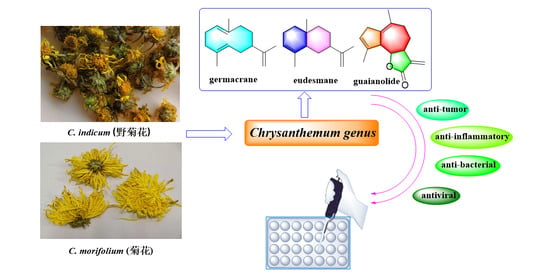
Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus
Abstract
1. Introduction
2. Sesquiterpenoids
2.1. Germacrane-Type Sesquiterpenoids
2.2. Eudesmane-Type Sesquiterpenoids
2.3. Guaianolide-Type Sesquiterpenoids
2.4. 1,10-Seco Guaianolide Sesquiterpenoids
2.5. Disesquiterpenoids and a Trisesquiterpenoid
2.6. Other Types of Sesquiterpenoids
3. Biosynthetic Pathway of Sesquiterpenoids
4. Pharmacological Activities
4.1. Antitumor Activity
4.2. Anti-Inflammatory Activity
4.3. Antibacterial Activity
4.4. Antiviral Activity
4.5. Antidiabetic and Antiobesity Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, P.L.; Wan, Q.; Guo, Y.P.; Yang, J.; Rao, G.Y. Phylogeny of the genus Chrysanthemum L.: Evidence from single-copy nuclear gene and chloroplast DNA sequences. PLoS ONE 2012, 7, e48970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.E.; Liu, Z.H.; Hu, X.; Yin, J.L.; Li, W.; Rao, G.Y.; Zhang, X.H.; Huang, C.L.; Anderson, N.; Zhang, Q.X.; et al. Chrysanthemum genetic resources and related genera of Chrysanthemum collected in China. Genet. Resour. Crop. Evol. 2009, 56, 937–946. [Google Scholar] [CrossRef]
- Ja, T.D.S. Chrysanthemum: advances in tissue culture, cryopreservation, postharvest technology, genetics and transgenic biotechnology. Biotechnol. Adv. 2003, 21, 715–766. [Google Scholar]
- Chatterjee, J.; Mandal, A.; Ranade, S.; Da Silva, J.A.T.; Datta, S. Molecular systematics in Chrysanthemum×grandiflorum (Ramat.) Kitamura. Sci. Hortic. 2006, 110, 373–378. [Google Scholar] [CrossRef]
- Zhou, H.P.; Ren, M.X.; Guan, J.Q.; Liu, Y.L.; Xiong, Y.X.; Zhong, Q.F.; Jiang, S.; Wu, S.X. Research progress on chemical constituents and pharmacological effects of Chrysanthemum morifolium and predictive analysis on quality markers. Chin. Tradit. Herbal Drugs 2019, 50, 4785–4795. [Google Scholar]
- Liu, L.L.; Xiao, Z.B. Chemical constituents from flowers of Chrysanthemum indicum. Chin. Tradit. Herbal Drugs 2018, 49, 29–33. [Google Scholar]
- Jin, J.Z.; Wen, M.; Shen, T.C. Research progress in chemical components in Chrysanthemum morifolium. Sci. Tech. Food Ind. 2014, 35, 386–389. [Google Scholar]
- Cai, H.F. The research progression of flos Chrysanthemi indici on chemical constituents and medicial application. Nat. Med. Front. China 2007, 2, 118–120. [Google Scholar]
- Li, Y.; Yang, P.; Luo, Y.; Gao, B.; Sun, J.; Lu, W.; Liu, J.; Chen, P.; Zhang, Y.; Yu, L.L. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019, 286, 8–16. [Google Scholar] [CrossRef]
- Gao, T.; Zhu, Z.Y.; Zhou, X.; Xie, M.L. Chrysanthemum morifolium extract improves hypertension-induced cardiac hypertrophy in rats by reduction of blood pressure and inhibition of myocardial hypoxia inducible factor-1alpha expression. Pharm. Biol. 2016, 54, 2895–2900. [Google Scholar] [CrossRef]
- Liu, L.L.; Ha, T.K.Q.; Ha, W.; Oh, W.K.; Yang, J.L.; Shi, Y.P. Sesquiterpenoids with various carbocyclic skeletons from the flowers of Chrysanthemum indicum. J. Nat. Prod. 2017, 80, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.F.; Feng, Z.M.; Yang, Y.N.; Jiang, J.S.; Zhang, P.C. Neuroprotective caffeoylquinic acid derivatives from the flowers of Chrysanthemum morifolium. J. Nat. Prod. 2017, 80, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.F.; Yang, Y.N.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. Six new compounds from the flowers of Chrysanthemum morifolium and their biological activities. Bioorganic Chem. 2019, 82, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Chang, J.; Zong, Y.; Hu, G.; Jia, J. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. Aromaticum using three extraction methods and two columns. Molecules 2018, 23, 576. [Google Scholar] [CrossRef] [PubMed]
- Han, X.B.; Zhao, J.; Cao, J.M.; Zhang, C.S. Essential oil of Chrysanthemum indicum L.: potential biocontrol agent against plant pathogen Phytophthora nicotianae. Environ. Sci. Pollut. Res. 2019, 26, 7013–7023. [Google Scholar] [CrossRef]
- Wang, J.S.; Zhou, J.; Kong, L.Y. Three new germacrane-type sesquiterpene stereoisomers from the flowers of Chrysanthemum indicum. Fitoterapia 2012, 83, 1675–1679. [Google Scholar] [CrossRef]
- Hu, L.; Chen, Z. Sesquiterpenoid alcohols from Chrysanthemum morifolium. Phytochemistry 1997, 44, 1287–1290. [Google Scholar] [CrossRef]
- Mareo, J.A.; Sanz, J.F.; Jakupovic, J.; Huneck, S. New sesquiterpene lactones and acetylenes from Chrysanthemum lavandulifolium. Tetrahedron 1990, 46, 6931–6938. [Google Scholar] [CrossRef]
- Shin, H.J.; Lee, S.Y.; Kim, J.S.; Lee, S.; Choi, R.J.; Chung, H.S.; Kim, Y.S.; Kang, S.S. Sesquiterpenes and other constituents from Dendranthema zawadskii var. latilobum. Chem. Pharm. Bull. 2012, 60, 306–314. [Google Scholar] [CrossRef]
- Guo, L.M.; Lyu, J.L.; Zhang, L.B. Research progress on anti-inflammatory mechanism of natural sesquiterpenoids. China J. Chin. Mater. Med. 2018, 43, 3989–3999. [Google Scholar]
- Liu, L.; Wang, R.; Yang, J.; Shi, Y. Five new sesquiterpenoids from Chrysanthemum indicum. Chin. J. Chem. 2012, 30, 1255–1260. [Google Scholar] [CrossRef]
- Zhao, F.Q.; Zhang, Q.Q.; Yan, Y.; Jia, H.Y.; Zhao, X.R.; Li, X.Y.; Zheng, L.H.; Han, G. Antioxidant constituents of Chrysanthemum ‘jinsidaju’ cultivated in Kaifeng. Fitoterapia 2019, 134, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Morikawa, T.; Toguchida, I.; Harima, S.; Matsuda, H. Medicinal flowers. II. Inhibitors of nitric oxide production and absolute stereostructures of five new germacrane-type sesquiterpenes, kikkanols D, D monoacetate, E, F, and F monoacetate from the flowers of Chrysanthemum indicum L. Chem. Pharm. Bull. 2000, 48, 651–656. [Google Scholar] [CrossRef]
- Toshihiko, O.; Akinori, S.; Saburo, T. Molecular structure and stereochemistry of chrysandiol, a novel sesquiterpene diol from Chrysanthemum morifolium. Tetrahedron Lett. 1974, 17, 1569–1572. [Google Scholar]
- Wang, X.L.; Peng, S.L.; Liang, J.; Yu, K.B. (3β,5α,6β,7β,14β)-Eudesmen-3,5,6,11-tetrol methanol solvate: a new sesquiterpenoid from Chrysanthemum indicum L. Acta. Crystallogr. E. 2010, 62, o3570–o3571. [Google Scholar] [CrossRef]
- Yang, J.L.; Liu, L.L.; Shi, Y.P. Two new eudesmane sesquiterpenoids from the flowers of Chrysanthemum indicum. Nat. Prod. Bioprospecting 2019, 9, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Q.; Xie, F.Z. Chemical constituents from flowers of Chrysanthemum indicum. Acta Pharm. Sin. 1987, 22, 837–840. [Google Scholar]
- Yoshikawa, M.; Morikawa, T.; Murakami, T.; Toguchida, I.; Harima, S.; Matsuda, H. Medicinal flowers. I. Aldose reductase inhibitors and three new eudesmane-type sesquiterpenes, kikkanols A, B, and C, from the flowers of Chrysanthemum indicum L. Chem. Pharm. Bull. 1999, 47, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Haruna, M.; Kato, M.; Ito, K.; Nikai, T.; Sugihara, H.; Muratat, H. Angeloylcumambrin-B, an antimicrobial sesquiterpene lactone from Chrysanthemum ornatum var. Spontaneum. Phytochemistry 1981, 20, 2583–2584. [Google Scholar] [CrossRef]
- Jang, D.S.; Park, K.H.; Choi, S.U.; Nam, S.H.; Yang, M.S. Antibacterial substances of the flower of Chrysanthemum zawadskii Herbich var. latilobum Kitamura. Agr. Chem. Biotechnol. 1997, 40, 85–88. [Google Scholar]
- Luo, P.; Cheng, Y.; Yin, Z.; Li, C.; Xu, J.; Gu, Q. Monomeric and dimeric cytotoxic guaianolide-type sesquiterpenoids from the aerial parts of Chrysanthemum indicum. J. Nat. Prod. 2019, 82, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, X.; Li, B.; Yang, K.; Wang, Y.; Sun, K.; Zhang, Y.; Zhu, W. A new sesquiterpenoid from Chrysanthemum indicum. Chem. Nat. Compd. 2019, 55, 1076–1079. [Google Scholar] [CrossRef]
- Chen, W.; Zeng, M.; Li, M.; Li, F.; Zhao, X.; Fan, H.; Zheng, X.; Feng, W. Four new sesquiterpenoids from Dendranthema morifolium (Ramat.) kitam flowers. Phytochem. Lett. 2018, 23, 52–56. [Google Scholar] [CrossRef]
- Feng, Z.M.; Song, S.; Xia, P.F.; Jiang, J.S.; Zhang, P.C. Three new sesquiterpenoids from Chrysanthemum indicum L. Helv. Chim. Acta 2009, 92, 1823–1828. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, M.; Li, M.; Chen, W.; Li, B.; Kan, Y.; Feng, W.; Zheng, X. Guaiane-type sesquiterpenoids from Dendranthema morifolium (Ramat.) S. Kitam flowers protect H9c2 cardiomyocyte from LPS-induced injury. Nat. Prod. Commun. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Mladenova, K.; Tsankova, E.; Van Hung, D. New sesquiterpenoids from Chrysanthemum indicum var. tuneful. Planta Med. 1988, 54, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.F.; Jia, L.; Shi, S.P.; Sun, X.L.; Chen, Y.Y.; Zhang, Y.B. New sesquiterpenes from the flowers of Chrysanthemum indicum L. Helv. Chim. Acta 2010, 93, 1953–1959. [Google Scholar] [CrossRef]
- Mladenova, K.; Tsankova, E.; Stoianova-Ivanova, B. Sesquiterpene lactones from Chrysanthemum indicum. Planta Med. 1985, 51, 284–285. [Google Scholar] [CrossRef]
- Xue, G.M.; Li, X.Q.; Chen, C.; Chen, K.; Wang, X.B.; Gu, Y.C.; Luo, J.G.; Kong, L.Y. Highly oxidized guaianolide sesquiterpenoids with potential anti-inflammatory activity from Chrysanthemum indicum. J. Nat. Prod. 2018, 81, 378–386. [Google Scholar] [CrossRef]
- Luyen, N.T.; Tram, L.H.; Hanh, T.T.H.; Binh, P.T.; Dang, N.H.; Minh, C.V.; Dat, N.T. Inhibitors of α-glucosidase, α-amylase and lipase from Chrysanthemum morifolium. Phytochem. Lett. 2013, 6, 322–325. [Google Scholar] [CrossRef]
- Xue, G.M.; Xue, J.F.; Zhao, C.G.; Zhao, Z.Z.; Zhi, Y.L.; Du, K.; Li, H.W.; Sun, Y.J.; Feng, W.S. 1,10-seco guaianolide-type sesquiterpenoids from Chrysanthemum indicum. J. Asian Nat. Prod. Res. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.S.; Zhang, Y.; Wang, P.R.; Guo, C.; Kong, L.Y. Disesquiterpenoid and sesquiterpenes from the flos of Chrysanthemum indicum. Chem. Pharm. Bull. 2012, 60, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, Y.; Cui, H.; Huang, D.; Zhou, J.; Wu, T.; Chen, Y.; Shi, L.; Xu, J. Chrysanolide A, an unprecedented sesquiterpenoid trimer from the flowers of Chrysanthemum indicum L. RSC Adv. 2013, 3, 10168–10172. [Google Scholar] [CrossRef]
- Zhang, W.J.; You, C.X.; Yang, K.; Wang, Y.; Su, Y.; Geng, Z.F.; Du, S.S.; Wang, C.F.; Deng, Z.W.; Wang, Y.Y. Bioactivity and chemical constituents of the essential oil from Dendranthema indicum (L.) Des Moul. against two stored insects. J. Oleo Sci. 2015, 64, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Si, M.; Tan, M.C.S.; Pelobello, D.H.; Don, M.J.; Shen, C.C. A new sesquiterpene from Dendranthema grandiflora flowers. Chem. Nat. Compd. 2020, 56, 436–439. [Google Scholar] [CrossRef]
- Engvild, K.C. Chlorine-containing natural compounds in higher plants. Phytochemistry 1986, 25, 781–791. [Google Scholar] [CrossRef]
- Youssef, D.; Frahm, A. Constituents of the Egyptian Centaurea scoparia; Chlorinated guaianolides of the aerial parts. Planta Med. 1994, 60, 267–271. [Google Scholar] [CrossRef]
- Xuan, G.M.; Xuan, J.F.; Du, K. Research progress on sesquiterpenoid dimers from Compositae. Nat. Prod. Res. Dev. 2019, 31, 2189–2196. [Google Scholar]
- Adio, A.M. Germacrenes A–E and related compounds: thermal, photochemical and acid induced transannular cyclizations. Tetrahedron 2009, 65, 1533–1552. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Saillard, N.; Yang, T.; Franssen, M.C.R.; Bouwmeester, H.J.; Jongsma, M.A. Biosynthesis of sesquiterpene lactones in Pyrethrum (Tanacetum cinerariifolium). PLoS ONE 2013, 8, e65030. [Google Scholar] [CrossRef]
- Kraker, J.W.d.; Bouwmeester, H.J.; Franssen, M.C.R.; Groot, A. (+)-Germacrene A synthesis in chicory (Cichorium intybus L.); the first step in sesquiterpene lactone biosynthesis. Acta Bot. Gallica 1999, 146, 111–115. [Google Scholar] [CrossRef]
- Xi, F.M.; Ma, S.G.; Liu, Y.B.; Li, L.; Yu, S.S. Artaboterpenoids A and B, bisabolene-derived sesquiterpenoids from Artabotrys hexapetalus. Org. Lett. 2016, 47, 3374–3377. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.S.; Li, H.; Kessler, S.; Solomon, P.S.; Piggott, A.M.; Chooi, Y.H. Bipolenins K–N: New sesquiterpenoids from the fungal plant pathogen Bipolaris sorokiniana. Beilstein J. Org. Chem. 2019, 15, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Liu, X.H.; Shi, Z.Z.; Miao, F.P.; Fang, S.T.; Ji, N.Y. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry 2018, 152, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.P.; Krichau, N.; Reichwald, K.; Simonsen, H.T. Guaianolides in apiaceae: perspectives on pharmacology and biosynthesis. Phytochem. Rev. 2009, 8, 581–599. [Google Scholar] [CrossRef]
- Andreas, S.; Oliver, R. Synthesis of biologically active guaianolides with a trans-annulated lactone moiety. Eur. J. Org. Chem. 2008, 14, 2253–2264. [Google Scholar]
- Xue, G.M.; Han, C.; Chen, C.; Li, L.N.; Wang, X.B.; Yang, M.H.; Gu, Y.C.; Luo, J.G.; Kong, L.Y. Artemisians A–D, diseco-guaianolide involved heterodimeric [4 + 2] adducts from Artemisia argyi. Org. Lett. 2017, 19, 5410–5413. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Gómez, A.; Ontiveros-Rodríguez, J.C.; Pablo-Pérez, S.S.; Vargas-Díaz, M.E.; Garduo-Siciliano, L. The potential role of sesquiterpene lactones isolated from medicinal plants in the treatment of the metabolic syndrome - A review. S. Afr. J. Bot. 2020, 135, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, J.L. Research progress on anticancer effects and mechanisms of three natural sesquiterpenoids. J. Jinzhou Med. Univ. 2020, 041, 109–112. [Google Scholar]
- Zhu, H.Y.; Pu, H.S. Research progress on anti-cancer mechanism of natural sesquiterpenoids. West China J. Pharm. Sci. 2015, 30, 381–383. [Google Scholar]
- Zhuo, F.; Zhang, C.; Xia, Y.; Xue, G.; Yang, L.; Kong, L. Chrysanthemulide A induces apoptosis through DR5 upregulation via JNK-mediated autophagosome accumulation in human osteosarcoma cells. J. Cell. Physiol. 2019, 234, 13191–13208. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Yang, Y.; Yu, M.; Han, Z.Z.; Wei, M.; Zhang, H.W.; Jia, H.M.; Zou, Z.M. Anti-inflammatory chemical constituents of Flos Chrysanthemi Indici determined by UPLC-MS/MS integrated with network pharmacology. Food Funct. 2020, 11, 6340–6351. [Google Scholar] [CrossRef] [PubMed]








| No. | Compounds | Species | Parts Used | Identification Methods | Ref. |
|---|---|---|---|---|---|
| Germacrane | |||||
| 1 | 1β,3α,5β-trihydroxyl-7-isopropenyl-germacren-4(15),10(14)-diene | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; X-ray | [16] |
| 2 | 1β,3β,5α-trihydroxyl-7-isopropenyl-germacren-4(15),10(14)-diene | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [16] |
| 3 | 1β,3β,5β-trihydroxyl-7-isopropenyl-germacren-4(15),10(14)-diene | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [16] |
| 4 | chrysanthemumin C | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 5 | chrysanthemumin D | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 6 | chrysanthediacetate B | C. morifolium | Flowers | 1D, 2D NMR; EIMS | [17] |
| 7 | chrysanthediacetate C | C. morifolium | Flowers | 1D, 2D NMR; EIMS | [17] |
| 8 | (3R,7R,9R)-3,9-dihydroxygermacra-4(15),10(14),11(12)-triene | C. morifolium | Flowers | 1D, 2D NMR; ESIMS | [22] |
| 9 | chrysanthediol A | C. morifolium | Flowers | 1D, 2D NMR; EIMS | [17] |
| 10 | kikkanol D | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [23] |
| 11 | kikkanol D monoacetate | C. indicum | Flowers | 1D, 2D NMR; HRFABMS | [23] |
| 12 | kikkanol E | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; Mosher | [23] |
| 13 | 1β-hydroxy-4(15),5E,10(14)-germacratriene | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 14 | chrysanthemumin I | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 15 | chrysandiol | C. morifolium | [24] | ||
| 16 | chrysanthemumin H | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; X-ray; ECD | [11] |
| 17 | 1β,3β-dihydroxygermacra-4Z,10(14)-dien-6β,7α,11αH-12,6-olide | C lavandulifolium | Aerial parts | 1D, 2D NMR; EIMS | [18] |
| 18 | 1β-hydroperoxy-3β-hydroxygermacra-4Z,10(14)-dien-6β,7α,11αH-12,6-olide | C lavandulifolium | Aerial parts | 1D, 2D NMR; EIMS | [18] |
| 19 | zawadskinolide D | C. zawadskii | Aerial parts | 1D, 2D NMR; HRFABMS | [19] |
| 20 | zawadskinolide E | C. zawadskii | Aerial parts | 1D, 2D NMR; HRCIMS | [19] |
| 21 | zawadskinolide F | C. zawadskii | Aerial parts | 1D, 2D NMR; HRCIMS | [19] |
| 22 | zawadskinolide A | C. zawadskii | Aerial parts | 1D, 2D NMR; HREIMS | [19] |
| 23 | zawadskinolide B | C. zawadskii | Aerial parts | 1D, 2D NMR; HREIMS | [19] |
| 24 | zawadskinolide C | C. zawadskii | Aerial parts | 1D, 2D NMR; HREIMS | [19] |
| 25 | kikkanol F | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [23] |
| 26 | kikkanol F monoacetate | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [23] |
| Eudesmane | |||||
| 27 | chrysanthemumol I | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 28 | chrysanthemumol J | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 29 | eudesm-4(14)-ene-3α,11-diol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [21] |
| 30 | (3β,5α,6β,7β,14β)-eudesmen-3,5,6,11-tetrol | C. indicum | Flowers | 1D NMR; X-ray | [25] |
| 31 | chrysanthemumin A | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 32 | 5α-hydroxy-β-eudesmol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 33 | chrysanthemumin D | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 34 | chrysanthemumin E | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 35 | chrysanthemumin B | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 36 | 7-epi-1β-hydroxy-β-eudesmol | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [26] |
| 37 | chrysanthemol | C. indicum | Flowers | 1D, 2D NMR; HREIMS | [27] |
| 38 | chrysanthemumin F | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; ECD | [11] |
| 39 | ligucyperonol | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 40 | chrysanthemumol K | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [21] |
| 41 | canusesnol E | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [21] |
| 42 | chrysanthemumin C | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [11] |
| 43 | β-dictyopterol | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [17] |
| 44 | 7-epi-eudesm-4(15),11(13)-diene-1β,3β-diol | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [26] |
| 45 | intermedeol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 46 | cyperusol C | C. morifolium | Flowers | 1D, 2D NMR; ESIMS | [22] |
| 47 | chrysantiloboside | C. zawadskii | Aerial parts | 1D, 2D NMR; FABMS | [19] |
| 48 | oplodiol 1-O-β-D-glucopyranoside | C. zawadskii | Aerial parts | 1D, 2D NMR; HREIMS | [19] |
| 49 | kikkanol C | C. indicum | Flowers | 1D, 2D NMR; HREIMS; Mosher | [28] |
| 50 | kikkanol B | C. indicum | Flowers | 1D, 2D NMR; HREIMS; Mosher | [28] |
| 51 | kikkanol A | C. indicum | Flowers | 1D, 2D NMR; HREIMS; Mosher | [28] |
| 52 | eudesm-4(15)-ene-1β,6α-diol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| Guaianolide | |||||
| 53 | angeloylcumambrin B | C. ornatum C. indicum C. zawadskii | Whole herbs Flowers Flowers | 1D, 2D NMR; ESIMS 1D, 2D NMR; ESIMS 1D, 2D NMR; ESIMS | [29] [11] [30] |
| 54 | cumambrin A | C.ornatum C. indicum C. zawadskii | Whole herbs Flowers Flowers | 1D, 2D NMR; ESIMS 1D, 2D NMR; ESIMS 1D, 2D NMR; ESIMS | [29] [11] [30] |
| 55 | cumambrin-B | C.ornatum | Whole herbs | 1D, 2D NMR; ESIMS | [29] |
| 56 | chrysanolide G | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 57 | tigloylcumambrin B | C. indicum | Aerial parts | 1D NMR | [31] |
| 58 | chrysanolide F | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 59 | 8-tigloylchrysanolide F | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 60 | chrysanolide B | C. indicum | Aerial parts | 1D NMR | [31] |
| 61 | 10α-hydroxy-8α-O-(β-D-glucopyranosyl)-1αH,5αH, 6βH,8βH,7αH,11βH,11α-methylguaia-3-enolide | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [32] |
| 62 | chrysanthemumin J | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; ECD | [11] |
| 63 | chrysanolide H | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [31] |
| 64 | 8-angeloylchrysanolide H | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 65 | chrysanthguaianolactone D | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [33] |
| 66 | chrysanthguaianolactone C | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [33] |
| 67 | 3α,4α,10β-trihydroxy-8α-acetoxyguai-1,11(13)-dien-6α,12-olide | C. morifolium | Flowers | 1D NMR | [33] |
| 68 | 3α,4α,10β-trihydroxy-8α-acetoxy-11βH-guai-1-en-6α,12-olide | C. morifolium | Flowers | 1D NMR | [33] |
| 69 | 1α,3α,4β-trihydroxy-8α-acetoxy-9-en-6α,12-olide | C. morifolium | Flowers | 1D NMR | [33] |
| 70 | chrysanthguaianolactone E | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [33] |
| 71 | 8α-(angelyloxy)-3β,4β-dihydroxy-5αH,6βH,7αH, 11αH-guai-1(10)-en-12,6-olide | C. morifolium | Flowers | 1D NMR | [33] |
| 72 | indicumolide A | C. indicum | Flowers | 1D, 2D NMR; HREIMS | [34] |
| 73 | indicumolide B | C. indicum | Flowers | 1D, 2D NMR; HREIMS | [34] |
| 74 | chrysanolide I | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [31] |
| 75 | 3β,4α-dihydroxy-8α-angelyloxy-1(10),11(13)-dien-6β,12-olide | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [35] |
| 76 | 11,13-dehydrodesacetylmatricarin | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 77 | matricarin | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 78 | 8β-angeloyloxy-1β,4β,10β-trihydroxy-guai-2-en-6α,12-olide | C. indicum | Aerial parts | 1D, 2D NMR; EIMS | [36] |
| 79 | chrysanthguaianolactone B | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [37] |
| 80 | (3α,6α,8α)-8-tigloyl-3,4-epoxyguai-1(10)-eno-12,6-lactone | C. indicum | Flowers | 1D NMR | [37] |
| 81 | Angeloylajad | C. indicum | Aerial parts | 1D NMR; EIMS | [38] |
| 82 | arteglasin A | C. indicum | Aerial parts | 1D NMR; EIMS | [38] |
| 83 | guaianolide ajadin | C. indicum | Aerial parts | 1D NMR; EIMS | [38] |
| 84 | apressin | C. indicum | Flowers | 1D NMR | [37] |
| 85 | athanadregeolid | C. indicum | Flowers | 1D NMR | [37] |
| 86 | chrysanthemulide H | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 87 | 8-tigloyldesacetylezomontanin | C. indicum | Aerial parts | 1D NMR | [39] |
| 88 | 10α-hydroxy-1α,4α-endoperoxy-guaia-2-en-12,6α-olide | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [40] |
| 89 | chrysanthemulide F | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 90 | chrysanthemulide G | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 91 | 10-epiajafinin | C. indicum | Aerial parts | 1D NMR | [39] |
| 92 | chrysanthemulide A | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 93 | chrysanthemulide B | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 94 | chrysanthemulide C | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 95 | chrysanthemulide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 96 | chrysanthemulide E | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 97 | chrysanthguaianolactone A | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [37] |
| 98 | isoseco-tanapartholide | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 99 | (-)-9-angeloyloxy-seco-tanapartholide B | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [41] |
| 100 | (-)-seco-tanapartholide B | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 101 | (-)-seco-tanapartholide A | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 102 | (+)-seco-tanapartholide A | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 103 | (+)-seco-tanapartholide B | C. indicum | Aerial parts | 1D, 2D NMR; ECD | [41] |
| 104 | handelin | C. ornatum C. indicum | Whole herbs Aerial parts | 1D, 2D NMR; ESIMS 1D NMR | [29] [31] |
| 105 | chrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; X-ray | [31] |
| 106 | chrysanolide E | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [31] |
| 107 | 8′-tigloylchrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 108 | 8-angeloyl-8′-hydroxychrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 109 | 8,8′-ditigloylchrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 110 | 8-tigloylchrysanolide D | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS | [31] |
| 111 | artanomalide C | C. indicum | Aerial parts | 1D NMR | [31] |
| 112 | - | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; X-ray | [42] |
| 113 | chrysanthemulide I | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 114 | chrysanthemulide J | C. indicum | Aerial parts | 1D, 2D NMR; HRESIMS; ECD | [39] |
| 115 | chrysanolide C | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [43] |
| 116 | chrysanolide A | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; ECD | [43] |
| Other types | |||||
| 117 | jinsidajuol A | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [22] |
| 118 | jinsidajuol B | C. morifolium | Flowers | 1D, 2D NMR; HRESIMS | [22] |
| 119 | chrysetuno | C. indicum | Aerial parts | 1D, 2D NMR; EIMS | [36] |
| 120 | tunefulin | C. indicum | Aerial parts | 1D, 2D NMR; EIMS | [36] |
| 121 | 11-hydroxy-1-oxo-4α,5α,7β,10β-eremophilane | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 122 | indicumolide C | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [34] |
| 123 | oplopanone | C. indicum | Flowers | 1D NMR; EIMS | [28] |
| 124 | spathulenol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 125 | caryolane 1,9β-dio | C. indicum | Flowers | 1D NMR; EIMS | [28] |
| 126 | chrysanthemumin G | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 127 | 11(7→6)abeo-14-norcarbrane-4,7-dione | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 128 | 6,8-cycloeudesm-4(15)-en-1-ol | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 129 | (4R,5R)-4,5-dihydroxycaryophyll-8(13)-ene | C. indicum | Flowers | 1D, 2D NMR; ESIMS | [11] |
| 130 | β-caryophyllene | C. indicum | Aerial parts | 1D NMR | [44] |
| 131 | grandiflorolide | C. grandiflora | Flowers | 1D, 2D NMR; HRESIMS | [45] |
| 132 | clovanediol | C. indicum | Flowers | 1D NMR; EIMS | [28] |
| 133 | - | C. indicum | Flowers | 1D, 2D NMR; HRESIMS | [42] |
| 134 | - | C. indicum | Flowers | 1D, 2D NMR; HRESIMS; X-ray | [42] |
| 135 | chamazulene | C. indicum | Aerial parts | 1D NMR | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Wang, M.; Jiang, Z.; Zafar, S.; Xie, Q.; Yang, Y.; Liu, Y.; Yuan, H.; Jian, Y.; Wang, W. Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus. Molecules 2021, 26, 3038. https://doi.org/10.3390/molecules26103038
Jiang S, Wang M, Jiang Z, Zafar S, Xie Q, Yang Y, Liu Y, Yuan H, Jian Y, Wang W. Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus. Molecules. 2021; 26(10):3038. https://doi.org/10.3390/molecules26103038
Chicago/Turabian StyleJiang, Sai, Mengyun Wang, Zichen Jiang, Salman Zafar, Qian Xie, Yupei Yang, Yang Liu, Hanwen Yuan, Yuqing Jian, and Wei Wang. 2021. "Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus" Molecules 26, no. 10: 3038. https://doi.org/10.3390/molecules26103038
APA StyleJiang, S., Wang, M., Jiang, Z., Zafar, S., Xie, Q., Yang, Y., Liu, Y., Yuan, H., Jian, Y., & Wang, W. (2021). Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus. Molecules, 26(10), 3038. https://doi.org/10.3390/molecules26103038