Inclined Substrate Deposition of Nanostructured TiO2 Thin Films for DSSC Application
Abstract
1. Introduction
2. Experimental Details
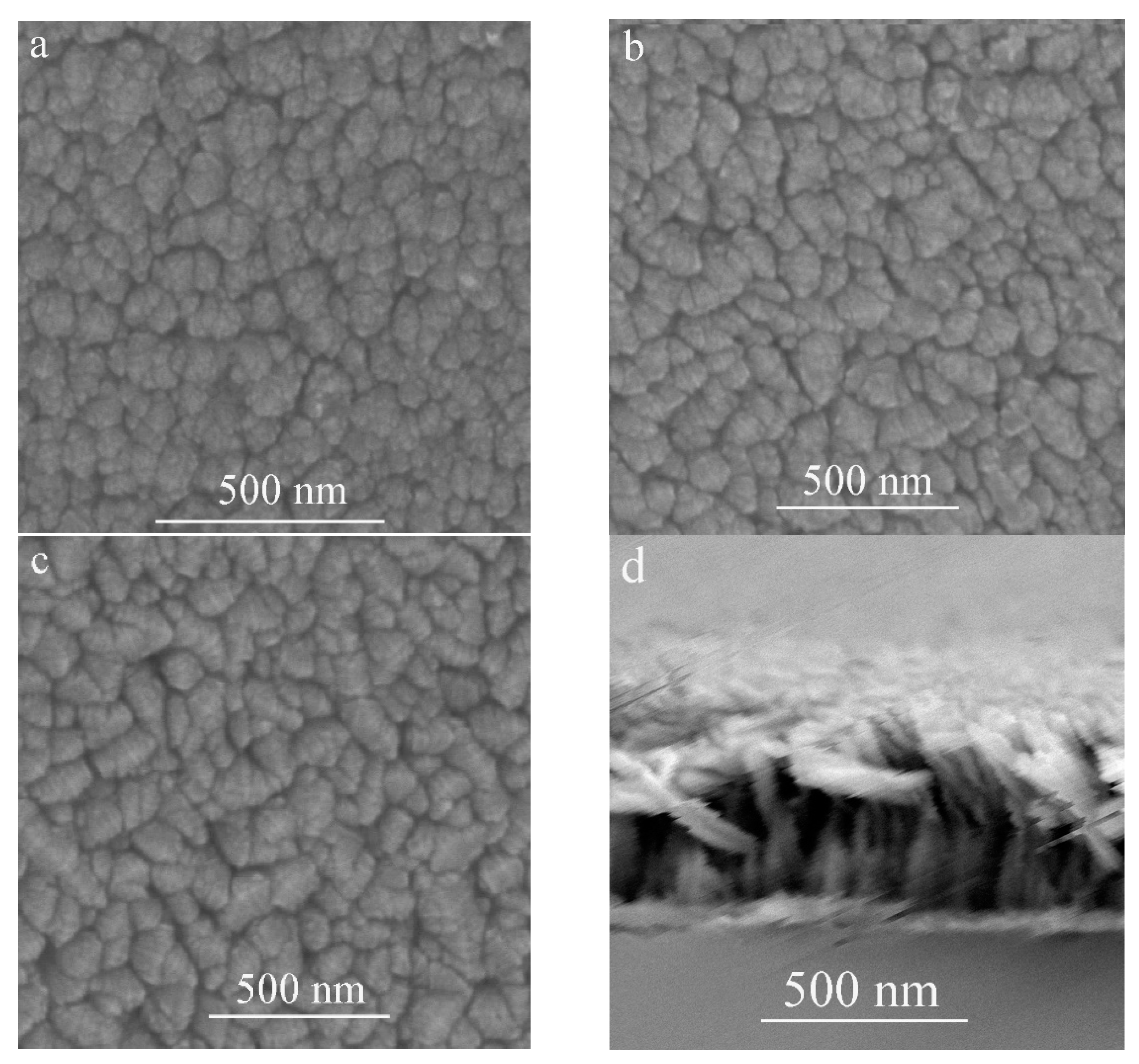
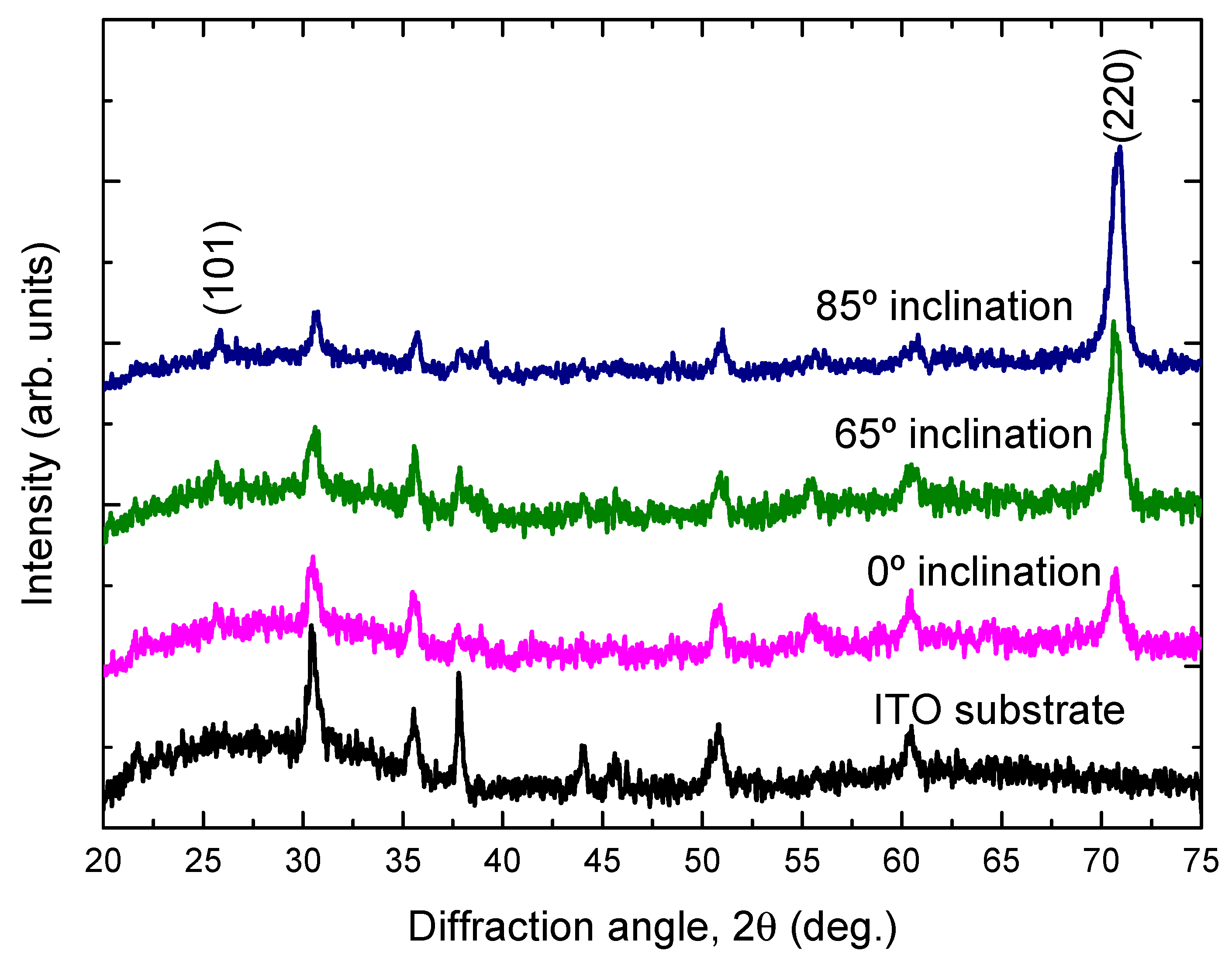
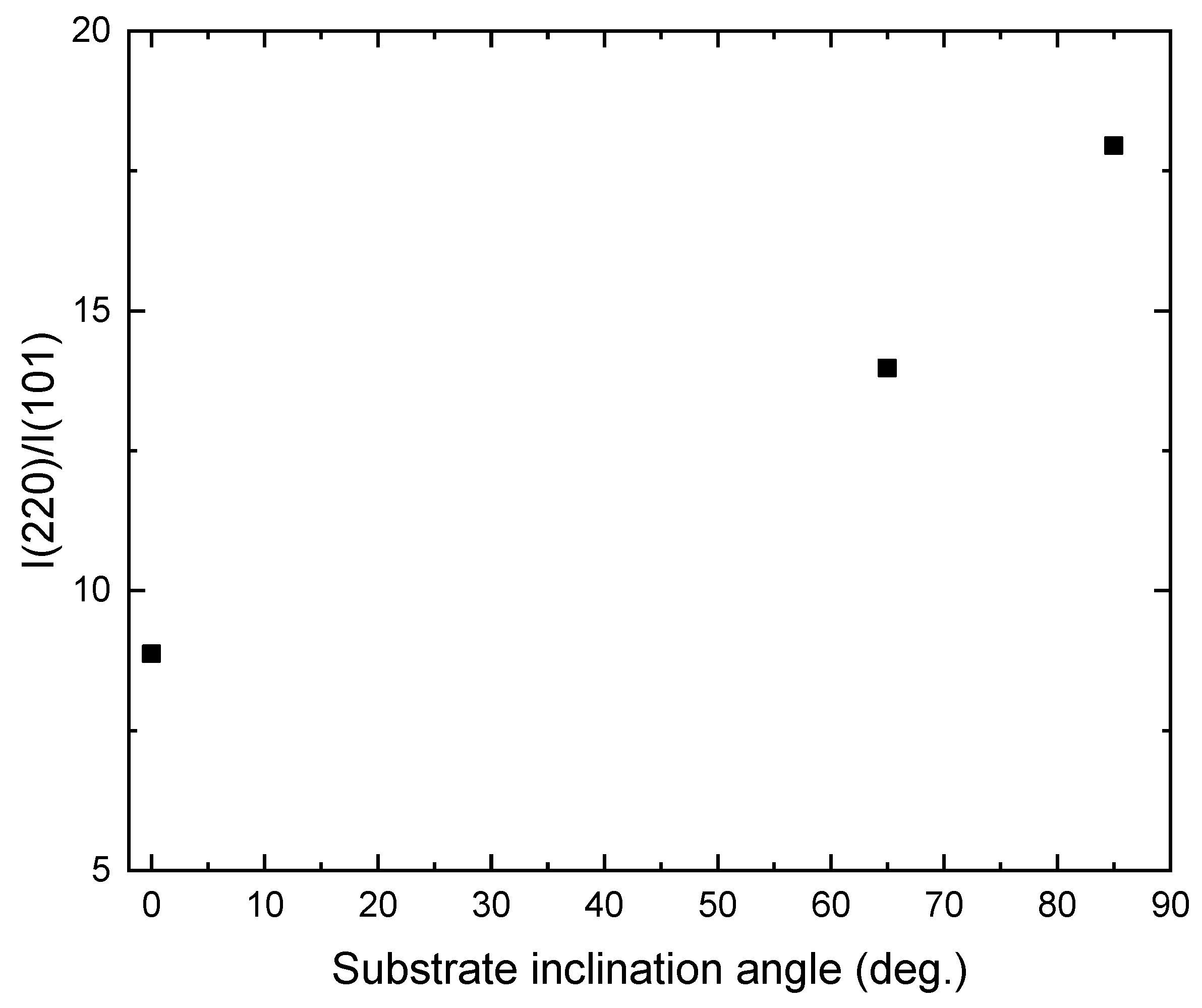
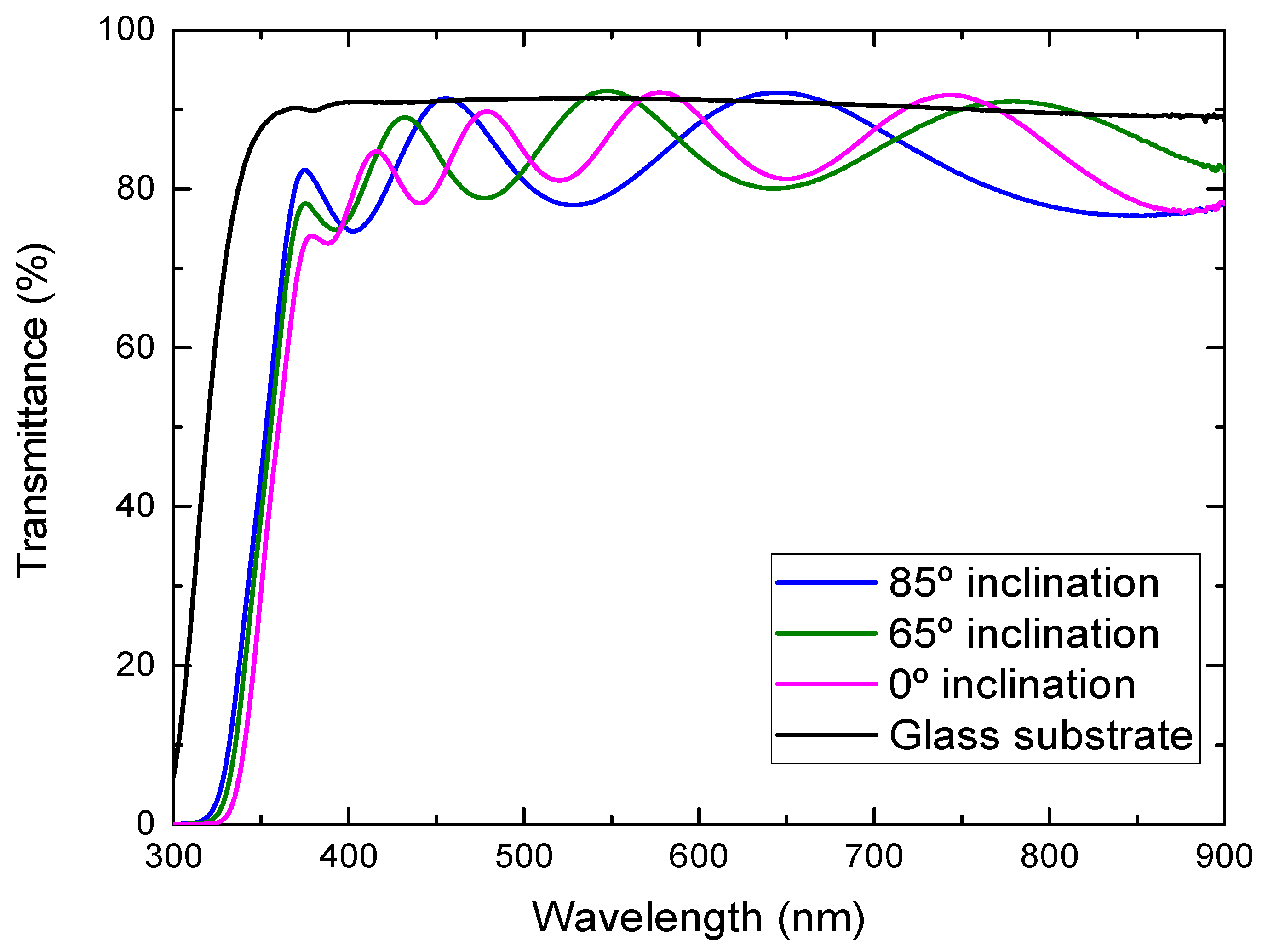
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guillén, C.; Montero, J.; Herrero, J. Anatase and rutile TiO2 thin films prepared by reactive DC sputtering at high deposition rates on glass and flexible polyimide substrates. J. Mater. Sci. 2014, 49, 5035–5042. [Google Scholar] [CrossRef]
- Jiang, W.; Cui, H.; Song, Y. Electrochemical corrosion behaviors of titanium covered by various TiO2 nanotube films in artificial saliva. J. Mater. Sci. 2018, 53, 15130–15141. [Google Scholar] [CrossRef]
- Zeinali, M.; Jaleh, B.; Vaziri, M.R.R.; Omidvar, A. Study of nonlinear optical properties of TiO2—polystyrene nanocomposite films. Quantum Electron. 2019, 49, 951–957. [Google Scholar] [CrossRef]
- Wang, X.; Lai, M.; Gao, R.; Huang, X.; Zhao, Z.; Yang, Y.; Zheng, G.; Ma, Y. Ultra-smooth TiO2 thin film based optical humidity sensor with a fast response and recovery. Appl. Opt. 2019, 58, 9740–9745. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, A.; Akl, A.A. Optical characterizations and refractive index dispersion parameters of annealed TiO2 thin films synthesized by RF-sputtering technique at different flow rates of the reactive oxygen gas. Phys. B Condens. Matter 2020, 576, 411718. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, W.F.; Koshy, P.; Sorrell, C.C. Enhanced photocatalytic performance of nanostructured TiO2 thin films through combined effects of polymer conjuga-tion and Mo-doping. J. Mater. Sci. 2019, 54, 5266–5279. [Google Scholar] [CrossRef]
- Tuckute, S.; Varnagiris, S.; Urbonavicius, M.; Lelis, M.; Sakalauskaitea, S. Tailoring of TiO2 film crystal texture for higher photocatalysis efficiency. Appl. Surf. Sci. 2019, 489, 576–583. [Google Scholar] [CrossRef]
- Sampaio, D.M.; Babu, R.S.; Costa, H.R.M.; De Barros, A.L.F. Investigation of nanostructured TiO2 thin film coatings for DSSCs application using natural dye extracted from jabuticaba fruit as photosensitizers. Ionics 2018, 25, 2893–2902. [Google Scholar] [CrossRef]
- Umale, S.; Sudhakar, V.; Sontakke, S.M.; Krishnamoorthy, K.; Pandit, A.B. Improved efficiency of DSSC using combustion synthesized TiO2. Mater. Res. Bull. 2019, 109, 222–226. [Google Scholar] [CrossRef]
- Hu, Z.; García-Martín, J.M.; Li, Y.; Billot, L.; Sun, B.; Fresno, F.; García-Martín, A.; González, M.U.; Aigouy, L.; Chen, Z. TiO2 Nanocolumn Arrays for More Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 5979–5989. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Borges, J.; Proença, M.; Pedrosa, P.; Martin, N.; Romanyuk, K.; Kholkin, A.L.; Vaz, F. Nanoplasmonic response of porous Au-TiO2 thin films prepared by oblique angle deposition. Nanotechnology 2019, 30, 225701. [Google Scholar] [CrossRef] [PubMed]
- Cormier, P.-A.; Dervaux, J.; Szuwarski, N.; Pellegrin, Y.; Odobel, F.; Gautron, E.; Boujtita, M.; Snyders, R.; Boujita, M. Single Crystalline-like and Nanostructured TiO2 Photoanodes for Dye Sensitized Solar Cells Synthesized by Reactive Magnetron Sputtering at Glancing Angle. J. Phys. Chem. C 2018, 122, 20661–20668. [Google Scholar] [CrossRef]
- Wang, B.; Qi, H.; Liu, Z.; Jin, Y.; Wang, H.; Yuan, J.; Zhao, J.; Shao, J. Structure, chemical state and photocatalytic activity of TiO2−x nanostructured thin films by glancing angle deposition technique. J. Alloys Compd. 2017, 716, 299–305. [Google Scholar] [CrossRef]
- Jeong, J.-A.; Kim, H.-K. Thickness effect of RF sputtered TiO2 passivating layer on the performance of dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 344–348. [Google Scholar] [CrossRef]
- Kang, S.H.; Kang, M.-S.; Kim, H.-S.; Kim, J.-Y.; Chung, Y.-H.; Smyrl, W.H.; Sung, Y.-E. Columnar rutile TiO2 based dye-sensitized solar cells by radio-frequency magnetron sputtering. J. Power Sources 2008, 184, 331–335. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, K.H.; Choi, H.W. Enhancing Efficiency of Dye-Sensitized Solar Cells Using TiO2 Composite Films and RF-Sputtered Passivating Layer. Mol. Cryst. Liq. Cryst. 2012, 567, 9–18. [Google Scholar] [CrossRef]
- Meng, L.; Ma, A.; Ying, P.; Feng, Z.; Li, C. Sputtered highly ordered TiO2 nanorod arrays and their applications as the electrode in dye-sensitized solar cells. J. Nanosci. Nanotechnol. 2011, 11, 929–934. [Google Scholar] [CrossRef][Green Version]
- Poxson, D.J.; Mont, F.W.; Schubert, M.F.; Kim, J.K.; Schubert, E.F. Quantification of porosity and deposition rate of nanoporous films grown by oblique-angle deposition. Appl. Phys. Lett. 2008, 93, 101914. [Google Scholar] [CrossRef]
- Meng, L.; Chen, H.; Li, C.; Dos Santos, M.P. Preparation and characterization of dye-sensitized TiO2 nanorod solar cells. Thin Solid Films 2015, 577, 103–108. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Thavasi, V.; Renugopalakrishnan, V.; Jose, R.; Ramakrishna, S. Controlled electron injection and transport at materials interfaces in dye sensitized solar cells. Mater. Sci. Eng. R Rep. 2009, 63, 81–99. [Google Scholar] [CrossRef]



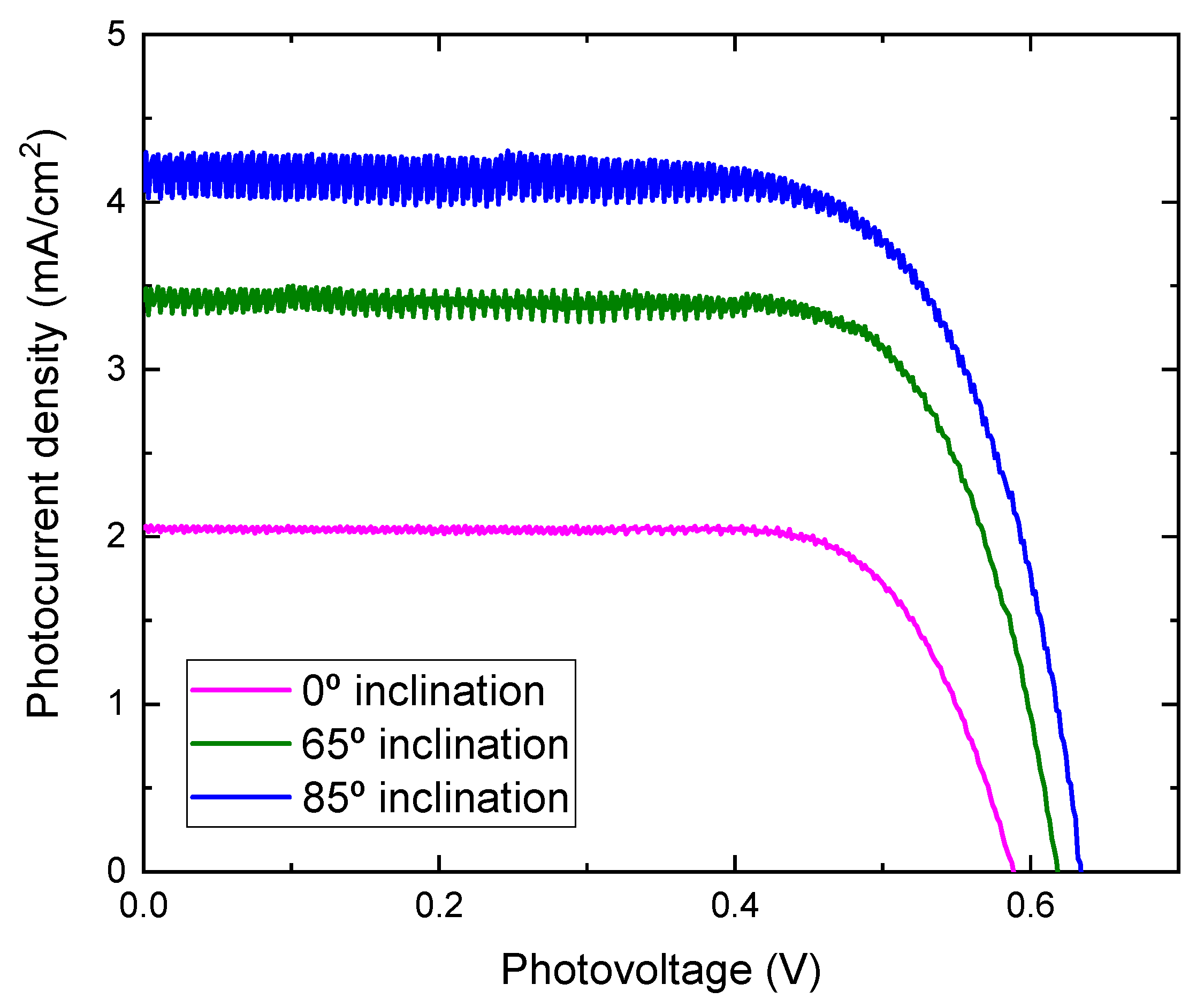
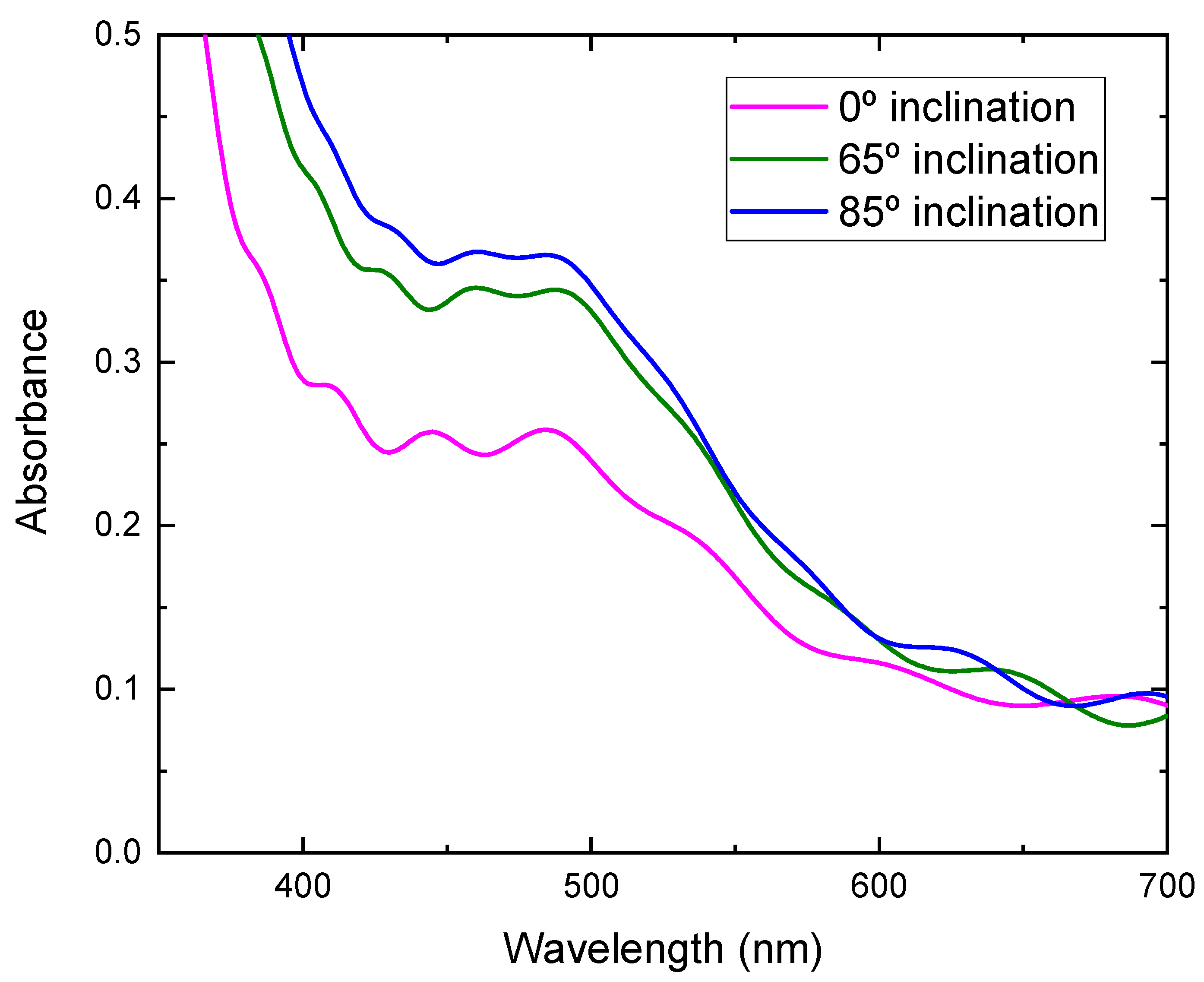
| Inclination Angle (°) | Voc (V) | Jsc (mA/cm2) | FF | η (%) | Thickness (nm) |
|---|---|---|---|---|---|
| 0 | 0.59 | 2.06 | 0.75 | 0.91 | 540 |
| 65 | 0.62 | 3.48 | 0.73 | 1.58 | 480 |
| 85 | 0.63 | 4.29 | 0.70 | 1.89 | 400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Yang, T. Inclined Substrate Deposition of Nanostructured TiO2 Thin Films for DSSC Application. Molecules 2021, 26, 3122. https://doi.org/10.3390/molecules26113122
Meng L, Yang T. Inclined Substrate Deposition of Nanostructured TiO2 Thin Films for DSSC Application. Molecules. 2021; 26(11):3122. https://doi.org/10.3390/molecules26113122
Chicago/Turabian StyleMeng, Lijian, and Tao Yang. 2021. "Inclined Substrate Deposition of Nanostructured TiO2 Thin Films for DSSC Application" Molecules 26, no. 11: 3122. https://doi.org/10.3390/molecules26113122
APA StyleMeng, L., & Yang, T. (2021). Inclined Substrate Deposition of Nanostructured TiO2 Thin Films for DSSC Application. Molecules, 26(11), 3122. https://doi.org/10.3390/molecules26113122






