Azo-Dyes-Grafted Oligosaccharides—From Synthesis to Applications
Abstract
1. Introduction
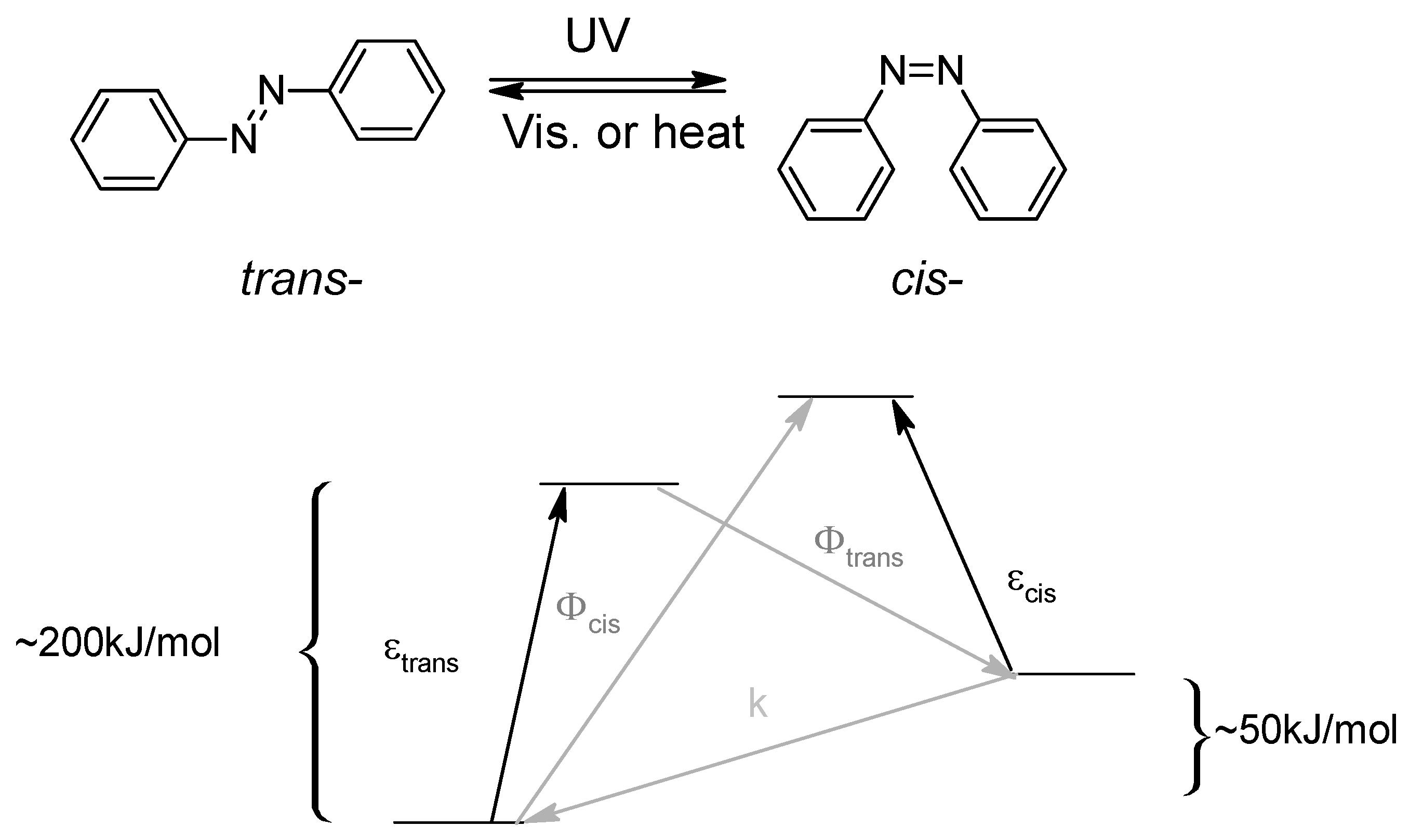
1.1. Azo Molecules
- Diazo coupling which implies the formation of a diazonium salt attacked by an electron-enriched aromaric.
- Mills reaction or oxidative/reductive coupling for in situ the formation of the diazo bond between two anilines/nitrobenzenes.
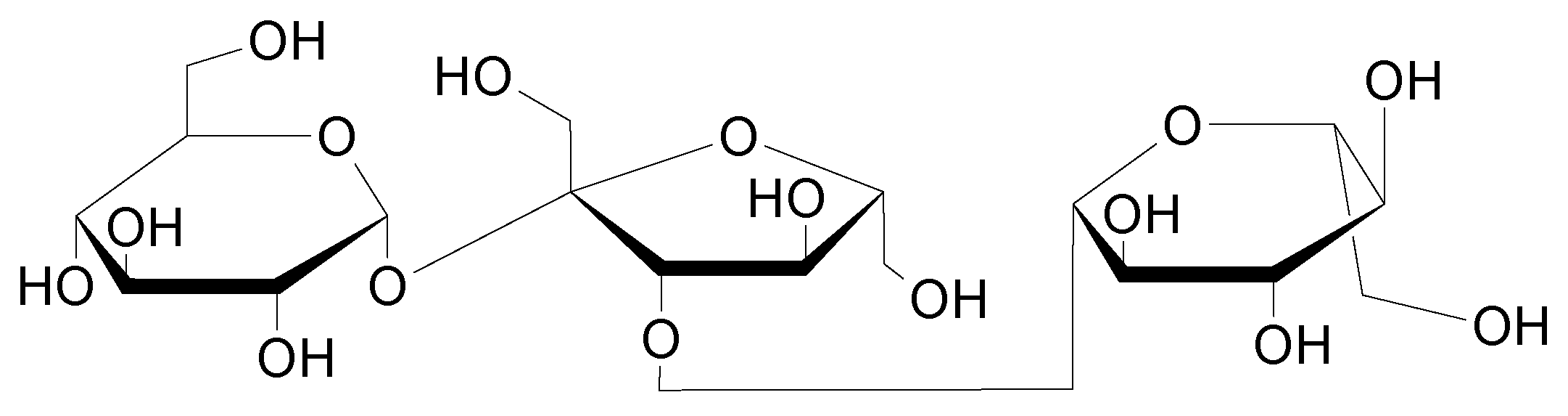
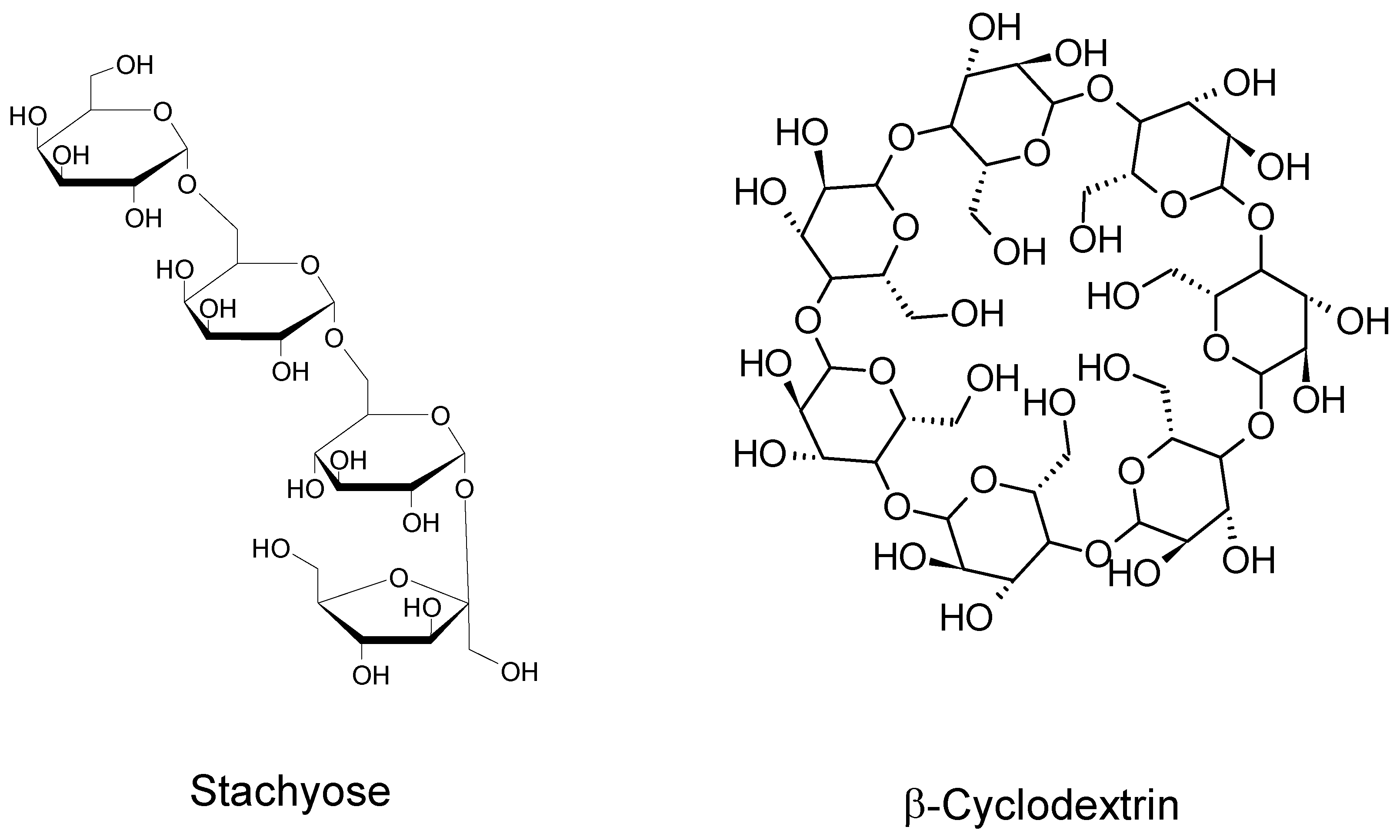
1.2. Oligosaccharides
2. Mono/Multivalent Sugar Photoswitch
3. Cellulose
3.1. Azo-Dye-Grafted or Coupled Cellulose
3.2. For Drug Delivery and Health
3.3. With Liquid or Nano Crystal Properties
3.4. For UV Protection
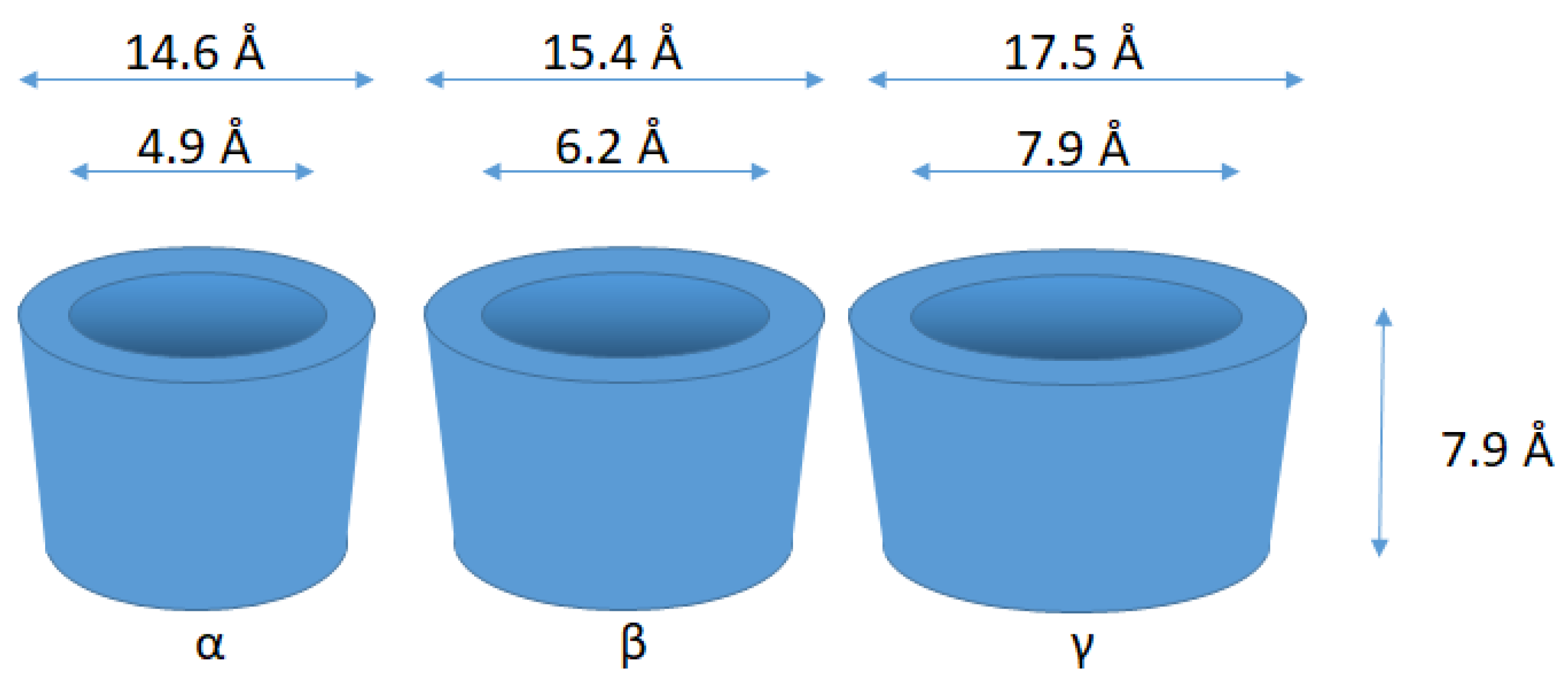
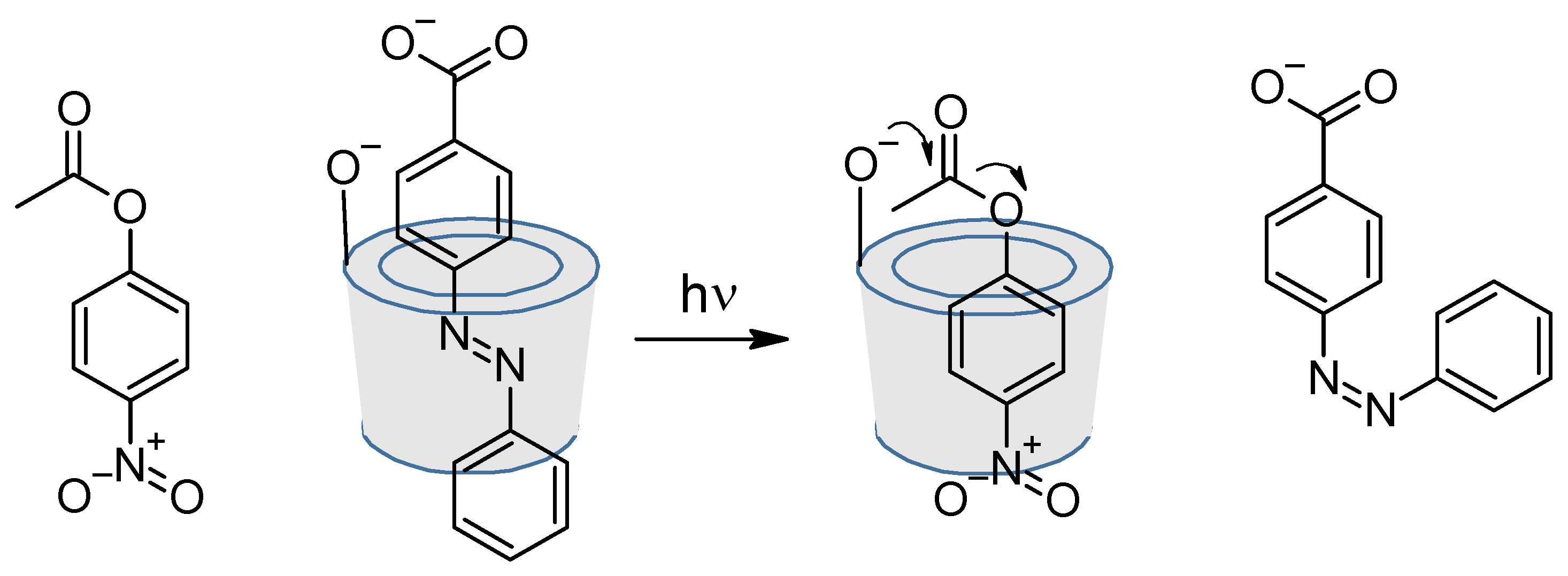
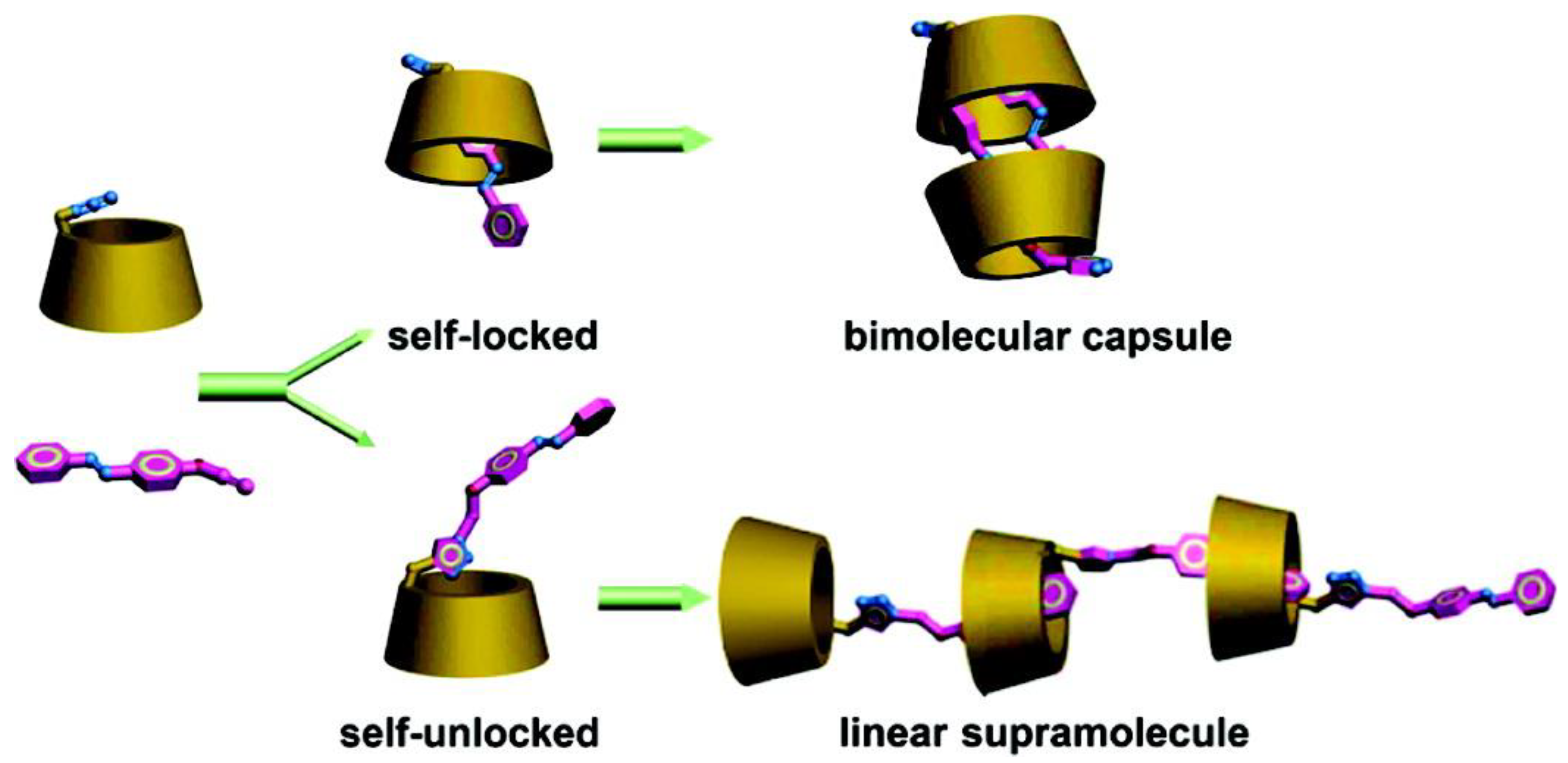
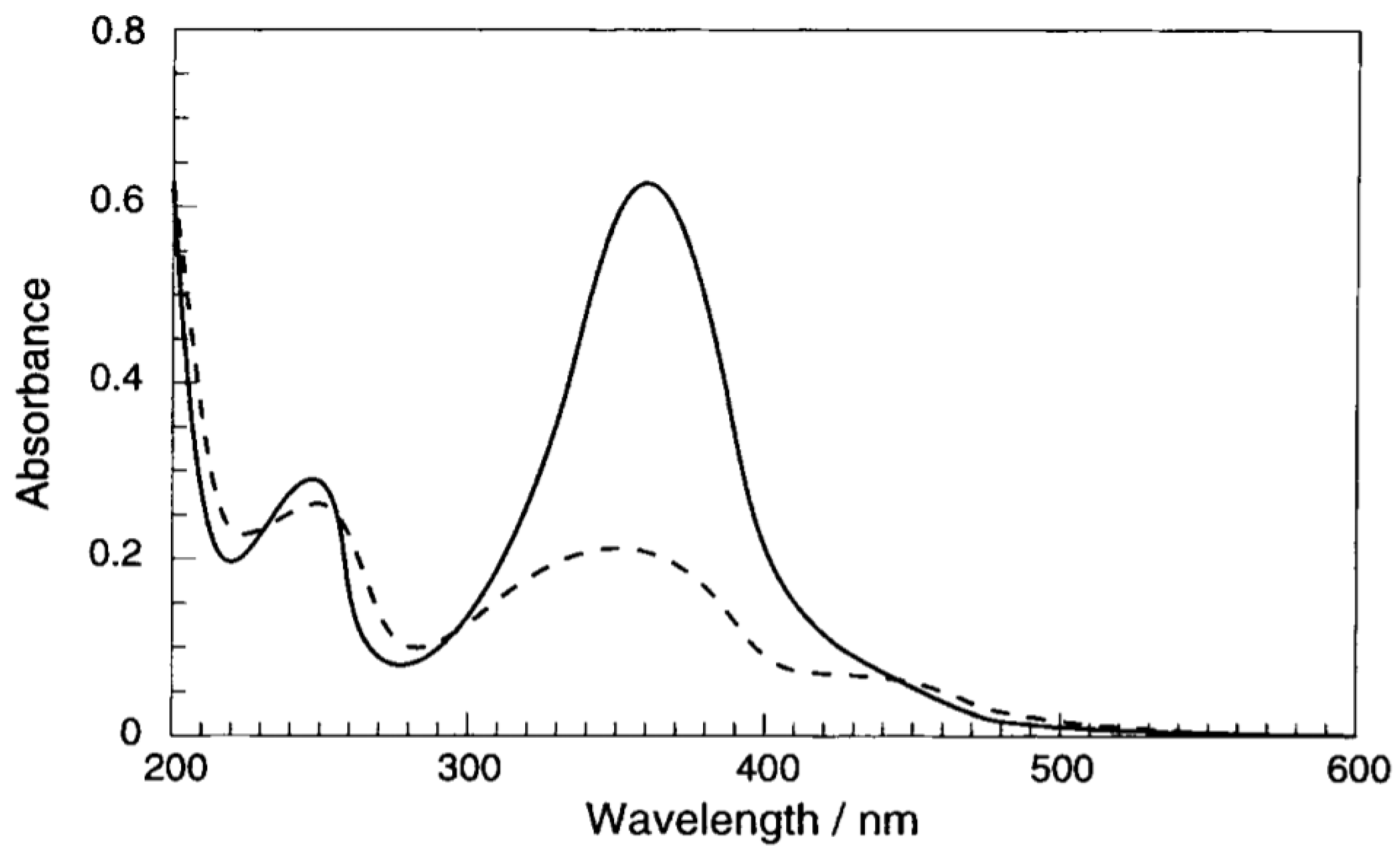
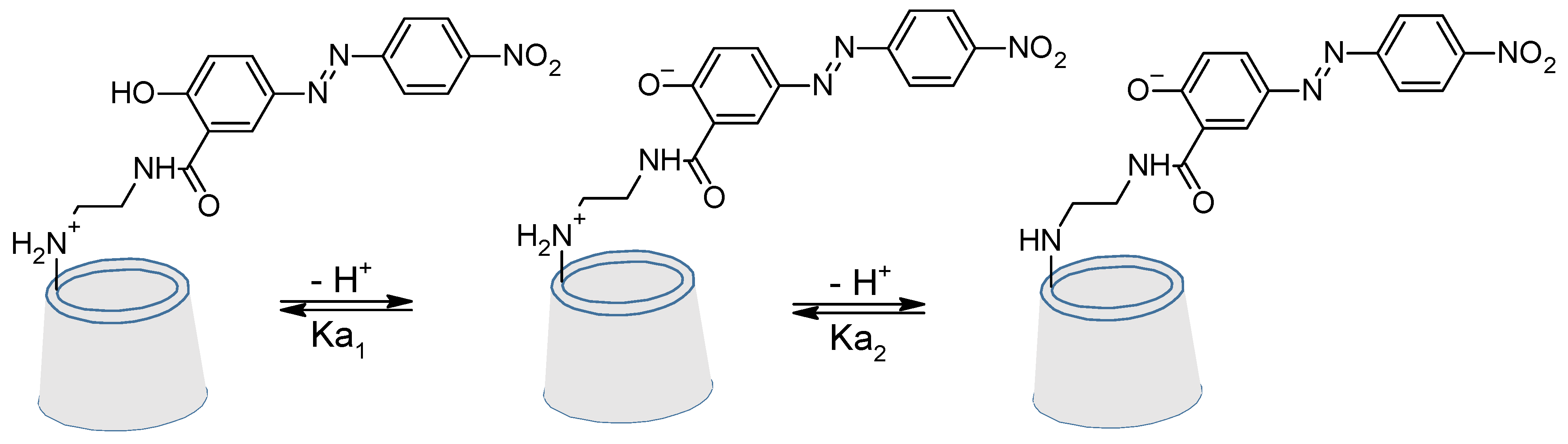
4. Cyclodextrins
4.1. Inclusion/Exclusion of Azobenzenes
4.2. Azo Functionalised Cyclodextrins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kortekaas, L.; Browne, W.R. The Evolution of Spiropyran: Fundamentals and Progress of an Extraordinarily Versatile Photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef] [PubMed]
- Pratap, R.; Ram, V.J. Natural and Synthetic Chromenes, Fused Chromenes, and Versatility of Dihydrobenzo[h]Chromenes in Organic Synthesis. Chem. Rev. 2014, 114, 10476–10526. [Google Scholar] [CrossRef] [PubMed]
- Kratochvılová, I.; Nešprek, S.; Šebera, J.; Záliš, S.; Pavelka, M.; Wang, G.; Sworakowski, J. New Organic FET-like Photoactive Device, Experiments and DFT Modeling. Eur. Phys. J. E 2008, 25, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Petermayer, C.; Dube, H. Indigoid Photoswitches: Visible Light Responsive Molecular Tools. Acc. Chem. Res. 2018, 51, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J. II. Photochemistry of Azobenzene and Its Derivatives. Chem. Soc. Rev. 1972, 1, 481–493. [Google Scholar] [CrossRef]
- Banaszak-Léonard, E. Les Photochromes Intégrant le Concept de Développement Durable; Editions Universitaires Européennes: Chisinau, Moldova, 2019; ISBN 613-8-45947-4. [Google Scholar]
- Yager, K.G.; Barrett, C.J. Novel Photo-Switching Using Azobenzene Functional Materials. J. Photochem. Photobiol. A 2006, 182, 250–261. [Google Scholar] [CrossRef]
- García-Amorós, J.; Velasco, D. Recent Advances towards Azobenzene-Based Light-Driven Real-Time Information-Transmitting Materials. Beilstein J. Org. Chem. 2012, 8, 1003–1017. [Google Scholar] [CrossRef]
- Granucci, G.; Persico, M. Excited State Dynamics with the Direct Trajectory Surface Hopping Method: Azobenzene and Its Derivatives as a Case Study. Theor. Chem. Acc. 2007, 117, 1131–1143. [Google Scholar] [CrossRef]
- Conti, I.; Garavelli, M.; Orlandi, G. The Different Photoisomerization Efficiency of Azobenzene in the Lowest Nπ* and Ππ* Singlets: The Role of a Phantom State. J. Am. Chem. Soc. 2008, 130, 5216–5230. [Google Scholar] [CrossRef] [PubMed]
- Klug, R.L.; Burcl, R. Rotational Barriers in Azobenzene and Azonaphthalene. J. Phys. Chem. A 2010, 114, 6401–6407. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Basa, P.N.; Yan, J.; Camire, C.E.; MacDonald, J.C.; Jackson, R.K.; Burdette, S.C. Systematic Modulation of Hydrogen Bond Donors in Aminoazobenzene Derivatives Provides Further Evidence for the Concerted Inversion Photoisomerization Pathway. Eur. J. Org. Chem. 2013, 2013, 4794–4803. [Google Scholar] [CrossRef]
- Loos, P.-F.; Preat, J.; Laurent, A.D.; Michaux, C.; Jacquemin, D.; Perpète, E.A.; Assfeld, X. Theoretical Investigation of the Geometries and UV-Vis Spectra of Poly(L-Glutamic Acid) Featuring a Photochromic Azobenzene Side Chain. J. Chem. Theory Comput. 2008, 4, 637–645. [Google Scholar] [CrossRef]
- Pederzoli, M.; Pittner, J.; Barbatti, M.; Lischka, H. Nonadiabatic Molecular Dynamics Study of the Cis–trans Photoisomerization of Azobenzene Excited to the S1 State. J. Phys. Chem. A 2011, 115, 11136–11143. [Google Scholar] [CrossRef] [PubMed]
- Weingart, O.; Lan, Z.; Koslowski, A.; Thiel, W. Chiral Pathways and Periodic Decay in Cis-Azobenzene Photodynamics. J. Phys. Chem. Lett. 2011, 2, 1506–1509. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Léonard, E.; Mangin, F.; Villette, C.; Billamboz, M.; Len, C. Azobenzenes and Catalysis. Catal. Sci. Technol. 2016, 6, 379–398. [Google Scholar] [CrossRef]
- Subala, S.S.; Sundar, B.S.; Sastry, S.S. Synthesis and Characterization of Nonsymmetric Liquid Crystal Dimer Containing Biphenyl and Azobenzene Moiety. J. Chem. 2013, 2013, e939406. [Google Scholar] [CrossRef]
- Paul, M.K.; Kalita, G.; Laskar, A.R.; Debnath, S.; Gude, V.; Sarkar, D.D.; Mohiuddin, G.; Varshney, S.K.; Nandiraju Rao, V.S. Synthesis and Mesomorphic Behaviour of Achiral Four-Ring Unsymmetrical Bent-Core Liquid Crystals: Nematic Phases. J. Mol. Struct. 2013, 1049, 78–89. [Google Scholar] [CrossRef]
- Barclay, T.G.; Constantopoulos, K.; Zhang, W.; Fujiki, M.; Petrovsky, N.; Matisons, J.G. Chiral Self-Assembly of Designed Amphiphiles: Influences on Aggregate Morphology. Langmuir 2013, 29, 10001. [Google Scholar] [CrossRef]
- Hayata, Y.; Nagano, S.; Takeoka, Y.; Seki, T. Photoinduced Volume Transition in Liquid Crystalline Polymer Gels Swollen by a Nematic Solvent. ACS Macro Lett. 2012, 1, 1357–1361. [Google Scholar] [CrossRef]
- Kageyama, Y.; Tanigake, N.; Kurokome, Y.; Iwaki, S.; Takeda, S.; Suzuki, K.; Sugawara, T. Macroscopic Motion of Supramolecular Assemblies Actuated by Photoisomerization of Azobenzene Derivatives. Chem. Commun. 2013, 49, 9386–9388. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamamoto, Y.; Hata, S.; Kondo, Y. Unusual Viscoelasticity Behaviour in Aqueous Solutions Containing a Photoresponsive Amphiphile. J. Colloid Interface Sci. 2013, 407, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, E.; Monteux, C.; Lequeux, F.; Tribet, C. Photofoams: Remote Control of Foam Destabilization by Exposure to Light Using an Azobenzene Surfactant. Langmuir 2012, 28, 2308–2312. [Google Scholar] [CrossRef]
- Menati, S.; Azadbakht, A.; Azadbakht, R.; Taeb, A.; Kakanejadifard, A. Synthesis, Characterization, and Electrochemical Study of Some Novel, Azo-Containing Schiff Bases and Their Ni(II) Complexes. Dyes Pigments 2013, 98, 499–506. [Google Scholar] [CrossRef]
- Pandhurnekar, C.P.; Meshram, E.M.; Chopde, H.N.; Batra, R.J. Synthesis, Characterization, and Biological Activity of 4-(2-Hydroxy-5-(Aryl-Diazenyl)Phenyl)-6-(Aryl)Pyrimidin-2-Ols Derivatives. Org. Chem. Int. 2013, 2013, e582079. [Google Scholar] [CrossRef]
- Guo, D.-C.; Li, K.-Y.; Li, Y.-L.; Tan, H.; Wang, L.-Y. Synthesis and Characterization of Carbazole-Based Azo Acylhydrazone Schiff Bases. Chem. Eng. Commun. 2013, 200, 1503–1512. [Google Scholar] [CrossRef]
- Amaravathi, M.; Kanakaraju, S.; Chandramouli, G.V.P. Synthesis and Characterization of Azobenzene-Porphyrins: Synthesis and Characterization of Azobenzene-Porphyrins. J. Heterocycl. Chem. 2013, 50, 268–271. [Google Scholar] [CrossRef]
- Sagar Vijay Kumar, P.; Suresh, L.; Vinodkumar, T.; Reddy, B.M.; Chandramouli, G.V.P. Zirconium Doped Ceria Nanoparticles: An Efficient and Reusable Catalyst for a Green Multicomponent Synthesis of Novel Phenyldiazenyl–Chromene Derivatives Using Aqueous Medium. ACS Sustain. Chem. Eng. 2016, 4, 2376–2386. [Google Scholar] [CrossRef]
- Leriche, G.; Budin, G.; Brino, L.; Wagner, A. Optimization of the Azobenzene Scaffold for Reductive Cleavage by Dithionite; Development of an Azobenzene Cleavable Linker for Proteomic Applications. Eur. J. Org. Chem. 2010, 2010, 4360–4364. [Google Scholar] [CrossRef]
- Rakesh, S.; Thulasiraman, V.; Sarojadevi, M. Synthesis, Characterization, and Thermal Properties of Cyanate Esters with Azo Linkages. Polym. Eng. Sci. 2015, 55, 47–53. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhang, X.; Li, H.; Niu, Z.; Shi, W.; Cheng, P. Dual-Functionalized Metal–Organic Frameworks Constructed from Hexatopic Ligand for Selective CO2 Adsorption. Inorg. Chem. 2015, 54, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Pamar, M.G.; Govender, P.; Muthusamy, K.; Krause, R.W.M.; Nanjundaswamy, H.M. Mg/Triethylammonium Formate: A Useful System for Reductive Dimerization of Araldehydes into Pinacols; Nitroarenes into Azoarenes and Azoarenes into Hydrazoarenes. Orient. J. Chem. 2013, 29, 969–974. [Google Scholar] [CrossRef]
- Hu, L.; Cao, X.; Chen, L.; Zheng, J.; Lu, J.; Sun, X.; Gu, H. Highly Efficient Synthesis of Aromatic Azos Catalyzed by Unsupported Ultra-Thin Pt Nanowires. Chem. Commun. 2012, 48, 3445–3447. [Google Scholar] [CrossRef] [PubMed]
- Combita, D.; Concepción, P.; Corma, A. Gold Catalysts for the Synthesis of Aromatic Azocompounds from Nitroaromatics in One Step. J. Catal. 2014, 311, 339–349. [Google Scholar] [CrossRef]
- Basheer, M.C.; Oka, Y.; Mathews, M.; Tamaoki, N. A Light-Controlled Molecular Brake with Complete ON–OFF Rotation. Chem. Eur. J. 2010, 16, 3489–3496. [Google Scholar] [CrossRef]
- Noureldin, N.A.; Bellegarde, J.W. A novel method. The synthesis of ketones and azobenzenes using supported permanganate. Synthesis 1999, 6, 939–942. [Google Scholar] [CrossRef]
- Flatt, A.K.; Dirk, S.M.; Henderson, J.C.; Shen, D.E.; Su, J.; Reed, M.A.; Tour, J.M. Synthesis and Testing of New End-Functionalized Oligomers for Molecular Electronics. Tetrahedron 2003, 59, 8555–8570. [Google Scholar] [CrossRef]
- Haberhauer, G.; Kallweit, C.; Wölper, C.; Bläser, D. An Azobenzene Unit Embedded in a Cyclopeptide as a Type-Specific and Spatially Directed Switch. Angew. Chem. Int. Ed. 2013, 52, 7879–7882. [Google Scholar] [CrossRef]
- Cannazza, G.; Perrone, S.; Rosato, F.; D’Accolti, L.; Parenti, C.; Troisi, L. One-Pot Synthesis of Azobenzene Derivatives by Oxidation of 2,3-Dihydrobenzothiadiazines. Synthesis 2014, 46, 962–966. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, N. Copper-Catalyzed Aerobic Oxidative Dehydrogenative Coupling of Anilines Leading to Aromatic Azo Compounds Using Dioxygen as an Oxidant. Angew. Chem. Int. Ed. 2010, 49, 6174–6177. [Google Scholar] [CrossRef]
- Hotta, Y.; Suiko, S.; Motoyanagi, J.; Onishi, H.; Ihozaki, T.; Arakawa, R.; Tsuda, A. A Physical Operation of Hydrodynamic Orientation of an Azobenzene Supramolecular Assembly with Light and Sound. Chem. Commun. 2014, 50, 5615–5618. [Google Scholar] [CrossRef] [PubMed]
- Commins, P.; Garcia-Garibay, M.A. Photochromic Molecular Gyroscope with Solid State Rotational States Determined by an Azobenzene Bridge. J. Org. Chem. 2014, 79, 1611–1619. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Zhi, C.; Luo, B.; Li, X.; Pan, Y.; Cao, X.; Gu, H. Highly Efficient Synthesis of Azos Catalyzed by the Common Metal Copper (0) through Oxidative Coupling Reactions. RSC Adv. 2014, 4, 16607–16611. [Google Scholar] [CrossRef]
- Bellotto, S.; Reuter, R.; Heinis, C.; Wegner, H.A. Synthesis and Photochemical Properties of Oligo-Ortho-Azobenzenes. J. Org. Chem. 2011, 76, 9826–9834. [Google Scholar] [CrossRef]
- Viehmann, P.; Hecht, S. Design and Synthesis of a Photoswitchable Guanidine Catalyst. Beilstein J. Org. Chem. 2012, 8, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Schönberger, M.; Trauner, D. A Photochromic Agonist for μ-Opioid Receptors. Angew. Chem. Int. Ed. 2014, 53, 3264–3267. [Google Scholar] [CrossRef]
- Takeuchi, T.; Mori, T.; Kuwahara, A.; Ohta, T.; Oshita, A.; Sunayama, H.; Kitayama, Y.; Ooya, T. Conjugated-Protein Mimics with Molecularly Imprinted Reconstructible and Transformable Regions That Are Assembled Using Space-Filling Prosthetic Groups. Angew. Chem. Int. Ed. 2014, 53, 12765–12770. [Google Scholar] [CrossRef] [PubMed]
- Rosales, A.M.; Mabry, K.M.; Nehls, E.M.; Anseth, K.S. Photoresponsive Elastic Properties of Azobenzene-Containing Poly(Ethylene-Glycol)-Based Hydrogels. Biomacromolecules 2015, 16, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Reuter, R.; Wegner, H.A. Meta-Oligoazobiphenyls–Synthesis via Site-Selective Mills Reaction and Photochemical Properties. Beilstein J. Org. Chem. 2012, 8, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.F.; Ibrahim, S.A.; El-Borai, M.A. Synthesis, Fastness Properties, Color Assessment and Antimicrobial Activity of Some Azo Reactive Dyes Having Pyrazole Moiety. Dyes Pigments 2015, 112, 86–92. [Google Scholar] [CrossRef]
- Teimouri, A.; Chermahini, A.N.; Ghorbani, M. Hassan. The Green Synthesis of New Azo Dyes Derived from Salicylic Acid Derivatives Catalyzed via Baker’s Yeast and Solid Acid Catalysis. Chemija 2013, 24, 59–66. [Google Scholar]
- Tanizaki, Y.; Kobayashi, T.; Hoshi, T. The Absorption Spectra of Dyes. XI. A Note on the Color Change in Some Azobenzene Dyes with Variation in the Acidity. BCSJ 1966, 39, 558–562. [Google Scholar] [CrossRef]
- Yamada, S.; Bessho, J.; Nakasato, H.; Tsutsumi, O. Color Tuning Donor–Acceptor-Type Azobenzene Dyes by Controlling the Molecular Geometry of the Donor Moiety. Dyes Pigments 2018, 150, 89–96. [Google Scholar] [CrossRef]
- Velema, W.A.; van der Berg, J.P.; Hansen, M.J.; Szymanski, W.; Driessen, A.J.M.; Feringa, B.L. Optical Control of Antibacterial Activity. Nat. Chem. 2013, 5, 924–928. [Google Scholar] [CrossRef]
- Wegener, M.; Hansen, M.J.; Driessen, A.J.M.; Szymanski, W.; Feringa, B.L. Photocontrol of Antibacterial Activity: Shifting from UV to Red Light Activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef] [PubMed]
- Banaszak-Leonard, E.; Fayeulle, A.; Franche, A.; Sagadevan, S.; Billamboz, M. Antimicrobial Azo Molecules: A Review. J. Iran. Chem. Soc. 2021. [Google Scholar] [CrossRef]
- Laprell, L.; Tochitsky, I.; Kaur, K.; Manookin, M.B.; Stein, M.; Barber, D.M.; Schön, C.; Michalakis, S.; Biel, M.; Kramer, R.H.; et al. Photopharmacological Control of Bipolar Cells Restores Visual Function in Blind Mice. J. Clin. Investig. 2017, 127, 2598–2611. [Google Scholar] [CrossRef]
- Tochitsky, I.; Helft, Z.; Meseguer, V.; Fletcher, R.B.; Telias, M.; Denlinger, B.; Malis, J.; Vessey, K.A.; Fletcher, E.L.; Kramer, R.H. How Azobenzene Photoswitches Restore Visual Responses to the Blind Retina. Neuron 2016, 92, 100–113. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, F.; Ma, C.; Han, H.; Sun, R.; Peng, H.; Xie, M. Enhanced Dielectric and Electrical Energy Storage Capability of Polymers with Combined Azobenzene and Triphenylamine Side Groups by Ring-Opening Metathesis Polymerization. Polymer 2019, 184, 121886. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zou, S.J.; Sun, X.H. Efficient Azobenzene Co-Sensitizer for Wide Spectral Absorption of Dye-Sensitized Solar Cells. RSC Adv. 2018, 8, 6212–6217. [Google Scholar] [CrossRef]
- Billamboz, M.; Mangin, F.; Drillaud, N.; Chevrin-Villette, C.; Banaszak-Léonard, E.; Len, C. Micellar Catalysis Using a Photochromic Surfactant: Application to the Pd-Catalyzed Tsuji–Trost Reaction in Water. J. Org. Chem. 2014, 79, 493–500. [Google Scholar] [CrossRef]
- Drillaud, N.; Banaszak-Léonard, E.; Pezron, I.; Len, C. Synthesis and Evaluation of a Photochromic Surfactant for Organic Reactions in Aqueous Media. J. Org. Chem. 2012, 77, 9553–9561. [Google Scholar] [CrossRef]
- Seidel, Z.P.; Lee, C.T. Enhanced Activity of the Cellulase Enzyme β-Glucosidase upon Addition of an Azobenzene-Based Surfactant. ACS Sustain. Chem. Eng. 2020, 8, 1751–1761. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Shimada, T. Oxidation of Toxic and Carcinogenic Chemicals by Human Cytochrome P-450 Enzymes. Chem. Res. Toxicol. 1991, 4, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Hanau, S.; Almugadam, S.H.; Sapienza, E.; Cacciari, B.; Manfrinato, M.C.; Trentini, A.; Kennedy, J.F. Schematic Overview of Oligosaccharides, with Survey on Their Major Physiological Effects and a Focus on Milk Ones. Carbohydr. Polym. Technol. Appl. 2020, 1, 100013. [Google Scholar] [CrossRef]
- Suzuki, M.; Kaneda, K.; Nakai, Y.; Kitaoka, M.; Taniguchi, H. Synthesis of Cellobiose from Starch by the Successive Actions of Two Phosphorylases. N Biotechnol. 2009, 26, 137–142. [Google Scholar] [CrossRef]
- Suzuki, M.; Kitaoka, M.; Taniguchi, H. Enzymatic Production of Cellobiose from Starch and Its Reduction to Cellobiitol. J. Appl. Glycosci. 2010, 57, 113–119. [Google Scholar] [CrossRef][Green Version]
- Bacon, J.S.D.; Dickinson, B. The Origin of Melezitose: A Biochemical Relationship between the Lime Tree (Tilia spp.) and an Aphis (Eucallipterus tiliae L.). Biochem. J. 1957, 66, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Forano, C. Environmental Remediation Involving Layered Double Hydroxides. In Interface Science and Technology; Wypych, F., Satyanarayana, K.G., Eds.; Clay Surfaces; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 425–458. [Google Scholar]
- Belorkar, S.A.; Gupta, A.K. Oligosaccharides: A Boon from Nature’s Desk. AMB Express 2016, 6. [Google Scholar] [CrossRef]
- Yilmaz, E. Chitosan: A Versatile Biomaterial. In Proceedings of the Biomaterials; Hasirci, N., Hasirci, V., Eds.; Springer: Boston, MA, USA, 2004; pp. 59–68. [Google Scholar]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. 1-Fundamentals of chitosan for biomedical applications. In Chitosan Based Biomaterials; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Shaston, UK, 2017; Volume 1, pp. 3–30. ISBN 978-0-08-100230-8. [Google Scholar]
- Atwood, J.L. Inclusion (Clathrate) Compounds. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 717–729. ISBN 978-0-12-227410-7. [Google Scholar]
- McQueen, R.H. 17-Odour control of medical textiles. In Handbook of Medical Textiles; Bartels, V.T., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Shaston, UK, 2011; pp. 387–416. ISBN 978-1-84569-691-7. [Google Scholar]
- Zhang, X.; Zhang, Y.; Armstrong, D.W. 8.10 Chromatographic Separations and Analysis: Cyclodextrin Mediated HPLC, GC and CE Enantiomeric Separations. In Comprehensive Chirality; Carreira, E.M., Yamamoto, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 177–199. ISBN 978-0-08-095168-3. [Google Scholar]
- Bricout, H.; Léonard, E.; Len, C.; Landy, D.; Hapiot, F.; Monflier, E. Impact of Cyclodextrins on the Behavior of Amphiphilic Ligands in Aqueous Organometallic Catalysis. Beilstein J. Org. Chem. 2012, 8, 1479–1484. [Google Scholar] [CrossRef]
- Ferreira, M.; Bricout, H.; Azaroual, N.; Landy, D.; Tilloy, S.; Hapiot, F.; Monflier, E. Cyclodextrin/Amphiphilic Phosphane Mixed Systems and Their Applications in Aqueous Organometallic Catalysis. Adv. Synth. Catal. 2012, 354, 1337–1346. [Google Scholar] [CrossRef]
- Gaspar, V.M.; Moreira, A.F.; de Melo-Diogo, D.; Costa, E.C.; Queiroz, J.A.; Sousa, F.; Pichon, C.; Correia, I.J. Chapter 6-Multifunctional nanocarriers for codelivery of nucleic acids and chemotherapeutics to cancer cells. In Nanobiomaterials in Medical Imaging; Grumezescu, A.M., Ed.; William Andrew Publishing: Shaston, UK, 2016; pp. 163–207. ISBN 978-0-323-41736-5. [Google Scholar]
- Gross, L. Bacterial Fimbriae Designed to Stay with the Flow. PLoS Biol. 2006, 4, e314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hartmann, M.; Lindhorst, T.K. The Bacterial Lectin FimH, a Target for Drug Discovery–Carbohydrate Inhibitors of Type 1 Fimbriae-Mediated Bacterial Adhesion. Eur. J. Org. Chem. 2011, 2011, 3583–3609. [Google Scholar] [CrossRef]
- Chandrasekaran, V.; Kolbe, K.; Beiroth, F.; Lindhorst, T.K. Synthesis and Testing of the First Azobenzene Mannobioside as Photoswitchable Ligand for the Bacterial Lectin FimH. Beilstein J. Org. Chem. 2013, 9, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Yoshiyama, C.; Kitaoka, T. Helical Assembly of Azobenzene-Conjugated Carbohydrate Hydrogelators with Specific Affinity for Lectins. Langmuir 2012, 28, 4404–4412. [Google Scholar] [CrossRef]
- Srinivas, O.; Mitra, N.; Surolia, A.; Jayaraman, N. Photoswitchable Cluster Glycosides as Tools to Probe Carbohydrate–Protein Interactions: Synthesis and Lectin-Binding Studies of Azobenzene Containing Multivalent Sugar Ligands. Glycobiology 2005, 15, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Gleich, G.J.; Allen, P.Z. Immunochemical Studies on Some Immune Systems Involving ß(1,4) Linked Glucose. Immunochemistry 1965, 2, 417–431. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, D.; Weng, J.; Chen, B.; Liu, H. Synthesis and Characterization of Photochromic Azobenzene Cellulose Ethers. Carbohydr. Polym. 2014, 99, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kang, H.; Li, G.; Wang, C.; Huang, Y.; Liu, R. Synthesis and Photosensitivity of Azobenzene Functionalized Hydroxypropylcellulose. RSC Adv. 2013, 3, 15909–15916. [Google Scholar] [CrossRef]
- Otsuka, I.; Barrett, C.J. Electrospinning of Photo-Responsive Azo-Cellulose: Towards Smart Fibrous Materials. Cellulose 2019, 26, 6903–6915. [Google Scholar] [CrossRef]
- Torque, C.; Bricout, H.; Hapiot, F.; Monflier, E. Substrate-Selective Aqueous Organometallic Catalysis. How Size and Chemical Modification of Cyclodextrin Influence the Substrate Selectivity. Tetrahedron 2004, 60, 6487–6493. [Google Scholar] [CrossRef]
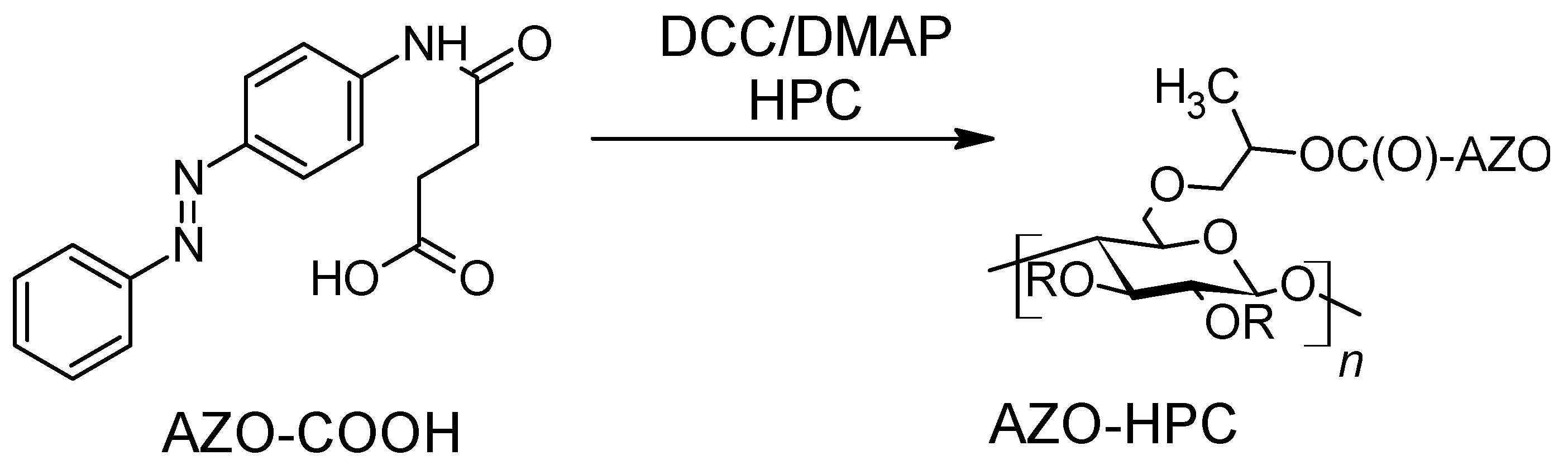
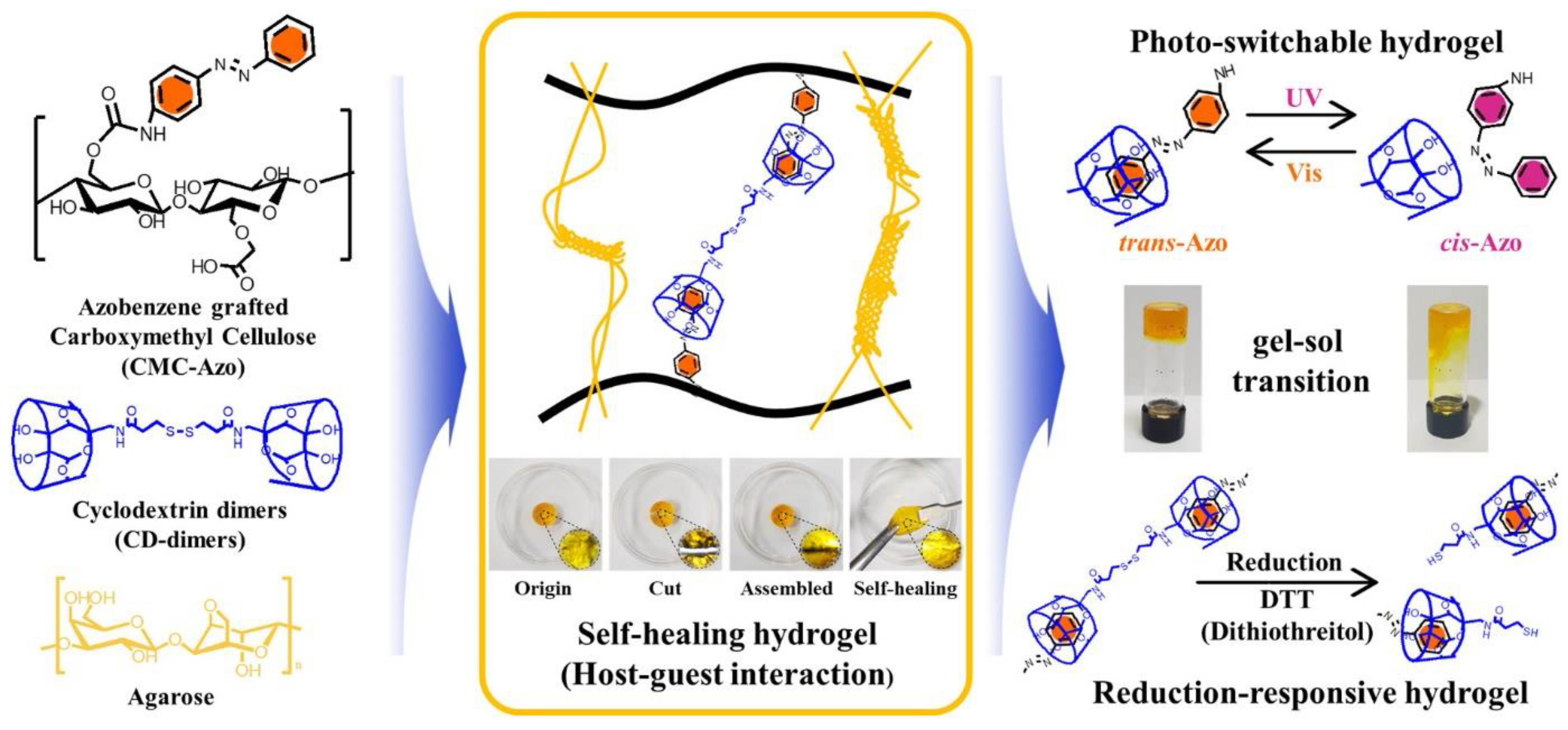
- Kim, Y.; Jeong, D.; Shinde, V.V.; Hu, Y.; Kim, C.; Jung, S. Azobenzene-Grafted Carboxymethyl Cellulose Hydrogels with Photo-Switchable, Reduction-Responsive and Self-Healing Properties for a Controlled Drug Release System. Int. J. Biol. Macromol. 2020, 163, 824–832. [Google Scholar] [CrossRef]
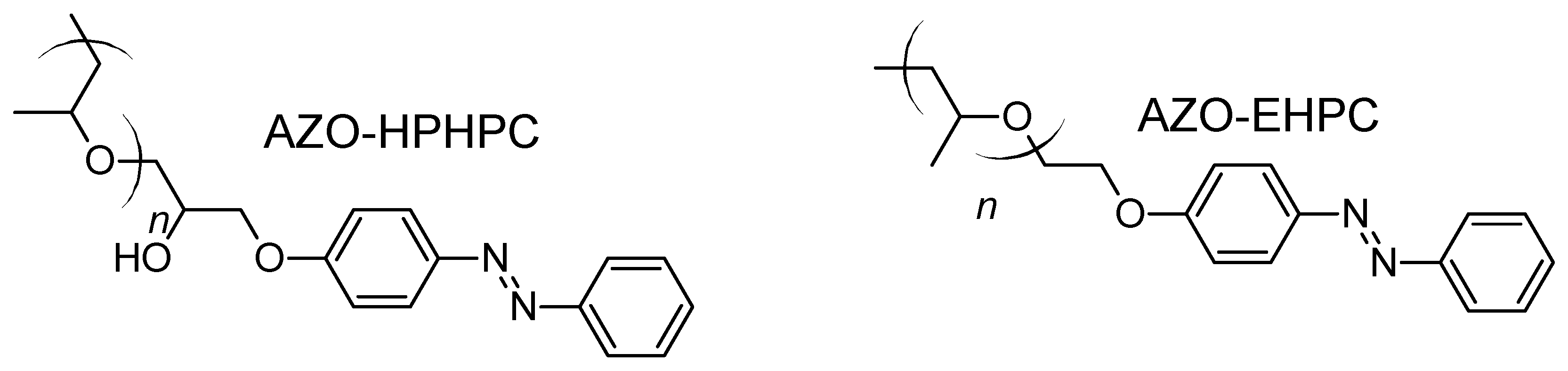
- Qin, W.; Li, Z.; Li, J.; Zhang, L.; Liu, R.; Liu, H. Synthesis and Characterization of Azobenzene Hydroxypropyl Cellulose with Photochromic and Thermotropic Liquid Crystal Properties. Cellulose 2015, 22, 203–214. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Liu, H. Thermal Inverse Phase Transition of Azobenzene Hydroxypropylcellulose in Aqueous Solutions. Cellulose 2016, 23, 1177–1188. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-Based Dynamic Materials. Chem. Soc. Rev. 2013, 43, 148–184. [Google Scholar] [CrossRef]
- Ueki, T.; Usui, R.; Kitazawa, Y.; Lodge, T.P.; Watanabe, M. Thermally Reversible Ion Gels with Photohealing Properties Based on Triblock Copolymer Self-Assembly. Macromolecules 2015, 48, 5928–5933. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Zheng, X.; Retulainen, E.; Fu, S. Dual Light- and PH-Responsive Composite of Polyazo-Derivative Grafted Cellulose Nanocrystals. Materials 2018, 11, 1725. [Google Scholar] [CrossRef]
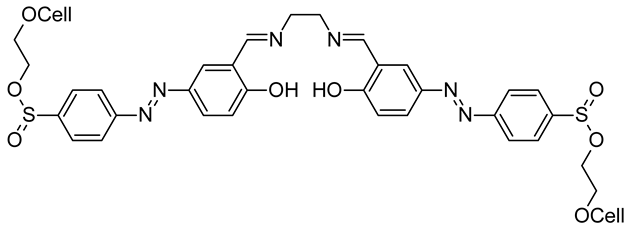
- Hou, A.; Zhang, C.; Wang, Y. Preparation and UV-Protective Properties of Functional Cellulose Fabrics Based on Reactive Azobenzene Schiff Base Derivative. Carbohydr. Polym. 2012, 87, 284–288. [Google Scholar] [CrossRef]
- Gerasimowicz, W.V.; Wojcik, J.F. Azo Dye-α-Cyclodextrin Adduct Formation. Bioorg. Chem. 1982, 11, 420–427. [Google Scholar] [CrossRef]
- Shibusawa, T.; Okamoto, J.; Abe, K.; Sakata, K.; Ito, Y. Inclusion of Azo Disperse Dyes by Cyclodextrins at Dyeing Temperature. Dyes Pigments 1998, 36, 79–91. [Google Scholar] [CrossRef]
- Suzuki, M.; Ohmori, H.; Kajtar, M.; Szejtli, J.; Vikmon, M. The Association of Inclusion Complexes of Cyclodextrins with Azo Dyes. J. Incl. Phenom. Macrocycl. Chem. 1994, 18, 255–264. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Wang, L.; Ding, L.; Tian, Y.; Jin, W.; Zhang, H. Study on the Inclusion Complexes of Cyclodextrin and Sulphonated Azo Dyes by Electrospray Ionization Mass Spectrometry. Int. J. Mass Spectrom. 2006, 252, 1–10. [Google Scholar] [CrossRef]
- Wu, M.; Yuguchi, Y.; Kumagai, T.; Endo, T.; Hirotsu, T. Nano-Complex Formation of Cyclodextrin and Azobenzene Using Supercritical Carbon Dioxide. Chem. Commun. 2004, 1288–1289. [Google Scholar] [CrossRef]
- Craig, M.R.; Claridge, T.D.W.; Anderson, H.L.; Hutchings, M.G. Synthesis of a Cyclodextrin Azo Dye [3]Rotaxane as a Single Isomer. Chem. Commun. 1999, 1537–1538. [Google Scholar] [CrossRef]
- Ueno, A.; Takahashi, K.; Osa, T. Photoregulation of Catalytic Activity of β-Cyclodextrin by an Azo Inhibitor. J. Chem. Soc. Chem. Commun. 1980, 837–838. [Google Scholar] [CrossRef]
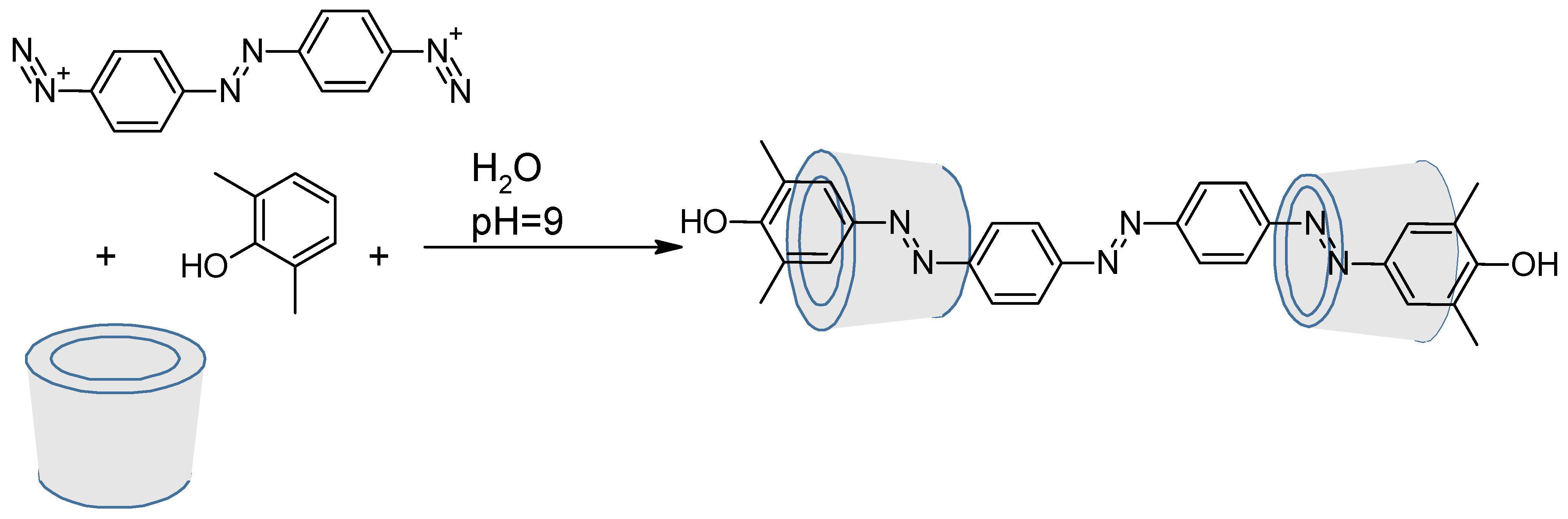
- Casas-Solvas, J.M.; Martos-Maldonado, M.C.; Vargas-Berenguel, A. Synthesis of [Beta]-Cyclodextrin Derivatives Functionalized with Azobenzene. Tetrahedron 2008, 64, 10919–10923. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Tian, H. Disparate Orientation of [1]Rotaxanes. Tetrahedron Lett. 2007, 48, 7112–7116. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.-X.; Chen, Y. Syntheses and Self-Assembly Behaviors of the Azobenzenyl Modified β-Cyclodextrins Isomers. J. Org. Chem. 2008, 73, 5298–5304. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Ueno, A.; Fukushima, M.; Osa, T. Synthesis and Photoisomerization of an Azobenzene Derivative Bearing Two β-Cyclodextrin Units at Both Ends. Macromol. Rapid Commun. 1998, 19, 103–105. [Google Scholar] [CrossRef]
- Kuwabara, T.; Shiba, K.; Nakajima, H.; Ozawa, M.; Miyajima, N.; Hosoda, M.; Kuramoto, N.; Suzuki, Y. Host−Guest Complexation Affected by PH and Length of Spacer for Hydroxyazobenzene-Modified Cyclodextrins. J. Phys. Chem. A 2006, 110, 13521–13529. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Nakamura, A.; Ikeda, H.; Ikeda, T.; Mihara, H.; Ueno, A. Alizarin Yellow-Modified β-Cyclodextrin as a Guest-Responsive Absorption Change Sensor. Anal. Chem. 1997, 69, 659–663. [Google Scholar] [CrossRef]
- Fukushima, M.; Osa, T.; Ueno, A. Photoswitchable Multi-Response Sensor of Azobenzene-Modified γ-Cyclodextrin for Detecting Organic Compounds. Chem. Lett. 1991, 20, 709–712. [Google Scholar] [CrossRef]
- Ueno, A.; Yoshimura, H.; Saka, R.; Osa, T. Photocontrol of Binding Ability of Capped Cyclodextrin. J. Am. Chem. Soc. 1979, 101, 2779–2780. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.-L.; Zhang, H.-Y.; Fan, Z.; Wen, G.-D.; Ding, F. Spectrophotometric Study of Inclusion Complexation of Aliphatic Alcohols by β-Cyclodextrins with Azobenzene Tether. J. Phys. Chem. B 2004, 108, 8836–8843. [Google Scholar] [CrossRef]
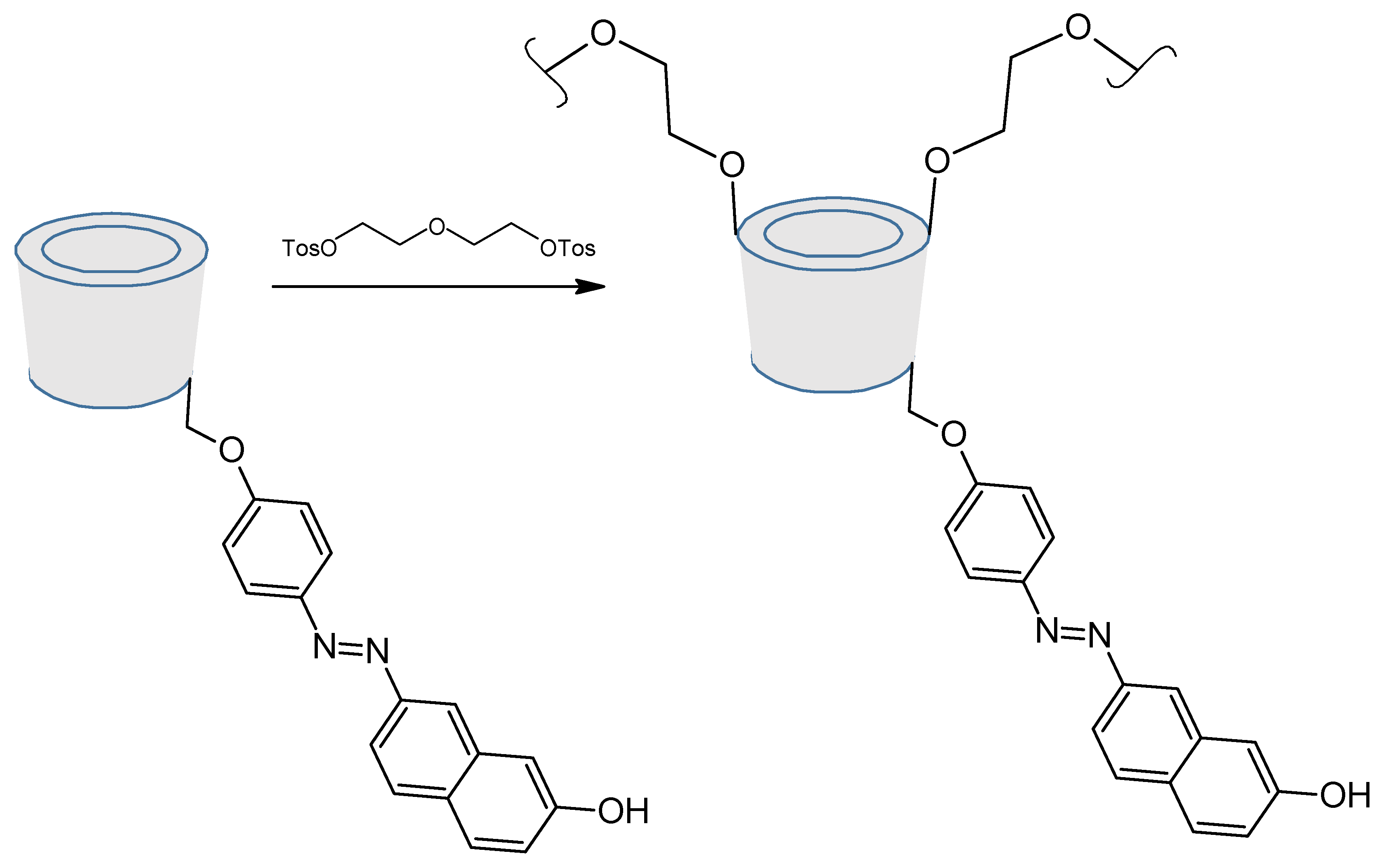
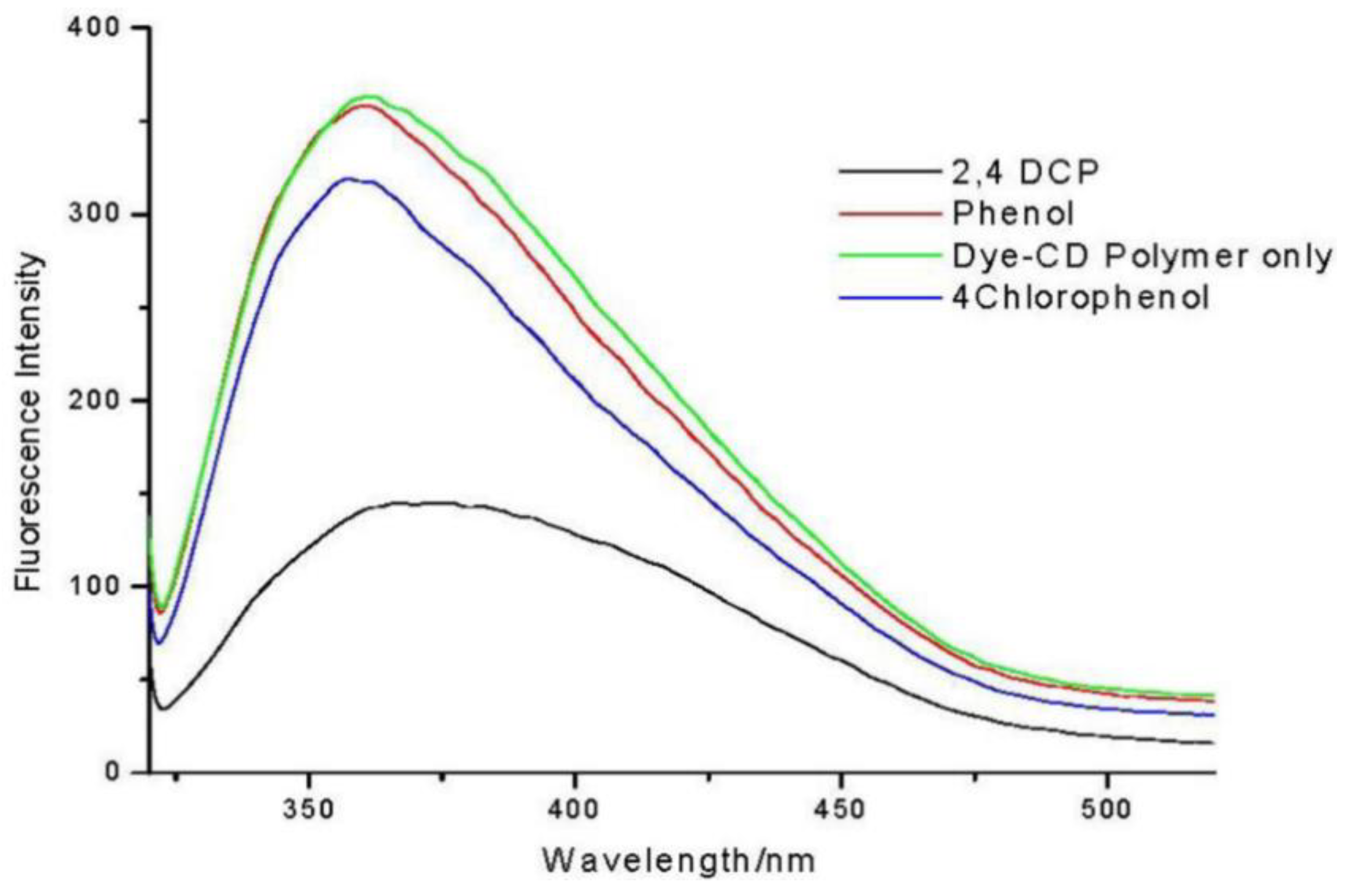
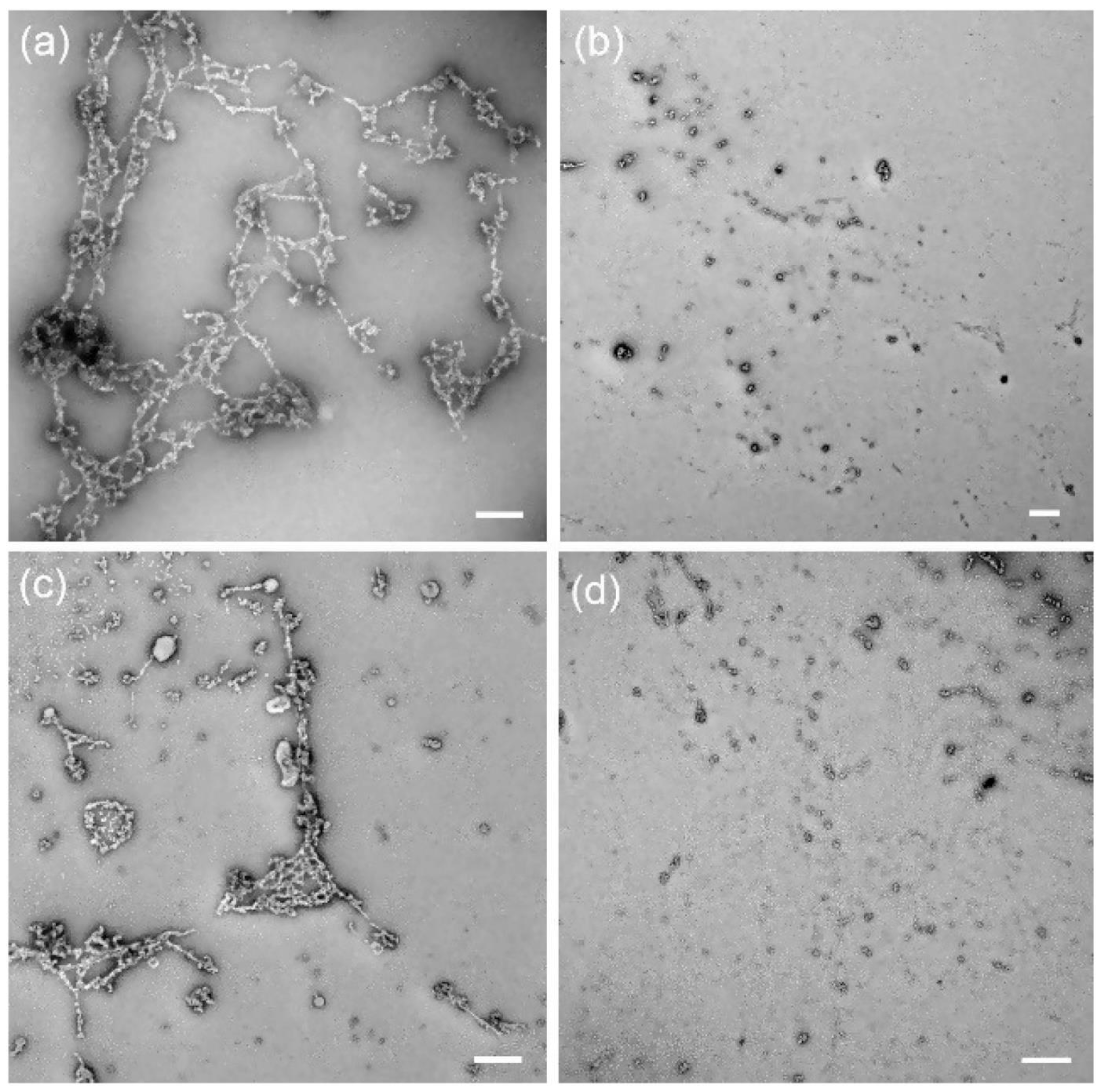
- Ncube, P.; Krause, R.W.; Mamba, B.B. Fluorescent Sensing of Chlorophenols in Water Using an Azo Dye Modified β-Cyclodextrin Polymer. Sensors 2011, 11, 4598–4608. [Google Scholar] [CrossRef]
- Kikuchi, T.; Narita, M.; Hamada, F. Synthesis of Bis Dansyl-Modified β-Cyclodextrin Dimer Linked with Azobenzene and Its Fluorescent Molecular Recognition. J. Incl. Phenom. 2002, 44, 329–334. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, B.; Zhou, J.; Zhao, L.; Xu, B.; Liu, C. Self-Assembly of β-Cyclodextrin-Derived Amphiphile with a Photo Responsive Guest. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123683. [Google Scholar] [CrossRef]



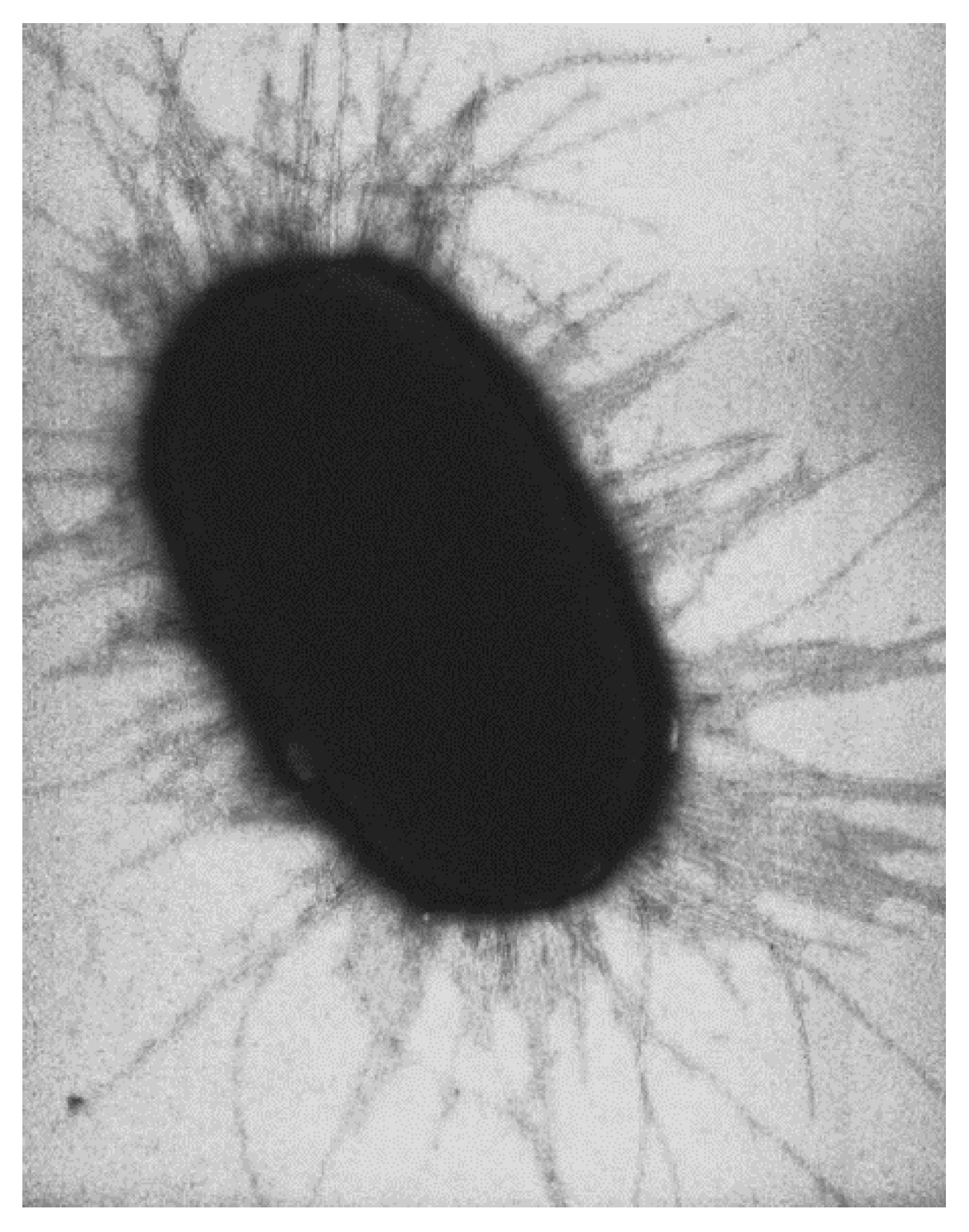
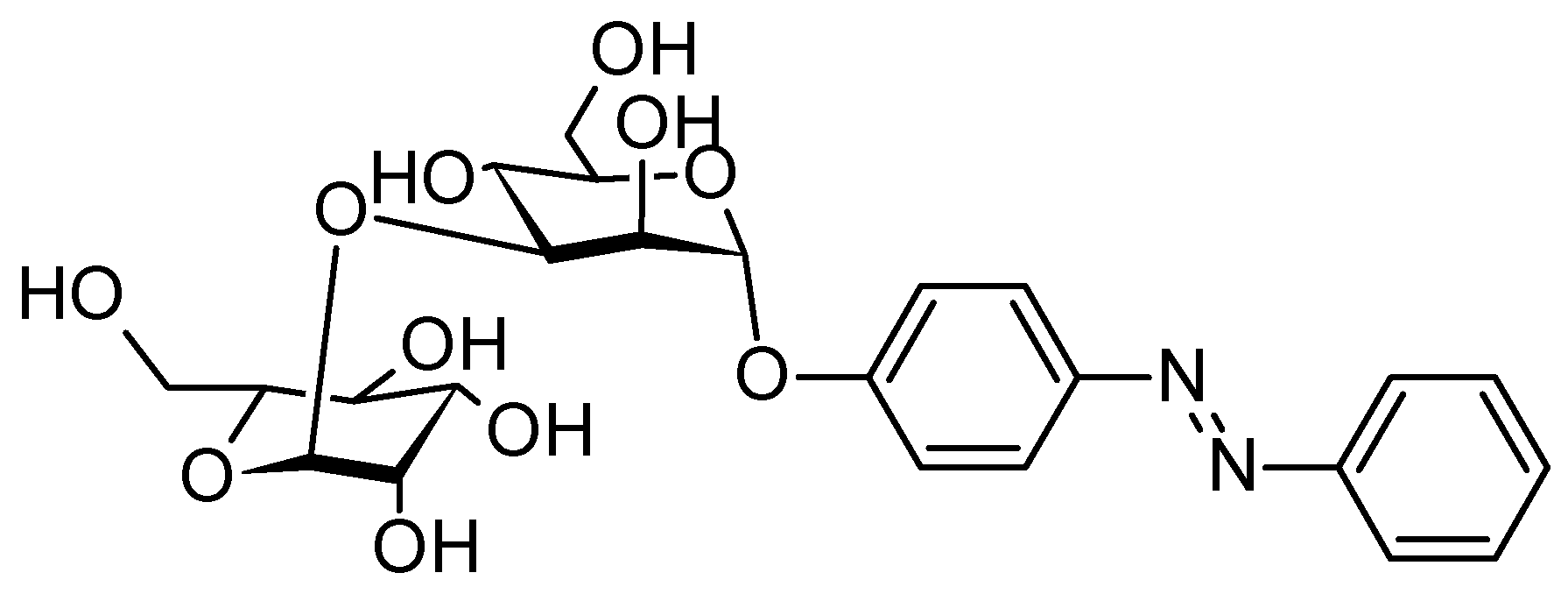
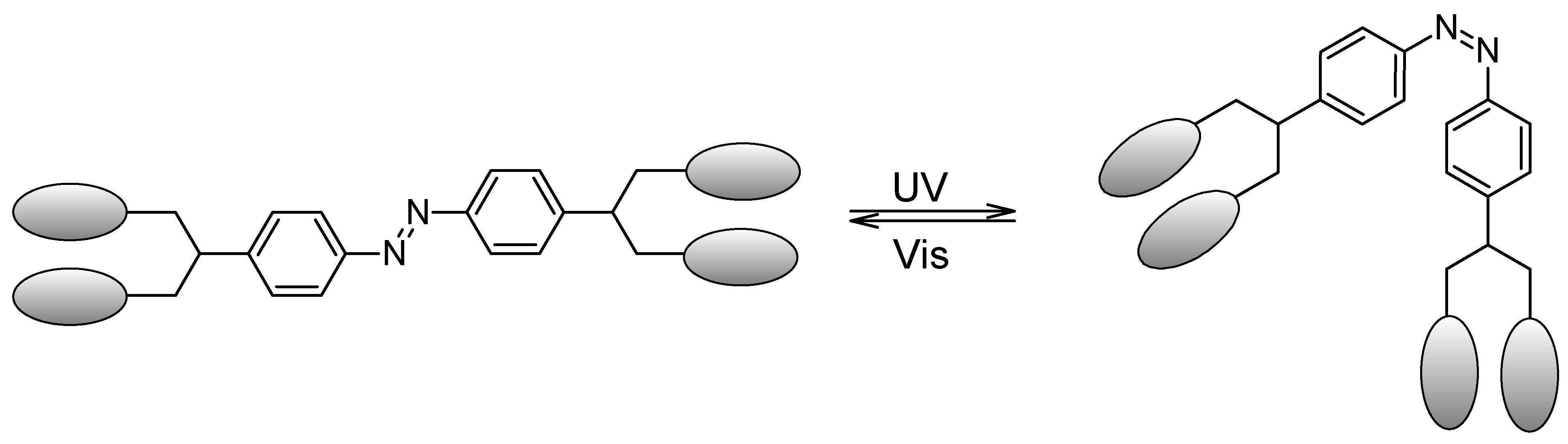
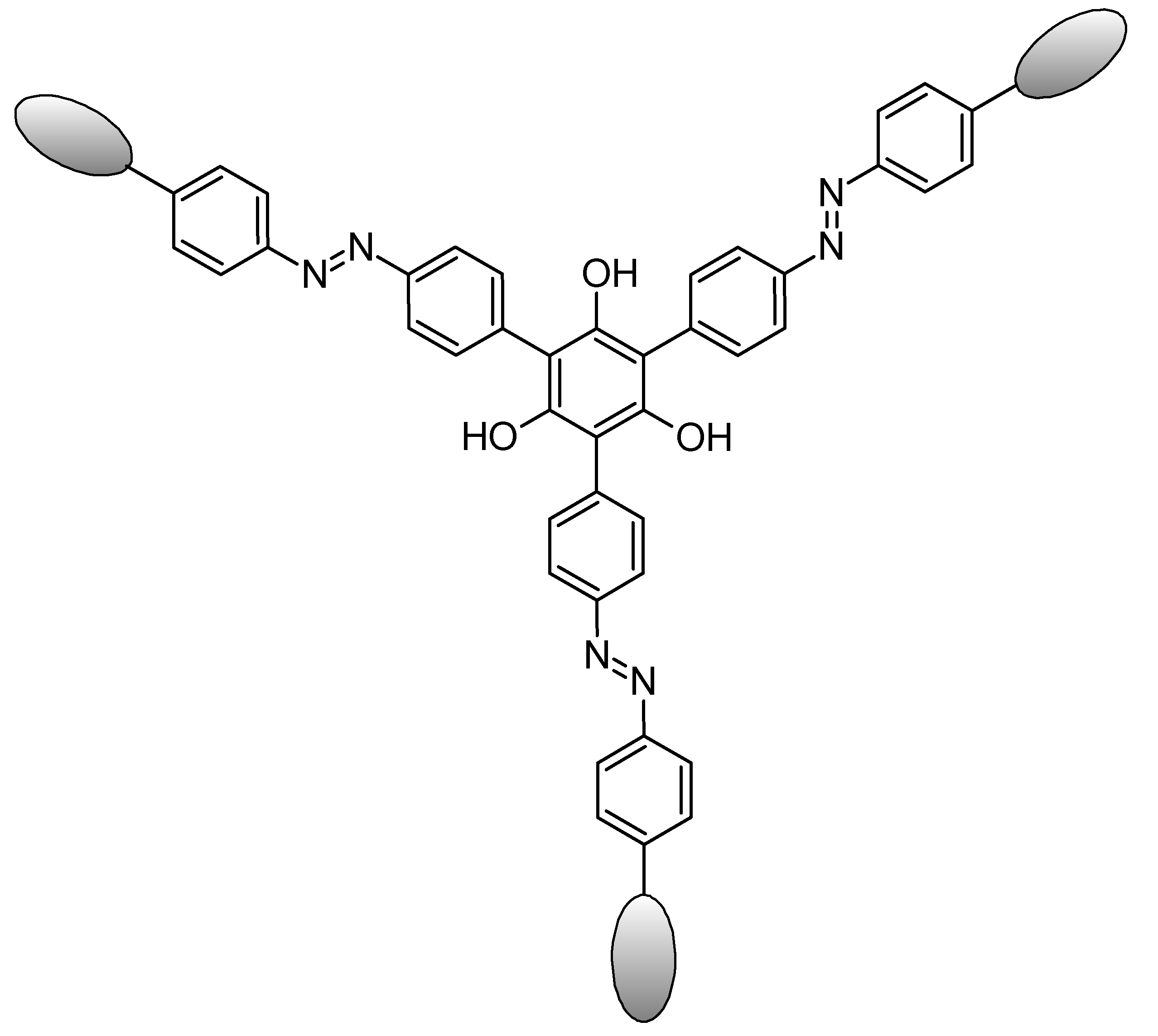






| Samples | Concentrations (g/L) | UPF | T(UVA) | T(UVB) |
|---|---|---|---|---|
| Control | 0 | 8.31 | 10.09 | 11.81 |
| FC-1 | 2 | 18.72 | 4.29 | 5.17 |
| FC-2 | 5 | 31.7 | 3.09 | 3.73 |

| Link | Main Reagents | Reference |
|---|---|---|
| Alkyle |
| [95] |
| Amide |
| [83] |
| [84] | |
| [90] | |
| [114] | |
| [115] | |
| Ester |
| [111] |
| Ether |
| [82] |
| [92] | |
| [96] | |
| [105] | |
| [107] | |
| [112] | |
| [113] | |
| Oxapentylamine |
| [110] |
| Triazole |
| [106] |
| Ether with in situ diazotation |
| [85] |
| Application | Reference (Year of Publication) |
|---|---|
| Inhibitory of cell surface adhesion, immunochemistry, UV protection | 82 (2013), 83 (2012), 84 (2005), 85 (1965), 96 (2012) |
| Hydrogels, liquid/nano crystals | 90 (2020), 92 (2016), 95 (2018) |
| Smart rotaxanes and self-assemblies | 102 (1999), 105 (2007), 106 (2008), 115 (2019) |
| Sensors | 110 (1991), 111 (1979), 112 (2004), 113 (2011), 114 (2002) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Léonard, E.; Fayeulle, A. Azo-Dyes-Grafted Oligosaccharides—From Synthesis to Applications. Molecules 2021, 26, 3063. https://doi.org/10.3390/molecules26113063
Léonard E, Fayeulle A. Azo-Dyes-Grafted Oligosaccharides—From Synthesis to Applications. Molecules. 2021; 26(11):3063. https://doi.org/10.3390/molecules26113063
Chicago/Turabian StyleLéonard, Estelle, and Antoine Fayeulle. 2021. "Azo-Dyes-Grafted Oligosaccharides—From Synthesis to Applications" Molecules 26, no. 11: 3063. https://doi.org/10.3390/molecules26113063
APA StyleLéonard, E., & Fayeulle, A. (2021). Azo-Dyes-Grafted Oligosaccharides—From Synthesis to Applications. Molecules, 26(11), 3063. https://doi.org/10.3390/molecules26113063







