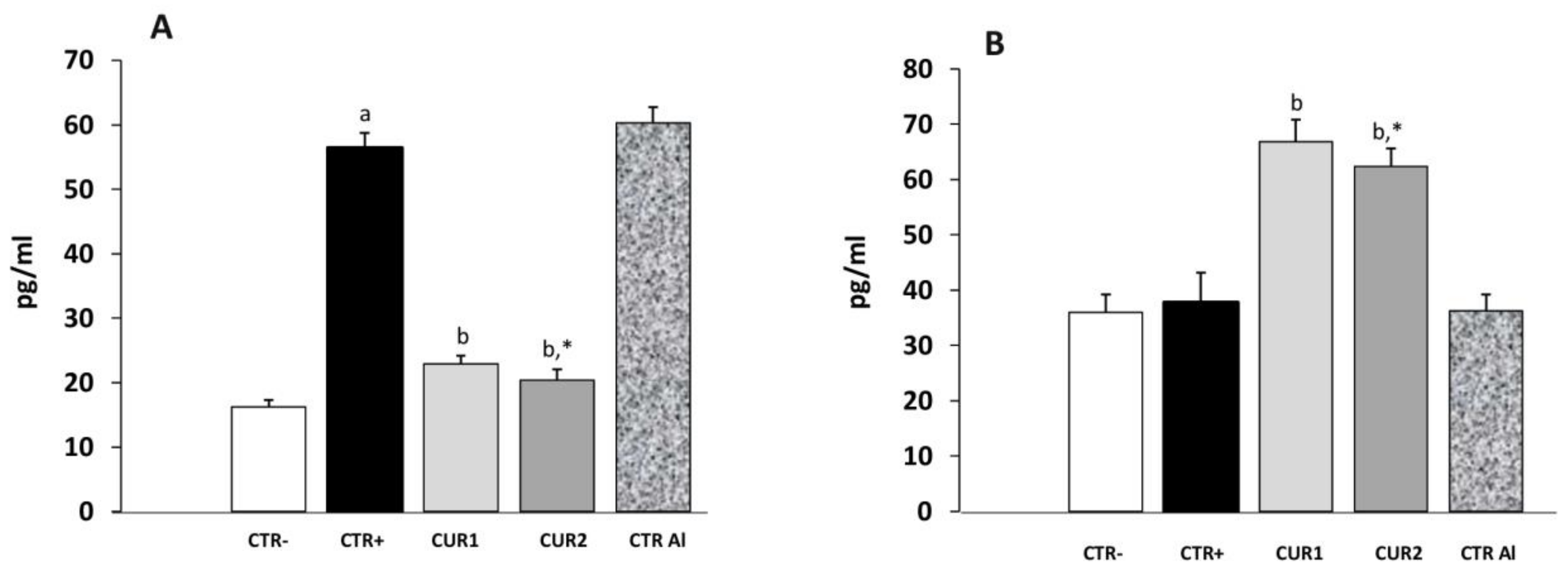
2.6. CUR Protects against AlCl3-Induced Neuroinflammation
2.7. CUR Enhances Viability of Hippocampus Cells in AlCl3-Exposed Rats
2.8. CUR Reduced Neurodegenetration in the Hippocampus of AlCl3-Exposed Rats
3. Discussion
4. Material and Methods
4.1. Animal Care and Chemicals
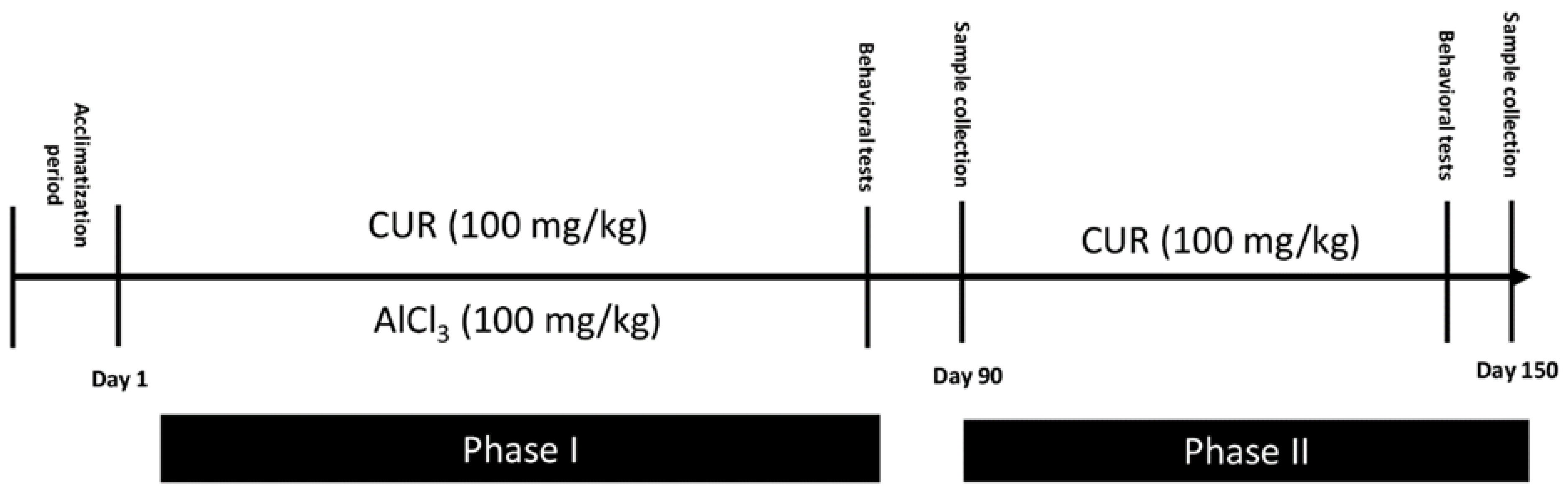
4.2. Induction of Neurotoxicity and Treatment Schedule
- Group CTR−: Six rats served as the control and provided only the vehicle solution (PBS; 1X) for 90 days.
- Group CTR+: Eighteen rats received AlCl3 (100 mg/kg b.w) diluted in 1 mL of PBS by oral gavage for 90 days to establish a model of neurotoxicity. At the end of AlCl3 treatment and AD induction, 6 of these rats were randomly sacrificed. These rats served as the positive control CTR+ of phase I. The other 12 rats continue the phase II of the experiment.
- Group CUR1: In this group, 6 rats were exposed daily to AlCl3 (100 mg/kg b.w) diluted in 1 mL of PBS by oral gavage, then treated, after 1 h, with CUR (100 mg/kg b.w) dissolved in 1 mL of corn oil by intragastric administration, for 90 days.
- Group CTR Al: Six rats were maintained without any other treatment for 60 days and served as a control for the CUR2 group.
- Group CUR2: Six rats were treated with 100 mg/kg of CUR dissolved in 1 mL of corn oil by intragastric administration for 60 days.
4.3. Evaluation of Neurodegenerescence
4.3.1. Body Weight Change
4.3.2. Behavioral Assessment
4.4. Hippocampus Preparation
4.4.1. Measurement of Hippocampus INF-γ and IL-4 Concentrations
4.4.2. Neurochemical Evaluation
4.4.3. Oxidative Stress Determination
4.4.4. MTT Cell Viability Assessment
4.4.5. Flow Cytometric Detection of Apoptotic Cells in the Hippocampus
4.4.6. Histological Examination
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Karran, E.; De Strooper, B.J. The amyloid cascade hypothesis: Are we poised for success or failure? Neurochemistry 2016, 139, 237–252. [Google Scholar] [CrossRef]
- Mendiola-Precoma, J.; Berumen, L.C.; Padilla, K.; Garcia-Alcocer, G. Therapies for Prevention and Treatment of Alzheimer’s Disease. Biomed. Res. Int. 2016, 258, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Colomina, M.T.; Peris-Sampedro, F. Aluminum and Alzheimer’s Disease. Adv. Neurobiol. Actions 2017, 18, 183–197. [Google Scholar]
- Sharma, S.; Wakode, S.; Sharma, A.; Nair, N.; Dhobi, M.; Wani, M.A.; Pottoo, F.H. Effect of environmental toxicants on neuronal functions. Environ. Sci. Pollut. Res. Int. 2020, 27, 44906–44921. [Google Scholar] [CrossRef] [PubMed]
- Maya, S.; Prakash, T.; Goli, D. Evaluation of neuroprotective effects of wedelolactone and gallic acid on aluminium-induced neurodegeneration: Relevance to sporadic amyotrophic lateral sclerosis. Eur. J. Pharmacol. 2018, 835, 41–51. [Google Scholar] [CrossRef]
- Olawuyi, T.S.; Akinola, K.B.; Adelakun, S.A.; Ogunlade, B.S.; Akingbade, G.T. Effects of Aqueous Leaf Extract of Lawsonia inermis on Aluminum-induced Oxidative Stress and Adult Wistar Rat Pituitary Gland Histology. JBRA Assist. Reprod. 2019, 23, 117–122. [Google Scholar]
- Ferreira, P.; Tonani, K.; Julião, F.; Cupo, P.; Domingo, J.; Segura-Muñoz, S. Aluminum concentrations in water of elderly people’s houses and retirement homes and its relation with elderly health. Bull. Environ. Contam. Toxicol. 2009, 83, 565–569. [Google Scholar] [CrossRef]
- Mocanu, C.S.; Jureschi, M.; Drochioiu, G. Aluminium Binding to Modified Amyloid-β Peptides: Implications for Alzheimer’s Disease. Molecules 2020, 25, 4536. [Google Scholar] [CrossRef] [PubMed]
- Mold, M.J.; O’Farrell, A.; Morris, B.; Exley, C. Aluminum and Neurofibrillary Tangle Co-Localization in Familial Alzheimer’s Disease and Related Neurological Disorders. J. Alzheimers Dis. 2020, 78, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. Aluminium and iron, but neither copper nor zinc, are key to the precipitation of beta-sheets of A beta(42) in senile plaque cores in Alzheimer’s disease. J. Alzheimers Dis. 2006, 10, 173–177. [Google Scholar] [CrossRef]
- Song, J. Animal Model of Aluminum-Induced Alzheimer’s Disease. Adv. Exp. Med. Biol. Actions 2018, 1091, 113–127. [Google Scholar]
- Inan-Eroglu, E.; Ayaz, A. Is aluminum exposure a risk factor for neurological disorders? J. Res. Med. Sci. 2018, 23, 51. [Google Scholar] [PubMed]
- Guieu, B.; Lecoutey, C.; Legay, R.; Davis, A.; Sopkova de Oliveira Santos, J.; Altomare, C.D.; Catto, M.; Rochais, C.; Dallemagne, P. First Synthesis of Racemic Trans Propargylamino-Donepezil, a Pleiotrope Agent Able to Both Inhibit AChE and MAO-B, with Potential Interest against Alzheimer’s Disease. Molecules 2020, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, F.; Abdallah, H.H.; Suroowan, S.; Jugreet, S.; Zhang, Y.; Hu, X. In silico Exploration of Bioactive Phytochemicals Against Neurodegenerative Diseases Via Inhibition of Cholinesterases. Pharm. Des. 2020, 26, 4151–4162. [Google Scholar] [CrossRef]
- ELBini Dhouib, I.; Annabi, A.; Doghri, R.; Rejeb, I.; Dallagi, Y.; Bdiri, Y.; Lasram, M.M.; Elgaaied, A.; Marrakchi, R.; Fazaa, S.; et al. Neuroprotective effects of curcumin against acetamiprid-induced neurotoxicity and oxidative stress in the developing male rat cerebellum: Biochemical, histological, and behavioral changes. Environ. Sci. Pollut. Res. Int. 2017, 24, 27515–27524. [Google Scholar] [CrossRef]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Curcumin Ameliorates Lead-Induced Hepatotoxicity by Suppressing Oxidative Stress and Inflammation, and Modulating Akt/GSK-3β Signaling Pathway. Biomolecules 2019, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Abo-Zaid, M.A.; Shaheen, E.S.; Ismail, A.H. Immunomodulatory effect of curcumin on hepatic cirrhosis in experimental rats. J. Food Biochem. 2020, 44, e13219. [Google Scholar] [CrossRef]
- Liao, K.K.; Wu, M.J.; Chen, P.Y.; Huang, S.W.; Chiu, S.J.; Ho, C.T.; Yen, J.H. Curcuminoids promote neurite outgrowth in PC12 cells through MAPK/ERK- and PKC-dependent pathways. J. Agric. Food Chem. 2012, 60, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Ashrafizadeh, M.; Azimi-Nezhad, M.; Samini, F.; Aschenr, M.; Samarghandian, S. Curcumin Efficacy in a Serum/glucose Deprivation-induced Neuronal PC12 Injury Model. Curr. Mol. Pharmacol. 2021, in press. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Cui, L.; Li, X.; Barish, P.A.; Foster, T.C.; Ogle, W.O. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007, 1162, 9–18. [Google Scholar] [CrossRef]
- Dong, S.; Zeng, Q.; Mitchell, E.S.; Xiu, J.; Duan, Y.; Li, C.; Tiwari, J.K.; Hu, Y.; Cao, X.; Zhao, Z. Curcumin enhances neurogenesis and cognition in aged rats: Implications for transcriptional interactions related to growth and synaptic plasticity. PLoS ONE 2012, 7, e31211. [Google Scholar] [CrossRef]
- Sadegh Malvajerd, S.; Izadi, Z.; Azadi, A.; Kurd, M.; Derakhshankhah, H.; Sharifzadeh, M.; Akbari Javar, H.; Hamidi, M. Neuroprotective Potential of Curcumin-Loaded Nanostructured Lipid Carrier in an Animal Model of Alzheimer’s Disease: Behavioral and Biochemical Evidence. J. Alzheimers Dis. 2019, 69, 671–686. [Google Scholar] [CrossRef]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin Coupled Lower Generation PAMAM Dendrimers for Brain Targeted Delivery of Memantine in Aluminum-Chloride-Induced Alzheimer’s Disease in Mice. Bioconjug. Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef]
- Prakash, D.; Sudhandiran, G. Dietary flavonoid fisetin regulates aluminium chloride-induced neuronal apoptosis in cortex and hippocampus of mice brain. J. Nutr. Biochem. 2015, 26, 1527–1539. [Google Scholar] [CrossRef]
- Thirunavukkarasu, S.V.; Venkataraman, S.; Raja, S.; Upadhyay, L. Neuroprotective effect of Manasamitra vatakam against aluminium induced cognitive impairment and oxidative damage in the cortex and hippocampus of rat brain. Drug Chem. Toxicol. 2012, 35, 104–115. [Google Scholar] [CrossRef]
- Bertrand, D.; Wallace, T.L. A Review of the Cholinergic System and Therapeutic Approaches to Treat Brain Disorders. Curr. Top Behav. Neurosci. 2020, 45, 1–28. [Google Scholar]
- Martinez, C.S.; Piagette, J.T.; Escobar, A.G.; Martín, Á.; Palacios, R.; Peçanha, F.M.; Vassallo, D.V.; Exley, C.; Alonso, M.J.; Salaices, M.; et al. Egg White Hydrolysate: A new putative agent to prevent vascular dysfunction in rats following long-term exposure to aluminum. Food Chem. Toxicol. 2019, 133, 110799. [Google Scholar] [CrossRef]
- Martinez, A.; Castro, A. Novel cholinesterase inhibitors as future effective drugs for the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 2006, 15, 1–12. [Google Scholar] [CrossRef]
- Eun, C.S.; Lim, J.S.; Lee, J.; Lee, S.P.; Yang, S.A. The protective effect of fermented Curcuma longa L. on memory dysfunction in oxidative stress-induced C6 gliomal cells, proinflammatory-activated BV2 microglial cells, and scopolamine-induced amnesia model in mice. BMC Complement. Altern. Med. 2017, 17, 367. [Google Scholar] [CrossRef]
- Gutierrez, M.E.Z.; Savall, A.S.P.; da Luz Abreu, E.; Nakama, K.A.; Dos Santos, R.B.; Guedes, M.C.M.; Ávila, D.S.; Luchese, C.; Haas, S.E.; Quines, C.B.; et al. Co-nanoencapsulated meloxicam and curcumin improves cognitive impairment induced by amyloid-beta through modulation of cyclooxygenase-2 in mice. Neural Regen. Res. 2021, 16, 783–789. [Google Scholar]
- Lee, E.H.; Lim, S.S.; Yuen, K.H.; Lee, C.Y. Curcumin and a hemi-analogue with improved blood-brain barrier permeability protect against amyloid-beta toxicity in Caenorhabditis elegans via SKN-1/Nrf activation. J. Pharm. Pharmacol. 2019, 71, 860–868. [Google Scholar] [CrossRef]
- Wong, K.Y.; Roy, J.; Fung, M.L.; Heng, B.C.; Zhang, C.; Lim, L.W. Relationships between Mitochondrial Dysfunction and Neurotransmission Failure in Alzheimer’s Disease. Aging Dis. 2020, 11, 1291–1316. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Clarke, R.M.; Lyons, A.; O’Connell, F.; Deighan, B.F.; Barry, C.E.; Anyakoha, N.G.; Nicolaou, A.; Lynch, M.A. A pivotal role for interleukin-4 in atorvastatin-associated neuroprotection in rat brain. J. Biol. Chem. 2008, 283, 1808–1817. [Google Scholar] [CrossRef]
- Nery-Flores, S.D.; Mendoza-Magaña, M.L.; Ramírez-Herrera, M.A.; Ramírez-Vázquez, J.J.; Romero-Prado, M.M.J.; Cortez-Álvarez, C.R.; Ramírez-Mendoza, A.A. Curcumin Exerted Neuroprotection against Ozone-Induced Oxidative Damage and Decreased NF-kappaB Activation in Rat Hippocampus and Serum Levels of Inflammatory Cytokines. Oxid. Med. Cell. Longev. 2018, 2018, 9620684. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Huang, P.; Chen, M.W. Curcumin attenuates cyclooxygenase-2 expression via inhibition of the NFkappaB pathway in lipopolysaccharide-stimulated human gingival fibroblasts. Cell Biol. Int. 2013, 37, 443–448. [Google Scholar] [CrossRef]
- Parada, E.; Buendia, I.; Navarro, E.; Avendano, C.; Egea, J.; Lopez, G.M. Microglial HO-1 induction by curcumin provides antioxidant, antineuroinflammatory, and glioprotective effects. Mol. Nutr. Food Res. 2015, 59, 1690–1700. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Chen, X.C.; Chen, Z.Z.; Zeng, Y.Q.; Shi, G.B.; Su, Y.H.; Peng, X. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol. Sin. 2004, 25, 1606–1612. [Google Scholar] [PubMed]
- Sood, P.K.; Nahar, U.; Nehru, B. Curcumin attenuates aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain. Neurotox Res. 2011, 20, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.M.; Zhang, J.; Zhang, C.L.; Zhang, Y.W.; Chen, X.C. Curcumin inhibits appoptosin-induced apoptosis via upregulating heme oxygenase-1 expression in SH-SY5Y cells. Acta Pharmacol. Sin. 2015, 36, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Lindström, T.; Kagedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimers Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef]
- Jiang, T.; Zhi, X.L.; Zhang, Y.H.; Pan, L.F.; Zhou, P. Inhibitory effect of curcumin on the Al(III)-induced Aβ₄₂ aggregation and neurotoxicity in vitro. Biochim. Biophys. Acta. 2012, 1822, 1207–1215. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, L.; Zhang, S.; Sun, P.C.; Ding, C.F.; Chu, Y.Q.; Ping, Z. Interaction of curcumin with Al(III) and its complex structures based on experiments and theoretical calculations. J. Mol. Struct. 2011, 1004, 163–173. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, W.; Liu, X.; Zhang, C.; Wang, P.; Zhao, X. Circulatory Levels of Toxic Metals (Aluminum, Cadmium, Mercury, Lead) in Patients with Alzheimer’s Disease: A Quantitative Meta-Analysis and Systematic Review. J. Alzheimers Dis. 2018, 62, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Aboelwafa, H.R.; El-kott, A.F.; Abd-Ella, E.M.; Yousef, H.N. The Possible Neuroprotective Effect of Silymarin against Aluminum Chloride-Prompted Alzheimer’s-Like Disease in Rats. Brain Sci. 2020, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Mathiyazahan, D.B.; Justin Thenmozhi, A.; Manivasagam, T. Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: A behavioural, biochemical and molecular approach. J. Funct. Foods 2015, 16, 423–435. [Google Scholar] [CrossRef]
- Yin, S.; Ran, Q.; Yang, J.; Zhao, Y.; Li, C. Nootropic effect of neferine on aluminium chloride-induced Alzheimer’s disease inexperimental models. J. Biochem. Mol. Toxicol. 2020, 34, e22429. [Google Scholar] [CrossRef] [PubMed]
- Mesole, S.B.; Alfred, O.O.; Yusuf, U.A.; Lukubi, L.; Ndhlovu, D. Apoptotic Inducement of Neuronal Cells by Aluminium Chloride and the Neuroprotective Effect of Eugenol in Wistar Rats. Oxid. Med. Cell. Longev. 2020, 2020, 8425643. [Google Scholar] [CrossRef]
- Giorgetti, M.; Gibbons, J.A.; Bernales, S.; Alfaro, I.E.; Drieu La Rochelle, C.; Cremers, T.; Altar, C.A.; Wronski, R.; Hutter-Paier, B.; Protter, A.A. Cognition-enhancing properties of dimebon in a rat novel object recognition task are unlikely to be associated with acetylcholinesterase inhibition or N-methyl-D-aspartate receptor antagonism. J. Pharm. Exp. Ther. 2010, 333, 748. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Báez, B.; Bordón, A.; Espinosa, L.; Martínez, E.; Pautassi, R.M. Short-term selection for high and low ethanol intake yields differential sensitivity to ethanol’s motivational effects and anxiety-like responses in adolescent Wistar rats. Prog. Neuropsychopharmacol. Biol Psychiatry. 2017, 79, 220–233. [Google Scholar] [CrossRef]
- Lakshmi, B.V.S.; Sudhakar, M.; Surya Prakash, K. Protective Effect of Selenium Against Aluminum Chloride-Induced Alzheimer’s Disease: Behavioral and Biochemical Alterations in Rats. Biol. Trace Elem. Res. 2015, 165, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Featherstone, R.M. A new and rapid colorimetrie determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Begue, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]


| Animal Body Weight (g) | Weight Gain (g) | Food Intake (g) | Food Efficiency Ratio (FER) | Hippocampus Weight (g) | ||
|---|---|---|---|---|---|---|
| Initial Body | Final Body | |||||
| CTR− | 142.45 ± 1.80 | 303.71 ± 2.03 | 62.83 ± 3.64 | 94.60 ± 3.42 | 0.19 ± 0.01 | 1.81 ± 0.67 |
| CTR+ | 148.75 ± 2.96 | 280.75 ± 3.40 a | 45.08 ± 2.66 a | 75.37 ± 2.75 | 0.19 ± 0.02 | 1.5 ± 0.23 a |
| CUR1 | 147.53 ± 1.67 | 275 ± 2.30 a | 36.083 ± 5.99 a,b | 66.43 ± 1.95 a,b | 0.18 ± 0.01 | 1.64 ± 0.26 |
| CTR Al | 280.75 ± 1.59 | 349.75 ± 2.40 a | 45.80 ± 2.50 a | 73.57 ± 2.75 | 0.18 ± 0.01 | 1.55 ± 0.19 a |
| CUR2 | 280.50 ± 1.23 | 388.21 ± 1.90 a,* | 30.84 ± 3.14 a,* | 62.13 ± 1.00 a,* | 0.18 ± 0.01 | 1.63 ± 0.29 |
| CTR− | CTR+ | CUR1 | CTRAl | CUR2 | |
|---|---|---|---|---|---|
| MDA | 0.62 ± 0.26 | 0.95 ± 0.05 a | 0.51 ± 0.20 b | 1.05 ± 0.09 | 0.60 ± 0.02 b,* |
| CAT | 0.76 ± 0.54 | 0.19 ± 0.03 a | 0.83 ± 0.26 b | 0.10 ± 0.09 | 0.89 ± 0.01 b,* |
| SOD | 0.10 ± 0.06 | 0.05 ± 0.01 a | 0.11 ± 0.081 b | 0.05 ± 0.01 | 0.15 ± 0.09 b,* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ELBini-Dhouib, I.; Doghri, R.; Ellefi, A.; Degrach, I.; Srairi-Abid, N.; Gati, A. Curcumin Attenuated Neurotoxicity in Sporadic Animal Model of Alzheimer’s Disease. Molecules 2021, 26, 3011. https://doi.org/10.3390/molecules26103011
ELBini-Dhouib I, Doghri R, Ellefi A, Degrach I, Srairi-Abid N, Gati A. Curcumin Attenuated Neurotoxicity in Sporadic Animal Model of Alzheimer’s Disease. Molecules. 2021; 26(10):3011. https://doi.org/10.3390/molecules26103011
Chicago/Turabian StyleELBini-Dhouib, Ines, Raoudha Doghri, Amenallah Ellefi, Imen Degrach, Najet Srairi-Abid, and Asma Gati. 2021. "Curcumin Attenuated Neurotoxicity in Sporadic Animal Model of Alzheimer’s Disease" Molecules 26, no. 10: 3011. https://doi.org/10.3390/molecules26103011
APA StyleELBini-Dhouib, I., Doghri, R., Ellefi, A., Degrach, I., Srairi-Abid, N., & Gati, A. (2021). Curcumin Attenuated Neurotoxicity in Sporadic Animal Model of Alzheimer’s Disease. Molecules, 26(10), 3011. https://doi.org/10.3390/molecules26103011






