Development of PE/PCL Bilayer Films Modified with Casein and Aluminum Oxide
Abstract
:1. Introduction
2. Results and Discussion
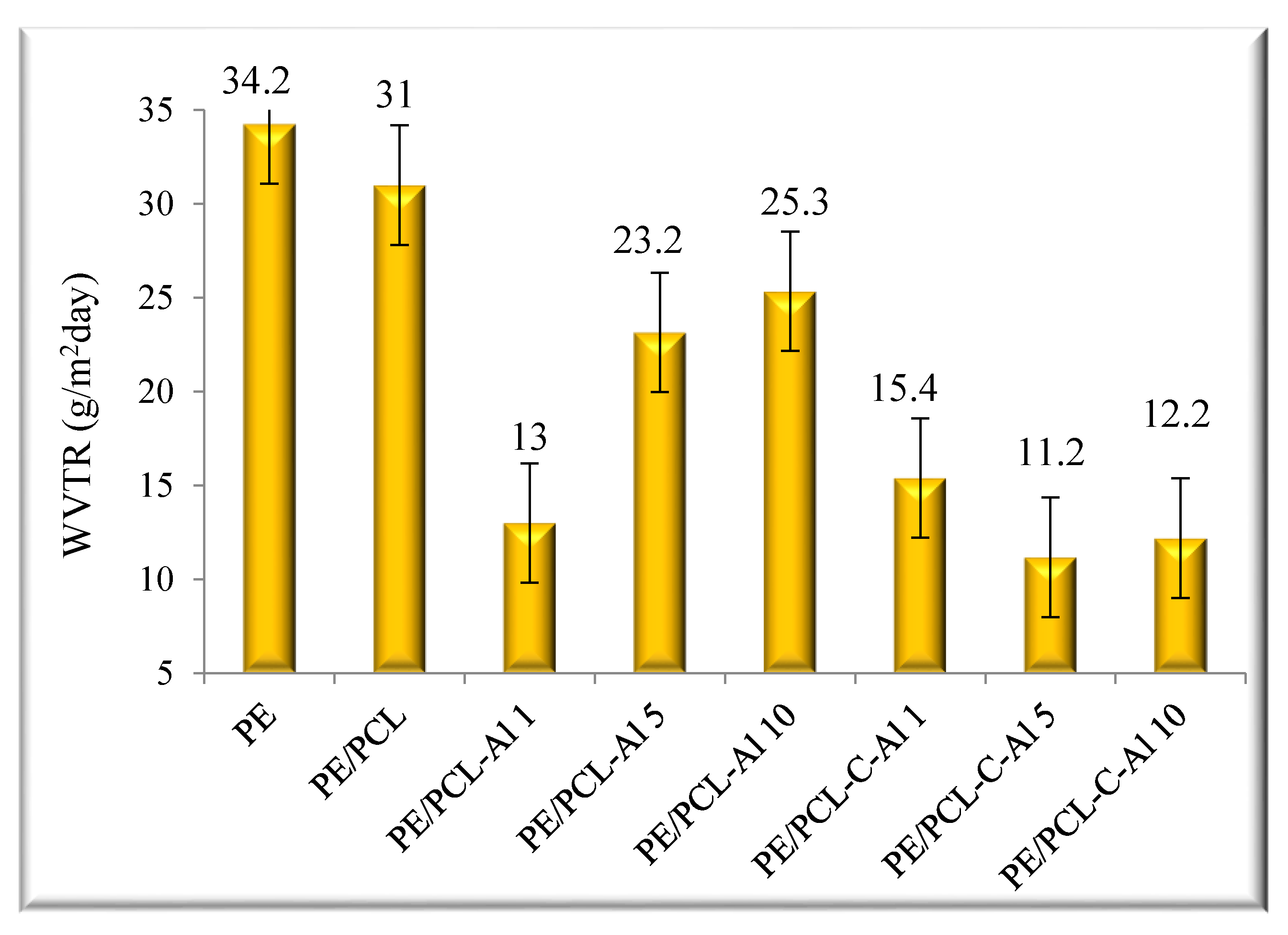
2.1. Water Vapor Permeability
2.2. TG Analysis
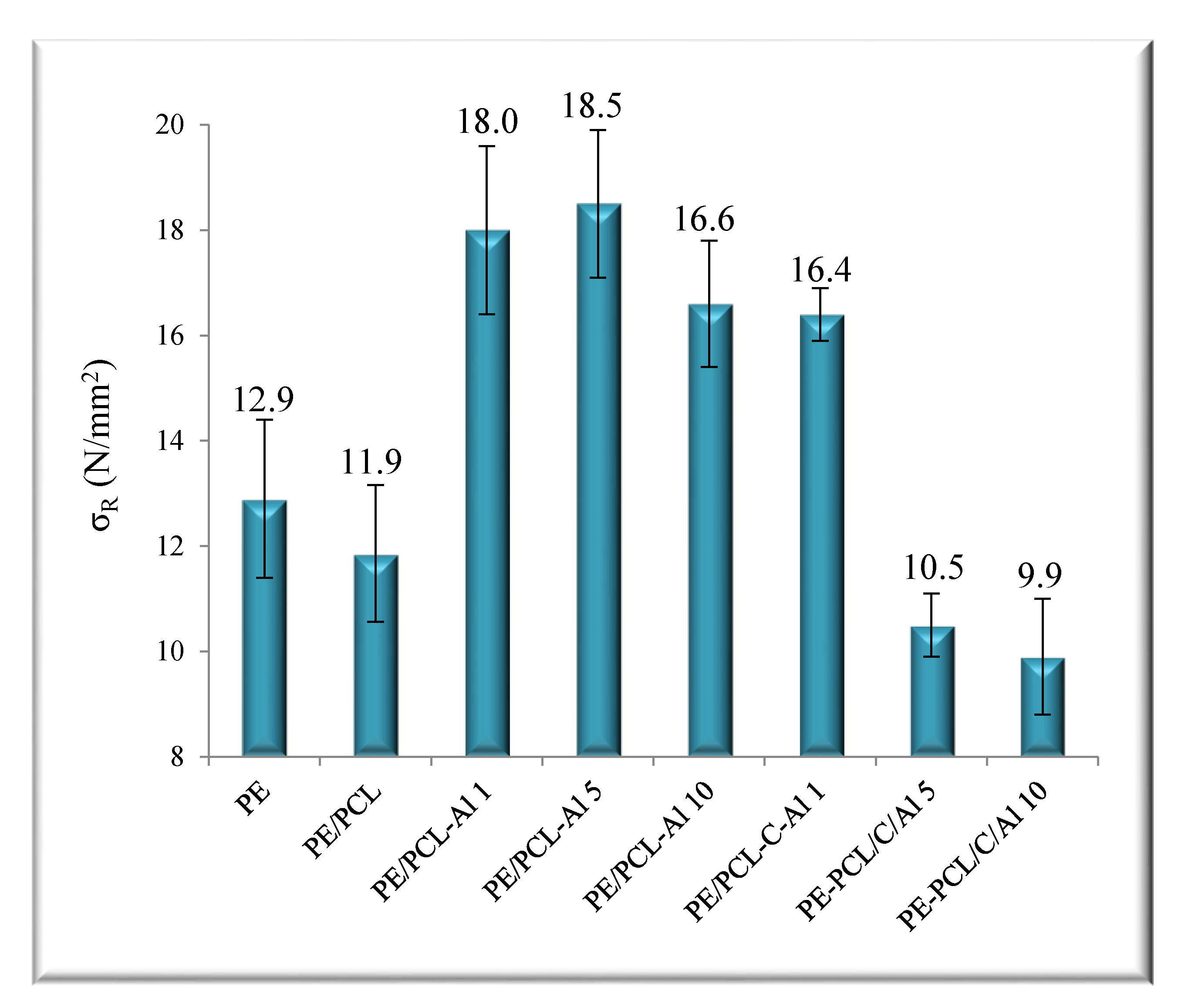
2.3. Mechanical Properties
2.4. FTIR Analysis
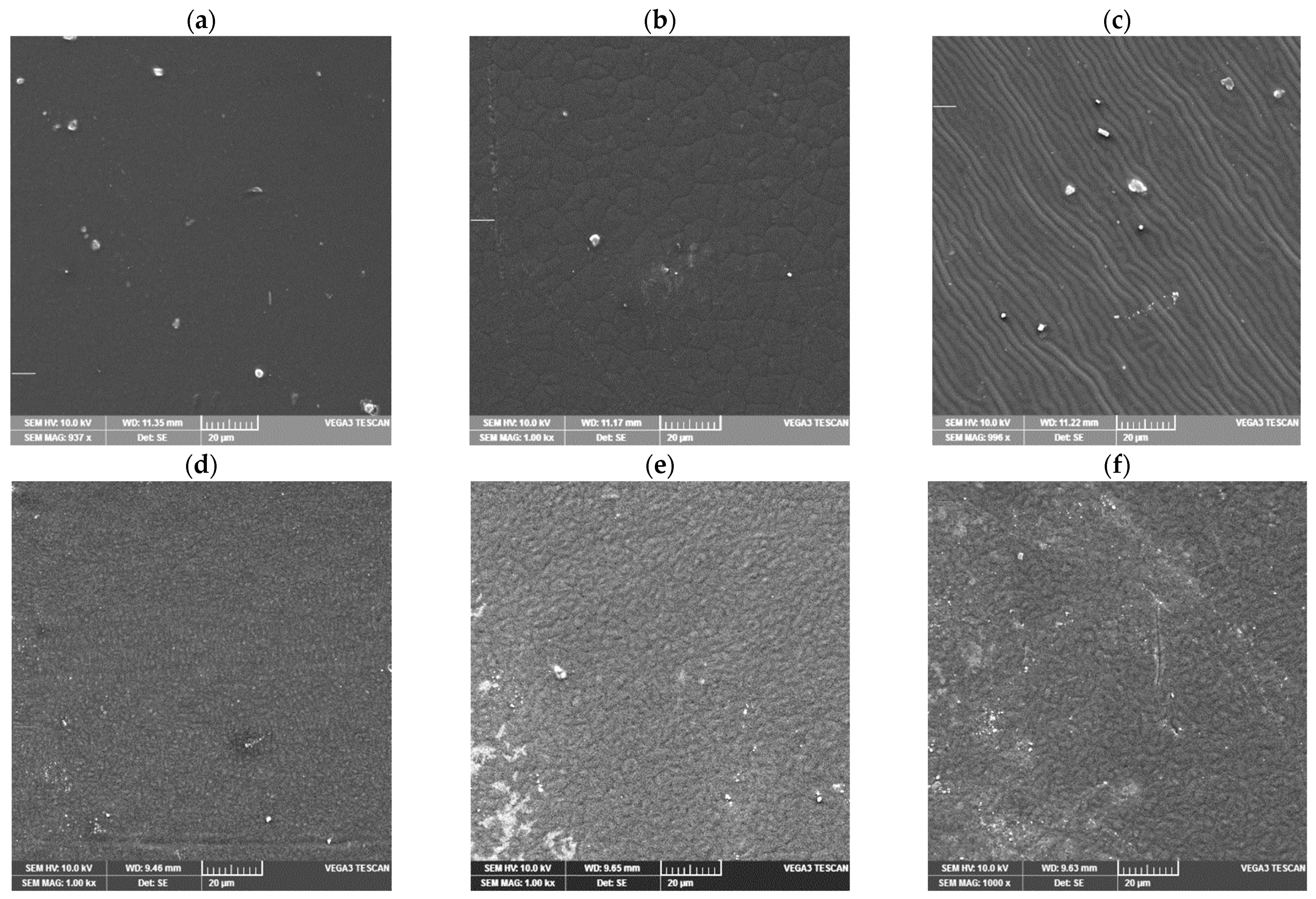
2.5. SEM Analysis
3. Experimental Part
3.1. Materials
3.2. Preparation of Casein/Aluminum Oxide Mixture
3.3. Preparation of Double Layer PE/PCL Films
3.4. Characterization
3.4.1. Water Vapor Permeability
- m0—mass of the device with water and specimen at the beginning (g)
- m2—mass of the device with water and a test tube after 24 h (g)
- m3—mass of the device with water and a test tube after 48 h (g)
3.4.2. TG Analysis
3.4.3. Mechanical Properties
3.4.4. FTIR Spectroscopy
3.4.5. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Huang, J.Y.; Limqueco, J.; Chieng, Y.Y.; Li, X.; Zhou, W. Performance evaluation of a novel food packaging material based on clay/polyvinyl alcohol nanocomposite. Innov. Food. Sci. Emerg. Technol. 2017, 43, 216–222. [Google Scholar] [CrossRef]
- Rezić, I.; Haramina, T.; Rezić, T. Metal nanoparticles and carbon nanotubes—perfect antimicrobial nanofillers in polymer-based food packaging materials. In Food Packaging: Nanotechnology in the Agri-Food Industry, 1st ed.; Grumezescu, A.M., Ed.; Academic Press: Amsterdam, The Netherlands, 2017; pp. 497–532. [Google Scholar]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. Part A 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Contreras, C.B.; Alvarez Igarzabal, C.I.; Strumia, M.C. Study of the structure/property relationship of nanomaterials for development of novel food packaging. In Food Packaging: Nanotechnology in the Agri-Food Industry, 1st ed.; Grumezescu, A.M., Ed.; Elsevier, Academic Press: Amsterdam, The Netherlands, 2017; pp. 265–294. [Google Scholar]
- Hrnjak-Murgić, Z.; Rešček, A.; Ptiček Siročić, A.; Kratofil Krehula, L.; Katančić, Z. Nanoparticles in Active Polymer Food Packaging, 1st ed.; Smithers Pira: Shawbury, UK, 2015; pp. 1–217. [Google Scholar]
- Riley, F.L. Aluminum Oxide. In Concise Encyclopedia of Advanced Ceramic Materials, 1st ed.; Brook, R.J., Ed.; Elsevier, Pergamon Press: Amsterdam, The Netherlands, 1991; pp. 10–13. [Google Scholar]
- Tammaro, L.; Vittoria, V.; Bugatti, V. Dispersion of modified layered double hydroxides in Poly(ethyleneterephthalate) by High Energy Ball Milling for food packaging applications. Eur. Polym. J. 2014, 52, 172–180. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; De Leon-Zapata, M.A.; Alvarez-Perez, O.B.; Torres-León, C.; Nieto-Oropeza, D.E.; Ventura Sobrevilla, J.M.; Aguilar, M.A.; Ruelas-Chacón, X.; Rojas, R.; Ramos-Aguiñaga, M.E.; et al. Basic and Applied Concepts of Edible Packaging for Foods. In Food Packaging and Preservation, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier, Academic Press: Amsterdam, The Netherlands, 2018; pp. 1–61. [Google Scholar]
- Vytejčková, S.; Vápenka, L.; Hradecký, J.; Dobiáš, J.; Hajšlová, J.; Loriot, C.; Vannini, L.; Poustka, J. Testing of polybutylene succinate based films for poultry meat packaging. Polym. Test. 2017, 60, 357–364. [Google Scholar] [CrossRef]
- Damari, S.P.; Cullari, L.; Nadiv, R.; Nir, Y.; Laredo, D.; Grunlan, J.; Regev, O. Graphene-induced enhancement of water vapor barrier in polymer nanocomposites. Compos. Part B Eng. 2018, 134, 218–224. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A review of the water barrier properties of polymer/clay and polymer/graphene nanocomposites. J. Membr. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Jost, V. Packaging related properties of commercially available biopolymers. An overview of the status quo. Express Polym. Lett. 2018, 12, 429–435. [Google Scholar] [CrossRef]
- Thissen, P.; Grundmeier, G.; Wippermann, S.; Schmidt, W.G. Water adsorption on the α-Al2O3 surface. Phys. Rev. B 2009, 80, 245403. [Google Scholar] [CrossRef]
- Finch, D.S.; Oreskovic, T.; Ramadurai, K.; Herrmann, C.F.; George, S.M.; Mahajan, R.L. Biocompatibility of Atomic Layer-Deposited Alumina Thin Films. J. Biomed. Mater. Res. A 2008, 87A, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirvikorpi, T.; Vaha-Nissi, M.; Harlin, A.; Salomaki, M.; Areva, S.; Korhonen, J.T.; Karppinen, M. Enhanced Water Vapor Barrier Properties for Biopolymer Films by Polyelectrolyte Multilayer and Atomic Layer Deposited Al2O3 double-coating. Appl. Surf. Sci. 2011, 257, 9451–9454. [Google Scholar] [CrossRef]
- Carcia, P.F.; McLean, R.S.; Reilly, M.H.; Groner, M.D.; George, S.M. Ca-tests of Al2O3 Gas Diffusion Barriers Grown By Atomic Layer Deposition on Polymers. Appl. Phys. Lett. 2006, 89, 031915. [Google Scholar] [CrossRef] [Green Version]
- Groner, M.D.; George, S.M.; McLean, R.S.; Carcia, P.F. Gas Diffusion Barriers on Polymers Using Al2O3 Atomic Layer Deposition. Appl. Phys. Lett. 2006, 88, 051907. [Google Scholar] [CrossRef]
- Langereis, E.; Creatore, M.; Heil, S.B.S.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition of Al2O3 Moisture Permeation Barriers on Polymers. Appl. Phys. Lett. 2006, 89, 081915. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vaha-Nissi, M.; Mustonen, T.; Iiskola, E.; Karppinen, M. Atomic Layer Deposited Aluminum Oxide Barrier Coatings for Packaging Materials. Thin Solid Films 2010, 518, 2654–2658. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vaha-Nissi, M.; Harlin, A.; Karppinen, M. Comparison of Some Coating Techniques to Fabricate Barrier Layers on Packaging Materials. Thin Solid Films 2010, 518, 5463–5466. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vaha-Nissi, M.; Harlin, A.; Marles, J.; Miikkulainen, V.; Karppinen, M. Effect of Corona Pre-Treatment on the Performance of Gas Barrier Layers Applied by Atomic Layer Deposition Onto Polymer Coated Paperboard. Appl. Surf. Sci. 2010, 257, 736–740. [Google Scholar] [CrossRef]
- Muller, K.; Jesdinszki, M.; Schmid, M. Modification of functional properties of whey protein isolate nanocomposite films and coatings with nanoclays. J. Nanomater. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lomakin, S.; Brevnov, P.; Koverzanova, E.; Usachev, S.; Shilkina, N.S.; Novokshonova, L.A.; Krasheninnikov, V.; Beriozkina, N.G.; Gajlewicz, I.; Lenartowicz-Klik, M. The effect of graphite nanoplates on the thermal degradation and combustion of polyethylene. J. Anal. Appl. Pyrol. 2017, 128, 275–280. [Google Scholar] [CrossRef]
- Ptiček Siročić, A.; Rešček, A.; Katančić, Z.; Hrnjak-Murgić, Z. Effect of modifiers; casein, zeolite and magnetiteon the properties of bilayer polyethylene/polycaprolactone films. J. Adhes. Sci. Technol. 2020, 34, 2537–2550. [Google Scholar] [CrossRef]
- Rešček, A.; Kratofil Krehula., L.; Katančić, Z.; Hrnjak-Murgić, Z. Active bilayer PE/PCL films for food packaging modified with zinc oxide and casein. Croat. Chem. Acta 2015, 88, 461–473. [Google Scholar] [CrossRef]
- Riley, A. Basics of polymer chemistry for packaging materials. In Packaging Technology Fundamentals, Materials And Processes, 1st ed.; Emblem, A., Emblem, H., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 262–286. [Google Scholar]
- Chrissafis, K.; Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochim. Acta 2011, 523, 1–24. [Google Scholar]
- Grassie, N.; Scott, G. Polymer Degradation and Stabilisation, 1st ed.; Cambridge University Press: Cambridge, UK, 1985; pp. 1–222. [Google Scholar]
- Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part II: Anoverview on thermal decomposition of polycondensation polymers. Thermochim. Acta 2011, 523, 25–45. [Google Scholar]
- Mirhosseini, M.M.; Haddadi-Asl, V.; Khordad, R. Molecular dynamics simulation, synthesis and characterization of polyurethane block polymers containing PTHF/PCL mixture as a soft segment. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Jianxiang, C.; Yuankun, W.; Zeren, Y.; Kam, C.T.; Defeng, W. Morphology and mechanical properties of poly(β-hydroxybutyrate)/poly(ε-caprolactone) blends controlled with cellulosic particles. Carbohydr. Polym. 2017, 174, 217–225. [Google Scholar]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Preparation and evaluation of nanocomposite LDPE films containing Ag and ZnO for food packaging applications. Adv. Mater. Res. 2010, 129–131, 1228–1232. [Google Scholar] [CrossRef]
- Conti, M.E. Heavy metals in food packagings. In Mineral Components in Food, 1st ed.; Nriagu, J., Szefer, P., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 339–362. [Google Scholar]
- Rešček, A.; Katančić, Z.; Kratofil Krehula, L.; Ščetar, M.; Hrnjak-Murgić, Z.; Galić, K. Development of Double-Layered PE/PCL Films for Food Packaging Modified with Zeolite and Magnetite. Adv. Polym. Technol. 2018, 37, 837–842. [Google Scholar] [CrossRef]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [Green Version]
- Canillas, M.; de Lima, G.G.; Rodriguez, M.A.; Nugent, M.J.D.; Devine, D.M. Bioactive composites fabricated by freezing-thawing method for bone regeneration applications. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 761–773. [Google Scholar] [CrossRef]
- Rešček, A.; Ščetar, M.; Hrnjak-Murgić, Z.; Dimitrov, N.; Galić, K. Polyethylene/Polycaprolatone Nanocomposite Films for Food Packaging Modified with Magnetite and Casein: Oxygen Barrier, Mechanical and Thermal Properties. Development of Double-Layered PE/PCL Films for Food Packaging Modified with Zeolite and Magnetite. Polym. Plast. Technol. Eng. 2016, 55, 1450–1459. [Google Scholar] [CrossRef]
- Naayi, S.A.; Hassan, A.I.; Salim, E.T. FTIR and X-ray Diffraction Analysis of Al2O3 Nanostructured Thin Film Prepared at Low Temperature Using Spray Pyrolysis Method. Int. J. Nanoelectron. Mater. 2018, 11, 1–6. [Google Scholar]
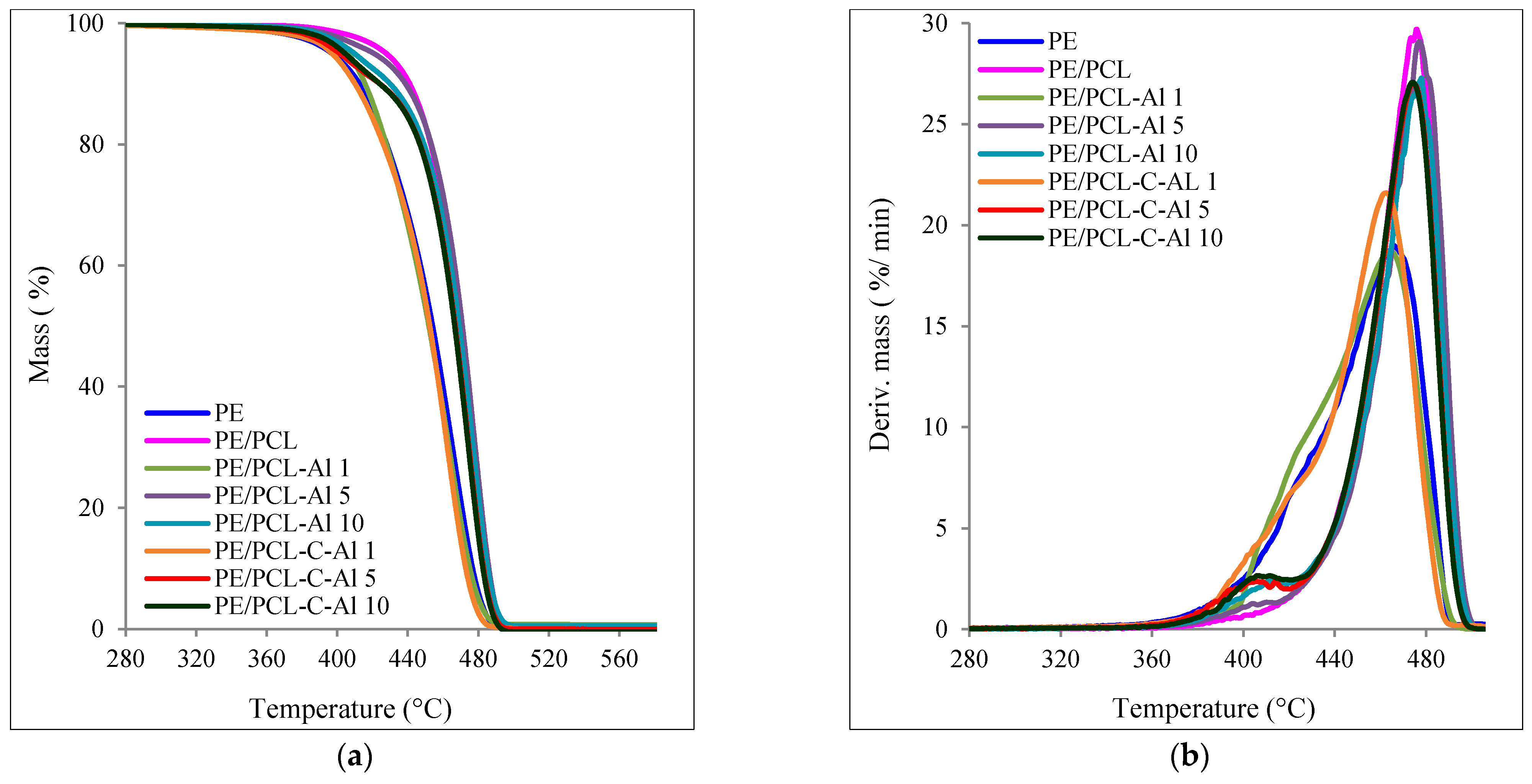


| Sample | T95 (°C) | Tmax1 (°C) | Tmax2 (°C) | mr550 °C (%) | vmax (%/min) |
|---|---|---|---|---|---|
| PE | 397.1 | 415–430 | 465.9 | 0.06 | 19.0 |
| PE/PCL | 427.9 | – | 475.1 | 0.01 | 29.3 |
| PE/PCL-Al 1 | 406.5 | 398–435 | 464.8 | 0.72 | 11.3 |
| PE/PCL-Al 5 | 421.5 | 385–425 | 477.1 | 0.83 | 29.1 |
| PE/PCL-Al 10 | 409.9 | 385–425 | 477.9 | 0.54 | 27.3 |
| PE/PCL-C-Al 1 | 397.4 | 385–427 | 463.1 | 0.14 | 21.6 |
| PE/PCL-C-Al 5 | 401.0 | 385–425 | 475.6 | 0.01 | 26.9 |
| PE/PCL-C-Al 10 | 404.5 | 385–425 | 474.8 | 0.50 | 27.0 |
| Sample | C | Al |
|---|---|---|
| C-Al 1 | 99.0 | 1.0 |
| C-Al 5 | 95.0 | 5.0 |
| C-Al 10 | 90.0 | 10.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptiček Siročić, A.; Rešček, A.; Katančić, Z.; Hrnjak-Murgić, Z. Development of PE/PCL Bilayer Films Modified with Casein and Aluminum Oxide. Molecules 2021, 26, 3090. https://doi.org/10.3390/molecules26113090
Ptiček Siročić A, Rešček A, Katančić Z, Hrnjak-Murgić Z. Development of PE/PCL Bilayer Films Modified with Casein and Aluminum Oxide. Molecules. 2021; 26(11):3090. https://doi.org/10.3390/molecules26113090
Chicago/Turabian StylePtiček Siročić, Anita, Ana Rešček, Zvonimir Katančić, and Zlata Hrnjak-Murgić. 2021. "Development of PE/PCL Bilayer Films Modified with Casein and Aluminum Oxide" Molecules 26, no. 11: 3090. https://doi.org/10.3390/molecules26113090
APA StylePtiček Siročić, A., Rešček, A., Katančić, Z., & Hrnjak-Murgić, Z. (2021). Development of PE/PCL Bilayer Films Modified with Casein and Aluminum Oxide. Molecules, 26(11), 3090. https://doi.org/10.3390/molecules26113090






