Ribes nigrum Leaf Extract Preferentially Inhibits IFN-γ-Mediated Inflammation in HaCaT Keratinocytes
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis
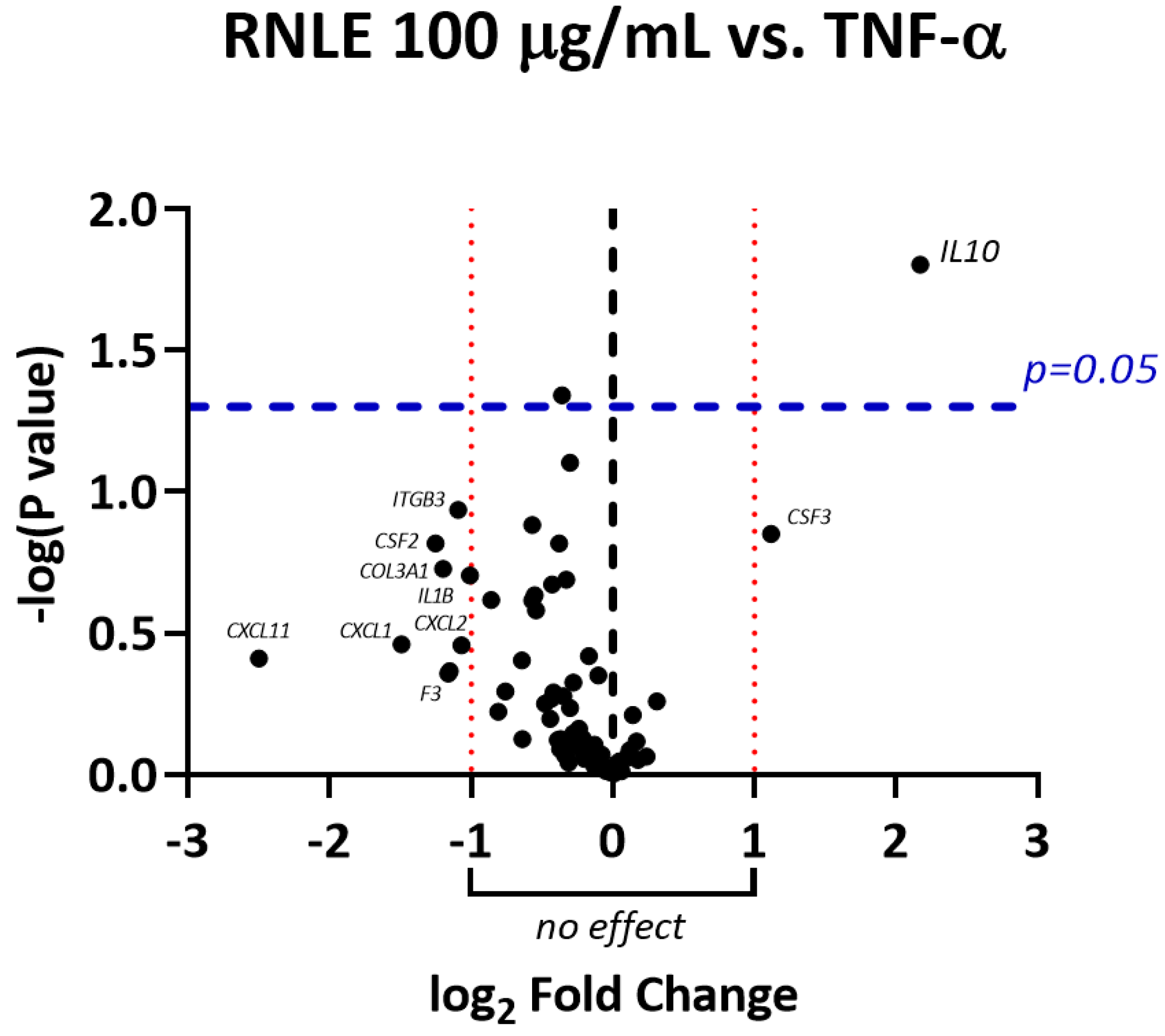
2.2. RNLE Increases IL-10 Expression in TNF-α-Induced HaCaT Cells
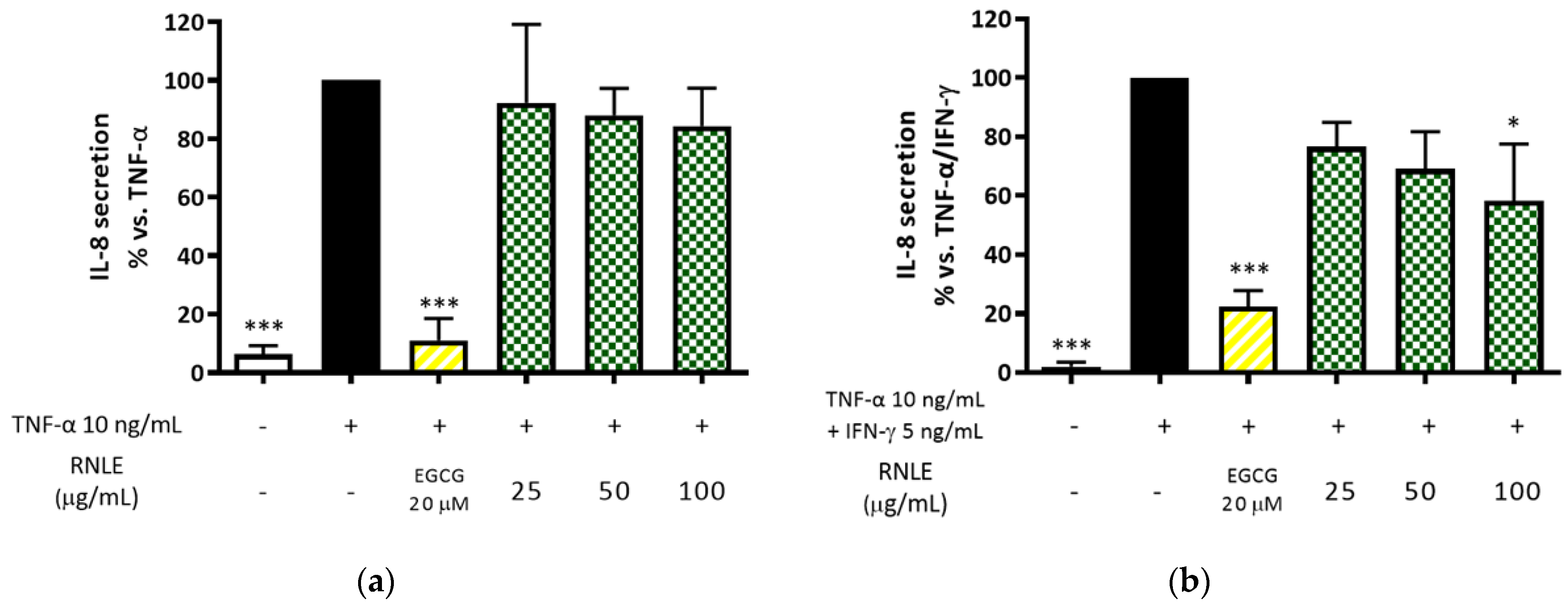
2.3. RNLE Inhibits TNF-α/IFN-γ- but Not TNF-α-Induced IL-8 Release in HaCaT Cells
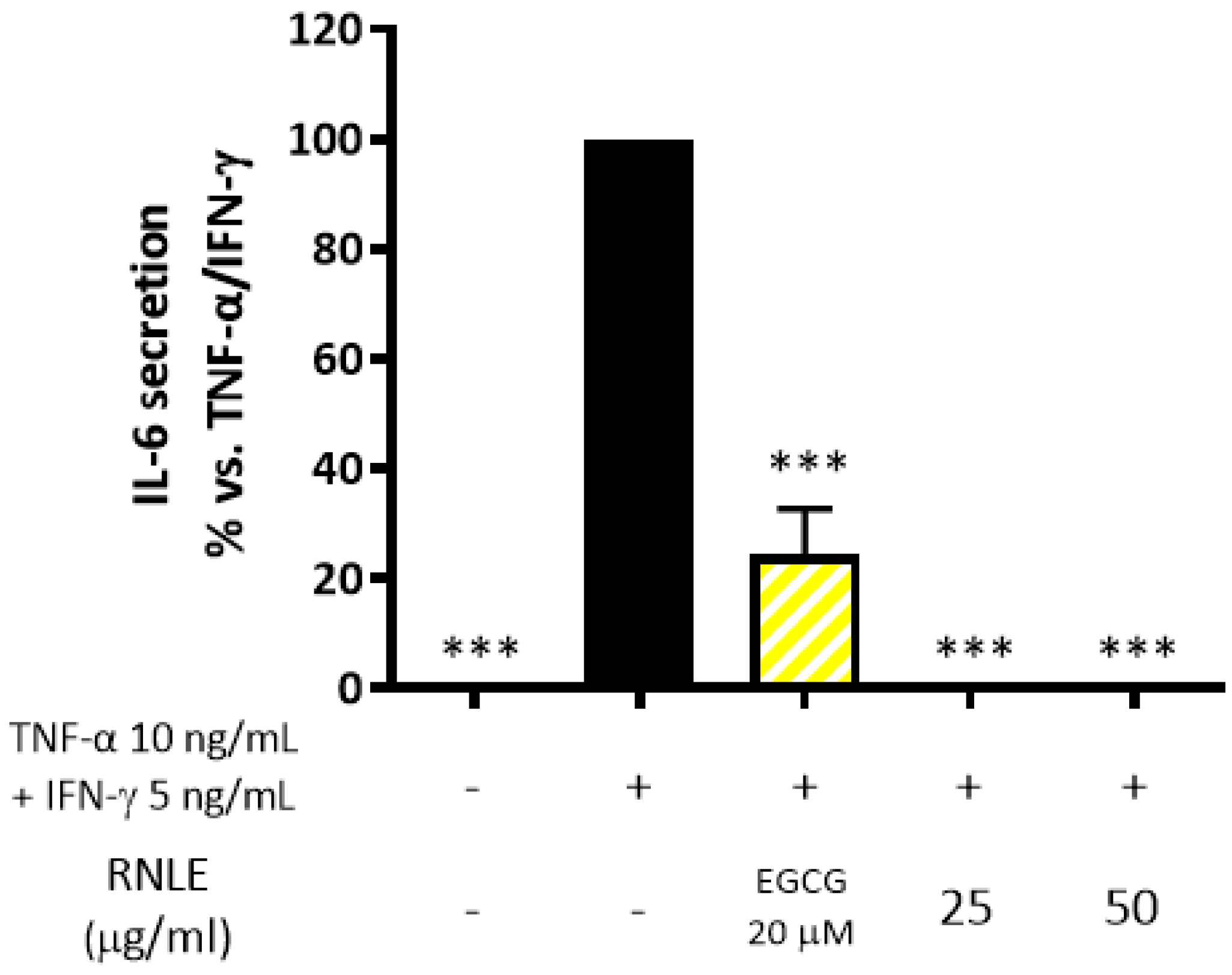
2.4. RNLE Inhibits TNF-α/IFN-γ-Induced IL-6 Release in HaCaT Cells
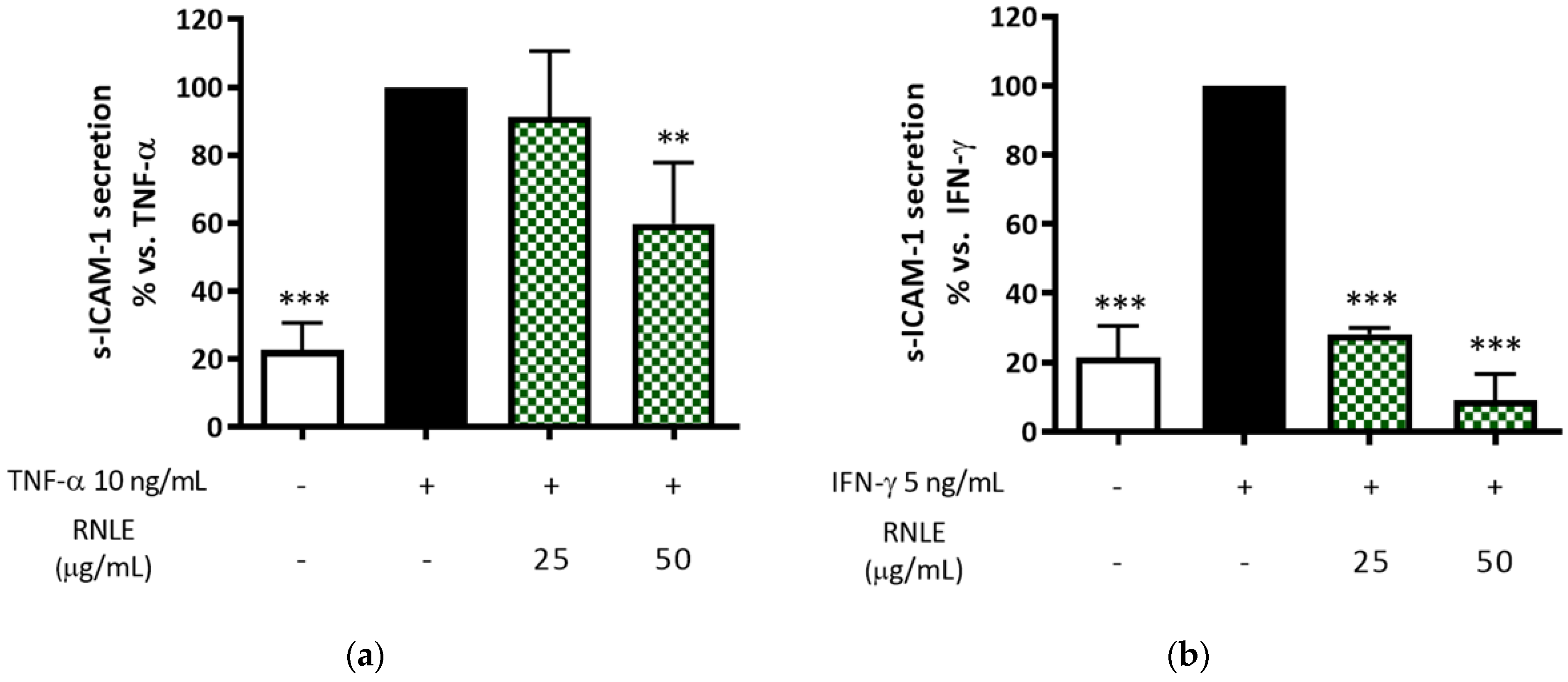
2.5. RNLE Preferentially Inhibits IFN-γ- over TNF-α-Induced s-ICAM-1 Release in HaCaT Cells
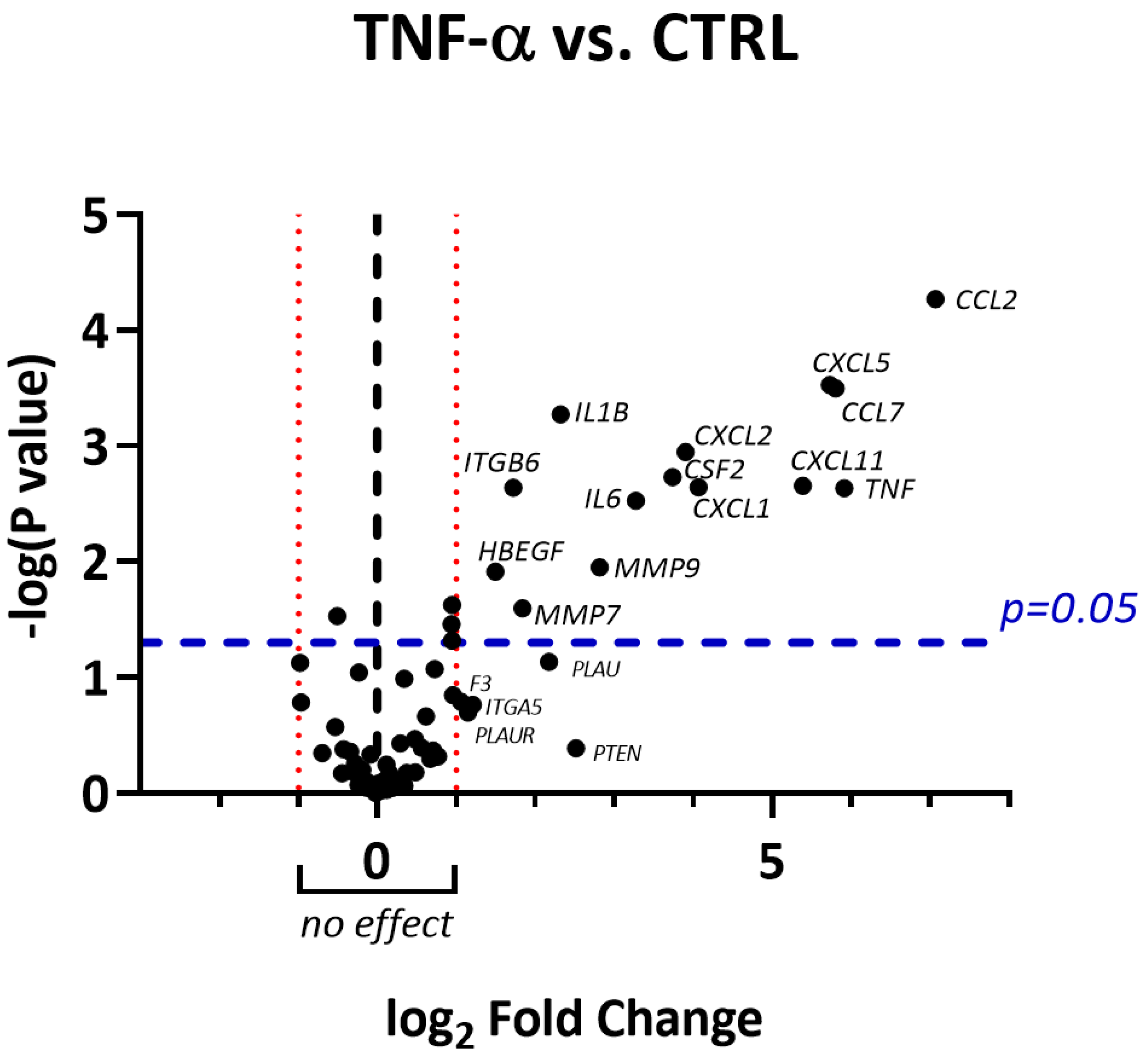
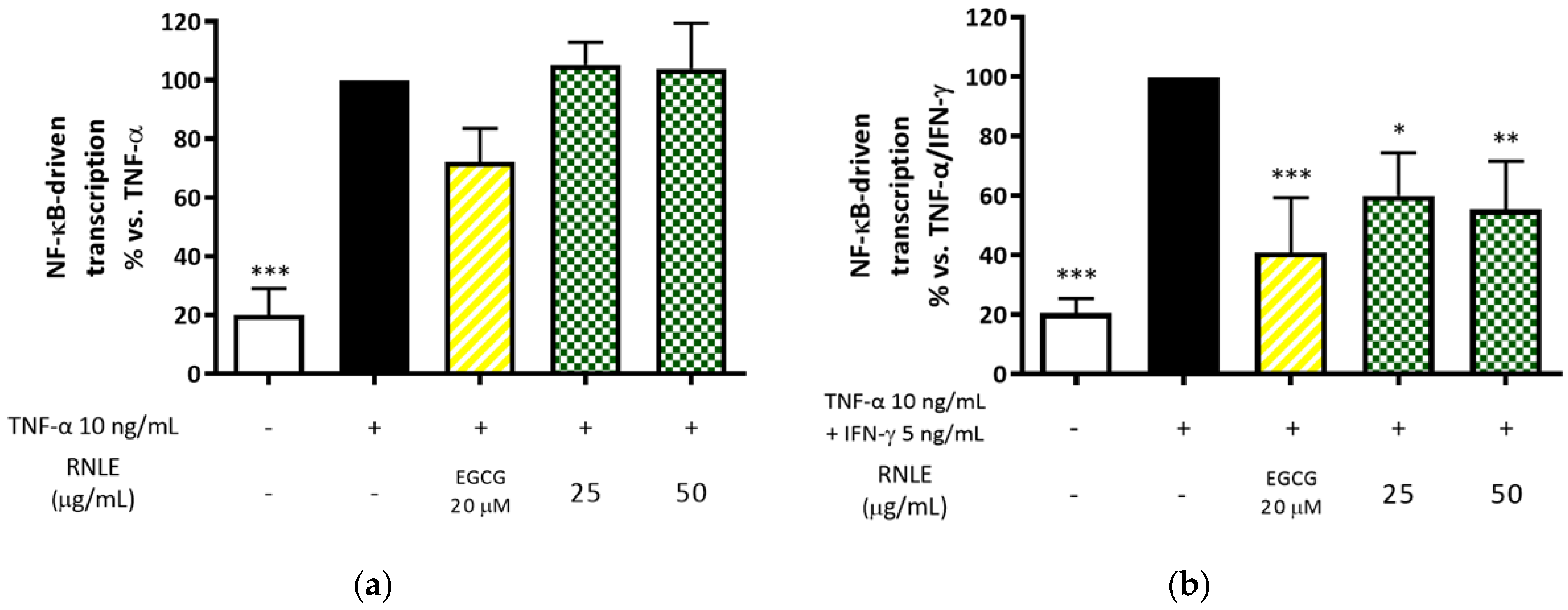
2.6. RNLE Inhibits TNF-α/IFN-γ-Induced NF-κB Activity in HaCaT Cells
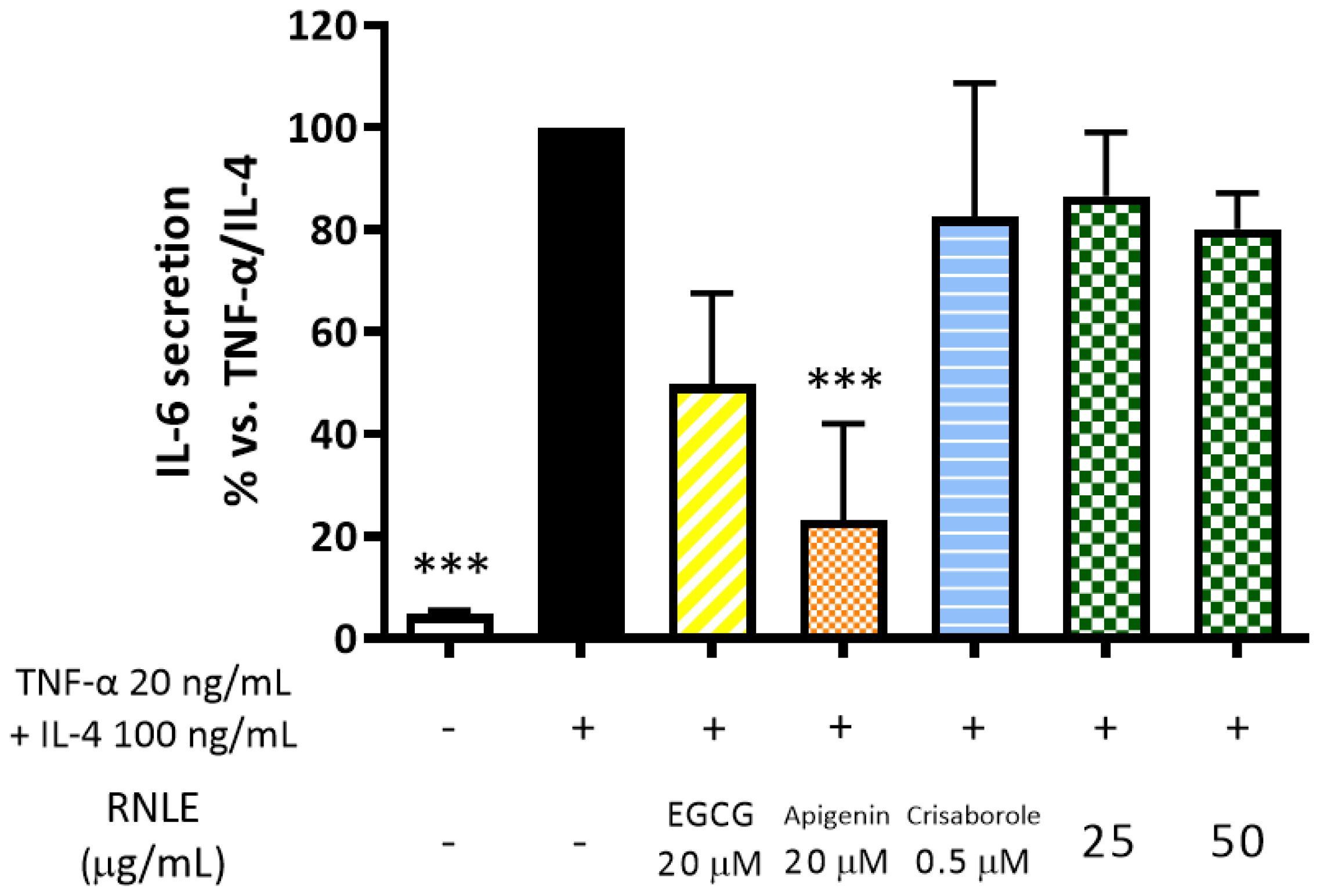
2.7. RNLE Is Not Able to Inhibit TNF-α/IL-4-Induced IL-6 Release in Differentiated HaCaT Cells
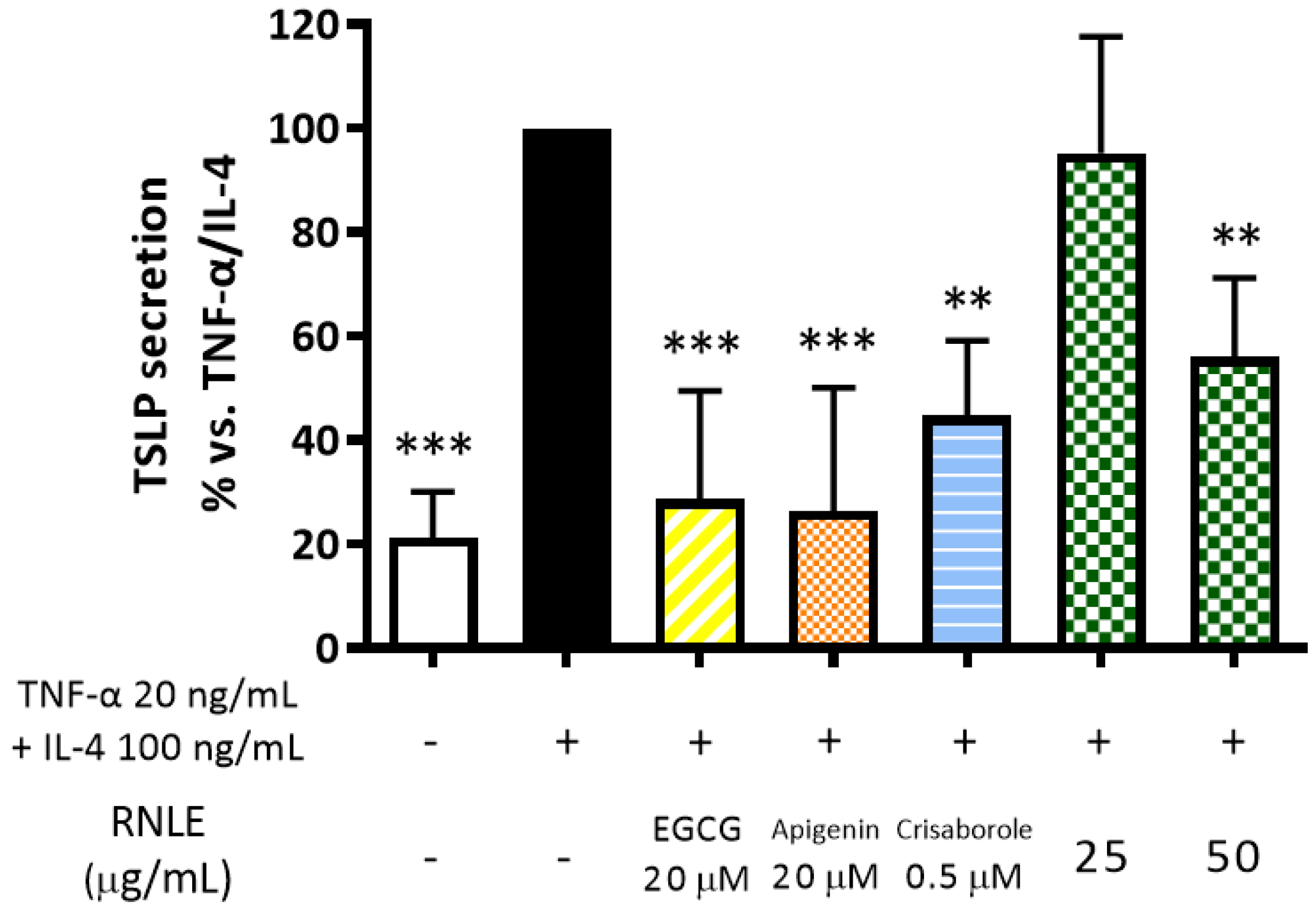
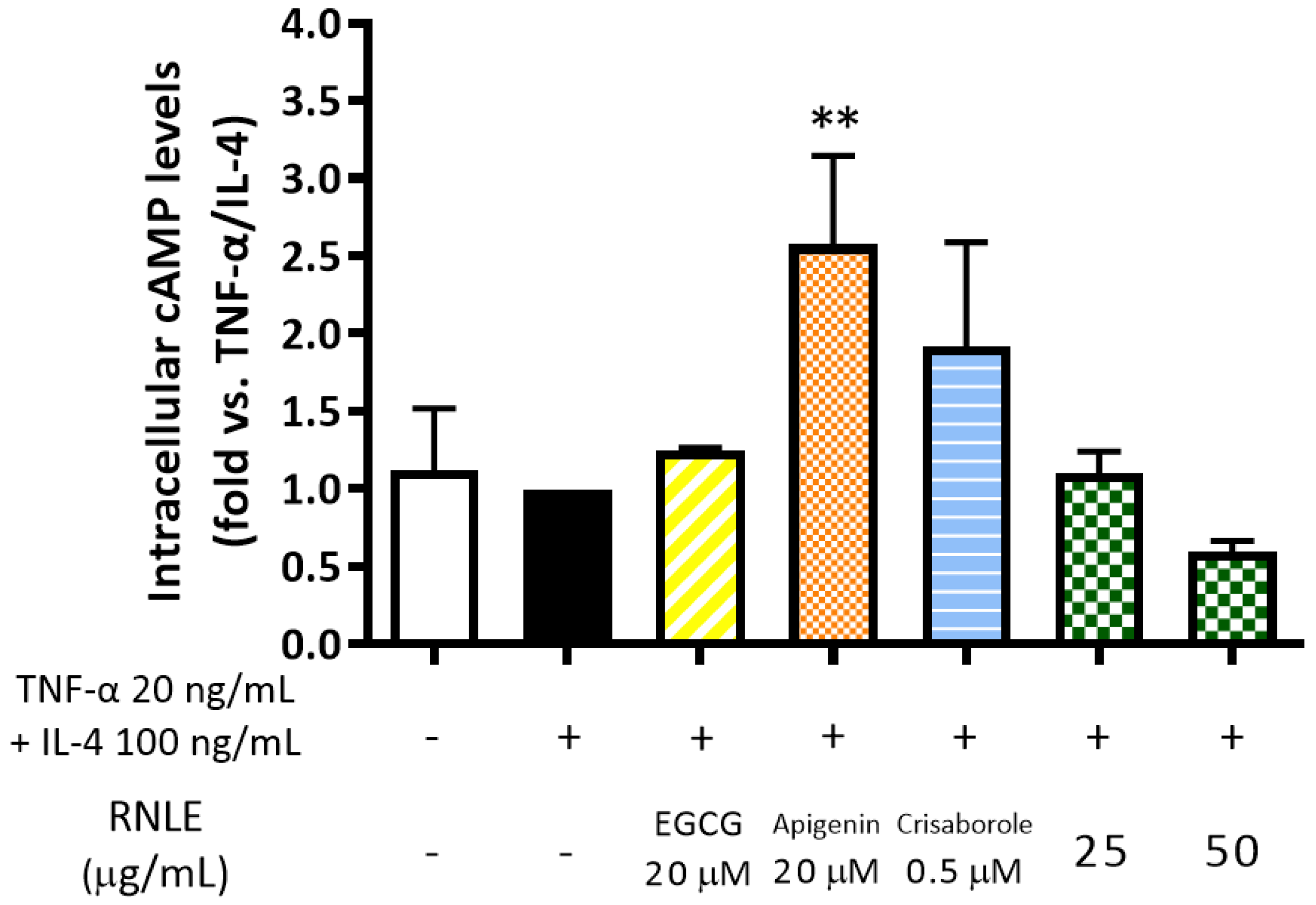
2.8. RNLE Inhibits TNF-α/IL-4-Induced TSLP Release in Differentiated HaCaT Cells with a cAMP-Independent Mechanism
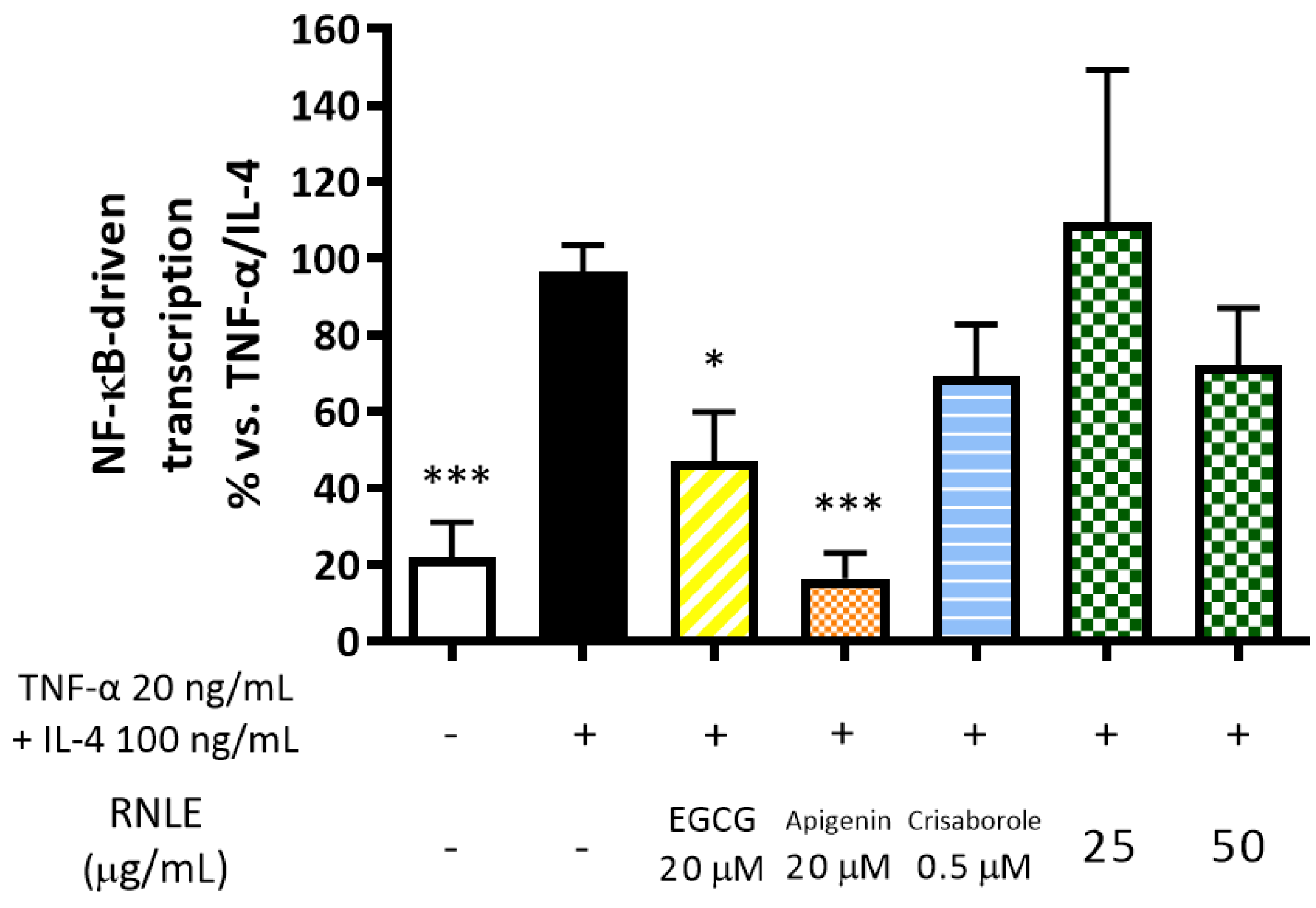
2.9. RNLE Slightly Inhibits TNF-α/IL-4-Induced NF-κB Activity in HaCaT Cells
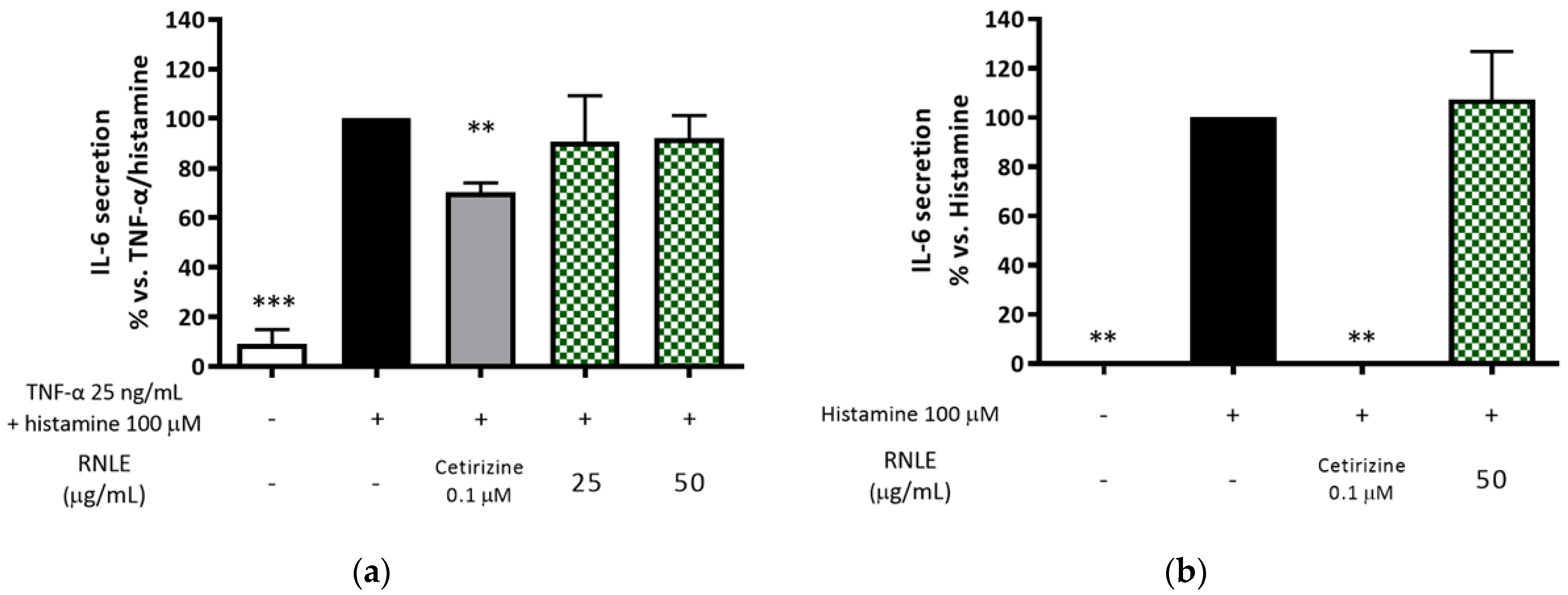
2.10. RNLE Is Not Able to Inhibit Histamine-Induced IL-6 Release in HaCaT Cells
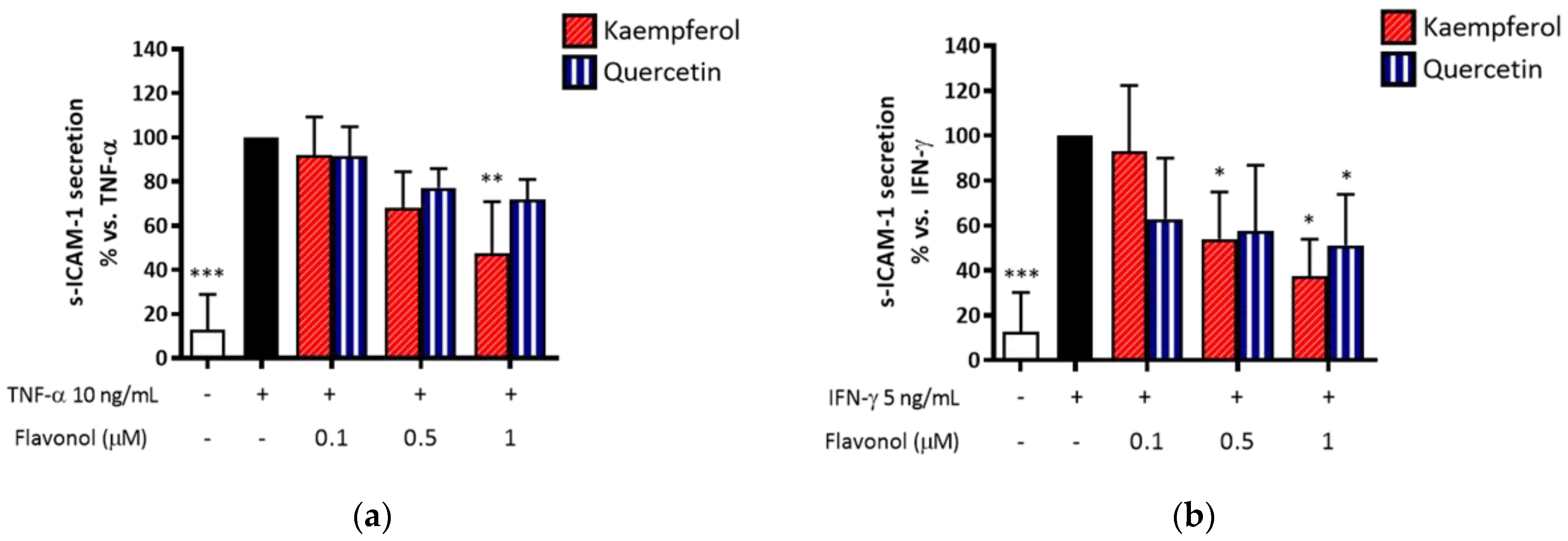
2.11. Quercetin and Kaempferol Preferentially Inhibit IFN-γ-Induced s-ICAM-1 Release in HaCaT Cells
3. Conclusions
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Treatments
- TNF-α 10 ng/mL, 6 h: assessment of gene expression; IL-8, IL-10, and s-ICAM-1 release; NF-κB-driven transcription.
- TNF-α 10 ng/mL, 24 h: assessment of s-ICAM-1 release.
- TNF-α 10 ng/mL + IFN-γ 5 ng/mL or IFN-γ 5 ng/mL, 24 h: assessment of IL-8, IL-6, and s-ICAM-1 release; NF-κB-driven transcription.
- TNF-α 25 ng/mL + histamine 100 µM or histamine 100 µM alone, 24 h: assessment of IL-6 release.
- TNF-α 20 ng/mL + IL-4 100 ng/mL, 24 h, differentiated cells: assessment of IL-6 and TSLP release; intracellular cAMP levels.
- TNF-α 20 ng/mL + IL-4 100 ng/mL, 6 h: assessment of NF-κB-driven transcription.
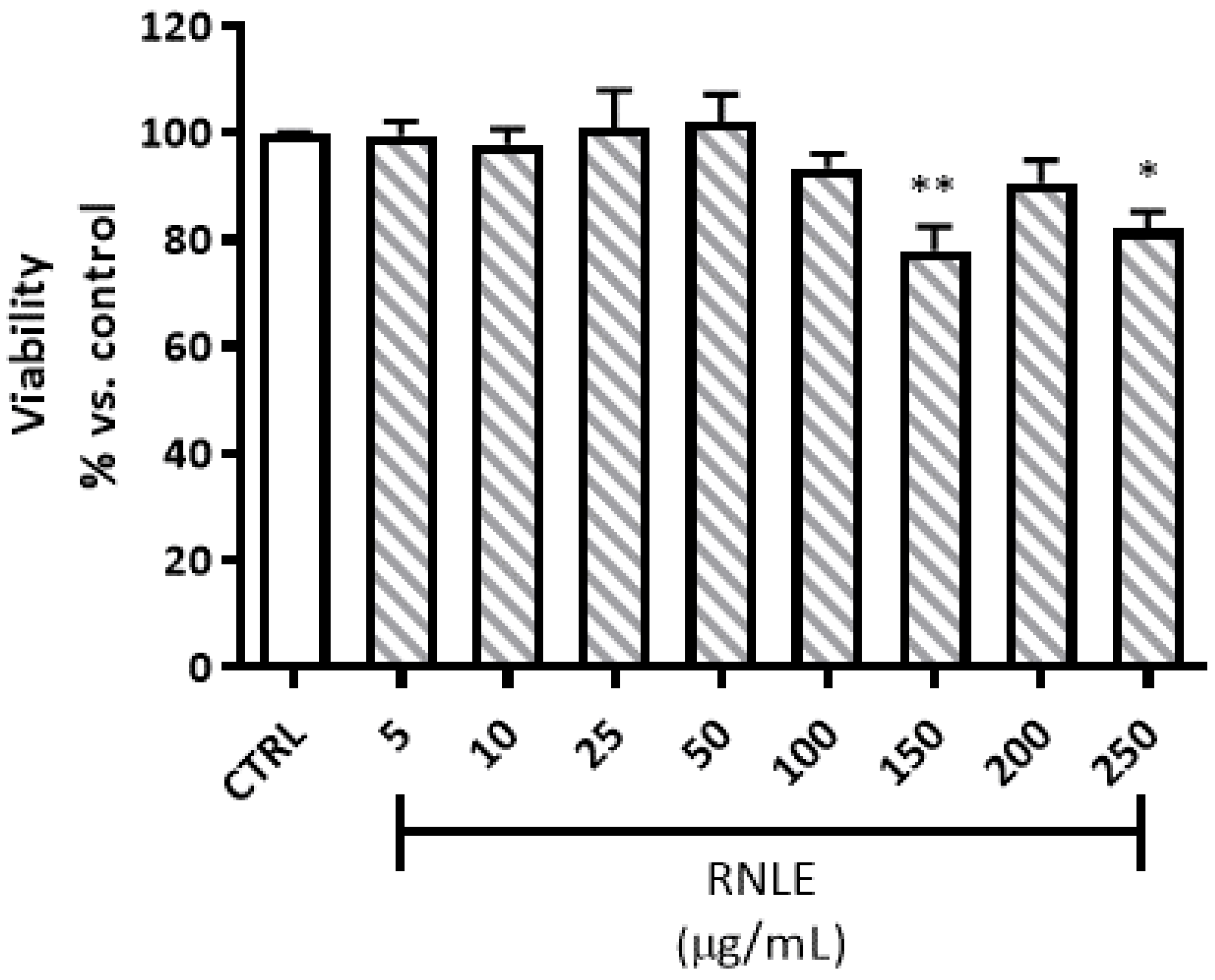
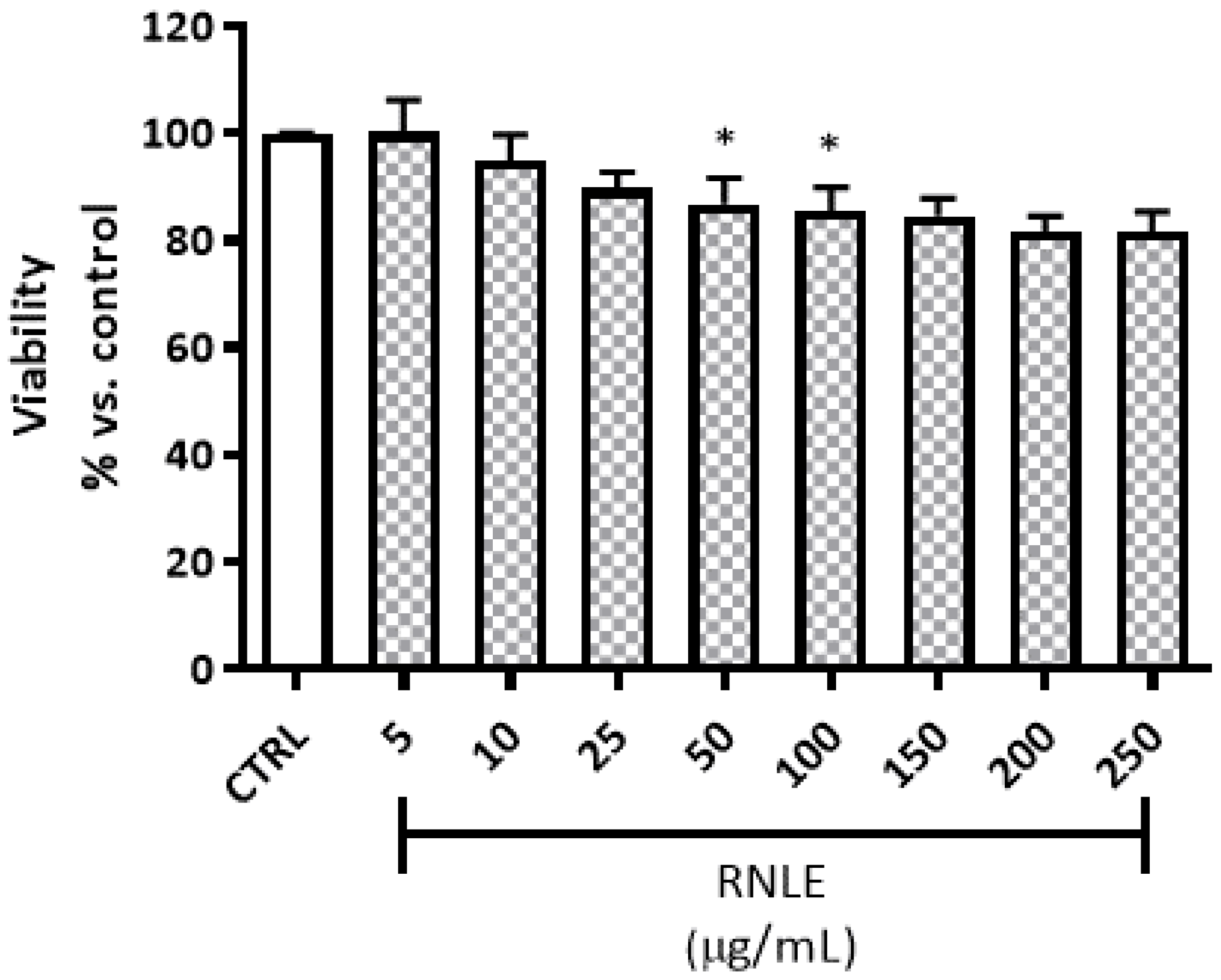
4.3. Cytotoxicity and Viability Assays
4.3.1. Neutral Red Uptake (NRU) Assay
4.3.2. LDH Assay
4.4. Phytochemical Characterization
4.4.1. Folin–Ciocȃlteu Assay
4.4.2. Mass Spectrometry
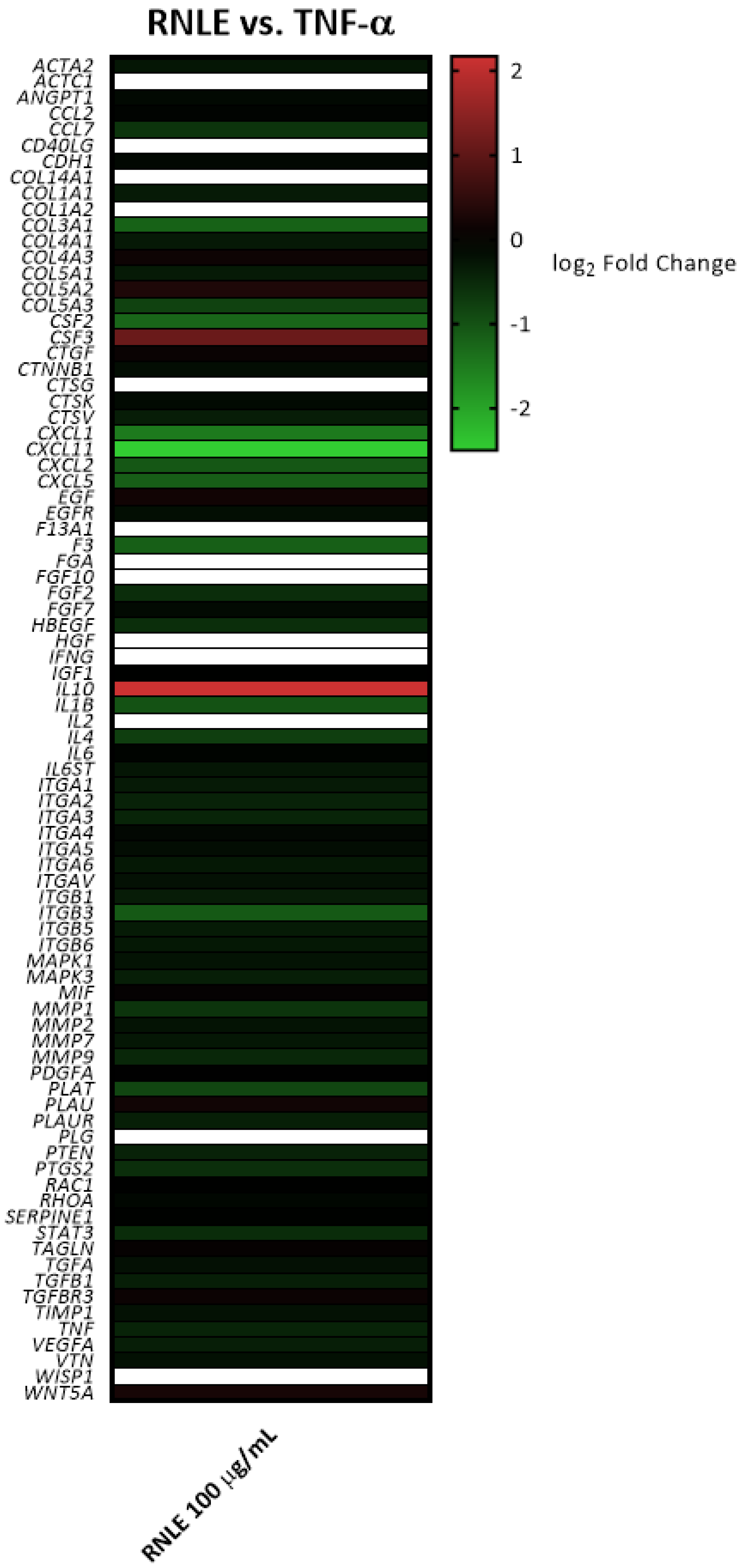
4.5. Gene Expression Analysis
4.5.1. RNA Extraction
4.5.2. cDNA Synthesis
4.5.3. qPCR
4.6. NF-κB-Driven Transcription
4.7. Measurement of IL-6, IL-8, IL-10, TSLP, and s-ICAM-1 Release
4.8. Measurement of Intracellular cAMP Levels
4.9. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Piazza, S.; Fumagalli, M.; Khalilpour, S.; Martinelli, G.; Magnavacca, A.; Dell’Agli, M.; Sangiovanni, E. A Review of the Potential Benefits of Plants Producing Berries in Skin Disorders. Antioxid. Basel 2020, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products (HMPC), European Union Herbal Monograph on Ribes Nigrum L. Folium. Available online: EMA/HMPC/745353/2016 (accessed on 1 April 2021).
- European Scientific Cooperative on Phytotherapy, ESCOP Monographs, The Scientific Foundation for Herbal Medicinal Products. Ribes Nigri Folium (Black Currant Leaf); Online Series; ESCOP: Exeter, UK, 2017. [Google Scholar]
- Liu, P.; Kallio, H.; Yang, B. Flavonol glycosides and other phenolic compounds in buds and leaves of different varieties of black currant (Ribes nigrum L.) and changes during growing season. Food Chem. 2014, 160, 180–189. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Evers, D.; Dommes, J. Ascorbic acid, phenolic acid, flavonoid, and carotenoid profiles of selected extracts from Ribes nigrum. J. Agric. Food Chem. 2011, 59, 4763–4770. [Google Scholar] [CrossRef]
- Garbacki, N.; Kinet, M.; Nusgens, B.; Desmecht, D.; Damas, J. Proanthocyanidins, from Ribes nigrum leaves, reduce endothelial adhesion molecules ICAM-1 and VCAM-1. J. Inflamm. (Lond.) 2005, 2, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mongold, J.J.; Susplugas, P.; Taillade, C.; Serrano, J.J. Anti-inflammatory activity of Ribes nigrum leaf extract in rats. Plantes Méd. Phytothér. 1993, 26, 109–116. [Google Scholar]
- Kendir, G.; Suntar, I.; Ceribasi, A.O.; Koroglu, A. Activity evaluation on Ribes species, traditionally used to speed up healing of wounds: With special focus on Ribes nigrum. J. Ethnopharmacol. 2019, 237, 141–148. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, S.J.; Ahn, E.M.; Oh, S.R.; Lee, H.J.; Jeong, J.A.; Lee, J.Y. Ribes fasciculatum var. chinense Attenuated Allergic Inflammation In Vivo and In Vitro. Biomol. Ther. (Seoul) 2014, 22, 547–552. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, J.S.; Cho, D.H.; Park, H.J. Molecular Mechanisms of Cutaneous Inflammatory Disorder: Atopic Dermatitis. Int. J. Mol. Sci. 2016, 17, 1234. [Google Scholar] [CrossRef]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Ziegler, S.F. TSLP: From allergy to cancer. Nat. Immunol. 2019, 20, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Fritz, Y.; Dawes, S.M.; Diaconu, D.; Al-Attar, P.M.; Guzman, A.M.; Chen, C.S.; Fu, W.; Gudjonsson, J.E.; McCormick, T.S.; et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J. Immunol. 2013, 190, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Cavani, A.; Girolomoni, G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: Synergistic or antagonist effects with IFN-gamma and TNF-alpha. J. Immunol. 1999, 162, 494–502. [Google Scholar]
- El Darzi, E.; Bazzi, S.; Daoud, S.; Echtay, K.S.; Bahr, G.M. Differential regulation of surface receptor expression, proliferation, and apoptosis in HaCaT cells stimulated with interferon-gamma, interleukin-4, tumor necrosis factor-alpha, or muramyl dipeptide. Int. J. Immunopathol. Pharmacol. 2017, 30, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Derocq, J.M.; Segui, M.; Poinot-Chazel, C.; Minty, A.; Caput, D.; Ferrara, P.; Casellas, P. Interleukin-13 stimulates interleukin-6 production by human keratinocytes. Similarity with interleukin-4. FEBS Lett. 1994, 343, 32–36. [Google Scholar] [CrossRef]
- Kohda, F.; Koga, T.; Uchi, H.; Urabe, K.; Furue, M. Histamine-induced IL-6 and IL-8 production are differentially modulated by IFN-gamma and IL-4 in human keratinocytes. J. Dermatol. Sci. 2002, 28, 34–41. [Google Scholar] [CrossRef]
- Hirano, T. Interleukin 6 and its receptor: Ten years later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef] [PubMed]
- Yuk, C.M.; Park, H.J.; Kwon, B.I.; Lah, S.J.; Chang, J.; Kim, J.Y.; Lee, K.M.; Park, S.H.; Hong, S.; Lee, S.H. Basophil-derived IL-6 regulates TH17 cell differentiation and CD4 T cell immunity. Sci. Rep. 2017, 7, 41744. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Baek, J.; Lee, J.R.; Roh, J.Y.; Jung, Y. Optimization of Cytokine Milieu to Reproduce Atopic Dermatitis-related Gene Expression in HaCaT Keratinocyte Cell Line. Immune Netw. 2018, 18, e9. [Google Scholar] [CrossRef]
- Cortez, R.E.; Gonzalez de Mejia, E. Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. J. Food Sci. 2019, 84, 2387–2401. [Google Scholar] [CrossRef] [PubMed]
- Boston University—Gene Resources NF-κB Target Genes. Available online: http://www.bu.edu/nf-kb/gene-resources/target-genes/ (accessed on 10 April 2021).
- Satoh, J.; Tabunoki, H. A Comprehensive Profile of ChIP-Seq-Based STAT1 Target Genes Suggests the Complexity of STAT1-Mediated Gene Regulatory Mechanisms. Gene Regul. Syst. Biol. 2013, 7, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Shimizu, T.; Tada, Y.; Watanabe, S. IL-18 enhances IFN-gamma-induced production of CXCL9, CXCL10, and CXCL11 in human keratinocytes. Eur. J. Immunol. 2007, 37, 338–350. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, M.S.; Jeong, G.S.; Yoon, J. Xanthii fructus extract inhibits TNF-alpha/IFN-gamma-induced Th2-chemokines production via blockade of NF-kappaB, STAT1 and p38-MAPK activation in human epidermal keratinocytes. J. Ethnopharmacol. 2015, 171, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, A.G.; Dige, A.; Krog, J.; Tonnesen, E.; Wogensen, L. Soluble adhesion molecules correlate with surface expression in an in vitro model of endothelial activation. Basic Clin. Pharmacol. Toxicol. 2013, 113, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Read, M.A.; Neish, A.S.; Whitley, M.Z.; Thanos, D.; Maniatis, T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995, 9, 899–909. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Bae, J.S. Barrier protective effects of rutin in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 3048–3055. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.J.; Frei, B. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 2016, 9, 104–113. [Google Scholar] [CrossRef]
- Bito, T.; Roy, S.; Sen, C.K.; Shirakawa, T.; Gotoh, A.; Ueda, M.; Ichihashi, M.; Packer, L. Flavonoids differentially regulate IFN gamma-induced ICAM-1 expression in human keratinocytes: Molecular mechanisms of action. FEBS Lett. 2002, 520, 145–152. [Google Scholar] [CrossRef]
- Son, E.D.; Kim, H.J.; Kim, K.H.; Bin, B.H.; Bae, I.H.; Lim, K.M.; Yu, S.J.; Cho, E.G.; Lee, T.R. S100A7 (psoriasin) inhibits human epidermal differentiation by enhanced IL-6 secretion through IkappaB/NF-kappaB signalling. Exp. Dermatol. 2016, 25, 636–641. [Google Scholar] [CrossRef]
- Omori-Miyake, M.; Yamashita, M.; Tsunemi, Y.; Kawashima, M.; Yagi, J. In vitro assessment of IL-4- or IL-13-mediated changes in the structural components of keratinocytes in mice and humans. J. Investig. Dermatol. 2014, 134, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Alexander, J.B.; Zhang, H.; Shen, K.; Chan, L.S. Interleukin-4 Downregulation of Involucrin Expression in Human Epidermal Keratinocytes Involves Stat6 Sequestration of the Coactivator CREB-Binding Protein. J. Interferon Cytokine Res. 2016, 36, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Matsui, K.; Tsuji, H.; Tamura, A.; Suzuki, K. Immunolocalisation of the janus kinases (JAK)—Signal transducers and activators of transcription (STAT) pathway in human epidermis. J. Anat. 2001, 198, 581–589. [Google Scholar] [CrossRef]
- Bogiatzi, S.I.; Fernandez, I.; Bichet, J.C.; Marloie-Provost, M.A.; Volpe, E.; Sastre, X.; Soumelis, V. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J. Immunol. 2007, 178, 3373–3377. [Google Scholar] [CrossRef]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef]
- Zhang, X.; Pathak, T.; Yoast, R.; Emrich, S.; Xin, P.; Nwokonko, R.M.; Johnson, M.; Wu, S.; Delierneux, C.; Gueguinou, M.; et al. A calcium/cAMP signaling loop at the ORAI1 mouth drives channel inactivation to shape NFAT induction. Nat. Commun. 2019, 10, 1971. [Google Scholar] [CrossRef]
- Vu, A.T.; Chen, X.; Xie, Y.; Kamijo, S.; Ushio, H.; Kawasaki, J.; Hara, M.; Ikeda, S.; Okumura, K.; Ogawa, H.; et al. Extracellular double-stranded RNA induces TSLP via an endosomal acidification- and NF-kappaB-dependent pathway in human keratinocytes. J. Investig. Dermatol. 2011, 131, 2205–2212. [Google Scholar] [CrossRef]
- Parry, G.C.; Mackman, N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J. Immunol. 1997, 159, 5450–5456. [Google Scholar]
- Dong, C.; Virtucio, C.; Zemska, O.; Baltazar, G.; Zhou, Y.; Baia, D.; Jones-Iatauro, S.; Sexton, H.; Martin, S.; Dee, J.; et al. Treatment of Skin Inflammation with Benzoxaborole Phosphodiesterase Inhibitors: Selectivity, Cellular Activity, and Effect on Cytokines Associated with Skin Inflammation and Skin Architecture Changes. J. Pharmacol. Exp. Ther. 2016, 358, 413–422. [Google Scholar] [CrossRef]
- Mammone, T.; Marenus, K.; Maes, D.; Lockshin, R.A. The induction of terminal differentiation markers by the cAMP pathway in human HaCaT keratinocytes. Skin Pharmacol. Appl. Skin Physiol. 1998, 11, 152–160. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Galli, G.V.; Bosisio, E. Inhibition of cGMP-phosphodiesterase-5 by biflavones of Ginkgo biloba. Planta Med. 2006, 72, 468–470. [Google Scholar] [CrossRef]
- Ko, W.C.; Shih, C.M.; Lai, Y.H.; Chen, J.H.; Huang, H.L. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochem. Pharmacol. 2004, 68, 2087–2094. [Google Scholar] [CrossRef]
- Chaabi, M.; Antheaume, C.; Weniger, B.; Justiniano, H.; Lugnier, C.; Lobstein, A. Biflavones of Decussocarpus rospigliosii as phosphodiesterases inhibitors. Planta Med. 2007, 73, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Jegal, J.; Park, N.J.; Park, S.A.; Bong, S.K.; Jegal, H.; Kim, S.N.; Yang, M.H. Juniperus chinensis Fruits Attenuate Oxazolone- and 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis Symptoms in Mice. Biol. Pharm. Bull. 2018, 41, 259–265. [Google Scholar] [CrossRef]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H.; Park, Y.J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J. Pharmacological PKA inhibition: All may not be what it seems. Sci. Signal. 2008, 1, re4. [Google Scholar] [CrossRef] [PubMed]
- Woo, T.E.; Kuzel, P. Crisaborole 2% Ointment (Eucrisa) for Atopic Dermatitis. Skin Ther. Lett. 2019, 24, 4–6. [Google Scholar]
- Beekmann, K.; de Haan, L.H.; Actis-Goretta, L.; van Bladeren, P.J.; Rietjens, I.M. Effect of Glucuronidation on the Potential of Kaempferol to Inhibit Serine/Threonine Protein Kinases. J. Agric. Food Chem. 2016, 64, 1256–1263. [Google Scholar] [CrossRef]
- Wang, X.F.; Song, S.D.; Li, Y.J.; Hu, Z.Q.; Zhang, Z.W.; Yan, C.G.; Li, Z.G.; Tang, H.F. Protective Effect of Quercetin in LPS-Induced Murine Acute Lung Injury Mediated by cAMP-Epac Pathway. Inflammation 2018, 41, 1093–1103. [Google Scholar] [CrossRef]
- Brenner, S.; Prosch, S.; Schenke-Layland, K.; Riese, U.; Gausmann, U.; Platzer, C. cAMP-induced Interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J. Biol. Chem. 2003, 278, 5597–5604. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Wolfle, U.; Haarhaus, B.; Schempp, C.M. Amarogentin Displays Immunomodulatory Effects in Human Mast Cells and Keratinocytes. Mediat. Inflamm. 2015, 2015, 630128. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. Protective effect of rutin on humoral and cell mediated immunity in rat model. Chem. Biol. Interact. 2017, 273, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Smith, S.M.; Wunder, M.B.; Norris, D.A.; Shellman, Y.G. A simple protocol for using a LDH-based cytotoxicity assay to assess the effects of death and growth inhibition at the same time. PLoS ONE 2011, 6, e26908. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, S.; Magnavacca, A.; Perego, F.; Fumagalli, M.; Sangiovanni, E.; Prato, M.; Dell’Agli, M.; Basilico, N. Effect of Hypoxia on Gene Expression in Cell Populations Involved in Wound Healing. Biomed. Res. Int. 2019, 2019, 2626374. [Google Scholar] [CrossRef]

| Compound | Molecular Weight (m/z of [M − H]−1) | Retention Time (min) | wt.% in RNLE |
|---|---|---|---|
| Kaempferol aglycone | 285 | 9.06 | 2.9% |
| Kaempferol-7-glucoside | 447 | 7.53 | 3.8% |
| Kaempferol-3-glucoside | 447 | 7.48 | 1.3% |
| Quercetin aglycone | 301 | 8.52 | 1.9% |
| Hyperoside | 463 | 7.26 | 0.8% |
| Rutin | 610 | 7.09 | 1.6% |
| Chlorogenic acid | 353 | 6.68 | 1.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnavacca, A.; Piazza, S.; Cammisa, A.; Fumagalli, M.; Martinelli, G.; Giavarini, F.; Sangiovanni, E.; Dell’Agli, M. Ribes nigrum Leaf Extract Preferentially Inhibits IFN-γ-Mediated Inflammation in HaCaT Keratinocytes. Molecules 2021, 26, 3044. https://doi.org/10.3390/molecules26103044
Magnavacca A, Piazza S, Cammisa A, Fumagalli M, Martinelli G, Giavarini F, Sangiovanni E, Dell’Agli M. Ribes nigrum Leaf Extract Preferentially Inhibits IFN-γ-Mediated Inflammation in HaCaT Keratinocytes. Molecules. 2021; 26(10):3044. https://doi.org/10.3390/molecules26103044
Chicago/Turabian StyleMagnavacca, Andrea, Stefano Piazza, Anna Cammisa, Marco Fumagalli, Giulia Martinelli, Flavio Giavarini, Enrico Sangiovanni, and Mario Dell’Agli. 2021. "Ribes nigrum Leaf Extract Preferentially Inhibits IFN-γ-Mediated Inflammation in HaCaT Keratinocytes" Molecules 26, no. 10: 3044. https://doi.org/10.3390/molecules26103044
APA StyleMagnavacca, A., Piazza, S., Cammisa, A., Fumagalli, M., Martinelli, G., Giavarini, F., Sangiovanni, E., & Dell’Agli, M. (2021). Ribes nigrum Leaf Extract Preferentially Inhibits IFN-γ-Mediated Inflammation in HaCaT Keratinocytes. Molecules, 26(10), 3044. https://doi.org/10.3390/molecules26103044







