Synthesis and Assessment of Two Malonyl Dihydrazide Derivatives as Corrosion Inhibitors for Carbon Steel in Acidic Media: Experimental and Theoretical Studies
Abstract
1. Introduction
2. Results
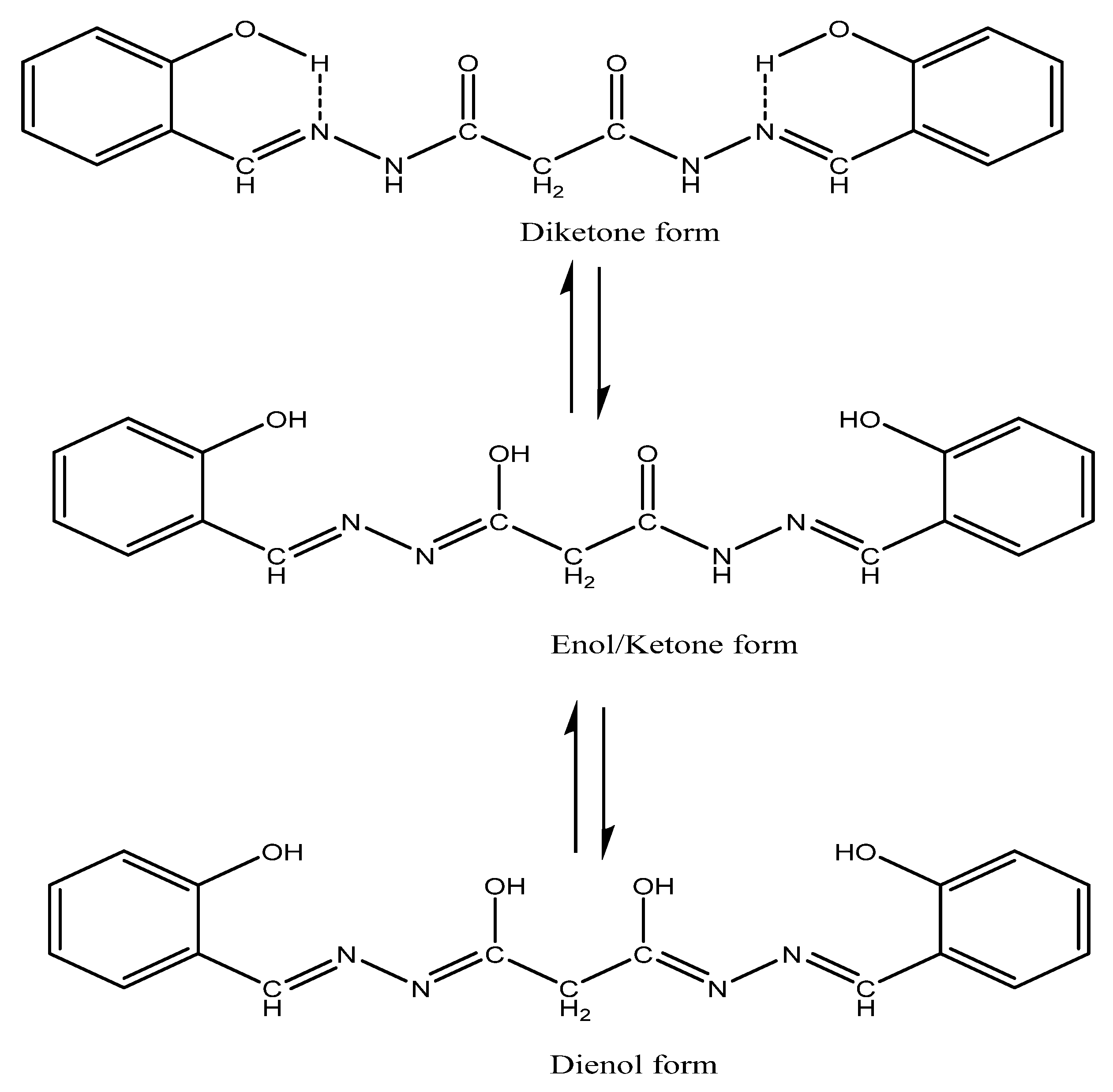
2.1. Characterisation of the Inhibitors
2.2. Weight Loss Measurements
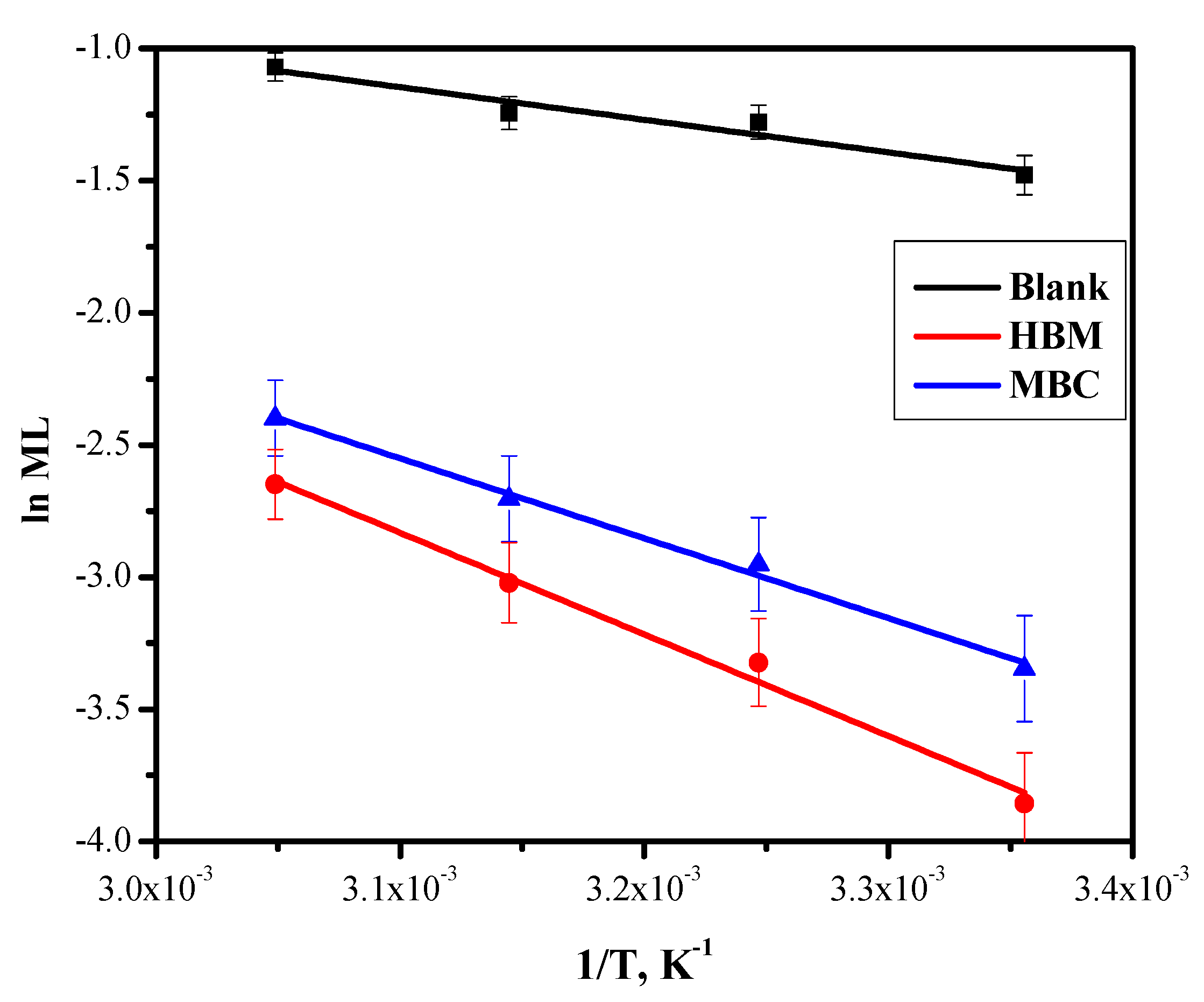
2.3. Temperature Effect
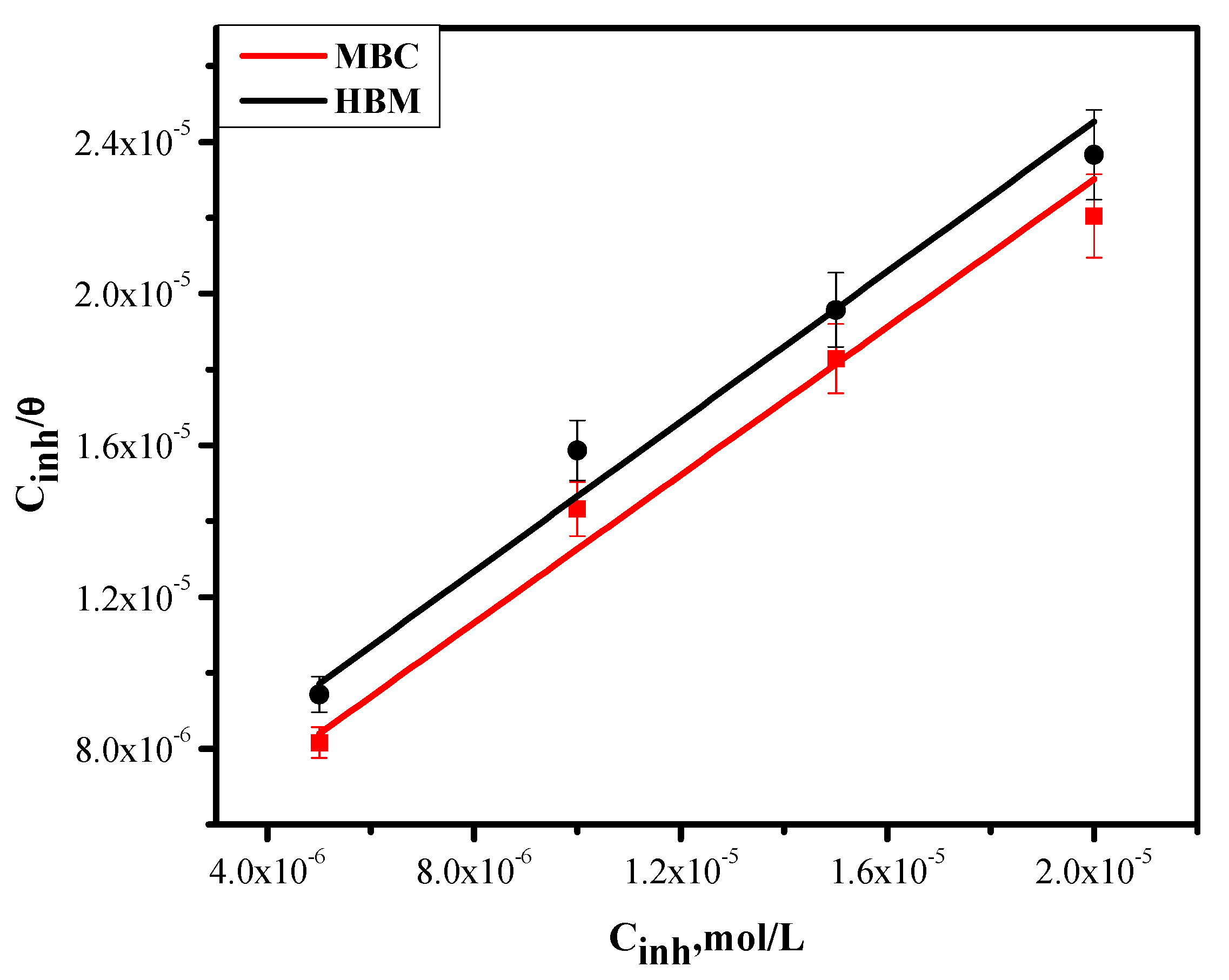
2.4. Adsorption Isotherm Model
2.5. Electrochemical Measurements
2.5.1. Potentiodynamic Polarisation
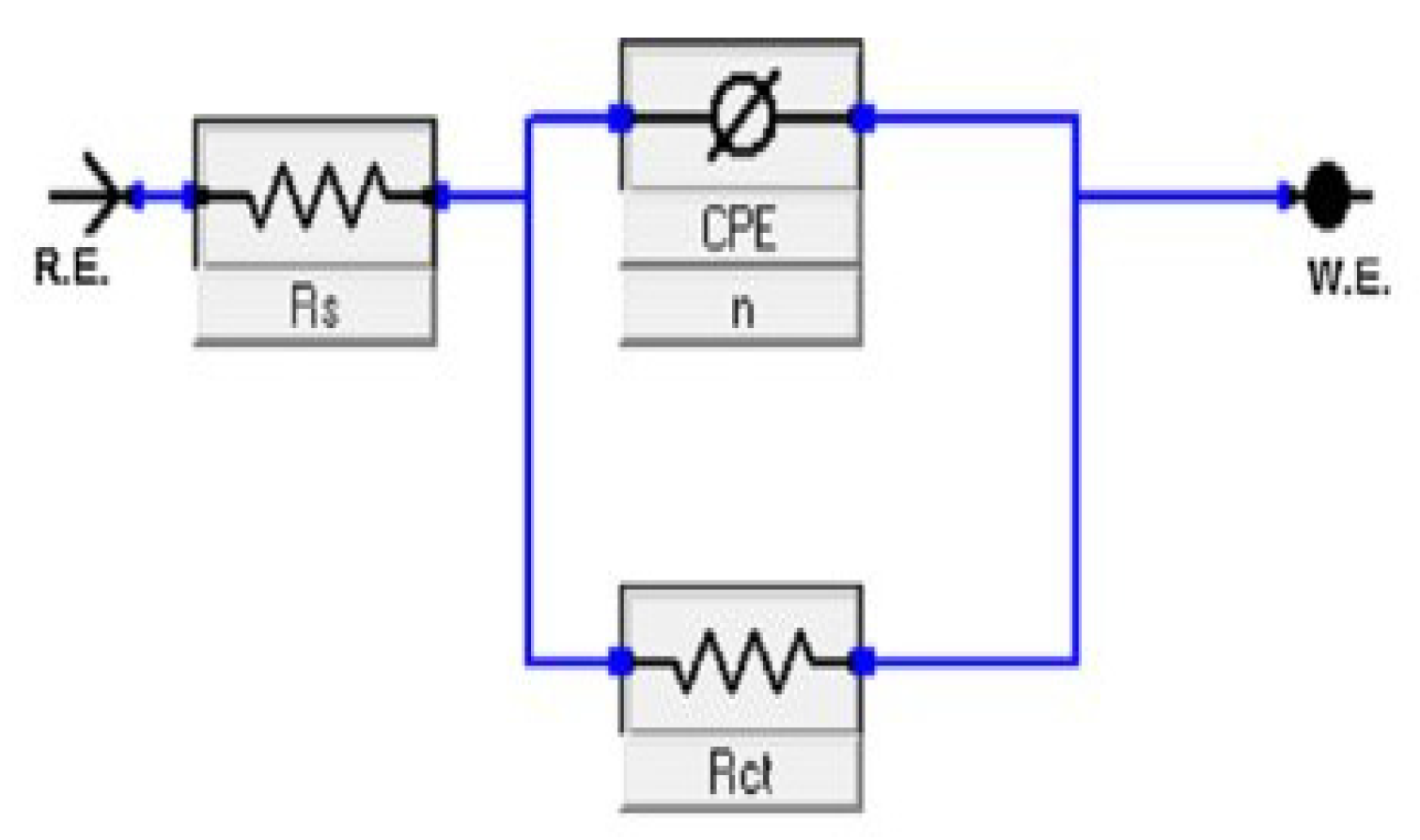
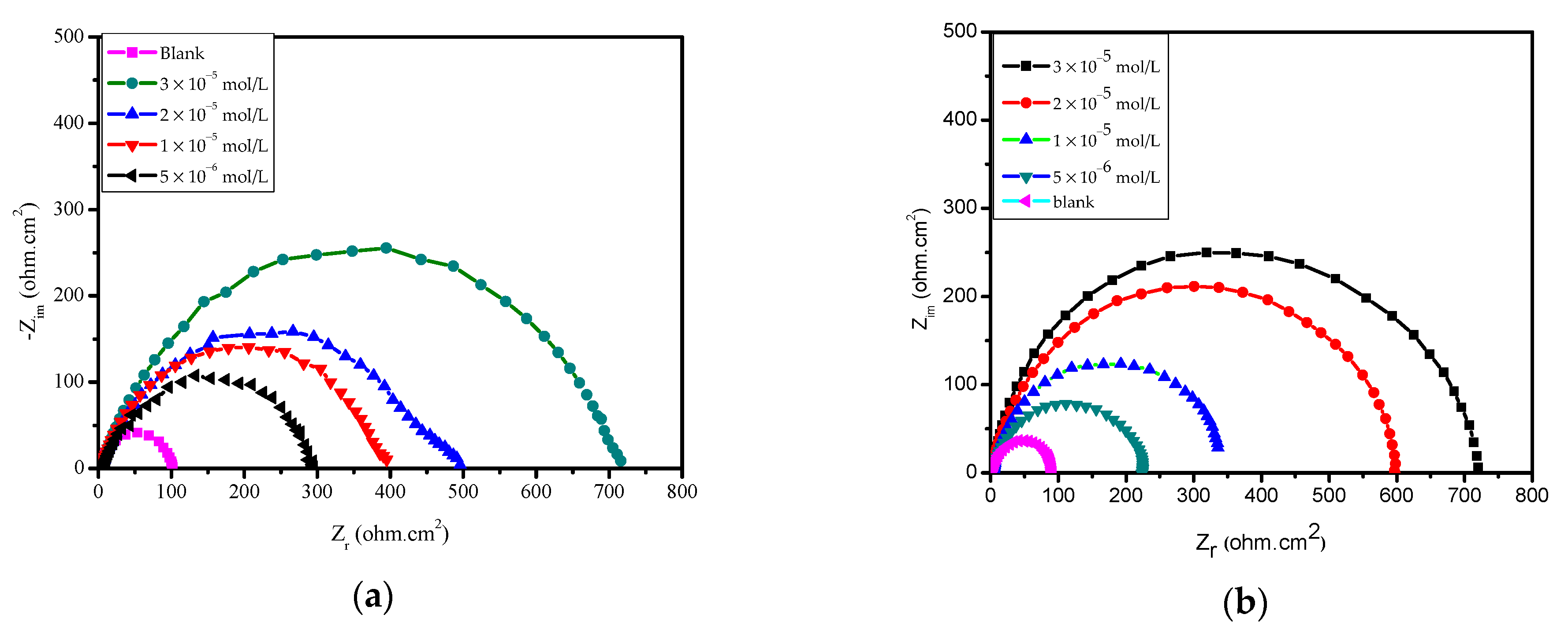
2.5.2. Electrochemical Impedance Spectroscopy EIS
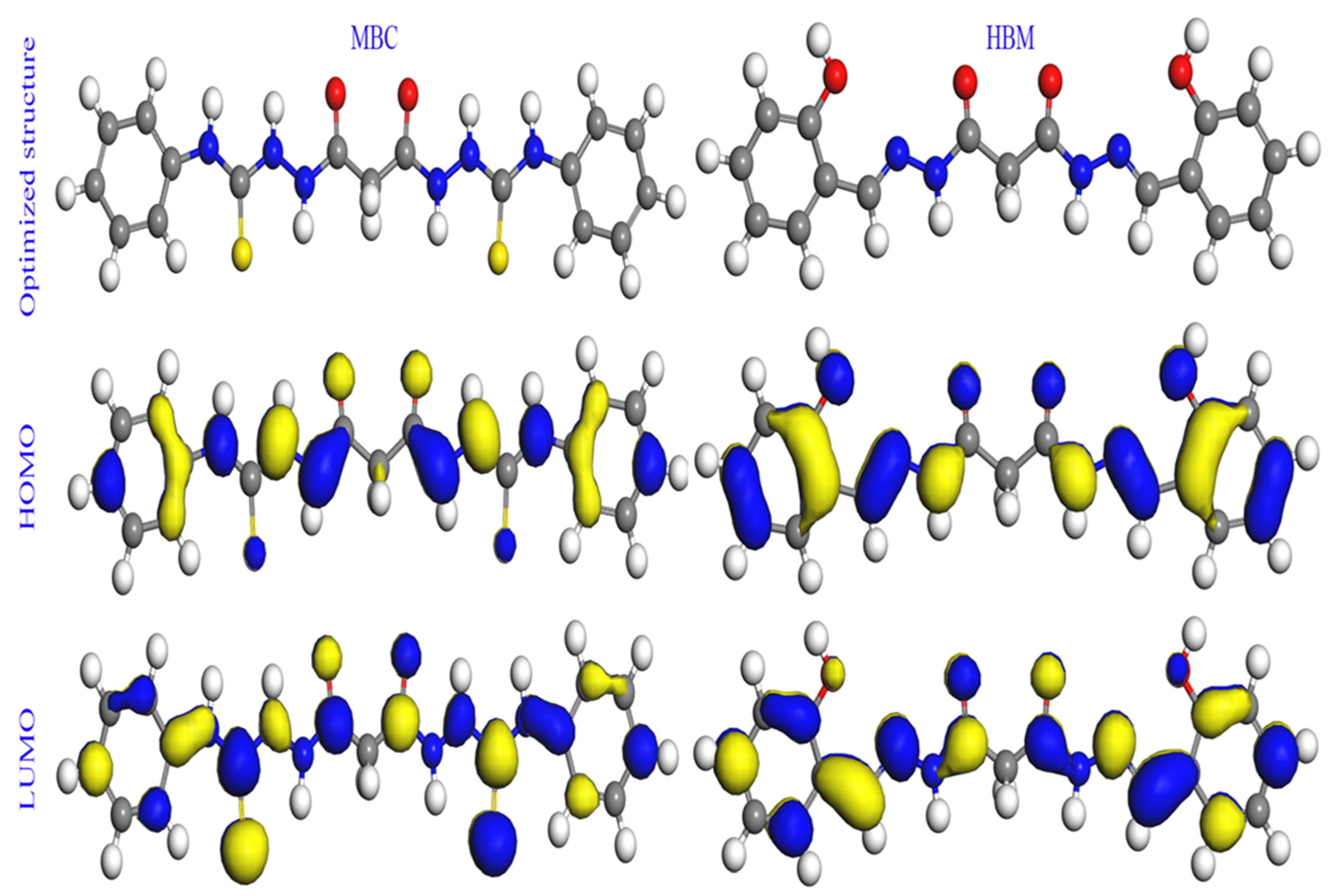
2.6. Quantum Chemical Calculations
2.6.1. Global Reactivity Descriptors
2.6.2. Local Reactivity: Fukui Functions
2.7. Monte Carlo Simulations
3. Materials and Methods
3.1. Materials
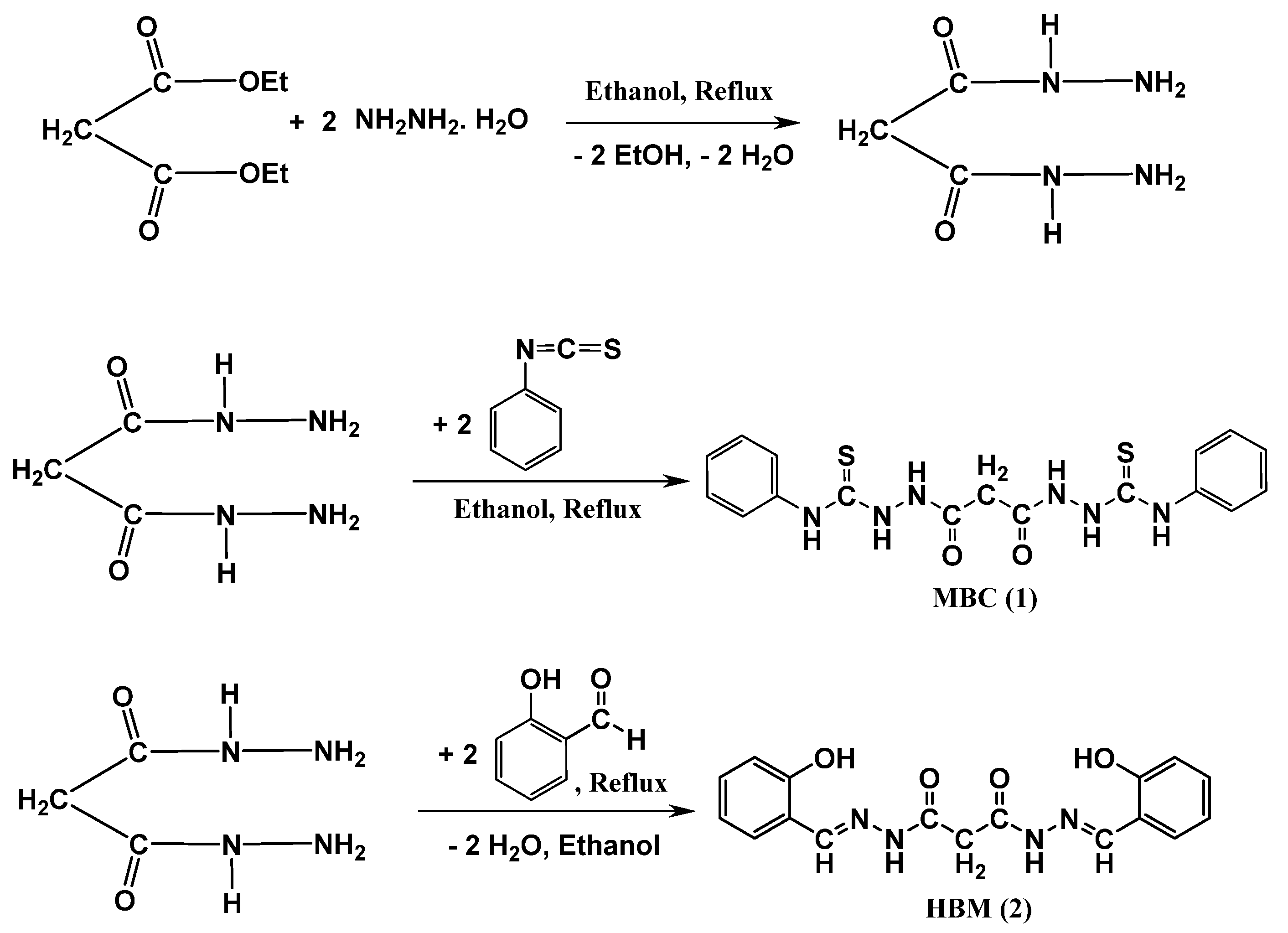
3.2. Synthesis of Malonyl Dihydrazide
3.3. Synthesis of 2,2’-Malonylbis(N-phenylhydrazine-1-carbothioamide) (MBC)
3.4. Synthesis of N’1, N’3-bis(2-Hydroxybenzylidene)malonohydrazide (HBM)
3.5. Electrochemical Measurements
3.6. Computational Study
4. Conclusions
- ❖
- MBC and HBM are suitable inhibitors for carbon steel corrosion in acidic media.
- ❖
- The PDP curves show that both MBC and HBM are mixed inhibitors.
- ❖
- EIS investigations proved that the addition of MBC and HBM to the corrosion medium increases the inhibition efficacy.
- ❖
- The study of the impact of temperature on the inhibition efficiency displays that it increases with the temperature increase.
- ❖
- This study revealed that the adsorption of both MBC and HBM molecules on the carbon steel surface has a chemical nature.
- ❖
- The theoretical studies for both MBC and HBM have further supported the experimental results.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Njoku, D.I.; Li, Y.; Lgaz, H.; Oguzie, E.E. Dispersive adsorption of Xylopia aethiopica constituents on carbon steel in acid-chloride medium: A combined experimental and theoretical approach. J. Mol. Liq. 2018, 249, 371–388. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Rodríguez, J.A.; Cruz-Borbolla, J.; Alvarado-Rodríguez, J.G.; Thangarasu, P. Development of a predictive model for corrosion inhibition of carbon steel by imidazole and benzimidazole derivatives. Corros. Sci. 2016, 108, 23–35. [Google Scholar] [CrossRef]
- Lgaz, H.; Saha, S.R.; Chaouiki, A.; Bhat, K.S.; Salghi, R.; Shubhalaxmi; Banerjee, P.; Ali, I.H.; Khan, M.I.; Chung, I.-M. Exploring the potential role of pyrazoline derivatives in corrosion inhibition of mild steel in hydrochloric acid solution: Insights from experimental and computational studies. Constr. Build. Mater. 2020, 233, 117320. [Google Scholar] [CrossRef]
- Naciri, M.; El Aoufir, Y.; Lgaz, H.; Lazrakd, F.; Ghanimi, A.; Guenboura, A.; Ali, I.H.; El Moudane, M.; Taoufik, J.; Chung, I.-M. Exploring the potential of a new 1,2,4-triazole derivative for corrosion protection of carbon steel in HCl: A computational and experimental evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124604. [Google Scholar] [CrossRef]
- Chaitra, T.K.; Mohana, K.N.S.; Tandon, H.C. Thermodynamic, electrochemical and quantum chemical evaluation of some triazole Schiff bases as mild steel corrosion inhibitors in acid media. J. Mol. Liq. 2015, 211, 1026–1038. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, Y.; Cang, H.; Xu, J.; Lu, G.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros. Sci. 2014, 83, 292–298. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Damej, M.; Lgaz, H.; Salghi, R.; Ali, I.H.; Benmessaoud, M.; Masroor, M.; Chung, I.-M. Bolaamphiphile-class surfactants as corrosion inhibitor model compounds against acid corrosion of mild steel. J. Mol. Liq. 2020, 309, 113070. [Google Scholar] [CrossRef]
- Morales-Gil, P.; Walczak, M.S.; Camargo, C.R.; Cottis, R.A.; Romero, J.M.; Lindsay, R. Corrosion inhibition of carbon-steel with 2-mercaptobenzimidazole in hydrochloric acid. Corros. Sci 2015, 101, 47–55. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, S.; Quan, H.; Huang, Z.; Xu, S. Synthesis and corrosion inhibition performance of alkyl triazole derivatives. Res. Chem. Intermed. 2015, 41, 2709–2724. [Google Scholar] [CrossRef]
- Geler, E.; Azambuja, D.S. Corrosion inhibition of copper in chloride solutions bipyrazole. Corros. Sci 2000, 42, 631–643. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Attia, S.K.; Rizk, S.A.; Essa, M.A.; Elkholy, A.E. Synthesis, characterisation and computational chemical study of novel pyrazole derivatives as anticorrosion and antiscalant agents. J. Mol. Struct. 2017, 1147, 714–724. [Google Scholar] [CrossRef]
- Ferkous, H.; Djellali, S.; Sahraoui, R.; Benguerba, Y.; Behloul, H.; Çukurovali, A. Corrosion inhibition of mild steel by 2-(2-methoxybenzylidene) hydrazine-1-carbothioamide in hydrochloric acid solution: Experimental measurements and quantum chemical calculations. J. Mol. Liq. 2020, 307, 11295. [Google Scholar] [CrossRef]
- Gouda, V.K.; Sayed, S.M. Corrosion inhibition of steel by hydrazine. Corros. Sci. 1973, 13, 647–652. [Google Scholar] [CrossRef]
- Gouda, V.K.; Shater, M.A. Corrosion inhibition of reinforcing steel using hydrazine hydrate. Corros. Sci. 1975, 15, 199–204. [Google Scholar] [CrossRef]
- Fouda, A.S.; El-Desoky, H.S.; Abdel-Galeil, M.A. Niclosamide and dichlorphenamide: New and effective corrosion inhibitors for carbon steel in 1M HCl solution. SN Appl. Sci. 2021, 3, 287–299. [Google Scholar] [CrossRef]
- Muthamma, K.; Kumari, P.; Lavanya, M. Corrosion Inhibition of Mild Steel in Acidic Media by N-[(3,4-Dimethoxyphenyl)Methyleneamino]-4-Hydroxy-Benzamide. J. Bio Tribo Corros. 2021, 7, 10–26. [Google Scholar] [CrossRef]
- Belghiti, M.E.; Echihi, S.; Dafali, A.; Karzazi, Y.; Bakasse, M.; Elalaoui-Elabdallaoui, H.; Olasunkanmi, L.O.; Ebenso, E.E.; Tabyaoui, M. Computational simulation and statistical analysis on the relationship between corrosion inhibition efficiency and molecular structure of some hydrazine derivatives in phosphoric acid on mild steel surface. Appl. Surf. Sci. 2019, 491, 707–722. [Google Scholar] [CrossRef]
- El-Faham, A.; Osman, S.M.; Al-Lohedan, H.A.; El-Mahdy, G.A. Hydrazino-methoxy-1,3,5-triazine Derivatives’ Excellent Corrosion Organic Inhibitors of Steel in Acidic Chloride Solution. Molecules 2016, 21, 714. [Google Scholar] [CrossRef]
- El-Haddad, M.A.M.; Radwan, B.A.; Sliem, M.H. Highly efficient eco-friendly corrosion inhibitor for mild steel in 5 M HCl at elevated temperatures: Experimental & molecular dynamics study. Sci. Rep. 2019, 9, 3695. [Google Scholar]
- Chafiq, M.; Chaouiki, A.; Albayati, M.R.; Lgaz, H.; Salghi, R.; AbdelRaheem, S.K.; Ali, I.H.; Mohamed, S.K.; Chung, I.-M. Unveiled understanding on corrosion inhibition mechanisms of hydrazone derivatives based on naproxen for mild steel in HCl: A joint experimental/theoretical study. J. Mol. Liq. 2020, 320, 114442. [Google Scholar] [CrossRef]
- Szlosek, D.; Currie, D. Application and Mechanism of Malonic Acid as a Green Alternative for Protein-Crosslinking. Green Sustain. Chem. 2016, 6, 110–115. [Google Scholar] [CrossRef]
- Weidlich, B.R. Condensation sproducte von Dihydraziden zweibasischer Säuren. Ber. Dtsch. Chem. Ges. 1906, 39, 3372–3377. [Google Scholar]
- Uygun, Y.; Bayrak, H.; Özkan, H. Synthesis and biological activities of methylenebis-4H-1,2,4-triazole derivatives. Turk. J. Chem. 2013, 37, 812–823. [Google Scholar] [CrossRef]
- Ranford, J.D.; Vittal, J.J.; Wang, Y.M. Dicopper(II) Complexes of the Antitumor Analogues Acylbis(salicylaldehyde hydrazones) and Crystal Structures of Monomeric [Cu2(1,3-propanedioyl bis(salicylaldehyde hydrazone))(H2O)2].(ClO4)2.3H2O and Polymeric [{Cu2(1,6-hexanedioyl bis(salicylaldehydehydrazone))(C2H5OH)2}m].(ClO4)2m.m(C2H5OH). Inorg. Chem. 1998, 37, 1226–1231. [Google Scholar]
- Zhang, C.; Rheinwald, G.; Lozan, V.; Wu, B.; Lassahn, P.-G.; Lang, H.; Janiak, C. Structural Study and Solution Integrity of Dioxomolybdenum(VI) Complexes with Tridentate Schiff Base and Azole Ligands. Z. Anorg. Allg. Chem. 2002, 628, 1259–1268. [Google Scholar] [CrossRef]
- Ali, I.H.; Suleiman, M.H.A. Effect of Acid Extract of Leaves of Juniperus procera on Corrosion Inhibition of Carbon Steel in HCl Solutions. Int. J. Electrochem. Sci 2018, 13, 3910–3922. [Google Scholar] [CrossRef]
- Ali, I.H.; Idris, A.M.; Suleiman, H.M.H.A. Evaluation of leaf and bark extracts of Acacia tortilis as corrosion inhibitors for mild steel in seawater: Experimental and studies. Int. J. Electrochem. Sci. 2019, 14, 6406–6419. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Al-Hadeethi, M.R.; Ali, I.H.; Mohamed, S.K.; Toumiat, K.; Salghi, R. Naproxen-Based Hydrazones as Effective Corrosion Inhibitors for Mild Steel in 1.0 M HCl. Coatings 2020, 10, 700. [Google Scholar] [CrossRef]
- Lgaz, H.; Masroor, S.; Chafiq, M.; Damej, M.; Brahmia, A.; Salghi, R.; Benmessaoud, B.; Ali, I.H.; Alghamdi, M.M.; Chaouiki, A.; et al. Evaluation of 2-mercaptobenzimidazole derivatives as corrosion inhibitors for mild steel in hydrochloric acid. Metals 2020, 10, 357. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, N.; Jain, V.; Rai, B. Amino acids as copper corrosion inhibitors: A density functional theory approach. Appl. Surf. Sci. 2020, 514, 145905. [Google Scholar] [CrossRef]
- Abuelela, A.M.; Bedair, M.A.; Zoghaib, W.M.; Wilson, L.D.; Mohamed, T.A. Molecular structure and mild steel/HCl corrosion inhibition of 4,5-Dicyanoimidazole: Vibrational, electrochemical and quantum mechanical calculations. J. Mol. Struct. 2021, 1230, 129647. [Google Scholar] [CrossRef]
- Shokry, H. Molecular dynamics simulation and quantum chemical calculations for the adsorption of some Azo-azomethine derivatives on mild steel. J. Mol. Struct. 2014, 1060, 80–87. [Google Scholar] [CrossRef]
- Gómez, B.; Likhanova, V.N.; Domínguez-Aguilar, A.M.; Martínez-Palou, R.; Vela, A.; Gázquez, L.J. Quantum Chemical Study of the Inhibitive Properties of 2-Pyridyl-Azoles. J. Phys. Chem. B 2006, 110, 8928–8934. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef]
- Badr, E.A.; Bedair, M.A.; Shaban, S.M. Adsorption and performance assessment of some imine derivatives as mild steel corrosion inhibitors in 1.0 M HCl solution by chemical, electrochemical and computational methods. Mater. Chem. Phys. 2018, 219, 444–460. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical Hardness; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1997. [Google Scholar]
- Padmanabhan, J.; Parthasarathi, R.; Sarkar, U.; Subramanian, V.; Chattaraj, P.K. Effect of solvation on the condensed Fukui function and the generalised philicity index. Chem. Phys. Lett. 2004, 383, 122–128. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbé, A. New Dual Descriptor for Chemical Reactivity. J. Phys. Chem. A 2005, 109, 205–212. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hou, B.S.; Xu, N.; Liu, H.F.; Zhang, G.A. Two novel thiadiazole derivatives as highly efficient inhibitors for the corrosion of mild steel in the CO2-saturated oilfield produced water. J. Taiwan Inst. Chem. Eng. 2019, 96, 588–598. [Google Scholar] [CrossRef]


| Inhibitor | 106 Concentration mol/L | Weight Loss (mg) | IEML (%) | Surface Coverage (θ) |
|---|---|---|---|---|
| Blank | -- | 67.9 | - | - |
| MBC | 5.0 | 26.3 | 61.3 | 0.6127 |
| 10.0 | 20.5 | 69.8 | 0.6981 | |
| 15.0 | 12.2 | 82.0 | 0.8203 | |
| 20.0 | 6.3 | 90.7 | 0.9072 | |
| HBM | 5.0 | 31.9 | 53.0 | 0.5301 |
| 10.0 | 25.1 | 63.0 | 0.6303 | |
| 15.0 | 16.0 | 76.4 | 0.7664 | |
| 20.0 | 10.5 | 84.5 | 0.8453 |
| Inhibitor | Temperature | Weight Loss (mg) | IE (η%) |
|---|---|---|---|
| Blank | 298 | 67.9 | - |
| 308 | 85.7 | - | |
| 318 | 91.6 | - | |
| 328 | 112.5 | - | |
| MBC | 298 | 6.3 | 90.7 |
| 308 | 11.1 | 87.0 | |
| 318 | 15.5 | 83.1 | |
| 328 | 23.2 | 79.4 | |
| HBM | 298 | 10.5 | 84.5 |
| 308 | 16.1 | 81.2 | |
| 318 | 21.3 | 76.7 | |
| 328 | 29.8 | 73.5 |
| Inhibitor | Ea (kJ/mol) | ∆H (kJ/mol) | ∆S (J/mol) |
|---|---|---|---|
| Blank | 12.9 | −10.3 | 175 |
| HBM | 27.7 | −25.1 | 144 |
| MBC | 34.6 | −40.3 | 122 |
| Inhibitor | ||
|---|---|---|
| HBM | 1.7 × 105 | −45.1 |
| MBC | 2.5 × 105 | −47.9 |
| Inhibitor | Concentration mol/L | −Ecorr (mV vs. SCE) | icorr (μA cm−2) | βa (mV dec−1) | βc (mV dec−1) | θ | IE% |
|---|---|---|---|---|---|---|---|
| Blank | --- | 335 | 327 | 62 | 124 | ||
| MBC | 5.0 | 378 | 129 | 81 | 126 | 0.6055 | 60.6 |
| 10 | 367 | 88 | 67 | 131 | 0.7309 | 73.1 | |
| 20.0 | 383 | 58 | 101 | 157 | 0.8226 | 82.3 | |
| 30.0 | 371 | 32 | 92 | 163 | 0.9021 | 90.2 | |
| MBC | 5.0 | 367 | 147 | 117 | 182 | 0.5504 | 55.0 |
| 10.0 | 379 | 97 | 92 | 127 | 0.7034 | 70.3 | |
| 20.0 | 356 | 71 | 88 | 136 | 0.7828 | 78.3 | |
| 30.0 | 341 | 49 | 82 | 125 | 0.8502 | 85.0 |
| Inhibitors | 106 × Conc. (mol/L) | RS, (Ω cm2) | 10−3 × Yο μ Ω−1 sn cm−1 | n | Rct (Ω cm2) | Θ | %IEEIS |
|---|---|---|---|---|---|---|---|
| Blank | -- | 11.9 | 134.10 | 0.799 | 19.3 ± 0.7 | - | - |
| MBC | 5.0 | 9.5 | 123.40 | 0.801 | 49.4 ± 1.7 | 0.6093 | 60.9 |
| 10.0 | 9.6 | 148.70 | 0.827 | 72.5 ± 3.1 | 0.7337 | 73.4 | |
| 20.0 | 1.5 | 33.22 | 0.866 | 111.8 ± 4.5 | 0.8274 | 82.7 | |
| 30.0 | 1.7 | 33.01 | 0.835 | 219.4 ± 7.8 | 0.9120 | 91.2 | |
| HBM | 5.0 | 2.7 | 85.72 | 0.887 | 43.1 ± 1.8 | 0.5522 | 55.2 |
| 10.0 | 10.0 | 90.12 | 0.750 | 64.4 ± 2.6 | 0.7003 | 70.0 | |
| 20.0 | 9.9 | 163.6 | 0.791 | 82.7 ± 3.7 | 0.7666 | 76.7 | |
| 30.0 | 1.4 | 39.83 | 0.862 | 140.7 ± 6.3 | 0.8628 | 86.3 |
| Molecule | EHOMO | ELUMO | ∆E | ∆Eback donation | T.E. | ε | σ | ω | χ | ∆N | ∆Nmax | η | IEEIS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (eV) | (eV) | (eV) | (eV) | (eV) | (eV−1) | (eV−1) | (eV) | (eV) | (e) | (e) | (eV) | (%) | ||
| Neutral | ||||||||||||||
| gas phase | MBC | −4.8178 | −1.8514 | 2.9664 | −0.3708 | −52,259.80 | 0.2668 | 0.6742 | 3.7484 | 3.3346 | 1.2356 | 1.1241 | 1.4832 | 91.2 |
| HBM | −5.0035 | −1.6499 | 3.3536 | −0.4192 | −31,753.34 | 0.3030 | 0.5964 | 3.3000 | 3.3267 | 1.0953 | 0.9920 | 1.6768 | 86.3 | |
| aqueous | MBC | −5.3499 | −2.3952 | 2.9547 | −0.3693 | −52,261.09 | 0.1970 | 0.6769 | 5.0754 | 3.8725 | 1.0585 | 1.3106 | 1.4774 | 91.2 |
| HBM | −5.2743 | −1.9849 | 3.2894 | −0.4112 | −31,755.13 | 0.2497 | 0.6080 | 4.0049 | 3.6296 | 1.0246 | 1.1034 | 1.6447 | 86.3 | |
| Protonated | ||||||||||||||
| gas phase | MBC-H2+ | −10.5274 | −9.4107 | 1.1167 | −0.1396 | −52,266.230 | 0.0112 | 1.7910 | 88.997 | 9.9691 | −2.6588 | 8.9273 | 0.5583 | 91.2 |
| HBM-H2+ | −10.7963 | −8.1222 | 2.6741 | −0.3343 | −31,769.026 | 0.0299 | 0.7479 | 33.460 | 9.4593 | −0.9196 | 3.5373 | 1.3371 | 86.3 | |
| aqueous | MBC-H2+ | −5.9557 | −4.1612 | 1.7945 | −0.2243 | −52,271.259 | 0.0701 | 1.1145 | 14.258 | 5.0584 | 1.0819 | 2.8188 | 0.8973 | 91.2 |
| HBM-H2+ | −6.1254 | −3.5374 | 2.5879 | −0.3235 | −31,776.083 | 0.1109 | 0.7728 | 9.0196 | 4.8314 | 0.8380 | 1.8669 | 1.2940 | 86.3 | |
| Atom | MBC | Atom | HBM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas Phase | Aqueous Phase | Gas Phase | Aqueous Phase | ||||||||||
| C (1) | 0.028 | 0.018 | 0.01 | 0.033 | 0.024 | 0.009 | C (1) | 0.025 | 0.015 | 0.01 | 0.032 | 0.019 | 0.013 |
| O (2) | 0.035 | 0.041 | −0.006 | 0.034 | 0.041 | −0.007 | O (2) | 0.039 | 0.037 | 0.002 | 0.04 | 0.036 | 0.004 |
| C (3) | 0.01 | 0.008 | 0.002 | 0.012 | 0.012 | 0 | C (3) | 0.009 | 0.007 | 0.002 | 0.012 | 0.009 | 0.003 |
| C (4) | 0.028 | 0.018 | 0.01 | 0.033 | 0.024 | 0.009 | C (4) | 0.025 | 0.015 | 0.01 | 0.032 | 0.019 | 0.013 |
| O (5) | 0.035 | 0.041 | −0.006 | 0.034 | 0.041 | −0.007 | O (5) | 0.039 | 0.037 | 0.002 | 0.04 | 0.036 | 0.004 |
| N (6) | 0.02 | 0.042 | −0.022 | 0.026 | 0.05 | −0.024 | N (6) | 0.014 | 0.038 | −0.024 | 0.019 | 0.039 | −0.02 |
| N (7) | 0.011 | 0.041 | −0.03 | 0.015 | 0.046 | −0.031 | N (7) | 0.014 | 0.038 | −0.024 | 0.019 | 0.04 | −0.021 |
| C (8) | 0.046 | 0.01 | 0.036 | 0.051 | 0.016 | 0.035 | N (8) | 0.044 | 0.029 | 0.015 | 0.053 | 0.039 | 0.014 |
| N (9) | 0.017 | 0.029 | −0.012 | 0.021 | 0.034 | −0.013 | N (9) | 0.044 | 0.029 | 0.015 | 0.053 | 0.039 | 0.014 |
| S (10) | 0.095 | 0.063 | 0.032 | 0.097 | 0.054 | 0.043 | C (10) | 0.049 | 0.032 | 0.017 | 0.057 | 0.032 | 0.025 |
| C (11) | 0.012 | 0.012 | 0 | 0.018 | 0.017 | 0.001 | C (11) | 0.049 | 0.032 | 0.017 | 0.058 | 0.032 | 0.026 |
| C (12) | 0.017 | 0.019 | −0.002 | 0.021 | 0.021 | 0 | C (12) | 0.017 | 0.024 | −0.007 | 0.022 | 0.031 | −0.009 |
| C (13) | 0.02 | 0.019 | 0.001 | 0.016 | 0.015 | 0.001 | C (13) | 0.017 | 0.024 | −0.007 | 0.023 | 0.032 | −0.009 |
| C (14) | 0.037 | 0.037 | 0 | 0.029 | 0.03 | −0.001 | C (14) | 0.02 | 0.027 | −0.007 | 0.024 | 0.032 | −0.008 |
| C (15) | 0.023 | 0.022 | 0.001 | 0.018 | 0.016 | 0.002 | C (15) | 0.026 | 0.023 | 0.003 | 0.022 | 0.022 | 0 |
| C (16) | 0.019 | 0.02 | −0.001 | 0.019 | 0.021 | −0.002 | C (16) | 0.048 | 0.04 | 0.008 | 0.041 | 0.034 | 0.007 |
| N (17) | 0.02 | 0.042 | −0.022 | 0.026 | 0.05 | −0.024 | C (17) | 0.026 | 0.037 | −0.011 | 0.021 | 0.036 | −0.015 |
| N (18) | 0.011 | 0.041 | −0.03 | 0.015 | 0.046 | −0.031 | C (18) | 0.029 | 0.021 | 0.008 | 0.03 | 0.02 | 0.01 |
| C (19) | 0.046 | 0.01 | 0.036 | 0.051 | 0.016 | 0.035 | C (19) | 0.029 | 0.02 | 0.009 | 0.03 | 0.021 | 0.009 |
| N (20) | 0.017 | 0.029 | −0.012 | 0.021 | 0.034 | −0.013 | C (20) | 0.026 | 0.037 | −0.011 | 0.022 | 0.036 | −0.014 |
| S (21) | 0.095 | 0.063 | 0.032 | 0.097 | 0.054 | 0.043 | C (21) | 0.048 | 0.04 | 0.008 | 0.041 | 0.034 | 0.007 |
| C (22) | 0.012 | 0.012 | 0 | 0.018 | 0.017 | 0.001 | C (22) | 0.026 | 0.023 | 0.003 | 0.022 | 0.022 | 0 |
| C (23) | 0.019 | 0.02 | −0.001 | 0.019 | 0.021 | −0.002 | C (23) | 0.02 | 0.027 | −0.007 | 0.024 | 0.033 | −0.009 |
| C (24) | 0.023 | 0.022 | 0.001 | 0.018 | 0.016 | 0.002 | O (24) | 0.016 | 0.034 | −0.018 | 0.019 | 0.041 | −0.022 |
| C (25) | 0.037 | 0.037 | 0 | 0.029 | 0.03 | −0.001 | O (25) | 0.016 | 0.034 | −0.018 | 0.018 | 0.04 | −0.022 |
| C (26) | 0.02 | 0.019 | 0.001 | 0.016 | 0.015 | 0.001 | |||||||
| C (27) | 0.017 | 0.019 | −0.002 | 0.021 | 0.021 | 0 | |||||||
| Inhibitor | Neutral | Protonated | ||
|---|---|---|---|---|
| MBC | HBM | MBC-H2+ | HBM-H2+ | |
| Total energy (kcal mol−1) | −6316.14 | −6315.12 | −6181.61 | −6164.68 |
| Adsorption energy (kcal mol−1) | −7285.79 | −6473.38 | −7245.47 | −6416.35 |
| Rigid adsorption energy (kcal mol−1) | −6358.46 | −6345.46 | −6318.53 | −6228.34 |
| Deformation energy (kcal mol−1) | −927.33 | −127.91 | −926.94 | −188.01 |
| (dEads/dNi) (kcal mol−1) | −1197.17 | −379.55 | −1378.37 | −629.96 |
| Binding energy (kcal mol−1) | 7285.79 | 6473.38 | 7245.47 | 6416.35 |
| IEEIS (%) | 91.2 | 86.3 | 91.2 | 86.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarfaji, S.S.; Ali, I.H.; Bani-Fwaz, M.Z.; Bedair, M.A. Synthesis and Assessment of Two Malonyl Dihydrazide Derivatives as Corrosion Inhibitors for Carbon Steel in Acidic Media: Experimental and Theoretical Studies. Molecules 2021, 26, 3183. https://doi.org/10.3390/molecules26113183
Alarfaji SS, Ali IH, Bani-Fwaz MZ, Bedair MA. Synthesis and Assessment of Two Malonyl Dihydrazide Derivatives as Corrosion Inhibitors for Carbon Steel in Acidic Media: Experimental and Theoretical Studies. Molecules. 2021; 26(11):3183. https://doi.org/10.3390/molecules26113183
Chicago/Turabian StyleAlarfaji, Saleh S., Ismat H. Ali, Mutasem Z. Bani-Fwaz, and Mahmoud A. Bedair. 2021. "Synthesis and Assessment of Two Malonyl Dihydrazide Derivatives as Corrosion Inhibitors for Carbon Steel in Acidic Media: Experimental and Theoretical Studies" Molecules 26, no. 11: 3183. https://doi.org/10.3390/molecules26113183
APA StyleAlarfaji, S. S., Ali, I. H., Bani-Fwaz, M. Z., & Bedair, M. A. (2021). Synthesis and Assessment of Two Malonyl Dihydrazide Derivatives as Corrosion Inhibitors for Carbon Steel in Acidic Media: Experimental and Theoretical Studies. Molecules, 26(11), 3183. https://doi.org/10.3390/molecules26113183








