Recent Progress in Transdermal Nanocarriers and Their Surface Modifications
Abstract
1. Introduction
2. Overview of Transdermal Drug Nanocarriers
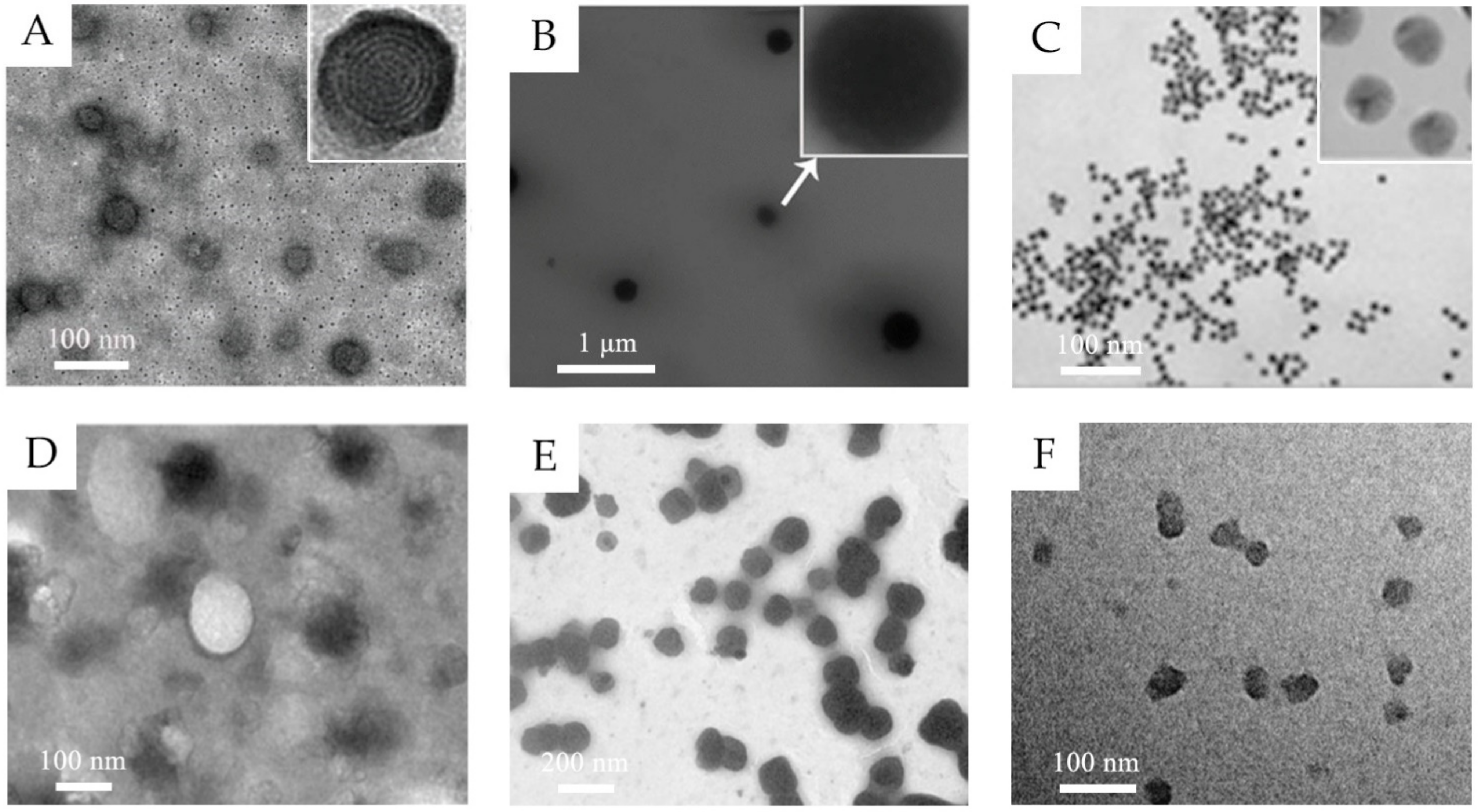
2.1. Lipid-Based Nanovesicles
2.2. Lipid Nanoparticles
2.3. Polymeric Nanoparticles
2.4. Inorganic Nanoparticles
2.5. Other Nanocarriers
3. Surface Modifications and Their Functions
3.1. Enhanced Penetration Efficiency
3.2. Controlled Release
3.3. Targeting Drug Delivery
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J. Microneedle Patches as Drug and Vaccine Delivery Platform. Curr. Med. Chem. 2017, 24, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Marepally, S.; Vemula, P.; Xu, C. Inorganic Nanoparticles for Transdermal Drug Delivery and Topical Application. In Nanoscience in Dermatology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–72. [Google Scholar]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Zhang, Y.; Zhou, J.; Sun, S.; Liu, Y. H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J. Mater. Chem. B 2017, 5, 8200–8208. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, C.; MacNeil, S.; Battaglia, G. Transdermal drug delivery: From micro to nano. Nanoscale 2012, 4, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Seah, B.C.; Teo, B.M. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Verma, S.; Singh, M.; Chalotra, T.; Utreja, P. Advanced Drug Delivery Systems for Transdermal Delivery of Non-Steroidal Anti-Inflammatory Drugs: A Review. Curr. Drug Deliv. 2018, 15, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Chen, Y.; Li, L.; Lan, P.; He, D.; Song, J.; Zhang, Y. Transdermal Delivery of 5-Aminolevulinic Acid by Nanoethosome Gels for Photodynamic Therapy of Hypertrophic Scars. ACS Appl. Mater. Interfaces 2019, 11, 3704–3714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Xu, H.; Wo, Y.; Zhang, Z.; Liu, Y.; Su, W.; Cui, D.; Zhang, Y. 5-Aminolevulinic acid loaded ethosomal vesicles with high entrapment efficiency for in vitro topical transdermal delivery and photodynamic therapy of hypertrophic scars. Nanoscale 2016, 8, 19270–19279. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Xin, Y.; Yu, Z.; Meng, X.; Zhang, Y.; He, D.; Zhang, Y. Functional Transdermal Nanoethosomes Enhance Photodynamic Therapy of Hypertrophic Scars via Self-Generating Oxygen. ACS Appl. Mater. Interfaces 2021, 13, 7955–7965. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Yanagi, M.; Hamada, H. Physical Enhancement? Nanocarrier? Current Progress in Transdermal Drug Delivery. Nanomaterials 2021, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yang, M.; Tang, X.; Wang, T.; Yang, D.; Zhai, G.; Liu, J. Lipid nanoparticles loading triptolide for transdermal delivery: Mechanisms of penetration enhancement and transport properties. J. Nanobiotechnol. 2018, 16, 68. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Gao, J.; Zhang, Z.; Wang, L.; Chen, X.; Mi, J.; Yao, Y.; Guan, D.; Chen, B.; et al. Transdermal Vascular Endothelial Growth Factor Delivery with Surface Engineered Gold Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 5173–5180. [Google Scholar] [CrossRef]
- Takeuchi, I.; Shimamura, Y.; Kakami, Y.; Kameda, T.; Hattori, K.; Miura, S.; Shirai, H.; Okumura, M.; Inagi, T.; Terada, H.; et al. Transdermal delivery of 40-nm silk fibroin nanoparticles. Colloids Surf. B Biointerfaces 2019, 175, 564–568. [Google Scholar] [CrossRef]
- Shakeel, F.; Ramadan, W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloids Surf. B Biointerfaces 2010, 75, 356–362. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Yao, X.; Zhang, Y.; Wu, W.; Jiang, X. Hyaluronic acid nanogels with enzyme-sensitive cross-linking group for drug delivery. J. Control. Release 2015, 205, 206–217. [Google Scholar] [CrossRef]
- Siler-Marinkovic, S. Liposomes as Drug Delivery Systems in Dermal and Transdermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; J.B. Metzler: Stuttgart, Germany, 2016; pp. 15–38. [Google Scholar]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes - novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef]
- Babaie, S.; Bakhshayesh, A.R.D.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A Novel Nanocarrier for Transdermal Drug Delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-formulations for transdermal drug delivery: A review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Takeuchi, I.; Takeshita, T.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for positively charged drugs. Colloids Surf. B Biointerfaces 2017, 160, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Sano, A.; Ohshima, H.; Terada, H.; Makino, K. In vitro permeation of gold nanoparticles through rat skin and rat intestine: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Giljohann, D.A.; Chen, D.L.; Massich, M.D.; Wang, X.Q.; Iordanov, H.; Mirkin, C.A.; Paller, A.S. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc. Natl. Acad. Sci. USA 2012, 109, 11975–11980. [Google Scholar] [CrossRef]
- Rao, Y.F.; Chen, W.; Liang, X.G.; Huang, Y.Z.; Miao, J.; Liu, L.; Lou, Y.; Zhang, X.G.; Wang, B.; Tang, R.K.; et al. Epirubicin-loaded superparamagnetic iron-oxide nanoparticles for transdermal delivery: Cancer therapy by circumventing the skin barrier. Small 2015, 11, 239–247. [Google Scholar] [CrossRef]
- Ramadan, S.; Guo, L.; Li, Y.; Yan, B.; Lu, W. Hollow copper sulfide nanoparticle-mediated transdermal drug delivery. Small 2012, 8, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Venuganti, V.V.; Perumal, O.P. Poly(amidoamine) dendrimers as skin penetration enhancers: Influence of charge, generation, and concentration. J. Pharm. Sci. 2009, 98, 2345–2356. [Google Scholar] [CrossRef]
- Atanasova, D.; Staneva, D.; Grabchev, I. Textile Materials Modified with Stimuli-Responsive Drug Carrier for Skin Topical and Transdermal Delivery. Materials 2021, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Möller, M.; Kalia, Y.N. Polymeric Micelles in Dermal and Transdermal Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; J.B. Metzler: Stuttgart, Germany, 2016; pp. 223–240. [Google Scholar]
- Yotsumoto, K.; Ishii, K.; Kokubo, M.; Yasuoka, S. Improvement of the skin penetration of hydrophobic drugs by polymeric micelles. Int. J. Pharm. 2018, 553, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 509–533. [Google Scholar] [CrossRef]
- Sabitha, M.; Sanoj Rejinold, N.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Development and evaluation of 5-fluorouracil loaded chitin nanogels for treatment of skin cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid vesicles: A versatile drug delivery platform for dermal and transdermal applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef]
- Natsheh, H.; Touitou, E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Zhang, Z.; Tsai, P.C.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef]
- Fathi, M.; Sahandi Zangabad, P.; Majidi, S.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Stimuli-responsive chitosan-based nanocarriers for cancer therapy. Bioimpacts 2017, 7, 269–277. [Google Scholar] [CrossRef]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Colloids Surf. B Biointerfaces 2011, 88, 706–710. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Delivery. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Al-Dhfyan, A.; Alanazi, F.K.; Alsarra, I.A. Chemoprevention of skin cancer using low HLB surfactant nanoemulsion of 5-fluorouracil: A preliminary study. Drug Deliv. 2015, 22, 573–580. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Pando, D.; Matos, M.; Gutierrez, G.; Pazos, C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf. B Biointerfaces 2015, 128, 398–404. [Google Scholar] [CrossRef]
- Shah, P.P.; Desai, P.R.; Singh, M. Effect of oleic acid modified polymeric bilayered nanoparticles on percutaneous delivery of spantide II and ketoprofen. J. Control. Release 2012, 158, 336–345. [Google Scholar] [CrossRef]
- Abdelgawad, R.; Nasr, M.; Moftah, N.H.; Hamza, M.Y. Phospholipid membrane tubulation using ceramide doping “Cerosomes”: Characterization and clinical application in psoriasis treatment. Eur. J. Pharm. Sci. 2017, 101, 258–268. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Mahmoud, N.N.; Ibrahim, L.H.; Al-Dabash, S.; Raschke, H.; Hergenroder, R. Tuning the Surface Chemistry of Melanin-Mimetic Polydopamine Nanoparticles Drastically Enhances Their Accumulation into Excised Human Skin. ACS Biomater. Sci. Eng. 2020, 6, 4424–4432. [Google Scholar] [CrossRef]
- Hiranphinyophat, S.; Otaka, A.; Asaumi, Y.; Fujii, S.; Iwasaki, Y. Particle-stabilized oil-in-water emulsions as a platform for topical lipophilic drug delivery. Colloids Surf. B Biointerfaces 2021, 197, 111423. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kim, S.Y.; Kong, B.J.; Kim, K.J.; Noh, G.Y.; Im, N.R.; Lim, J.W.; Ha, J.H.; Kim, J.; Park, S.N. Cell penetrating peptide conjugated liposomes as transdermal delivery system of Polygonum aviculare L. extract. Int. J. Pharm. 2015, 483, 26–37. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, D.; Niu, J.; Wang, H.; Wang, L.; Li, Q.; Li, C.; Song, H.; Chang, J.; Zhang, L. Development of an efficient transdermal drug delivery system with TAT-conjugated cationic polymeric lipid vesicles. J. Mater. Chem. B 2014, 2, 877–884. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, T.; Li, T.; Ma, Y.; Shen, S.; He, B.; Mo, R. Enhanced Transdermal Drug Delivery by Transfersome-Embedded Oligopeptide Hydrogel for Topical Chemotherapy of Melanoma. ACS Nano 2018, 12, 9693–9701. [Google Scholar] [CrossRef]
- Silva, C.O.; Rijo, P.; Molpeceres, J.; Figueiredo, I.V.; Ascensao, L.; Fernandes, A.S.; Roberto, A.; Reis, C.P. Polymeric nanoparticles modified with fatty acids encapsulating betamethasone for anti-inflammatory treatment. Int. J. Pharm. 2015, 493, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Pashirova, T.N.; Sapunova, A.S.; Lukashenko, S.S.; Burilova, E.A.; Lubina, A.P.; Shaihutdinova, Z.M.; Gerasimova, T.P.; Kovalenko, V.I.; Voloshina, A.D.; Souto, E.B.; et al. Synthesis, structure-activity relationship and biological evaluation of tetracationic gemini Dabco-surfactants for transdermal liposomal formulations. Int. J. Pharm. 2020, 575, 118953. [Google Scholar] [CrossRef] [PubMed]
- Shekh, M.I.; Amirian, J.; Stadler, F.J.; Du, B.; Zhu, Y. Oxidized chitosan modified electrospun scaffolds for controllable release of acyclovir. Int. J. Biol. Macromol. 2020, 151, 787–796. [Google Scholar] [CrossRef]
- Fujimoto, T.; Ito, M.; Ito, S.; Kanazawa, H. Fractional laser-assisted percutaneous drug delivery via temperature-responsive liposomes. J. Biomater. Sci. Polym. Ed. 2017, 28, 679–689. [Google Scholar] [CrossRef]
- Rancan, F.; Giulbudagian, M.; Jurisch, J.; Blume-Peytavi, U.; Calderon, M.; Vogt, A. Drug delivery across intact and disrupted skin barrier: Identification of cell populations interacting with penetrated thermoresponsive nanogels. Eur. J. Pharm. Biopharm. 2017, 116, 4–11. [Google Scholar] [CrossRef]
- Zhang, K.; Zhuang, Y.; Zhang, W.; Guo, Y.; Liu, X. Functionalized MoS2-nanoparticles for transdermal drug delivery of atenolol. Drug Deliv. 2020, 27, 909–916. [Google Scholar] [CrossRef]
- Kong, M.; Park, H.; Feng, C.; Hou, L.; Cheng, X.; Chen, X. Construction of hyaluronic acid noisome as functional transdermal nanocarrier for tumor therapy. Carbohydr. Polym. 2013, 94, 634–641. [Google Scholar] [CrossRef]
- Beack, S.; Kong, W.H.; Jung, H.S.; Do, I.H.; Han, S.; Kim, H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Photodynamic therapy of melanoma skin cancer using carbon dot–chlorin e6–hyaluronate conjugate. Acta Biomater. 2015, 26, 295–305. [Google Scholar] [CrossRef]
- Ruan, R.; Chen, M.; Sun, S.; Wei, P.; Zou, L.; Liu, J.; Gao, D.; Wen, L.; Ding, W. Topical and Targeted Delivery of siRNAs to Melanoma Cells Using a Fusion Peptide Carrier. Sci. Rep. 2016, 6, 29159. [Google Scholar] [CrossRef]
- Huang, X.; Chen, C.; Zhu, X.; Zheng, X.; Li, S.; Gong, X.; Xiao, Z.; Jiang, N.; Yu, C.; Yi, C. Transdermal BQ-788/EA@ZnO quantum dots as targeting and smart tyrosinase inhibitors in melanocytes. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 45–52. [Google Scholar] [CrossRef]
- Gu, T.W.; Wang, M.Z.; Niu, J.; Chu, Y.; Guo, K.R.; Peng, L.H. Outer membrane vesicles derived from E. coli as novel vehicles for transdermal and tumor targeting delivery. Nanoscale 2020, 12, 18965–18977. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J. Recent advances in follicular drug delivery of nanoparticles. Expert Opin. Drug Deliv. 2020, 17, 49–60. [Google Scholar] [CrossRef]
- Valjakka-Koskela, R.; Hirvonen, J.; Monkkonen, J.; Kiesvaara, J.; Antila, S.; Lehtonen, L.; Urtti, A. Transdermal delivery of levosimendan. Eur. J. Pharm. Sci. 2000, 11, 343–350. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Al-Qaoud, K.M.; Al-Bakri, A.G.; Alkilany, A.M.; Khalil, E.A. Colloidal stability of gold nanorod solution upon exposure to excised human skin: Effect of surface chemistry and protein adsorption. Int. J. Biochem. Cell Biol. 2016, 75, 223–231. [Google Scholar] [CrossRef]
- Nasrollahi, S.A.; Taghibiglou, C.; Azizi, E.; Farboud, E.S. Cell-penetrating peptides as a novel transdermal drug delivery system. Chem. Biol. Drug Des. 2012, 80, 639–646. [Google Scholar] [CrossRef]
- Gao, S.; Tian, B.; Han, J.; Zhang, J.; Shi, Y.; Lv, Q.; Li, K. Enhanced transdermal delivery of lornoxicam by nanostructured lipid carrier gels modified with polyarginine peptide for treatment of carrageenan-induced rat paw edema. Int. J. Nanomed. 2019, 14, 6135–6150. [Google Scholar] [CrossRef]
- Ference, J.D.; Last, A.R. Choosing topical corticosteroids. Am. Fam. Physician 2009, 79, 135–140. [Google Scholar]
- Kebebe, D.; Liu, Y.; Wu, Y.; Vilakhamxay, M.; Liu, Z.; Li, J. Tumor-targeting delivery of herb-based drugs with cell-penetrating/tumor-targeting peptide-modified nanocarriers. Int. J. Nanomed. 2018, 13, 1425–1442. [Google Scholar] [CrossRef]
- Lestini, B.J.; Sagnella, S.M.; Xu, Z.; Shive, M.S.; Richter, N.J.; Jayaseharan, J.; Case, A.J.; Kottke-Marchant, K.; Anderson, J.M.; Marchant, R.E. Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J. Control. Release 2002, 78, 235–247. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chung, H.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 2010, 31, 106–114. [Google Scholar] [CrossRef] [PubMed]
| Classification | Typical Components | Structure | Transdermal Delivery Mechanism | Ref. | |
|---|---|---|---|---|---|
| Lipid-based nanovesicles | Liposomes | Phospholipid, cholesterol | Spherical vesicles with one or more lipid bilayers and aqueous inner core | The phospholipid component interacts with the lipids of the SC. | [16] |
| Ethosomes | Phospholipid, ethanol (high concentration up to 20–50% w/w) | Ethanol increases the fluidity of phospholipid bilayers and disrupts the membrane barrier of SC. | [17] | ||
| Transfersomes | Phospholipid, edge activators | The edge activators increase the flexibility and deformability for passing through the narrow pores. | [18] | ||
| Niosomes | Nonionic surfactants, cholesterol | Similar to liposome The nonionic surfactants enhance the drug encapsulation efficiency. | [19] | ||
| Glycerosomes | Phospholipid, glycerol, cholesterol | Similar to transfersome The glycerol improves the elasticity and deformability. | [20] | ||
| Invasomes | Phospholipid, ethanol (low concentration as 3% w/w), terpenes | Ethanol and terpenes disrupt the SC lipid structure and increase the membrane elasticity. | [21] | ||
| Lipid nanoparticles | Solid lipid nanoparticles | Solid lipids | Solid particles with non-aqueous core | The lipid nanoparticles form a mono-layer lipid film to enlarge the inter-keratinocyte gap. The lipid components and incorporated surfactant could disrupt skin structure and increase the intercellular space. | [11,22] |
| Nanostructured lipid carriers | Solid and liquid lipids | ||||
| Polymeric nanoparticles | Natural polymers and synthetic polymers | Solid colloidal carriers | They create a drug concentration gradient to enhance the drug permeation. Some small or positively charged polymeric nanoparticles can penetrate through epidermal barrier through paracellular route and hair-follicle route. | [13,22,23] | |
| Inorganic nanoparticles | Gold nanoparticles | Au | Solid and rigid particles | Some small nanoparticles can penetrate the skin through the lipidic matrix of the stratum corneum and through hair follicle orifices. | [24,25] |
| Fe3O4 nanoparticles | Fe3O4 | They penetrate into deeper dermis via transfollicular route. | [26] | ||
| CuS nanoparticles | CuS | The near-infrared absorption induces the localized thermal ablation of SC and facilitate the penetration. | [27] | ||
| Dendrimers | Poly (amidoamine) and other polymers | High-branched polymeric nanocarriers | Serving as penetration enhancer through interaction with skin lipid bilayers | [28] | |
| Micelles | Amphiphilic polymers, surfactants | Spherical or irregular monolayer structure | Improving the water solubility of drugs | [29,30,31] | |
| Nanoemulsions | Water, oil, surfactants | Dispersions of water and oil | Disrupting the skin lipid bilayers; Increasing solubility for both liposoluble and water-soluble drugs | [22] | |
| Nanogels | Polymers | Cross-linked network structure | The nanocarrier dispersions prolong the topical contact duration and increase localized drug concentration. Cationically charged nanogels interact with epidermis. | [32,33,34] | |
| Functions | Surface Modifier | Nanocarrier | Achieved Improvement | Ref. | ||
|---|---|---|---|---|---|---|
| Enhanced penetration efficiency | Oleic acid | Niosomes; polymeric nanoparticles | Increasing penetration depth | [46,47] | ||
| Ceramides | Vesicular phospholipid system | Increasing drug deposition in the skin | [48] | |||
| Polymers | PEG | PDA NPs | Preventing aggregation; Increasing penetration depth | [49] | ||
| PIPP | Cellulose nanocrystal (CNC)-stabilized emulsions | Increasing surface hydrophobicity and stability; Increasing penetration depth | [50] | |||
| Cell-penetrating peptide | Lipid-based vesicles | Increasing cell uptake and internalization; Improving skin penetration and permeation | [51,52,53] | |||
| Controlled release | Sustained drug release | Oleic acid | Polymeric nanoparticles | Controlled release lasting 72 h | [54] | |
| Dabco surfactants | Liposomal system | Sustained release of 50% loaded drug in 12 h | [55] | |||
| Oxidized chitosan | Nanofibers | Sustained release of 50% loaded drug in 9 h | [56] | |||
| Activated modulated release | Thermosensitive poly-N-isopropylacrylamide (PNIPAAm) | Liposomes | Drug release and skin permeation begin from 37 °C | [57] | ||
| Thermosensitive polyglycerol (tPG) | Nanogels | Drug release and skin permeation begin from 40 °C | [58] | |||
| Cationic hydroxyethyl cellulose (JR400) | MoS2 nanoparticles | Irritated by near-infrared 808 nm laser | [59] | |||
| Targeting drug delivery | Hyaluronic acid and derivatives | Niosomes; Cdot-Ce6 | Cancer cell targeting | [60,61] | ||
| Epidermal growth factor | Fusion peptide carrier | Melanoma cell targeting | [62] | |||
| BQ-788 (endothelin ETB receptor ligand) | ZnO quantum dots | Melanocyte targeting | [63] | |||
| Integrin αvβ3 ligand | Escherichia coli derived outer membrane vesicles | Melanoma cell targeting | [64] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Meng, X.; Zhang, S.; Chen, Y.; Zhang, Z.; Zhang, Y. Recent Progress in Transdermal Nanocarriers and Their Surface Modifications. Molecules 2021, 26, 3093. https://doi.org/10.3390/molecules26113093
Yu Z, Meng X, Zhang S, Chen Y, Zhang Z, Zhang Y. Recent Progress in Transdermal Nanocarriers and Their Surface Modifications. Molecules. 2021; 26(11):3093. https://doi.org/10.3390/molecules26113093
Chicago/Turabian StyleYu, Zhixi, Xinxian Meng, Shunuo Zhang, Yunsheng Chen, Zheng Zhang, and Yixin Zhang. 2021. "Recent Progress in Transdermal Nanocarriers and Their Surface Modifications" Molecules 26, no. 11: 3093. https://doi.org/10.3390/molecules26113093
APA StyleYu, Z., Meng, X., Zhang, S., Chen, Y., Zhang, Z., & Zhang, Y. (2021). Recent Progress in Transdermal Nanocarriers and Their Surface Modifications. Molecules, 26(11), 3093. https://doi.org/10.3390/molecules26113093






