Blockade of NF-κB Translocation and of RANKL/RANK Interaction Decreases the Frequency of Th2 and Th17 Cells Capable of IL-4 and IL-17 Production, Respectively, in a Mouse Model of Allergic Asthma
Abstract
:1. Introduction
2. Results
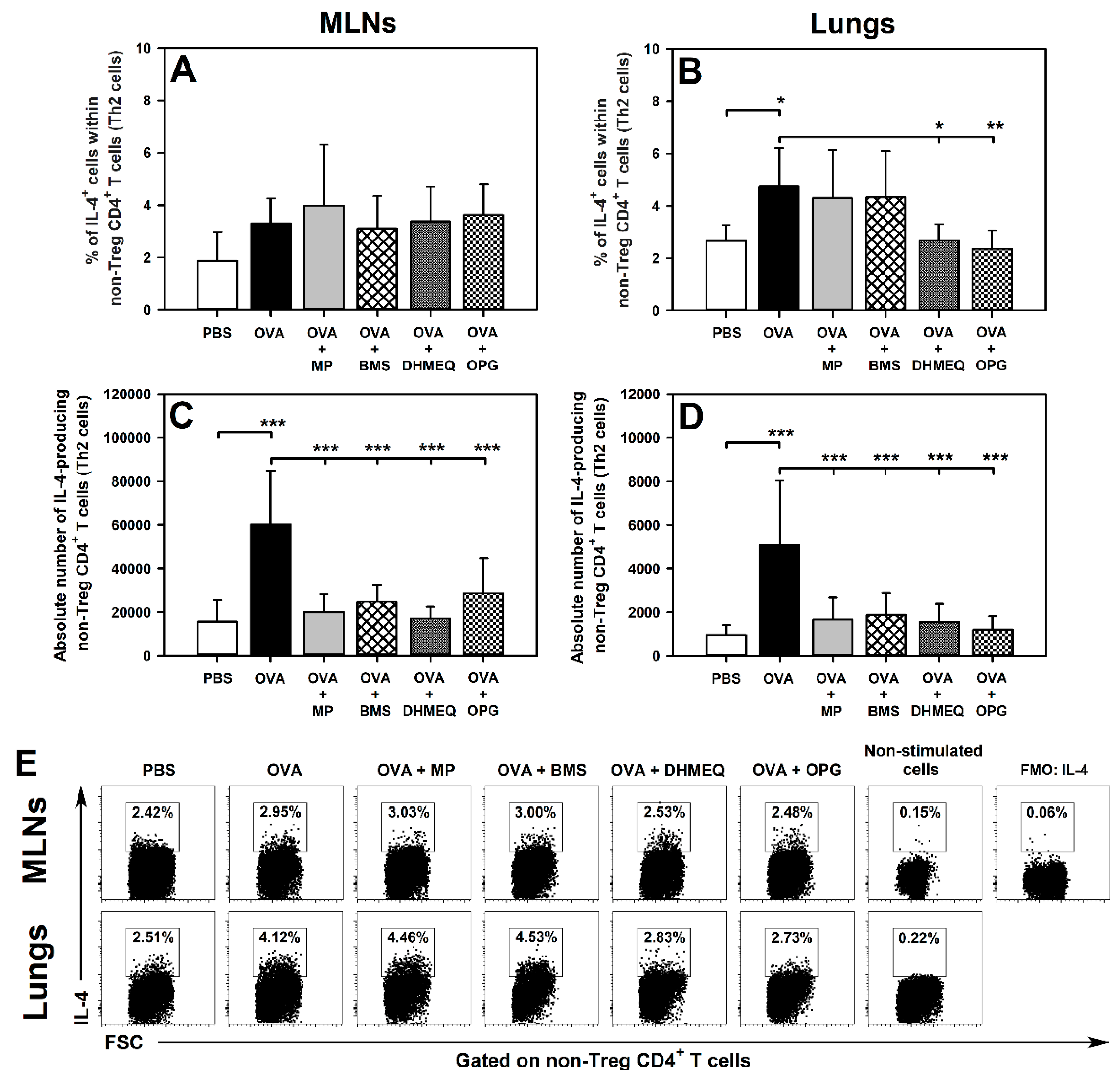
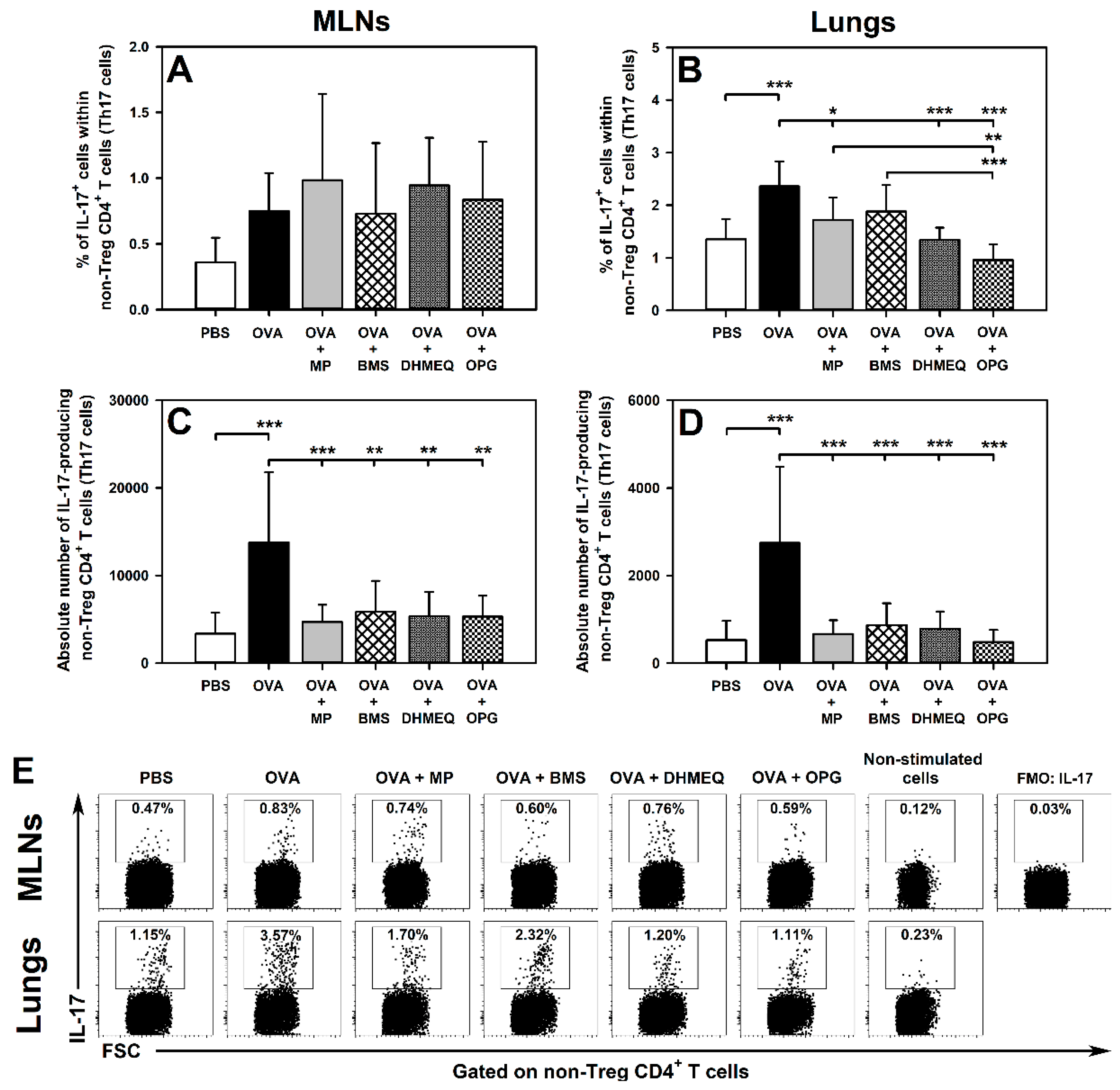
2.1. DHMEQ and OPG Prevent OVA-Induced Increase in the Relative and Absolute Counts of IL-4-, IL-10- and IL-17-Producing Non-Treg CD4+ T Cells in the Lungs and Administration of BMS Prevents the Absolute, but Not Relative, Increase in the Number of These Cells
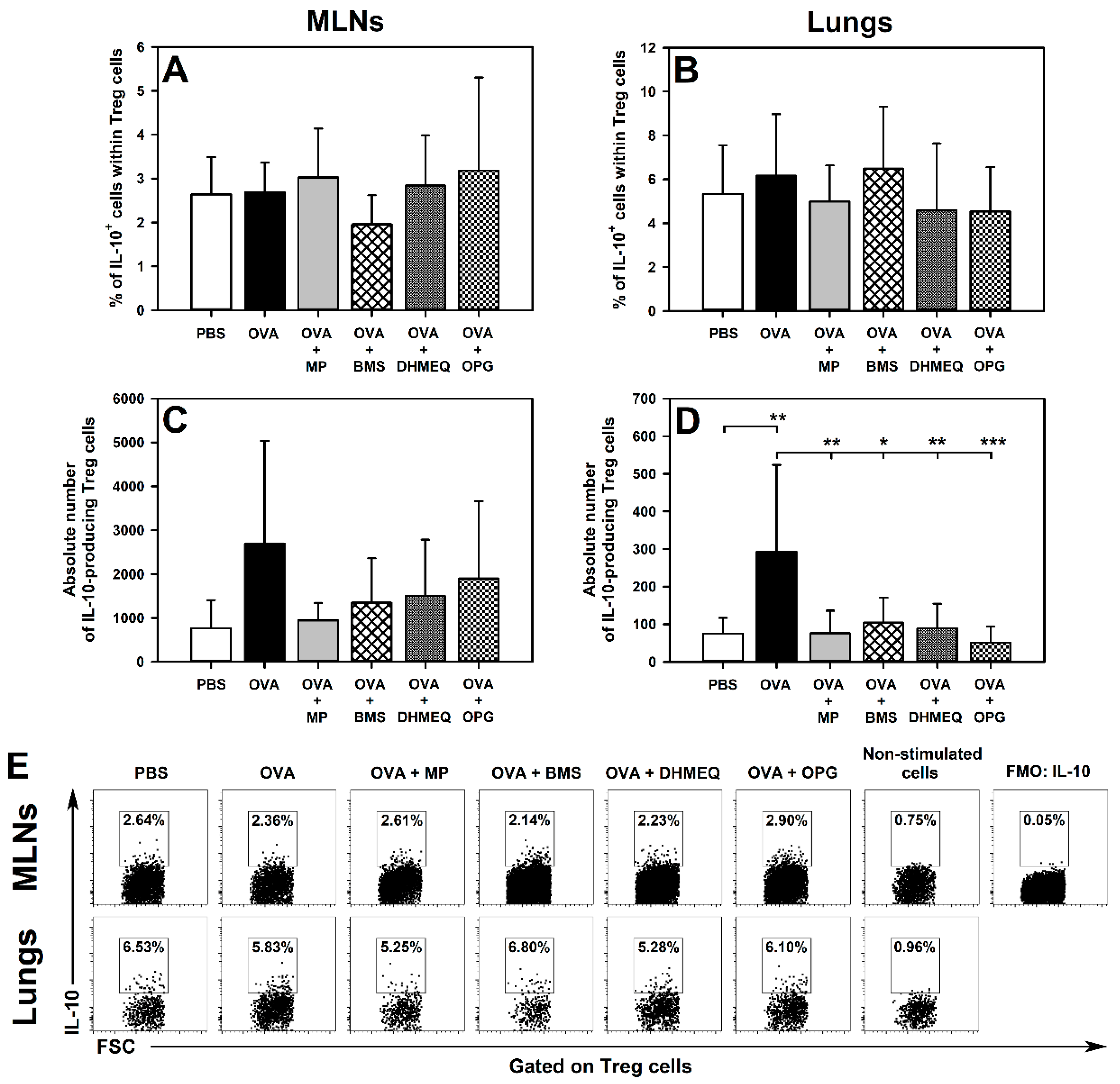
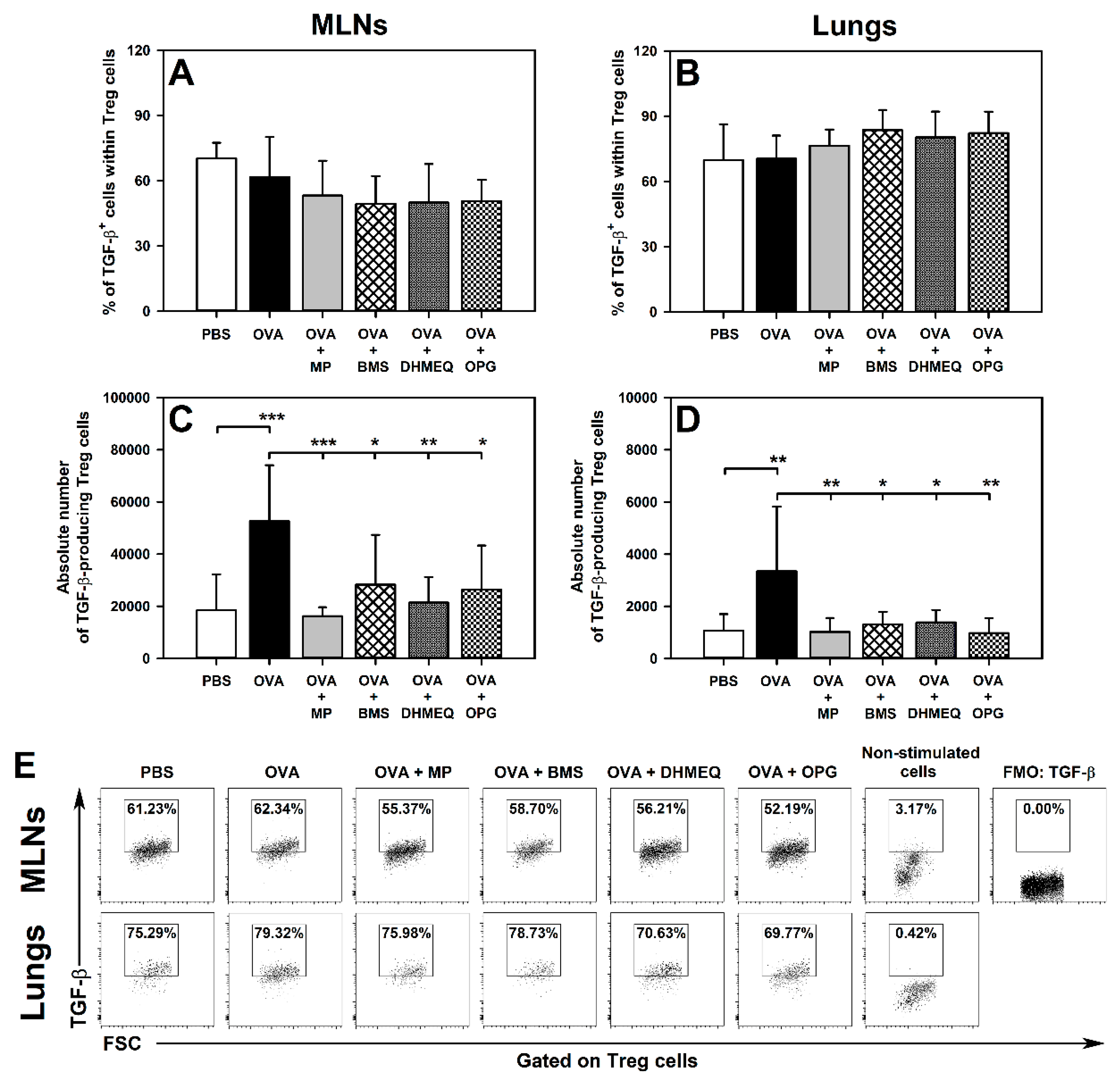
2.2. BMS, DHMEQ and OPG Prevent OVA-Induced Increase in the Absolute Counts of IL-10- and TGF-β-Producing Treg Cells in the Lungs
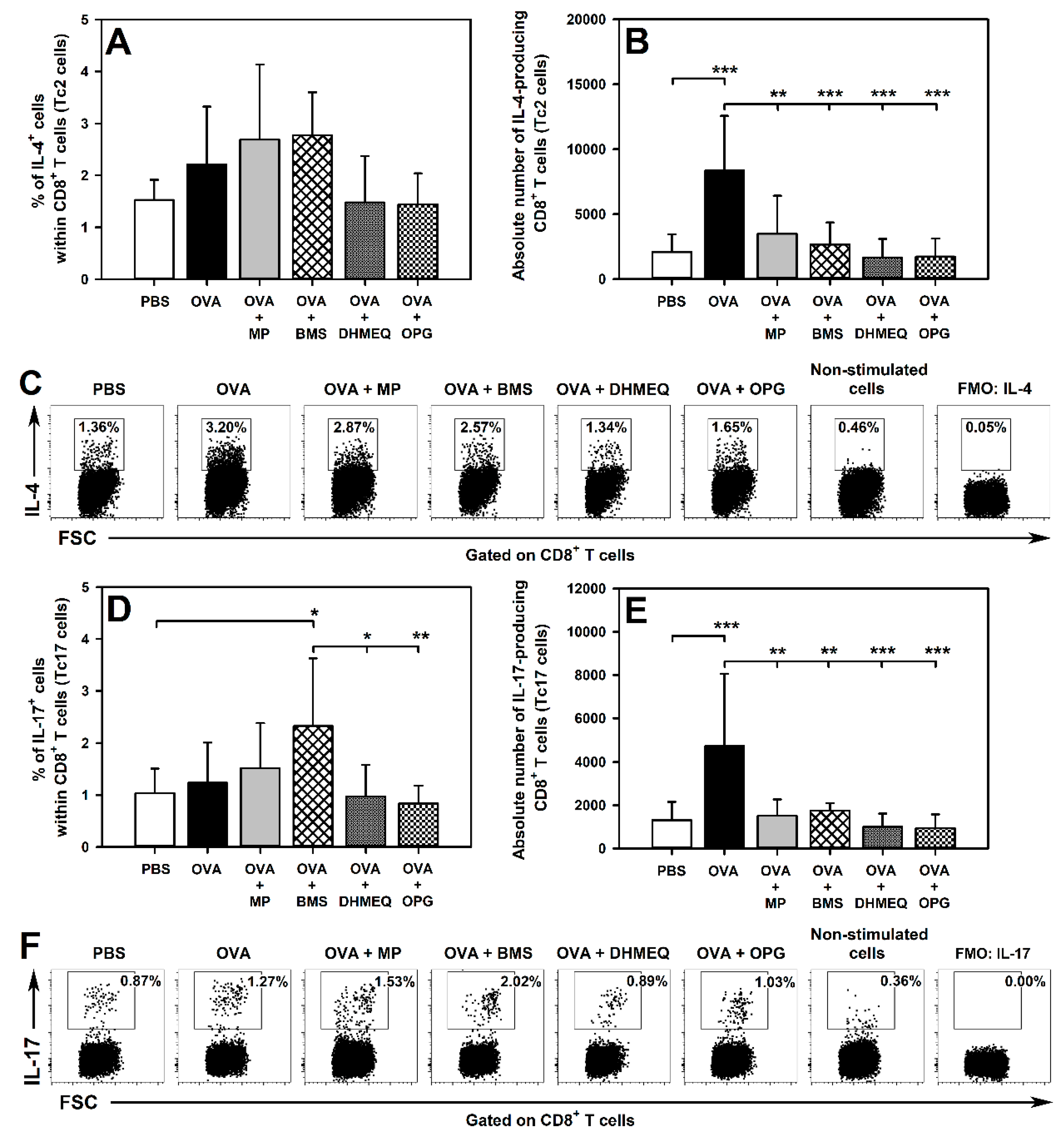
2.3. BMS, DHMEQ and OPG Prevent OVA-Induced Increase in the Absolute Counts of IL-4- and IL-17-Producing CD8+ T Cells in the Lungs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Antigen Immunization, Airway Challenge and Treatment Protocol
4.3. Isolation of Inductive and Effector Site Lymphocytes and Culture Conditions
4.3.1. MLNs
4.3.2. Lungs
4.3.3. Ex Vivo Stimulation of Cytokine Production
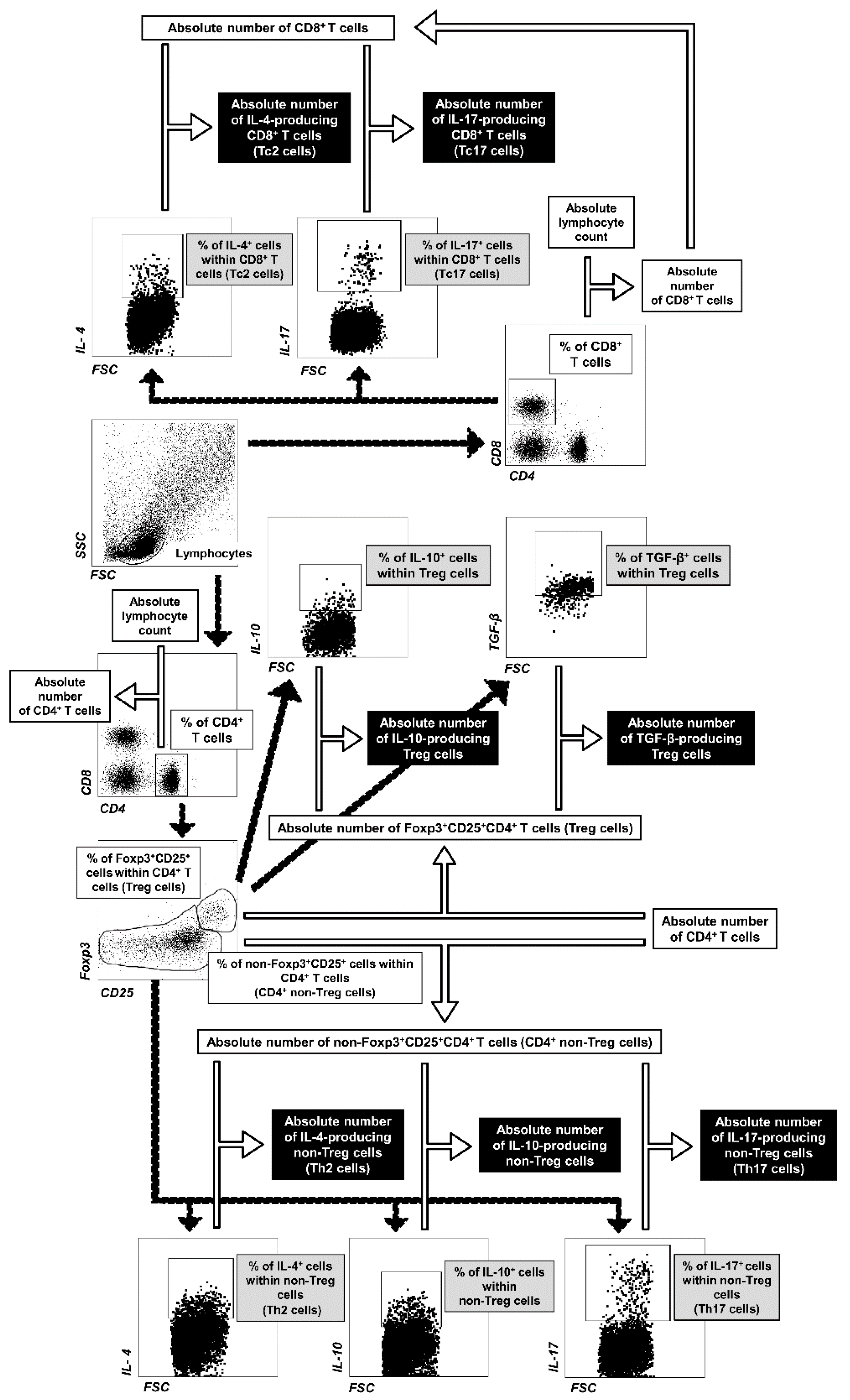
4.4. Flow Cytometry
4.4.1. Extracellular Staining
4.4.2. Intracellular Staining
4.5. FACS Acquisition and Data Analysis
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Adcock, I.M.; Ford, P.A.; Bhavsar, P.; Ahmad, T.; Chung, K.F. Steroid resistance in asthma: Mechanisms and treatment options. Curr. Allergy Asthma Rep. 2008, 8, 171–178. [Google Scholar] [CrossRef]
- Edwards, M.R.; Bartlett, N.W.; Clarke, D.; Birrell, M.; Belvisi, M.; Johnston, S.L. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol. Ther. 2009, 121, 1–13. [Google Scholar] [CrossRef]
- Cheng, M.L.; Fong, L. Effects of RANKL-targeted therapy in immunity and cancer. Front. Oncol. 2014, 3, 329. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Schwarz, E.M.; O’Keefe, R.J.; Ma, L.; Boyce, B.F.; Xing, L. RANK signalling is not required for TNF alpha-mediated increase in CD11hi osteoclast precursors but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. J. Bone Miner. Res. 2004, 19, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Seshasayee, D.; Wang, H.; Lee, W.P.; Gribling, P.; Ross, J.; Van Bruggen, N.; Carano, R.; Grewal, I.S. A novelin vivorole for osteoprotegerin ligand in activation of monocyte effector function and inflammatory response. J. Biol. Chem. 2004, 279, 30202–30209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorczyk, I.; Maślanka, T. Blockade of RANKL/RANK and NF-ĸB signalling pathways as novel therapeutic strategies for allergic asthma: A comparative study in a mouse model of allergic airway inflammation. Eur. J. Pharmacol. 2020, 879, 173129. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M.; Giaisi, M.; Baumann, S.; Pálfi, K.; Krammer, P.H. NF-kappa B synergizes with NF-AT and NF-IL6 in activation of the IL-4 gene in T cells. Eur. J. Immunol. 2004, 34, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Molinero, L.L.; Cubre, A.; Mora-Solano, C.; Wang, Y.; Alegre, M.-L. T cell receptor/CARMA1/NF- B signaling controls T-helper (Th) 17 differentiation. Proc. Natl. Acad. Sci. USA 2012, 109, 18529–18534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.-H.; Weaver, M.T. Airway cytokine responses to acute and repeated stress in a murine model of allergic asthma. Biol. Psychol. 2010, 84, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. Taking our breath away: Dendritic cells in the pathogenesis of asthma. Nat. Rev. Immunol. 2003, 3, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Tian, J.J.; Ko, W.S.; Shih, C.J.; Chiou, Y.L. Oligo-fucoidan improved unbalance the Th1/Th2 and Treg/Th17 ratios in asthmatic patients: An ex vivo study. Exp. Ther. Med. 2019, 17, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rensen, E.L.J.; Sont, J.K.; Evertse, C.E.; Willems, L.N.A.; Mauad, T.; Hiemstra, P.S.; Sterk, P.J. Bronchial CD8 Cell Infiltrate and Lung Function Decline in Asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Leggat, J.A.; Gibbons, D.L.; Haque, S.F.Y.; Smith, A.L.; Wells, J.W.; Choy, K.; Lloyd, C.M.; Hayday, A.C.; Noble, A. Innate responsiveness of CD8 memory T-cell populations nonspecifically inhibits allergic sensitization. J. Allergy Clin. Immunol. 2008, 122, 1014–1021.e4. [Google Scholar] [CrossRef] [Green Version]
- Otter, I.D.; Willems, L.N.; Van Schadewijk, A.; Van Wijngaarden, S.; Janssen, K.; De Jeu, R.C.; Sont, J.K.; Sterk, P.J.; Hiemstra, P.S. Lung function decline in asthma patients with elevated bronchial CD8, CD4 and CD3 cells. Eur. Respir. J. 2016, 48, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Wang, Z.; Cao, Y.; Bunjhoo, H.; Zhu, J.; Chen, Y.; Xiong, S. The study of the ratio and distribution of Th17 cells and Tc17 cells in asthmatic patients and the mouse model. Asian Pac. J. Allergy Immunol. 2013, 31, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, Q.-Z.; Wang, W.; Zhang, G.-Q.; Yang, J. Increased IL-4- and IL-17-producing CD8+ cells are related to decreased CD39+CD4+Foxp3+ cells in allergic asthma. J. Asthma 2017, 55, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Ruan, G.; Wang, D.; Li, Y.; Wang, Z.; Yin, G. Imbalance of Peripheral Th17 and Regulatory T Cells in Children with Allergic Rhinitis and Bronchial Asthma. Iran. J. Allergy Asthma Immunol. 2015, 14, 273–279. [Google Scholar]
- McGee, H.S.; Agrawal, D.K. Naturally Occurring and Inducible T-Regulatory Cells Modulating Immune Response in Allergic Asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M. Healthy immune response to allergens: T regulatory cells and more. Curr. Opin. Immunol. 2006, 18, 738–744. [Google Scholar] [CrossRef]
- Maślanka, T.; Jaroszewski, J.J. In vitro effects of dexamethasone on bovine CD25+CD4+ and CD25−CD4+ cells. Res. Vet. Sci. 2012, 93, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Maślanka, T.; Otrocka-Domagała, I.; Zuśka-Prot, M.; Gesek, M. Beneficial effects of rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, in a mouse allergic asthma model is not associated with the recruitment or generation of Foxp3-expressing CD4+ regulatory T cells. Eur. J. Pharmacol. 2019, 848, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; Giorgini, A.; Leggat, J.A. Cytokine-induced IL-10-secreting CD8 T cells represent a phenotypically distinct sup-pressor T-cell lineage. Blood 2006, 107, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.W.; Cowled, C.J.; Giorgini, A.; Kemeny, D.M.; Noble, A. Regulation of allergic airway inflammation by class I–restricted allergen presentation and CD8 T-cell infiltration. J. Allergy Clin. Immunol. 2007, 119, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Verhagen, J.; Taylor, A.; Karamloo, F.; Karagiannidis, C.; Crameri, R.; Thunberg, S.; Deniz, G.; Valenta, R.; Fiebig, H.; et al. Immune Responses in Healthy and Allergic Individuals Are Characterized by a Fine Balance between Allergen-specific T Regulatory 1 and T Helper 2 Cells. J. Exp. Med. 2004, 199, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Shahzad, M.; Raza Asim, M.B.; Imran, M.; Shabbir, A. Zingiber officinale ameliorates allergic asthma via sup-pression of Th2-mediated immune response. Pharm. Biol. 2015, 53, 359–367. [Google Scholar] [CrossRef]
- McIntyre, K.W.; Shuster, D.J.; Gillooly, K.M.; Dambach, D.M.; Pattoli, M.A.; Lu, P.; Zhou, X.D.; Qiu, Y.; Zusi, F.C.; Burke, J.R. A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in colla-gen-induced arthritis in mice. Arthritis Rheum. 2003, 48, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Li, C.; Wang, X.; Dian, L.; Zhang, X.; Li, L.; Chen, S.; Cao, R.; Li, L.; Huang, N.; et al. Ainsliadimer A se-lectively inhibits IKKα/β by covalently binding a conserved cysteine. Nat. Commun. 2015, 6, 6522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, T.; Kondo, Y.; Shinozaki, S.; Kaneko, E.; Ishigami, A.; Maruyama, N.; Umezawa, K.; Shimokado, K. A Selective NFκB Inhibitor, DHMEQ, Reduced Atherosclerosis in ApoE-deficient mice. J. Atheroscler. Thromb. 2006, 13, 308–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Konno, S.; Ozaki, M.; Umezawa, K.; Yamashita, K.; Todo, S.; Nishimura, M. Dehydroxymethylepoxyquinomicin (DHMEQ), a novel NF-kappaB inhibitor, inhibits allergic inflammation and airway remodelling in murine models of asthma. Clin. Exp. Allergy 2012, 42, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, S.S.; Dumont, N.A.; Bouchard, P.; Lavergne, É.; Penninger, J.M.; Frenette, J. Osteoprotegerin Protects against Muscular Dystrophy. Am. J. Pathol. 2015, 185, 920–926. [Google Scholar] [CrossRef]
- Shin, M.; Matsuo, K.; Tada, T.; Fukushima, H.; Furuta, H.; Ozeki, S.; Kadowaki, T.; Yamamoto, K.; Okamoto, M.; Jimi, E. The inhibition of RANKL/RANK signaling by osteoprotegerin suppresses bone invasion by oral squamous cell carcinoma cells. Carcinogenesis 2011, 32, 1634–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawayama, T.; O’Byrne, P.M.; Watson, R.M.; Killian, K.J.; Duong, M.; Yoshida, M.; Gauvreau, G.M. Effects of inhaled ci-clesonide on circulating T-helper type 1/T-helper type 2 cells in atopic asthmatics after allergen challenge. Clin. Exp. Allergy 2006, 36, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Caucheteux, S.M.; Hu-Li, J.; Mohammed, R.N.; Ager, A.; Paul, W.E. Cytokine regulation of lung Th17 response to airway immunization using LPS adjuvant. Mucosal Immunol. 2016, 10, 361–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregorczyk, I.; Jasiecka-Mikołajczyk, A.; Maślanka, T. Blockade of NF-κB Translocation and of RANKL/RANK Interaction Decreases the Frequency of Th2 and Th17 Cells Capable of IL-4 and IL-17 Production, Respectively, in a Mouse Model of Allergic Asthma. Molecules 2021, 26, 3117. https://doi.org/10.3390/molecules26113117
Gregorczyk I, Jasiecka-Mikołajczyk A, Maślanka T. Blockade of NF-κB Translocation and of RANKL/RANK Interaction Decreases the Frequency of Th2 and Th17 Cells Capable of IL-4 and IL-17 Production, Respectively, in a Mouse Model of Allergic Asthma. Molecules. 2021; 26(11):3117. https://doi.org/10.3390/molecules26113117
Chicago/Turabian StyleGregorczyk, Izabela, Agnieszka Jasiecka-Mikołajczyk, and Tomasz Maślanka. 2021. "Blockade of NF-κB Translocation and of RANKL/RANK Interaction Decreases the Frequency of Th2 and Th17 Cells Capable of IL-4 and IL-17 Production, Respectively, in a Mouse Model of Allergic Asthma" Molecules 26, no. 11: 3117. https://doi.org/10.3390/molecules26113117
APA StyleGregorczyk, I., Jasiecka-Mikołajczyk, A., & Maślanka, T. (2021). Blockade of NF-κB Translocation and of RANKL/RANK Interaction Decreases the Frequency of Th2 and Th17 Cells Capable of IL-4 and IL-17 Production, Respectively, in a Mouse Model of Allergic Asthma. Molecules, 26(11), 3117. https://doi.org/10.3390/molecules26113117





