The Latest Trends in Electric Vehicles Batteries
Abstract
1. Introduction
2. The Positive Electrode (Cathode)
2.1. Cathode Chemistry and Microstructure
2.2. Lithium-Iron-Phosphate (LFP) and Lithium-Manganese Oxide (LMO)
2.3. Layered Oxides
2.4. Lithium Cobalt Oxide, LiCoO
2.5. Lithium Nickel Oxide, LiNiO
2.6. Lithium Manganese Oxide, LiMnO
2.7. Lithium Nickel Manganese Cobalt Oxide, NMC, and Lithium Nickel Cobalt Aluminum Oxide, NCA
Problems Associated with Ni-rich LiNiMnCoO Cathodes
- Electrolyte oxidation is more severe for NMC cathodes, owing to both increased rate of Mn dissolution, opposed to Al dissociation from NCA and higher susceptibility for irreversible phase transitions, mainly from layered oxide to NiO rock-salt phase. Even though this transformation occurs for both types of cathodes, the tendency of Mn-based layers to form a spinel-like structure promotes the formation of this phase.
- Particle pulverization, or separation of primary particles, is caused by severe volume changes during cycling. The transition from H2 to H3 and vice-versa introduces severe anisotropic lattice changes, which serve as a driving force for intergranular cracks. NCA suffers larger volume changes than NMC cathodes, suggesting that Mn is more effective than Al at mitigating volume changes.
- The migration of cathode dissolution to graphitic anodes produces damages at the solid electrolyte interphase on the anode side, leading to a capacity decay. Again, the couple NMC/graphite seems to be more susceptible to this degradation mechanism in comparison with NCA/graphite. It was also concluded [69] that dissolution/crossover of transition metals and irreversible phase transformation in NMC outweighs the susceptibility to particle pulverization of NCA, leading to a superior capacity decay of the former type of electrode. Reducing the chemical activity of the electrolyte-electrode interface is a key factor towards achieving enhanced cyclability of NMC-based LIBs. On the other hand, intergranular cracks (particle pulverization) of both Ni-rich cathodes severely affect the structural integrity, and, thus, the cycle life of the cell.
2.8. Solutions for Mitigating Low Ionic Conductivity and Capacity Fading of Ni-Rich Cathodes
3. The Negative Electrode (Anode)
- Low chemical potential (Li)-(LiC) ≈ 0.1 eV, meaning that a lithiated graphite (LiC) anode shows an equilibrium plateau discharge voltage that is 0.1 V lower than a Li-metal anode, for a cell containing a similar cathode and internal resistance.
- Significant worldwide reserves.
- Good electrochemical stability [75].
- Safer than Lithium in case of fire.
3.1. Silicon Anodes
3.2. Importance of Particle Size for Si Nanoparticles (SiNPs) Anodes
3.3. Design of Nano-Si/Carbon Composite Anodes
3.4. Progress in Si Composite Anodes Design
3.5. Other Alternatives for Improving Si Anodes
4. Future Prospects
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Linder, C. Elon Musk Finally Reveals His Grand Plans to Revolutionize the Battery. 2020. Available online: https://www.popularmechanics.com/science/energy/a34114885/elon-musk-tesla-battery-day-recap/ (accessed on 25 September 2020).
- Lyons, K. Here are Tesla’s Biggest Announcements from Battery Day. 2020. Available online: https://www.theverge.com/2020/9/22/21450840/tesla-battery-day-production-elon-musk-tabless-range-cathode-cobalt-plaid (accessed on 25 September 2020).
- Kelly, A. Pollution Causing Birth Defects in Children of DRC Cobalt Miners–Study. 2020. Available online: https://www.theguardian.com/global-development/2020/may/06/pollution-causing-birth-defects-in-children-of-drc-cobalt-miners-study (accessed on 13 June 2020).
- CLowes, W. DRC Moves to Monopolise about 25 Percent of All cobalt Export. 2020. Available online: https://www.aljazeera.com/economy/2020/1/30/drc-moves-to-monopolise-about-25-percent-of-all-cobalt-exports (accessed on 13 June 2020).
- Hu, J.; Wu, B.; Cao, X.; Bi, Y.; Chae, S.; Niu, C.; Xiao, B.; Tao, J.; Zhang, J.; Xiao, J. Evolution of the rate-limiting step: From thin film to thick Ni-rich cathodes. J. Power Sources 2020, 454, 227966. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, M.; Li, J.; Zhang, D.; Yan, Y.; Li, Z. Recent progress in coatings and methods of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials: A short review. Ceram. Int. 2020, 46, 21888–21901. [Google Scholar] [CrossRef]
- Kim, Y.; Seong, W.M.; Manthiram, A. Cobalt-free, high-nickel layered oxide cathodes for lithium-ion batteries: Progress, challenges, and perspectives. Energy Storage Mater. 2021, 34, 250–259. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Wade, A.; Tan, C.; Parker, J.E.; Matras, D.; Leach, A.S.; Robinson, J.B.; Llewellyn, A.; Dimitrijevic, A.; Jervis, R.; et al. Identifying the Origins of Microstructural Defects Such as Cracking within Ni-Rich NMC811 Cathode Particles for Lithium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2002655. [Google Scholar] [CrossRef]
- KrannichSolarGermany. LG Chem ESS Cell. 2018. Available online: https://www.youtube.com/watch?v=CU0RGPnwYYA (accessed on 13 June 2020).
- Jürgens, J. This is Why NMC is the Preferable Cathode Material for Li-Ion Batteries. 2019. Available online: https://lghomebatteryblog.eu/en/this-is-why-ncm-is-the-preferable-cathode-material-for-li-ion-batteries/ (accessed on 13 June 2020).
- Commission, E. Commission Publishes Information on CO2 Emissions from Maritime Transport. 2019. Available online: https://ec.europa.eu/clima/news/commission-publishes-information-co2-emissions-maritime-transport_en (accessed on 14 June 2020).
- Commission, E. Reducing Emissions from the Shipping Sector. 2020. Available online: https://ec.europa.eu/clima/policies/transport/shipping_en (accessed on 13 June 2020).
- Helgesen, S.; Aarseth Langli, A. Electrical Energy Storage for Ships; EMSA Report. 2020. Available online: http://www.emsa.europa.eu/publications/item/3895-study-on-electrical-energy-storage-for-ships.html (accessed on 19 May 2021).
- Airbus. This New Airbus Facility will Help Zero-Emission Technologies to Take Flight. 2019. Available online: https://www.airbus.com/newsroom/news/en/2019/10/new-airbus-facility-will-help-zero-emission-technologies-to-take-flight.html (accessed on 16 June 2020).
- Airbus. Zephyr-Pioneering the Stratosphere. 2020. Available online: https://www.airbus.com/defence/uav/zephyr.html (accessed on 16 June 2020).
- Downing, S. 6 Electric Aviation Companies to Watch. 2019. Available online: https://www.greenbiz.com/article/6-electric-aviation-companies-watch (accessed on 16 June 2020).
- Baraniuk, C. The Largest Electric Plane Ever to Fly. 2020. Available online: Available online: https://www.bbc.com/future/article/20200617-the-largest-electric-plane-ever-to-fly (accessed on 18 June 2020).
- Airbus. Airbus, Rolls-Royce, and Siemens Team up for Electric Future Partnership Launches E-Fan X Hybrid-Electric Flight Demonstrator. 2017. Available online: https://www.airbus.com/newsroom/press-releases/en/2017/11/airbus–rolls-royce–and-siemens-team-up-for-electric-future-par.html (accessed on 16 June 2020).
- Kaminski-Morrow, D. Airbus and Rolls-Royce cancel E-Fan X hybrid-Electric RJ100 Experiment. 2020. Available online: https://www.flightglobal.com/air-transport/airbus-and-rolls-royce-cancel-e-fan-x-hybrid-electric-rj100-experiment/138067.article (accessed on 16 June 2020).
- Shahan, C. Airbus and Rolls-Royce End E-Fan X Electric Demo Project, Still Working To Decarbonize Aviation. 2020. Available online: 16 Jun. 2020 Available online: https://cleantechnica.com/2020/06/26/airbus-rolls-royce-end-e-fan-x-electric-demo-project-still-working-to-decarbonize-aviation/ (accessed on 18 June 2020).
- Bailey, J. Could The Emissions Free Airbus Of The Future Be The A220? 2020. Available online: Available online: https://simpleflying.com/airbus-emissions-free-aircraft/ (accessed on 2 July 2020).
- Wheeler, E. Electric Vehicles to Set New Market Share Record in 2020. 2020. Available online: https://www.spglobal.com/marketintelligence/en/news-insights/latest-news-headlines/electric-vehicles-to-set-new-market-share-record-in-2020-59050766 (accessed on 18 June 2020).
- Gorner, M.; Paoli, L. How Global Electric Car Sales Defied Covid-19 in 2020. 2021. Available online: https://www.iea.org/commentaries/how-global-electric-car-sales-defied-covid-19-in-2020 (accessed on 14 February 2021).
- Energy, U.; Batteries for Hybrid and plug-In Electric Vehicles. Alternative Fuels Data Center 2020. 2020. Available online: https://afdc.energy.gov/vehicles/electric_batteries.html (accessed on 13 June 2020).
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Kane, M. LG Chem Is Now The Biggest xEV Battery Maker By Capacity. 2020. Available online: https://insideevs.com/news/408484/lg-chem-biggest-xev-battery-maker-capacity/ (accessed on 8 June 2020).
- Zart, N. The state of EV Batteries: LG Chem, SK Innovations, & Tesla-Panasonic Improvements. 2018. Available online: https://smartcity.lv/the-state-of-ev-batteries-lg-chem-sk-innovation-tesla-panasonic-improvements/ (accessed on 8 June 2020).
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles andr Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-Art and Energy Management System of Lithium-Ion Batteries in Electric Vehicle Applications: Issues and Recommendations. IEEE Access 2018, 6, 19362–19378. [Google Scholar] [CrossRef]
- Battery University. BU-301a: Types of Battery Cells. 2011. Available online: https://batteryuniversity.com/learn/article/types_of_battery_cells (accessed on 20 May 2020).
- Lima, P. Renault 5 Electric Will Be Truly Affordable Thanks to LFP Batteries. 2021. Available online: https://pushevs.com/2021/02/13/renault-5-electric-will-be-truly-affordable-thanks-to-lfp-batteries/ (accessed on 18 February 2021).
- October, D. Here Are the Raw Materials We Need. 2016. Available online: https://www.businessinsider.com/materials-needed-to-fuel-electric-car-boom-2016-10 (accessed on 13 June 2020).
- Lima, P. Comparison of different EV batteries in 2020. 2020. Available online: https://pushevs.com/2020/04/04/comparison-of-different-ev-batteries-in-2020/ (accessed on 13 June 2020).
- Nissan, G. High Capacity Lithium-Ion Battery in a Lightweight, Compact Design. 2020. Available online: https://www.nissan-global.com/EN/TECHNOLOGY/OVERVIEW/li_ion_ev.html (accessed on 13 June 2020).
- Walz, E. Mercedes Benz Scales Back Production of the Electric EQC Due to Battery Shortage. 2020. Available online: https://www.futurecar.com/3744/Mercedes-Benz-Scales-Back-Production-of-the-Electric-EQC-Due-to-Battery-Shortage (accessed on 18 June 2020).
- Lima, P. Tesla Model 3 Cobalt-Free LFP Version Details. 2020. Available online: https://pushevs.com/2020/06/11/tesla-model-3-cobalt-free-lfp-version-details/ (accessed on 18 June 2020).
- Ueki, K.; Toriyama, J.; Seyama, Y.; Nishiyama, K. Development of Large-Sized Long-Life Type Lithium-ion Cells for Eletric Vehicles. 2012. Available online: https://www.gs-yuasa.com/en/technic/vol9/pdf/009_01_026.pdf (accessed on 13 June 2020).
- Lima, P.; GS Yuasa’s Improved Cells: LEV50 vs. LEV50N. 2015. Available online: Available online: https://pushevs.com/2015/11/04/gs-yuasa-improved-cells-lev50-vs-lev50n/ (accessed on 13 June 2020).
- Kane, M. Here is the Nissan Leaf e+ 62kWh Battery. 2019. Available online: Available online: https://insideevs.com/news/342009/here-is-the-nissan-leaf-e-62-kwh-battery-video/ (accessed on 13 June 2020).
- Albatts, P. ALBATTS|Results. 2020. Available online: https://www.project-albatts.eu/en/results (accessed on 15 January 2021).
- Home, A. Tesla’s Reluctant Commitment to Cobalt a Warning to Others-Andy Home. 2020. Available online: https://www.reuters.com/article/us-tesla-cobalt-ahome-idUSKBN23U20Q (accessed on 2 July 2020).
- Phadatare, M.; Patil, R.; Blomquist, N.; Forsberg, S.; Örtegren, J.; Hummelgård, M.; Meshram, J.; Hernández, G.; Brandell, D.; Leifer, K.; et al. Silicon-Nanographite Aerogel-Based Anodes for High Performance Lithium Ion Batteries. Sci. Rep. 2019, 9, 14621. [Google Scholar] [CrossRef]
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery Technologies for Grid-Level Large-Scale Electrical Energy Storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef]
- Gong, C.; Xue, Z.; Wen, S.; Ye, Y.; Xie, X. Advanced carbon materials/olivine LiFePO4 composites cathode for lithium ion batteries. J. Power Sources 2016, 318, 93–112. [Google Scholar] [CrossRef]
- Zhang, W.J. Structure and performance of LiFePO4 cathode materials: A review. J. Power Sources 2011, 196, 2962–2970. [Google Scholar] [CrossRef]
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M.; et al. Lithium-ion batteries – Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Mohamed, N.; Allam, N.K. Recent advances in the design of cathode materials for Li-ion batteries. RSC Adv. 2020, 10, 21662–21685. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, G.S.; Jung, C.; Ko, D.S.; Park, S.Y.; Kim, H.G.; Hong, S.H.; Zhu, Y.; Kim, M. Revisiting Primary Particles in Layered Lithium Transition-Metal Oxides and Their Impact on Structural Degradation. Adv. Sci. 2019, 6, 1800843. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; et al. Kinetics Tuning of Li-Ion Diffusion in Layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef] [PubMed]
- Delmas, C.; Carlier, D.; Guignard, M. The Layered Oxides in Lithium and Sodium-Ion Batteries: A Solid-State Chemistry Approach. Adv. Energy Mater. 2021, 11, 2001201. [Google Scholar] [CrossRef]
- Lu, Y.C.; Gallant, B.M.; Kwabi, D.G.; Harding, J.R.; Mitchell, R.R.; Whittingham, M.S.; Shao-Horn, Y. Lithium–oxygen batteries: Bridging mechanistic understanding and battery performance. Energy Environ. Sci. 2013, 6, 750–768. [Google Scholar] [CrossRef]
- Dahn, J.R.; von Sacken, U.; Michal, C.A. Structure and electrochemistry of Li1±yNiO2 and a new Li2NiO2 phase with the Ni (OH)2 structure. Solid State Ionics 1990, 44, 87–97. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, L.; Zhu, C.; Ren, W.; Kong, L.; Wan, Y. Nano LiFePO4 coated Ni rich composite as cathode for lithium ion batteries with high thermal ability and excellent cycling performance. J. Power Sources 2020, 464, 228235. [Google Scholar] [CrossRef]
- Chen, G.; Peng, B.; Han, R.; Chen, N.; Wang, Z.; Wang, Q. A robust carbon coating strategy toward Ni-rich lithium cathodes. Ceram. Int. 2020, 46, 20985–20992. [Google Scholar] [CrossRef]
- Kim, U.H.; Park, N.Y.; Park, G.T.; Kim, H.; Yoon, C.S.; Sun, Y.K. High-Energy W-Doped Li[Ni0.95Co0.04Al0.01]O2 Cathodes for Next-Generation Electric Vehicles. Energy Storage Mater. 2020, 33, 399–407. [Google Scholar] [CrossRef]
- Tang, L.b.; Liu, Y.; Wei, H.x.; Yan, C.; He, Z.j.; Li, Y.j.; Zheng, J.c. Boosting cell performance of LiNi0.8Co0.1Mn0.1O2 cathode material via structure design. J. Energy Chem. 2021, 55, 114–123. [Google Scholar] [CrossRef]
- Jeong, M.; Kim, H.; Lee, W.; Ahn, S.J.; Lee, E.; Yoon, W.S. Stabilizing effects of Al-doping on Ni-rich LiNi0.80Co0.15Mn0.05O2 cathode for Li rechargeable batteries. J. Power Sources 2020, 474, 228592. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, Q.; Li, B.; Manthiram, A. An in-depth understanding of the effect of aluminum doping in high-nickel cathodes for lithium-ion batteries. Energy Storage Mater. 2021, 34, 229–240. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Jin, B.S.; Kim, H.S. Preparation and electrochemical performances of Ni-rich LiNi0.91Co0.06Mn0.03O2 cathode for high-energy LIBs. Int. J. Hydrogen Energy 2019, 44, 13684–13689. [Google Scholar] [CrossRef]
- Fan, X.; Hu, G.; Zhang, B.; Ou, X.; Zhang, J.; Zhao, W.; Jia, H.; Zou, L.; Li, P.; Yang, Y. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Lin, Q.; Guan, W.; Zhou, J.; Meng, J.; Huang, W.; Chen, T.; Gao, Q.; Wei, X.; Zeng, Y.; Li, J.; et al. Ni–Li anti-site defect induced intragranular cracking in Ni-rich layer-structured cathode. Nano Energy 2020, 76, 105021. [Google Scholar] [CrossRef]
- Zhu, L.; Bao, C.; Xie, L.; Yang, X.; Cao, X. Review of synthesis and structural optimization of LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium-ion batteries applications. J. Alloy. Compd. 2020, 831, 154864. [Google Scholar] [CrossRef]
- Hu, D.; Su, Y.; Chen, L.; Li, N.; Bao, L.; Lu, Y.; Zhang, Q.; Wang, J.; Chen, S.; Wu, F. The mechanism of side reaction induced capacity fading of Ni-rich cathode materials for lithium ion batteries. J. Energy Chem. 2020, 58, 1–8. [Google Scholar] [CrossRef]
- Guan, P.; Zhou, L.; Yu, Z.; Sun, Y.; Liu, Y.; Wu, F.; Jiang, Y.; Chu, D. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. J. Energy Chem. 2020, 43, 220–235. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich NMC-Based Lithium-Ion Batteries. Electrochem. Energy Rev. 2020, 3, 43–80. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity Fading of Ni-Rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Yang, Z. A review on cathode materials for advanced lithium ion batteries: microstructure designs and performance regulations. Nanotechnology 2019, 31, 012001. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, X.; Celio, H.; Smith, P.; Dolocan, A.; Chi, M.; Manthiram, A. Mn versus Al in Layered Oxide Cathodes in Lithium-Ion Batteries: A Comprehensive Evaluation on Long-Term Cyclability. Adv. Energy Mater. 2018, 8, 1703154. [Google Scholar] [CrossRef]
- Schmidt, D.; Kamlah, M.; Knoblauch, V. Highly densified NCM-cathodes for high energy Li-ion batteries: Microstructural evolution during densification and its influence on the performance of the electrodes. J. Energy Storage 2018, 17, 213–223. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, B.; Su, S.L.; Ming, L.; Zhao, Y.; Wang, C.H.; Ou, X. Comparison of monocrystalline and secondary LiNi0.5Co0.2Mn0.3O2 cathode material for high-performance lithium-ion batteries. J. Alloy. Compd. 2020, 845, 156202. [Google Scholar] [CrossRef]
- Wu, Y.S.; Pham, Q.T.; Yang, C.C.; Chern, C.S.; Musuvadhi Babulal, L.; Seenivasan, M.; Brunklaus, G.; Placke, T.; Hwang, B.J.; Winter, M. Study of electrochemical performance and thermal property of LiNi0.5Co0.2Mn0.3O2 cathode materials coated with a novel oligomer additive for high-safety lithium-ion batteries. Chem. Eng. J. 2021, 405, 126727. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material – fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Kwon, H.J.; Hwang, J.Y.; Shin, H.J.; Jeong, M.G.; Chung, K.Y.; Sun, Y.K.; Jung, H.G. Nano/Microstructured Silicon–Carbon Hybrid Composite Particles Fabricated with Corn Starch Biowaste as Anode Materials for Li-Ion Batteries. Nano Lett. 2020, 20, 625–635. [Google Scholar] [CrossRef]
- Mao, C.; Wood, M.; David, L.; An, S.J.; Sheng, Y.; Du, Z.; Meyer, H.M.; Ruther, R.E.; Wood, D.L. Selecting the Best Graphite for Long-Life, High-Energy Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A1837–A1845. [Google Scholar] [CrossRef]
- Scott, A. In the Battery Materials World, the Anode’s Time Has Come. 2019. Available online: https://cen.acs.org/materials/energy-storage/battery-materials-world-anodes-time/97/i14 (accessed on 2 July 2020).
- Wu, Z.; Kong, D. Comparative life cycle assessment of lithium-ion batteries with lithium metal, silicon nanowire, and graphite anodes. Clean Technol. Environ. Policy 2018, 20, 1233–1244. [Google Scholar] [CrossRef]
- Dose, W.M.; Xu, C.; Grey, C.P.; De Volder, M.F. Effect of Anode Slippage on Cathode Cutoff Potential and Degradation Mechanisms in Ni-Rich Li-Ion Batteries. Cell Rep. Phys. Sci. 2020, 1, 100253. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Zhang, H.L.; Liu, S.H.; Li, F.; Bai, S.; Liu, C.; Tan, J.; Cheng, H.M. Electrochemical performance of pyrolytic carbon-coated natural graphite spheres. Carbon 2006, 44, 2212–2218. [Google Scholar] [CrossRef]
- Al Hassan, M.R.; Sen, A.; Zaman, T.; Mostari, M.S. Emergence of graphene as a promising anode material for rechargeable batteries: A review. Mater. Today Chem. 2019, 11, 225–243. [Google Scholar] [CrossRef]
- Altairnano. Products. 2020. Available online: https://altairnano.com/products/ (accessed on 2 July 2020).
- Leclanche. Cells. Available online: https://www.leclanche.com/our-technologies/cell/ (accessed on 2 July 2020).
- Toshiba. What is SciB? 2020. Available online: https://www.scib.jp/en/about/index.htm (accessed on 13 June 2020).
- Toyohara. Toshiba IR Day 2019 Battery Division. 2019. Available online: https://www.toshiba.co.jp/about/ir/en/pr/pdf/tpr20191114_7e.pdf (accessed on 13 June 2020).
- Li, P.; Zhao, G.; Zheng, X.; Xu, X.; Yao, C.; Sun, W.; Dou, S.X. Recent progress on silicon-based anode materials for practical lithium-ion battery applications. Energy Storage Mater. 2018, 15, 422–446. [Google Scholar] [CrossRef]
- Son, Y.; Kim, N.; Lee, T.; Lee, Y.; Ma, J.; Chae, S.; Sung, J.; Cha, H.; Yoo, Y.; Cho, J. Calendering-Compatible Macroporous Architecture for Silicon–Graphite Composite toward High-Energy Lithium-Ion Batteries. Adv. Mater. 2020, 32, 2003286. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Kang, W.; Ye, Y.; Pan, Q.; Zhang, X.; Ke, Y.; Wang, C.; Qiu, Z.; Tang, Y. A review on silicon nanowire-based anodes for next-generation high-performance lithium-ion batteries from a material-based perspective. Sustain. Energy Fuels 2020, 4, 1577–1594. [Google Scholar] [CrossRef]
- Amprius. 100% Silicon Nanowire Batteries from Amprius Technologies. 2020. Available online: https://www.amprius.com/technology/ (accessed on 2 July 2020).
- Li, C.; Zhu, W.; Lao, B.; Huang, X.; Yin, H.; Yang, Z.; Wang, H.; Chen, D.; Xu, Y. Lithium Difluorophosphate as an Effective Additive for Improving the Initial Coulombic Efficiency of a Silicon Anode. ChemElectroChem 2020, 7, 3743–3751. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Bhandakkar, T.K.; Gao, H. Cohesive modeling of crack nucleation in a cylindrical electrode under axisymmetric diffusion induced stresses. Int. J. Solids Struct. 2011, 48, 2304–2309. [Google Scholar] [CrossRef]
- McDowell, M.T.; Ryu, I.; Lee, S.W.; Wang, C.; Nix, W.D.; Cui, Y. Studying the Kinetics of Crystalline Silicon Nanoparticle Lithiation with In Situ Transmission Electron Microscopy. Adv. Mater. 2012, 24, 6034–6041. [Google Scholar] [CrossRef]
- Domi, Y.; Usui, H.; Sugimoto, K.; Sakaguchi, H. Effect of Silicon Crystallite Size on Its Electrochemical Performance for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1800946. [Google Scholar] [CrossRef]
- Morita, T.; Takami, N. Nano Si Cluster-SiOx-C Composite Material as High-Capacity Anode Material for Rechargeable Lithium Batteries. J. Electrochem. Soc. 2006, 153, A425–A430. [Google Scholar] [CrossRef]
- Hwang, S.W.; Yoon, W.Y. Effect of Li Powder-Coated Separator on Irreversible Behavior of SiOx-C Anode in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A1753–A1758. [Google Scholar] [CrossRef]
- Zhao, J.; Lee, H.W.; Sun, J.; Yan, K.; Liu, Y.; Liu, W.; Lu, Z.; Lin, D.; Zhou, G.; Cui, Y. Metallurgically lithiated SiO<sub>x</sub> anode with high capacity and ambient air compatibility. Proc. Natl. Acad. Sci. USA 2016, 113, 7408. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, J.K.; Yin, Y.X.; Guo, Y.G. Facile Synthesis of Blocky SiOx/C with Graphite-Like Structure for High-Performance Lithium-Ion Battery Anodes. Adv. Funct. Mater. 2018, 28, 1705235. [Google Scholar] [CrossRef]
- Tao, Z.; Lijun, F.; Jie, G.; Lichun, Y.; Yuping, W.; Hoqing, W. Core-shell Si/C nanocomposite as anode material for lithium ion batteries. Pure Appl. Chem. 2006, 78, 1889–1896. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Xu, Z.; Wang, J.; Nuli, Y.; Zhuang, X.; Feng, X. Silicon anodes protected by a nitrogen-doped porous carbon shell for high-performance lithium-ion batteries. Nanoscale 2017, 9, 8871–8878. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Zhang, C.; Park, J.D.; Razmjooei, F.; Yu, J.S. Silicon core-mesoporous shell carbon spheres as high stability lithium-ion battery anode. J. Colloid Interface Sci. 2019, 534, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wu, H.; McDowell, M.; Yao, Y.; Wang, C.; Cui, Y. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 2012, 12, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, K.; Hou, X.; Zhang, G.; Wang, S.; Chen, F.; Fu, L.; Qin, H.; Xia, Y.; Zhou, G. Si-based anode with hierarchical protective function and hollow ring-like carbon matrix for high performance lithium ion batteries. Appl. Surf. Sci. 2019, 470, 496–506. [Google Scholar] [CrossRef]
- Ma, Q.; Xie, H.; Qu, J.; Zhao, Z.; Zhang, B.; Song, Q.; Xing, P.; Yin, H. Tailoring the Polymer-Derived Carbon Encapsulated Silicon Nanoparticles for High-Performance Lithium-Ion Battery Anodes. ACS Appl. Energy Mater. 2020, 3, 268–278. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Wei, H.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Porosity- and Graphitization-Controlled Fabrication of Nanoporous Silicon@Carbon for Lithium Storage and Its Conjugation with MXene for Lithium-Metal Anode. Adv. Funct. Mater. 2020, 30, 1908721. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, M.; Yuan, Y.; Wu, Q.; Wang, H.; He, Y.; Lin, Z.; Zhou, F.; Ling, M.; Qian, C.; et al. Accommodation of Silicon in an Interconnected Copper Network for Robust Li-Ion Storage. Adv. Funct. Mater. 2020, 30, 1910249. [Google Scholar] [CrossRef]
- Hu, L.; Luo, B.; Wu, C.; Hu, P.; Wang, L.; Zhang, H. Yolk-shell Si/C composites with multiple Si nanoparticles encapsulated into double carbon shells as lithium-ion battery anodes. J. Energy Chem. 2019, 32, 124–130. [Google Scholar] [CrossRef]
- Cai, X.; Liu, W.; Yang, S.; Zhang, S.; Gao, Q.; Yu, X.; Li, J.; Wang, H.; Fang, Y. Dual-Confined SiO Embedded in TiO2 Shell and 3D Carbon Nanofiber Web as Stable Anode Material for Superior Lithium Storage. Adv. Mater. Interfaces 2019, 6, 1801800. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, P.; Chen, M.; Jin, H.; Bai, Y.; Li, S.; Ma, F.; Xu, H.; Lian, K. In Situ Synthesis of Multilayer Carbon Matrix Decorated with Copper Particles: Enhancing the Performance of Si as Anode for Li-Ion Batteries. ACS Nano 2019, 13, 3054–3062. [Google Scholar] [CrossRef]
- Majeed, M.K.; Ma, G.; Cao, Y.; Mao, H.; Ma, X.; Ma, W. Metal–Organic Frameworks-Derived Mesoporous Si/SiOx@NC Nanospheres as a Long-Lifespan Anode Material for Lithium-Ion Batteries. Chem. A Eur. J. 2019, 25, 11991–11997. [Google Scholar] [CrossRef]
- Shi, M.; Nie, P.; Fu, R.; Fang, S.; Li, Z.; Dou, H.; Zhang, X. Catalytic Growth of Graphitic Carbon-Coated Silicon as High-Performance Anodes for Lithium Storage. Energy Technol. 2019, 7, 1900502. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Dong, B.; Yan, Y.; Zerrin, T.; Ozkan, M.; Ozkan, C.S. Scalable coral-like silicon powders with three-dimensional interconnected structures for lithium ion battery anodes. Energy Storage 2020, 2, e187. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.X.; Chou, S.L.; Zhang, R.; Xu, Y.; Fan, J.; Zhang, W.X.; Kun Liu, H.; Zhao, D.; Xue Dou, S. Yolk-shell silicon-mesoporous carbon anode with compact solid electrolyte interphase film for superior lithium-ion batteries. Nano Energy 2015, 18, 133–142. [Google Scholar] [CrossRef]
- Smrekar, S.; Bracamonte, M.V.; Primo, E.N.; Luque, G.L.; Thomas, J.; Barraco, D.E.; Leiva, E. A Mapping of the Physical and Electrochemical Properties of Composite Lithium-Ion Batteries Anodes Made from Graphite, Sn, and Si. Batter. Supercaps 2020, 3, 1248–1256. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.; Park, C.M.; Jeong, G. In situ fabrication of nanohybrid carbon/polyamide film providing robust binding and conductive network in silicon anode for lithium-ion battery. J. Power Sources 2019, 410–411, 25–30. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Liu, T.; Gao, X.; Li, S.; Ling, M.; Liang, C.; Zheng, J.; Lin, Z. Silicon Anode with High Initial Coulombic Efficiency by Modulated Trifunctional Binder for High-Areal-Capacity Lithium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1903110. [Google Scholar] [CrossRef]
- Guan, X.; Yong, Y.; Wu, Q.; Zhang, X.; Guo, X.; Li, C.; Xu, J. Metal-Chelated Biomimetic Polyelectrolyte as a Powerful Binder for High-Performance Micron Silicon Anodes. Energy Technol. 2020, 8, 2000278. [Google Scholar] [CrossRef]
- Jia, H.; Zou, L.; Gao, P.; Cao, X.; Zhao, W.; He, Y.; Engelhard, M.H.; Burton, S.D.; Wang, H.; Ren, X.; et al. High-Performance Silicon Anodes Enabled By Nonflammable Localized High-Concentration Electrolytes. Adv. Energy Mater. 2019, 9, 1900784. [Google Scholar] [CrossRef]
- Xiaohui, Z.; Gouri, C.; Changhyeon, K.; Kwon-Koo, C.; Hyo-Jun, A.; Ki-Won, K.; Jou-Hyeon, A. Lithium/Sulfur Secondary Batteries: A Review. J. Electrochem. Sci. Technol 2016, 7, 97–114. [Google Scholar]
- He, J.; Manthiram, A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater. 2019, 20, 55–70. [Google Scholar] [CrossRef]
- Zhou, L.; Utetiwabo, W.; Chen, R.; Yang, W. 2.12-Layer by Layer Assemble of Colloid Nanomaterial and Functional✩Multilayer Films for Energy Storage and Conversion. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Oxford, UK, 2019; pp. 255–278. [Google Scholar] [CrossRef]
- Azimi, N.; Xue, Z.; Zhang, S.; Zhang, Z. 5-Materials and technologies for rechargeable lithium–sulfur batteries. In Rechargeable Lithium Batteries; Franco, A.A., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Cambridge, UK, 2015; pp. 117–147. [Google Scholar] [CrossRef]
- Chapter 2-Technologies of Energy Storage Systems. In Grid-scale Energy Storage Systems and Applications; Wu, F.B., Yang, B., Ye, J.L., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 17–56. [Google Scholar] [CrossRef]
- Danylenko, M. What Materials are Behind the EV Battery Revolution? 2018. Available online: https://matmatch.com/blog/what-materials-are-behind-the-ev-battery-revolution/ (accessed on 23 March 2021).
- Targray. LiPF6 Battery Electrolyte for Li-ion Cell Manufacturers. 2020. Available online: https://www.targray.com/li-ion-battery/electrolyte (accessed on 23 March 2021).
- staff writers, N. Toyota’s Game-Changing Solid-State Battery en Route for 2021 Debut. 2020. Available online: https://asia.nikkei.com/Spotlight/Most-read-in-2020/Toyota-s-game-changing-solid-state-battery-en-route-for-2021-debut (accessed on 24 March 2021).
- Billington, J. Daimler Busts EV Battery Myths and Discusses Future Battery Technologies. 2020. Available online: https://www.electrichybridvehicletechnology.com/features/daimler-busts-ev-battery-myths-and-discusses-future-battery-technologies.html (accessed on 24 March 2021).
- AV, V.; Powerful and Scalable: The New ID. Battery System. 2020. Available online: https://www.volkswagenag.com/en/news/stories/2018/10/powerful-and-scalable-the-new-id-battery-system.html (accessed on 24 March 2021).
- Willems, S. Nissan Charging Forward on Electric AWD, Solid-state Batteries. 2019. Available online: https://www.thetruthaboutcars.com/2019/10/nissan-charging-forward-on-electric-awd/ (accessed on 24 March 2021).
- Gearino, D. The Solid-State Race: Legacy Automakers Reach for Battery Breakthrough. 2021. Available online: Available online: https://insideclimatenews.org/news/19032021/solid-state-batteries-electric-vehicles-automakers/ (accessed on 23 March 2021).
- Nanotechnologies, S. The Future of Energy Storage. 2020. Available online: Available online: https://silanano.com/news/futureofenergystorage/ (accessed on 22 September 2020).
- Braga, M.H.; Ferreira, J.A.; Stockhausen, V.; Oliveira, J.E.; El-Azab, A. Novel Li3ClO based glasses with superionic properties for lithium batteries. J. Mater. Chem. A 2014, 2, 5470–5480. [Google Scholar] [CrossRef]
- Braga, M.H.; Oliveira, J.E.; Murchison, A.J.; Goodenough, J.B. Performance of a ferroelectric glass electrolyte in a self-charging electrochemical cell with negative capacitance and resistance. Appl. Phys. Rev. 2020, 7, 011406. [Google Scholar] [CrossRef]
- Braga, M.H.; Grundish, N.S.; Murchison, A.J.; Goodenough, J.B. Alternative strategy for a safe rechargeable battery. Energy Environ. Sci. 2017, 10, 331–336. [Google Scholar] [CrossRef]
- Sakai, M. A Reaction Model for Li Deposition at the Positive Electrode of the Braga-Goodenough Li-S Battery. J. Electrochem. Soc. 2020, 167, 160540. [Google Scholar] [CrossRef]
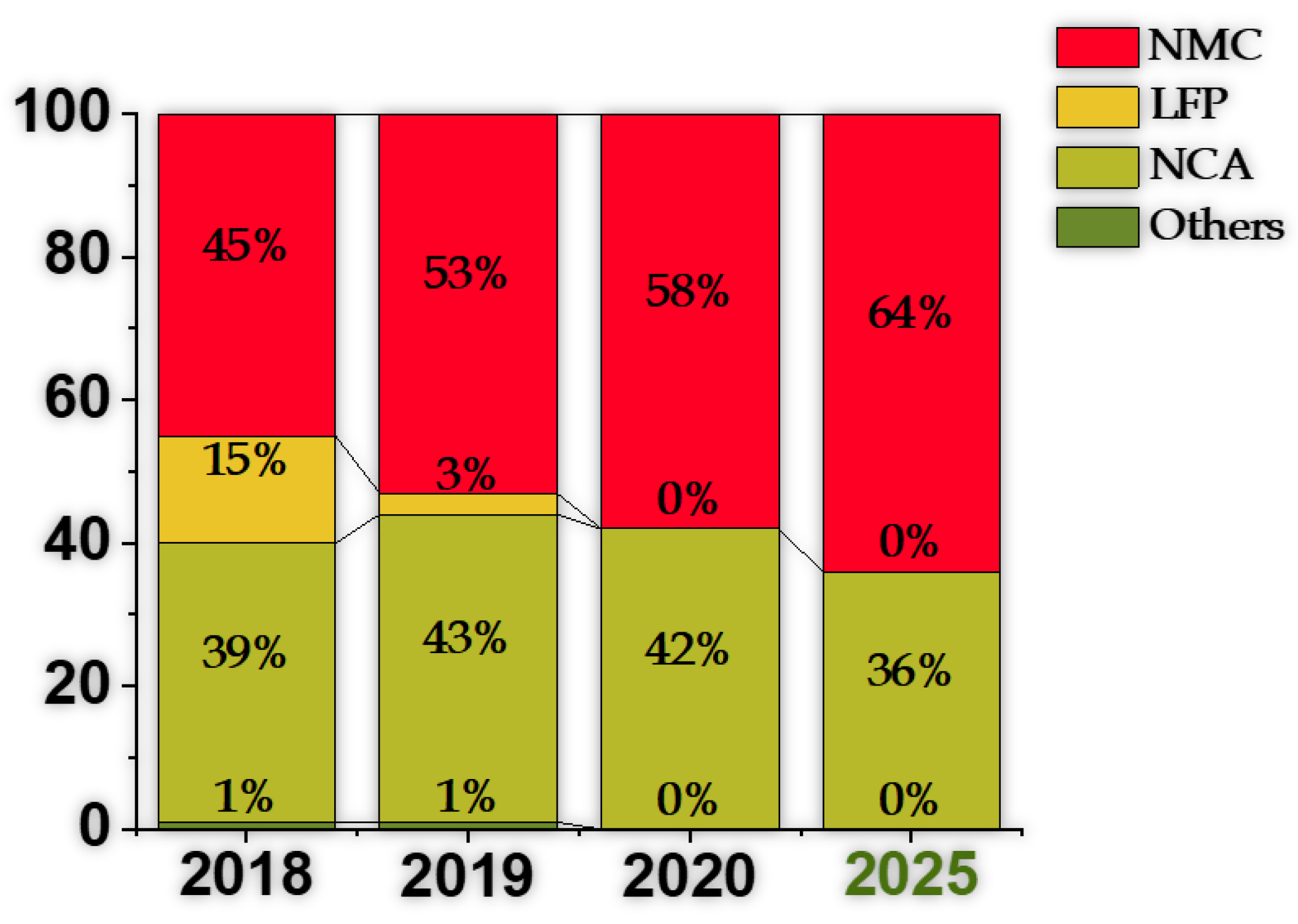
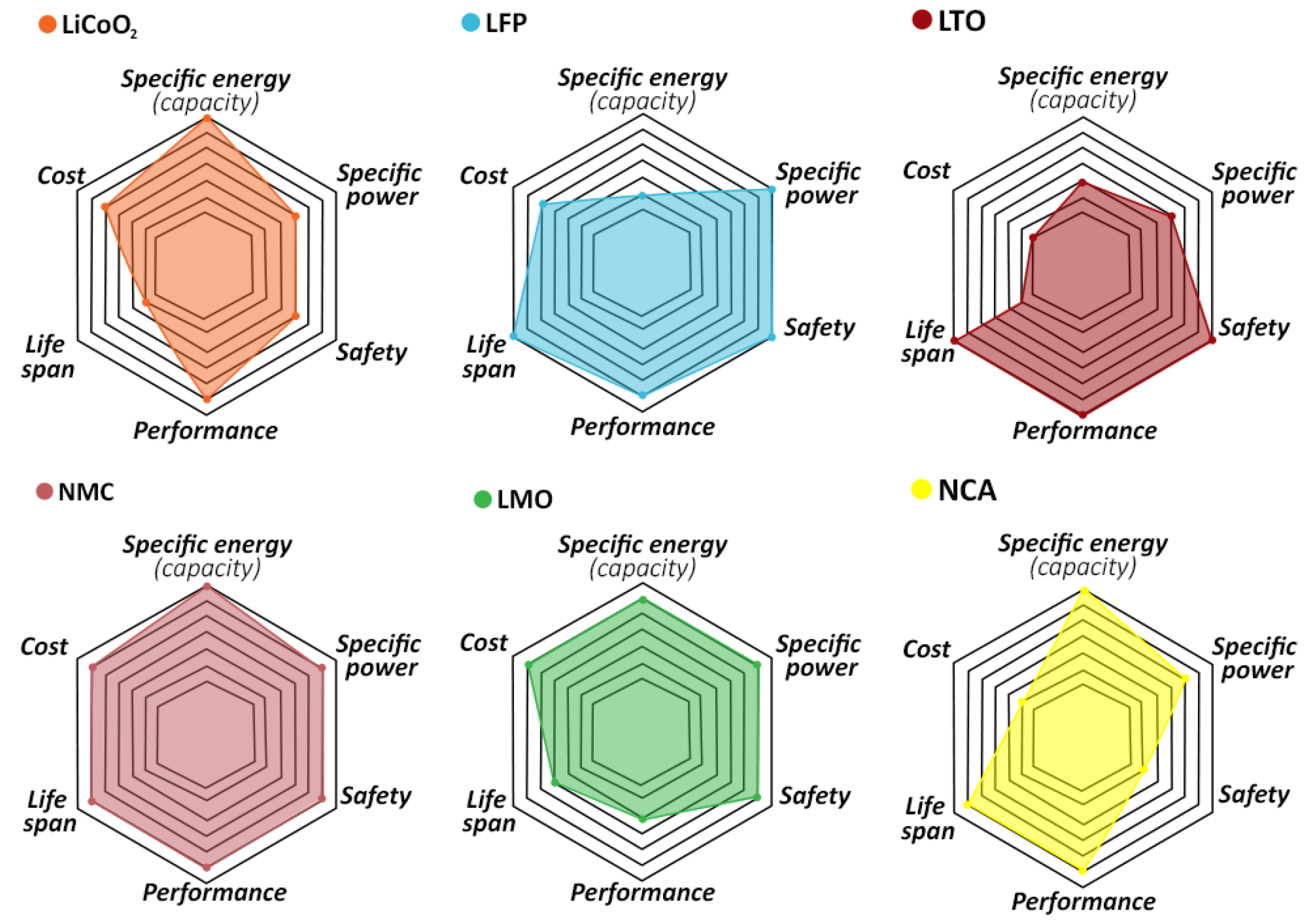
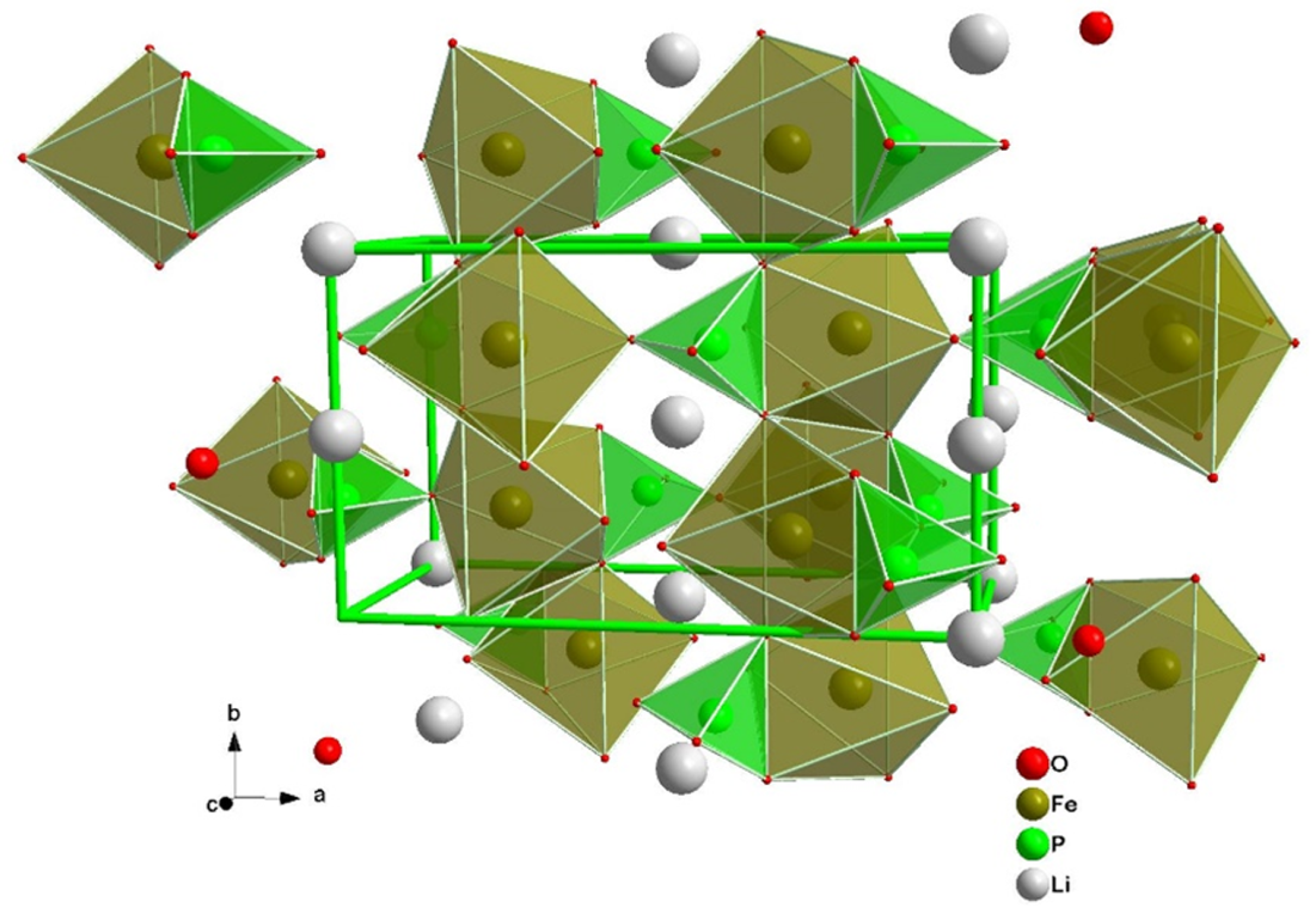
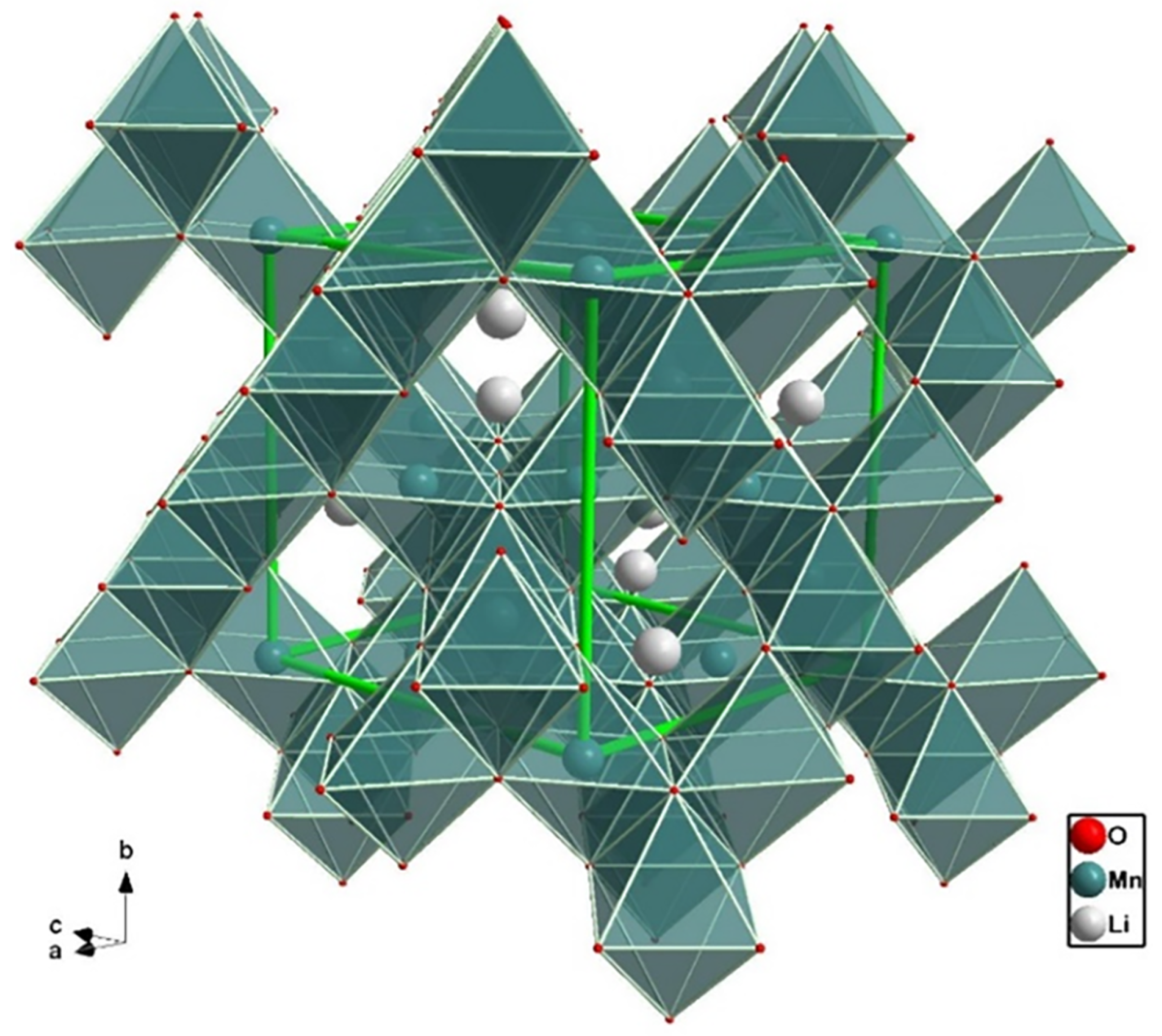
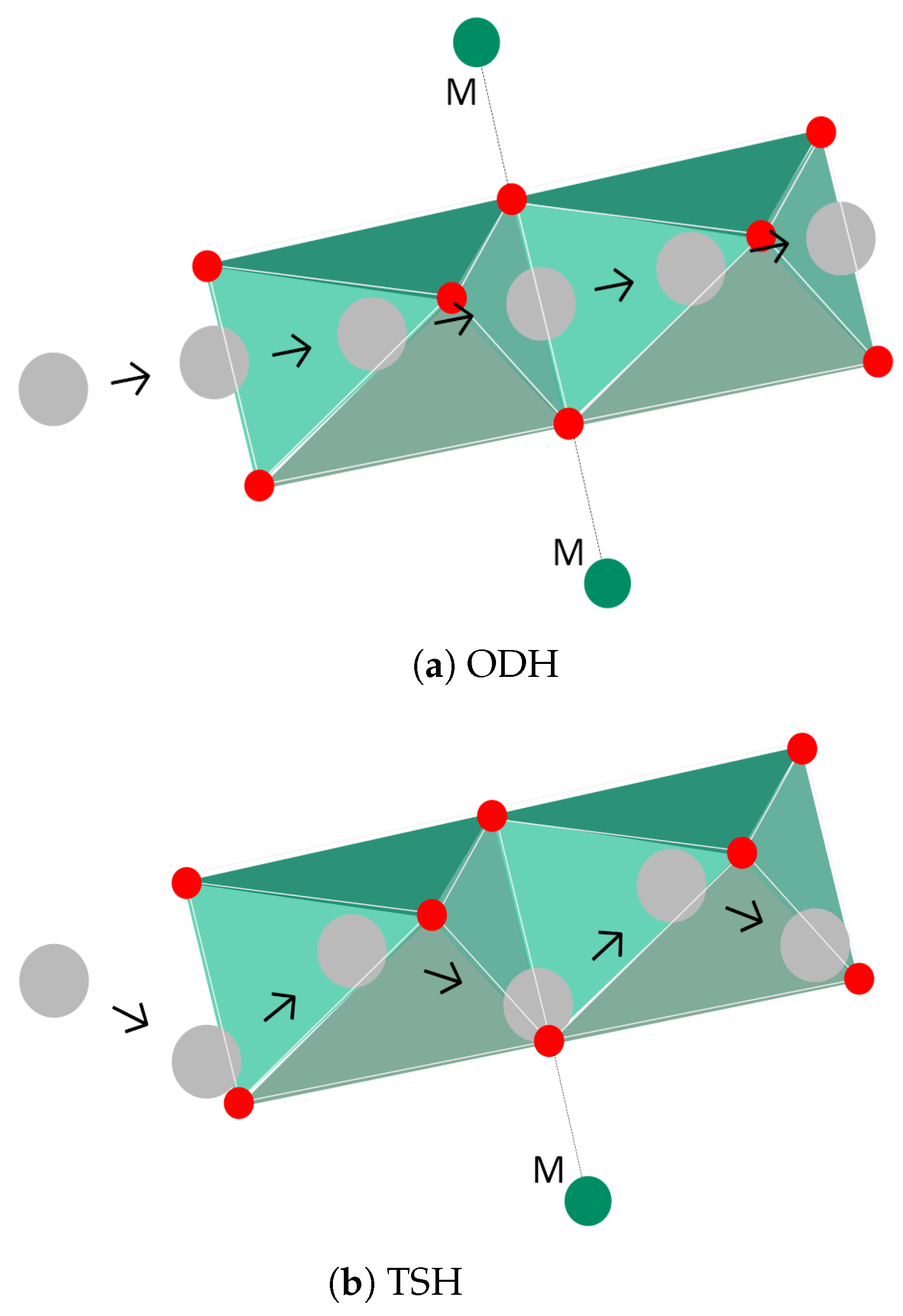
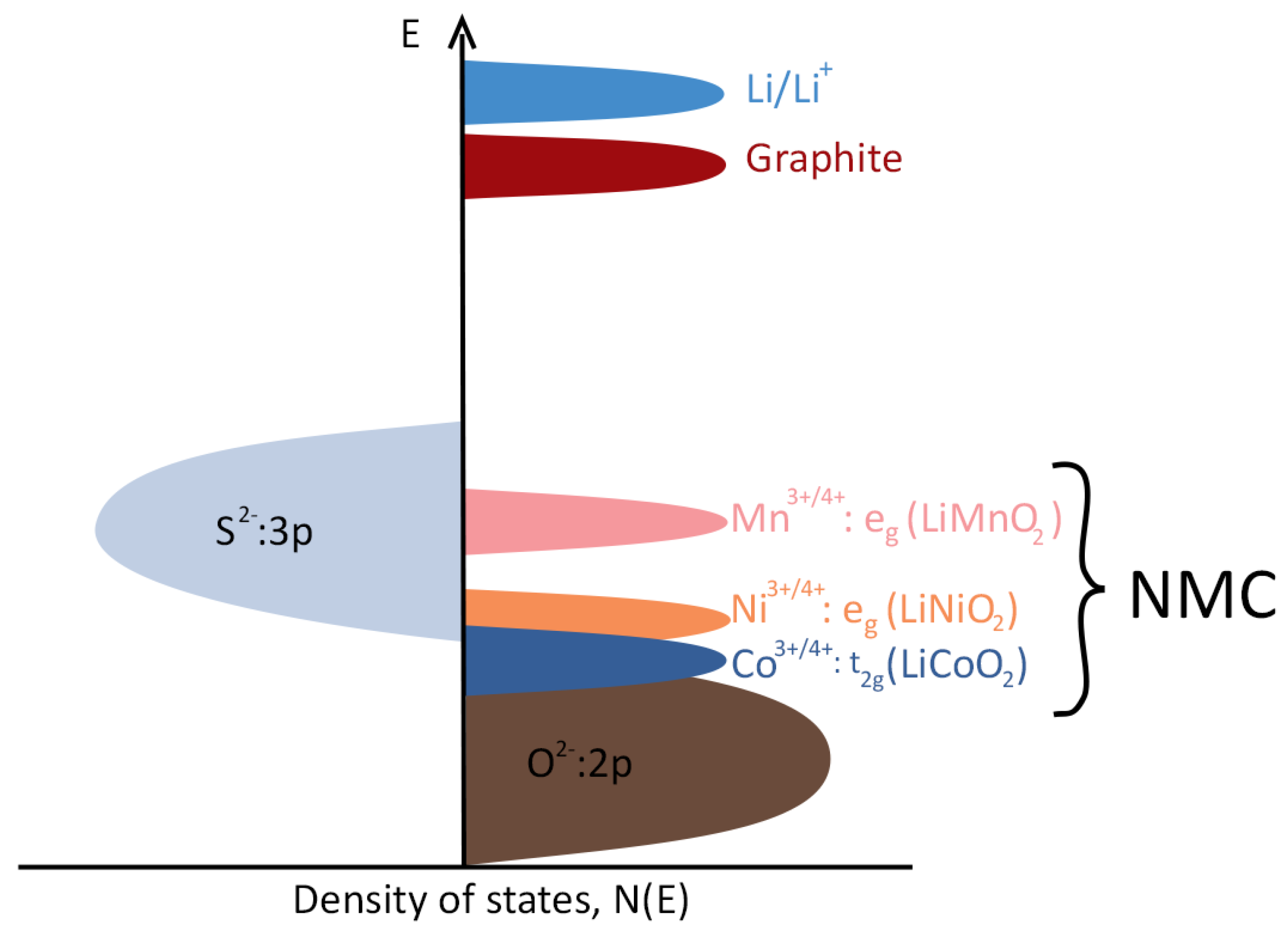
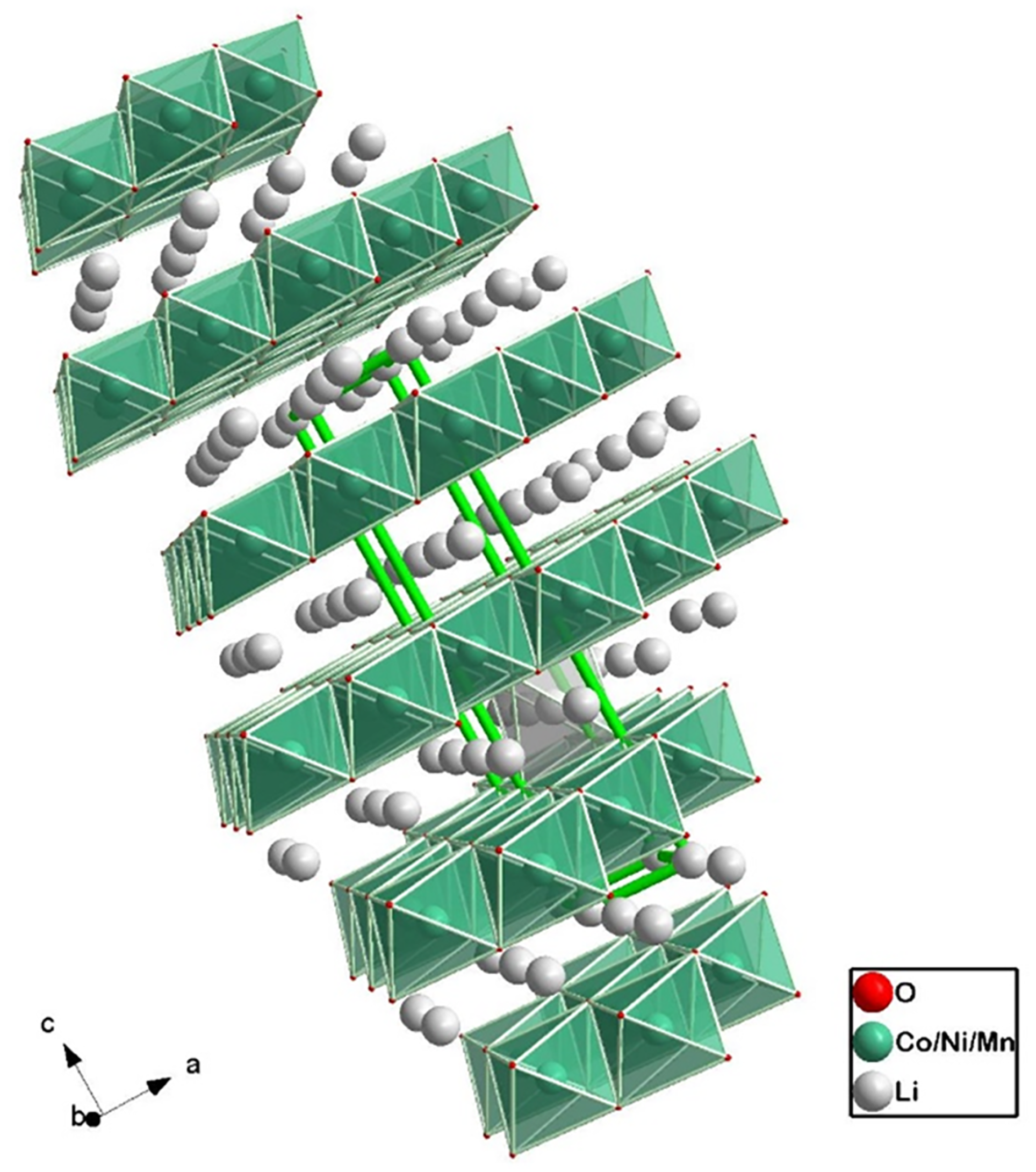
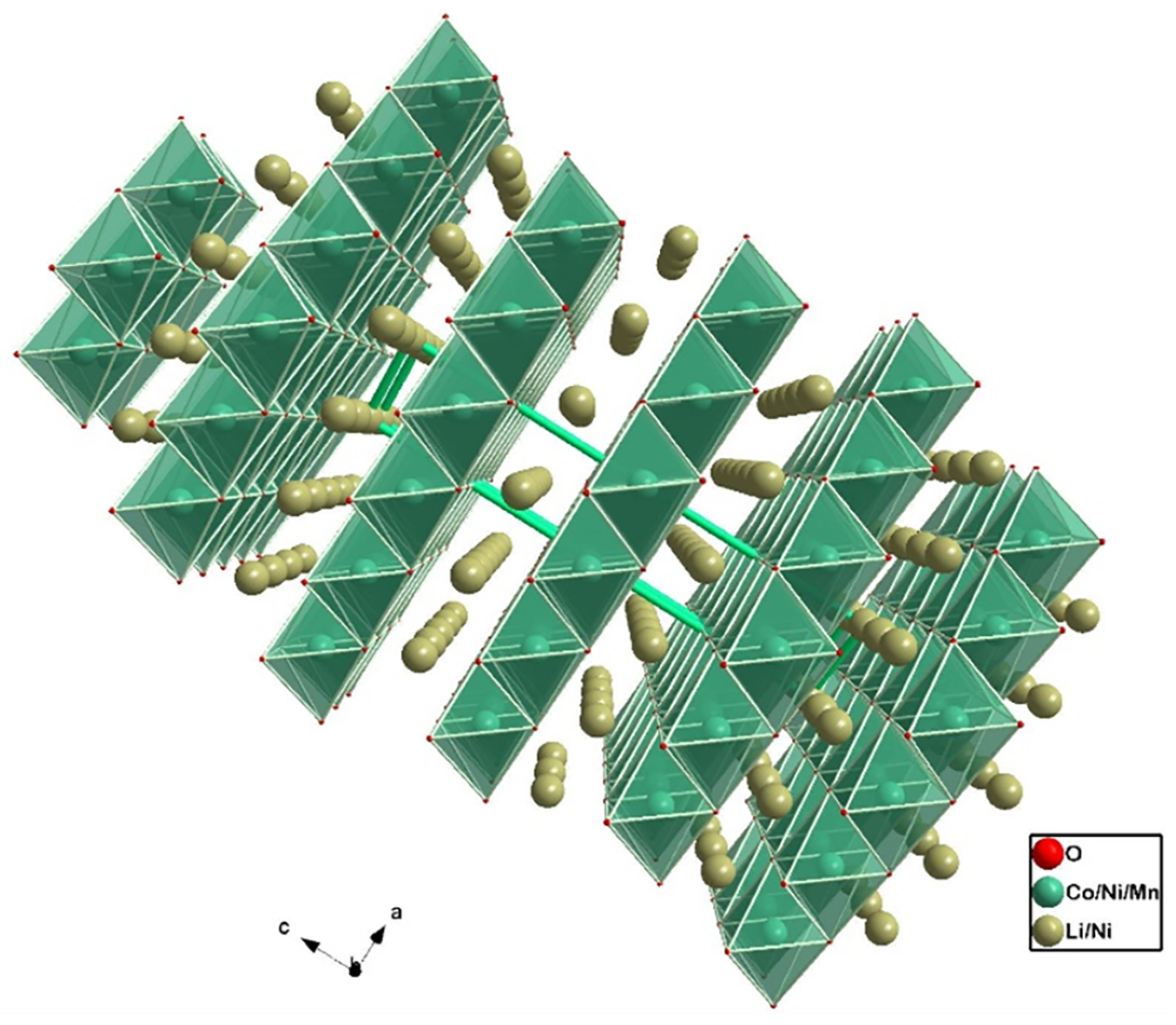
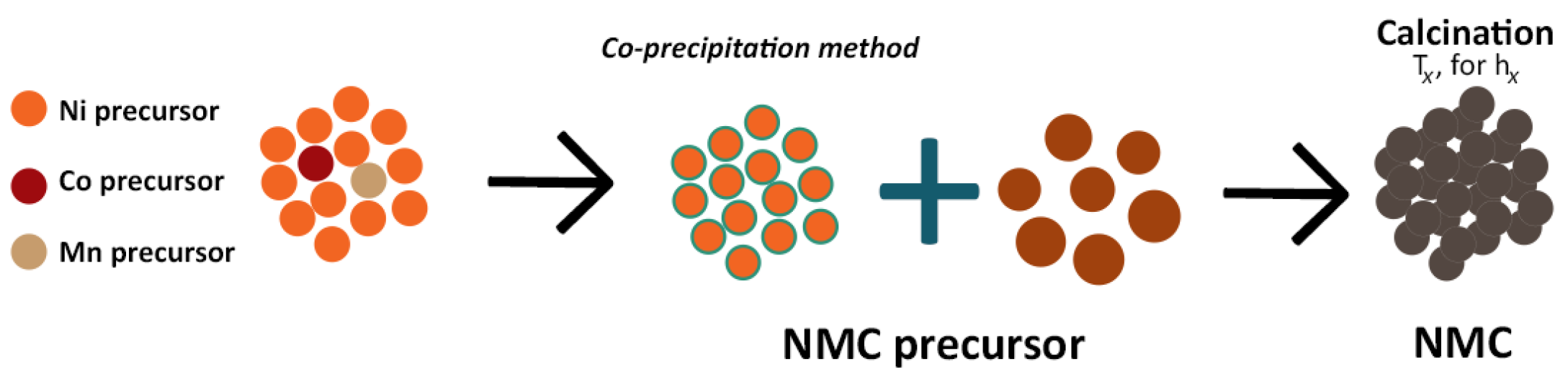
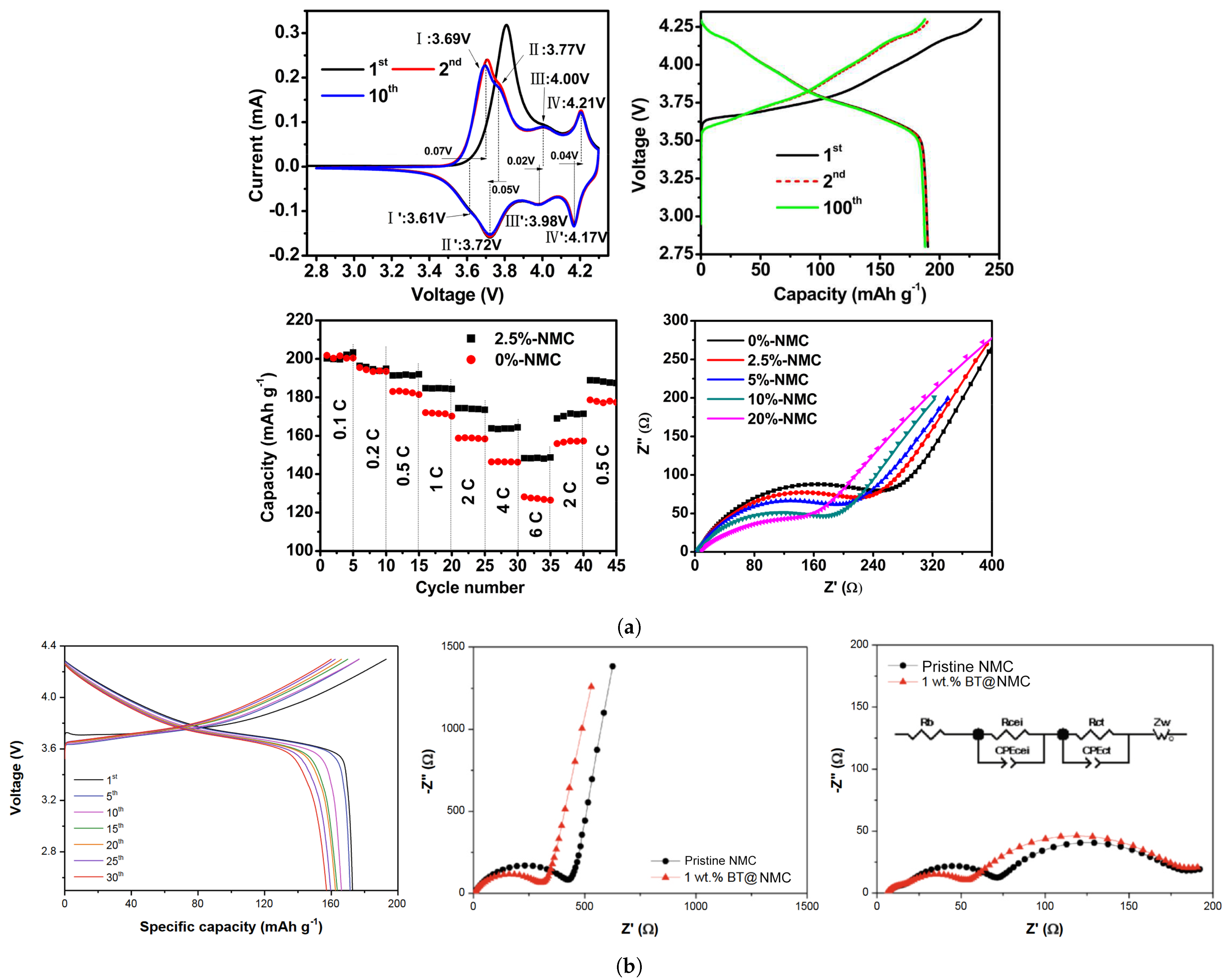

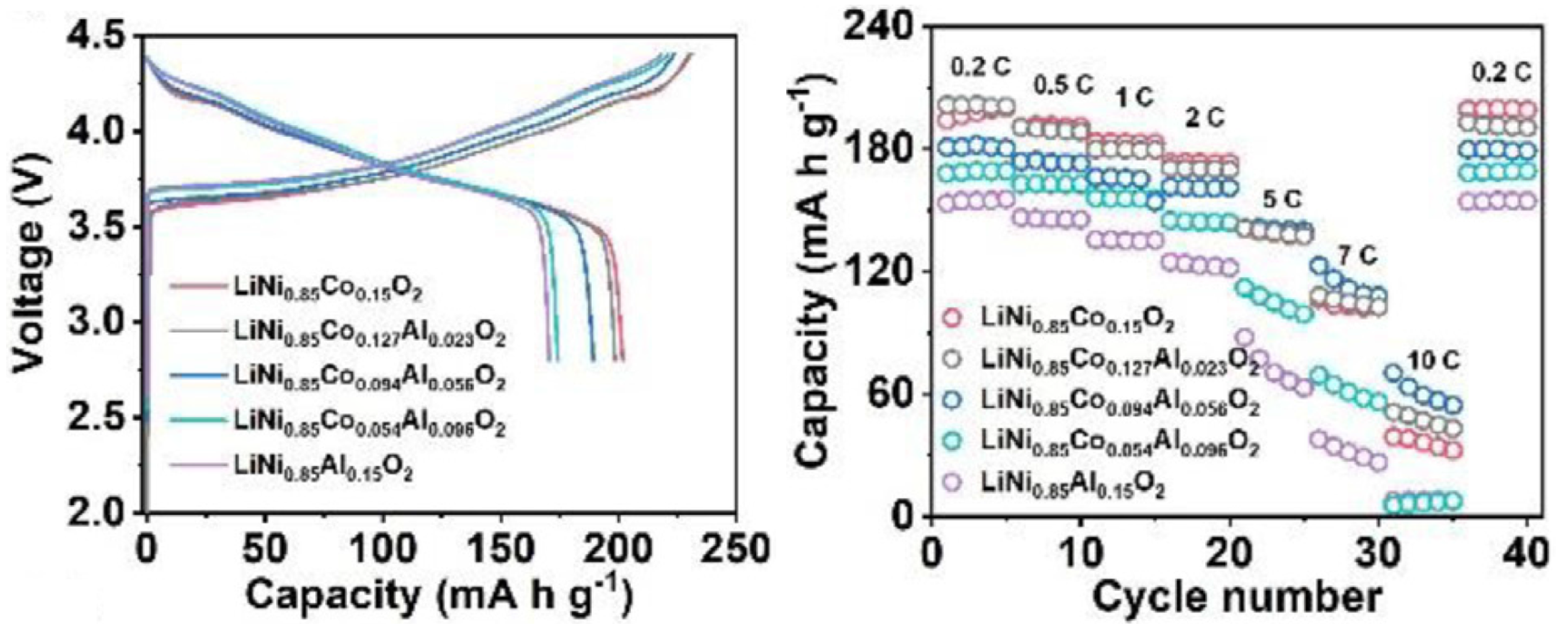
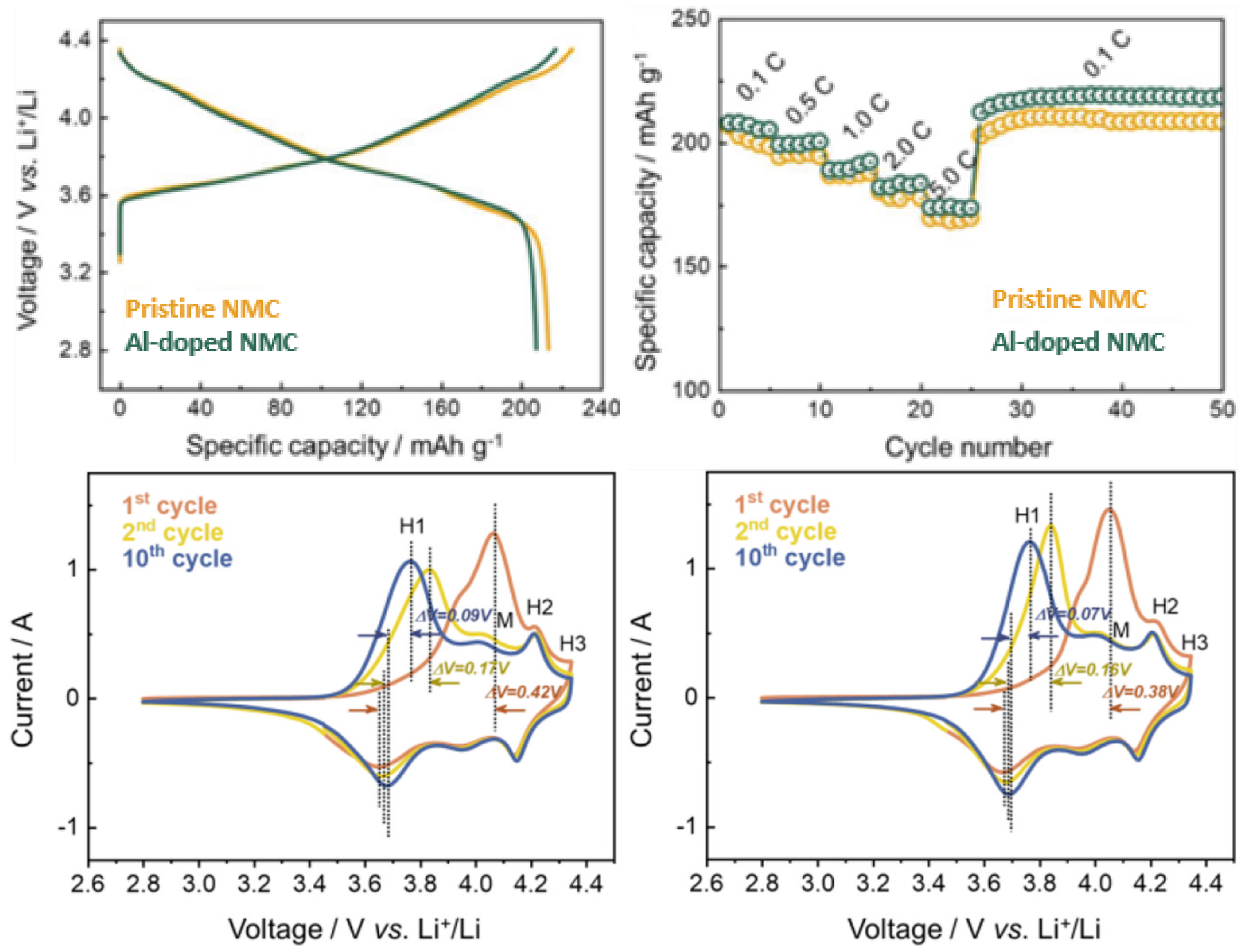
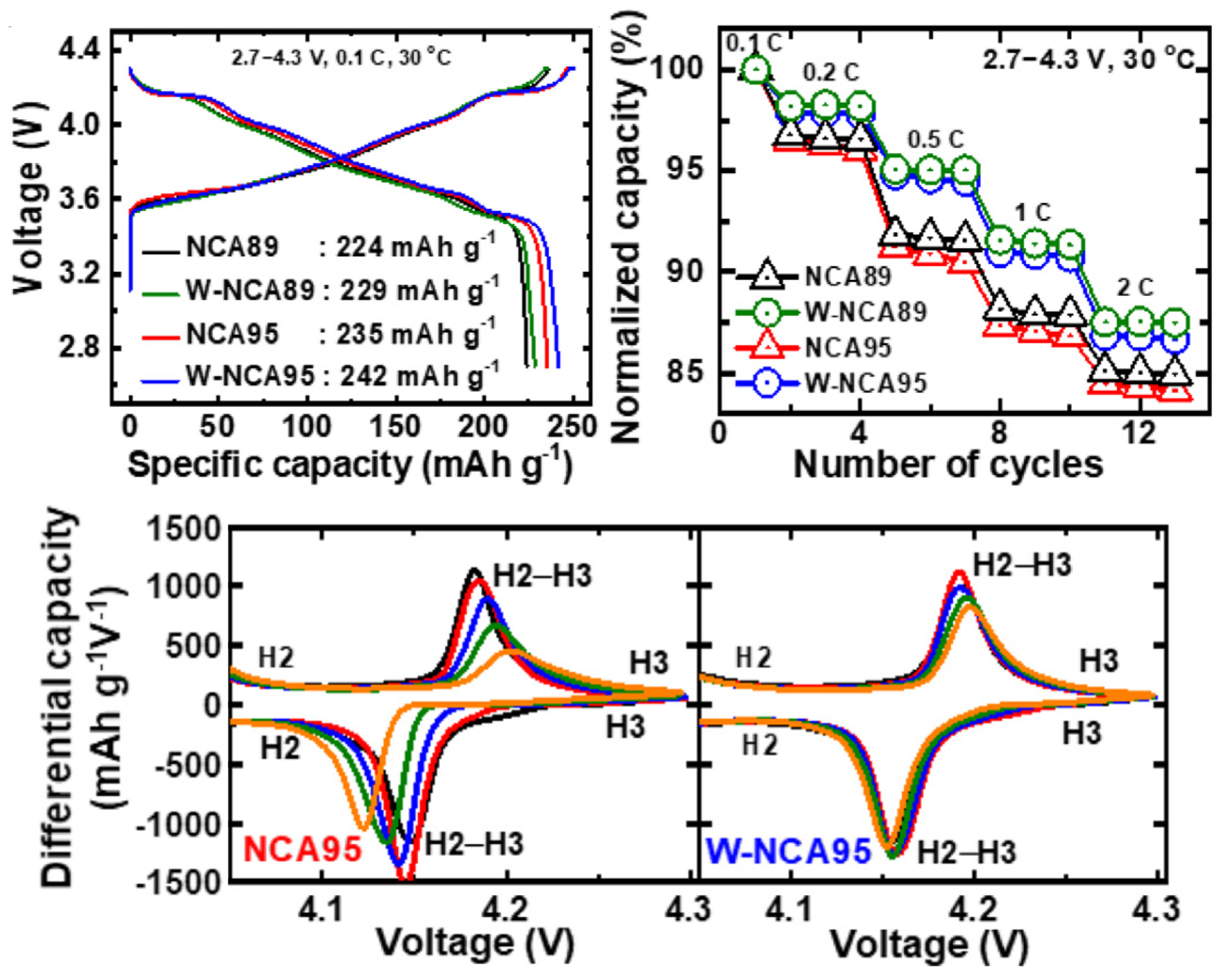
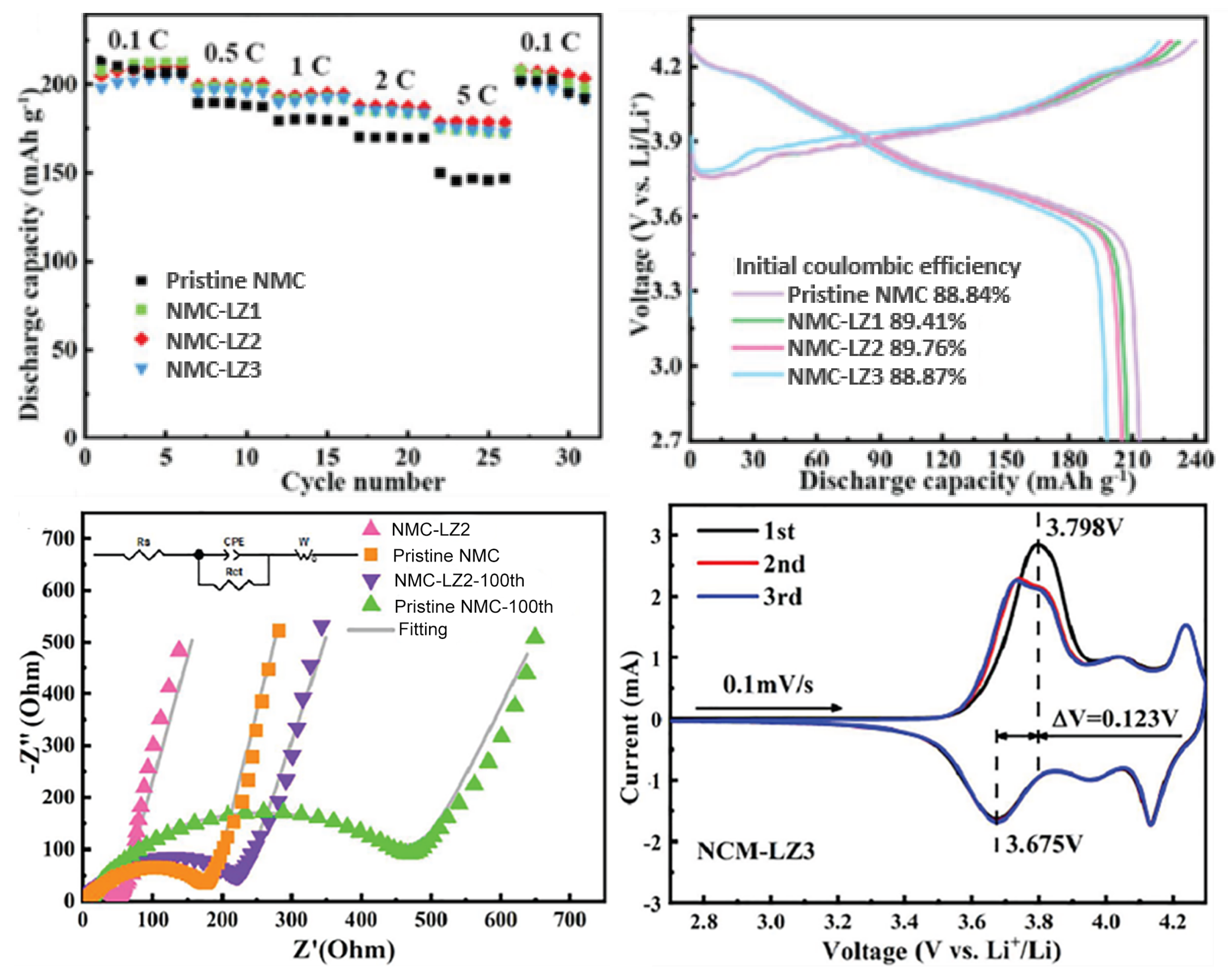
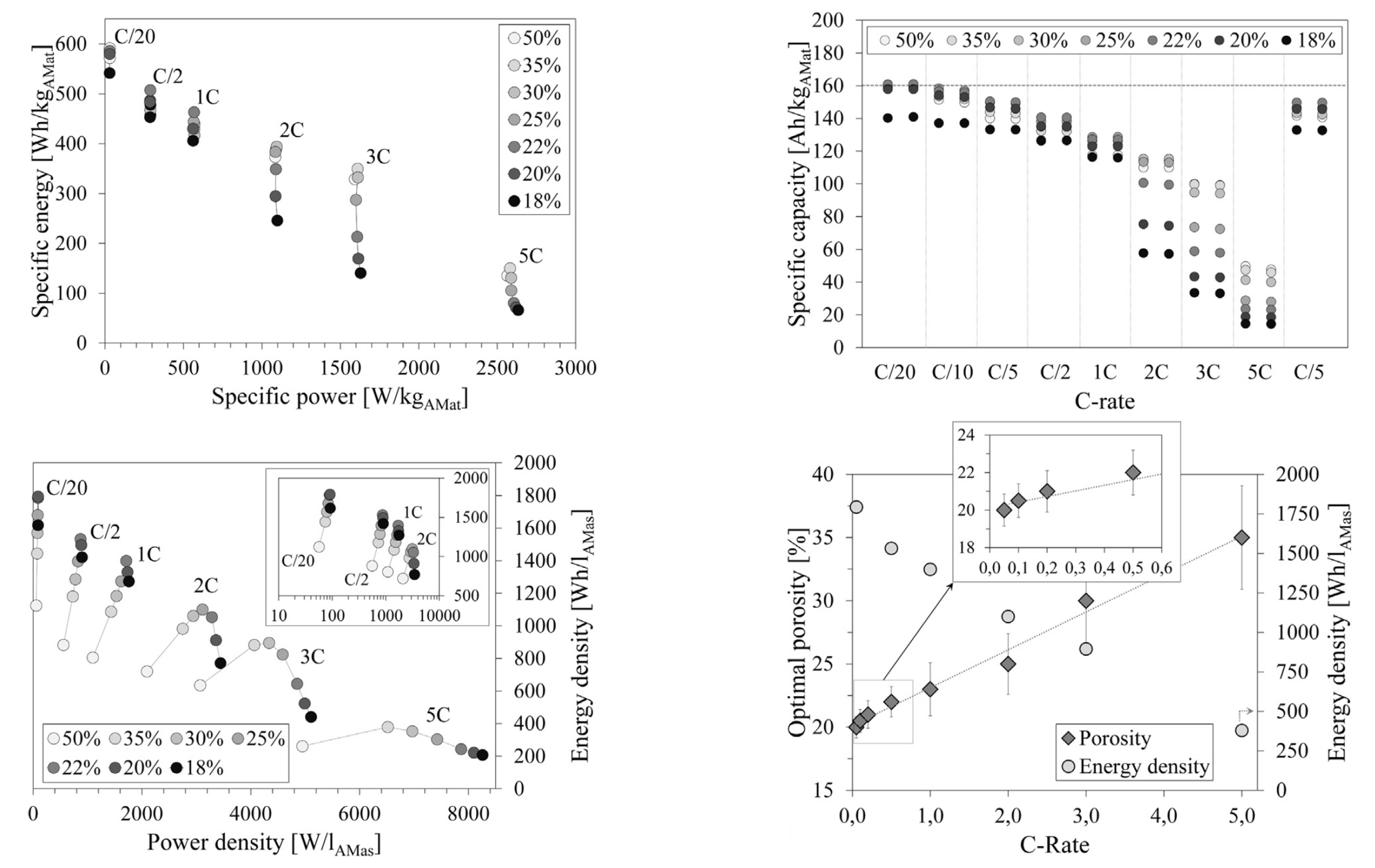

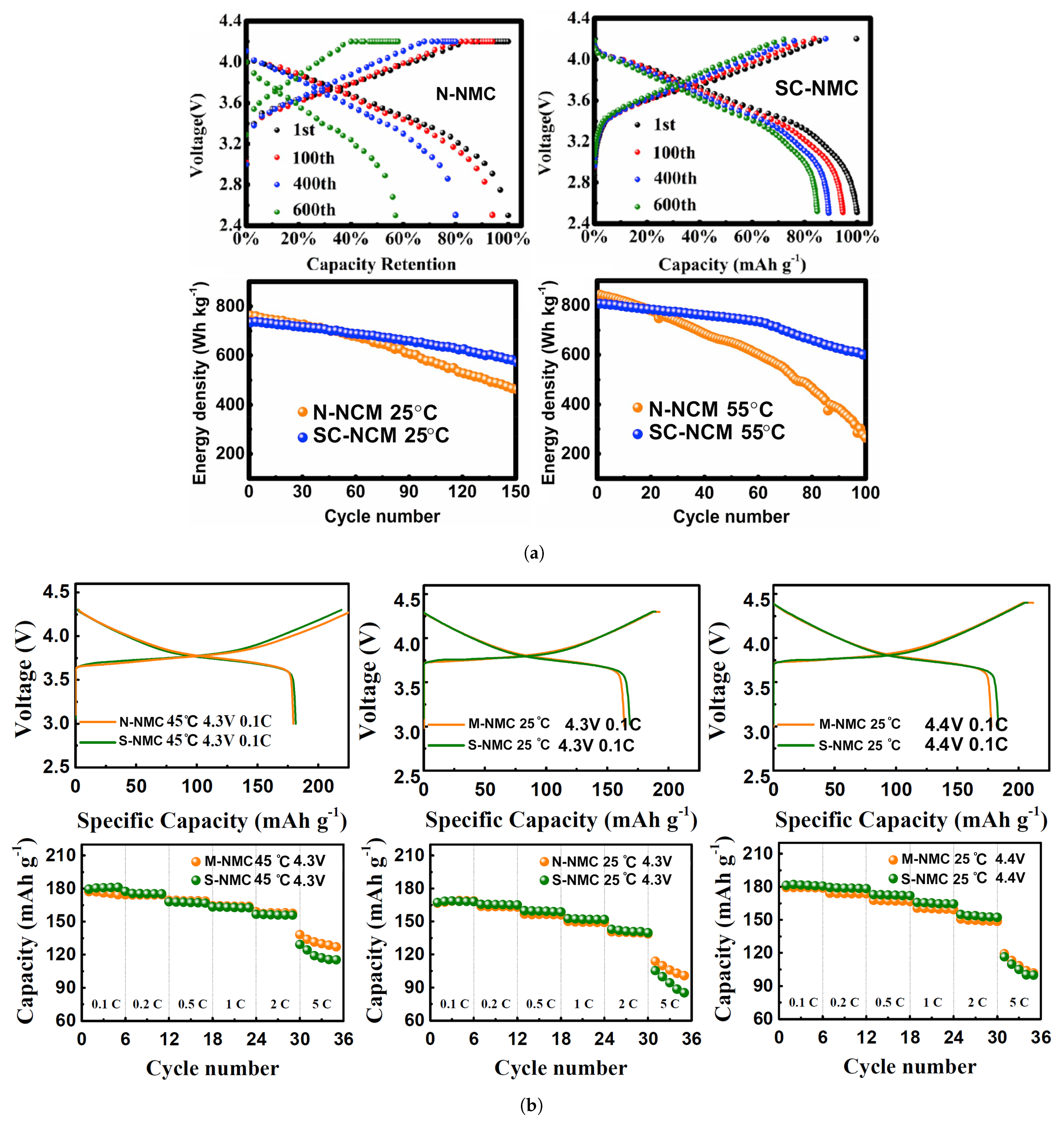
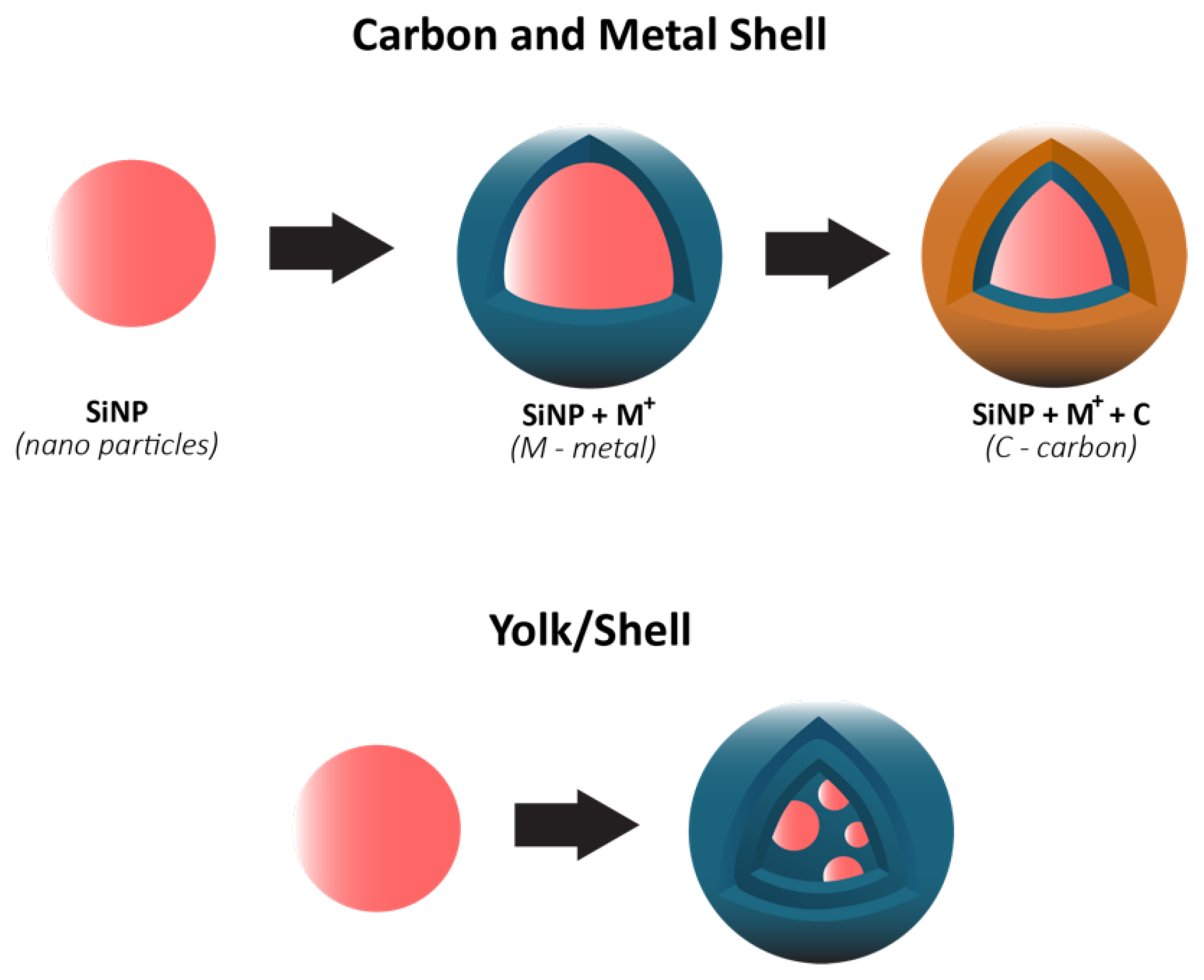

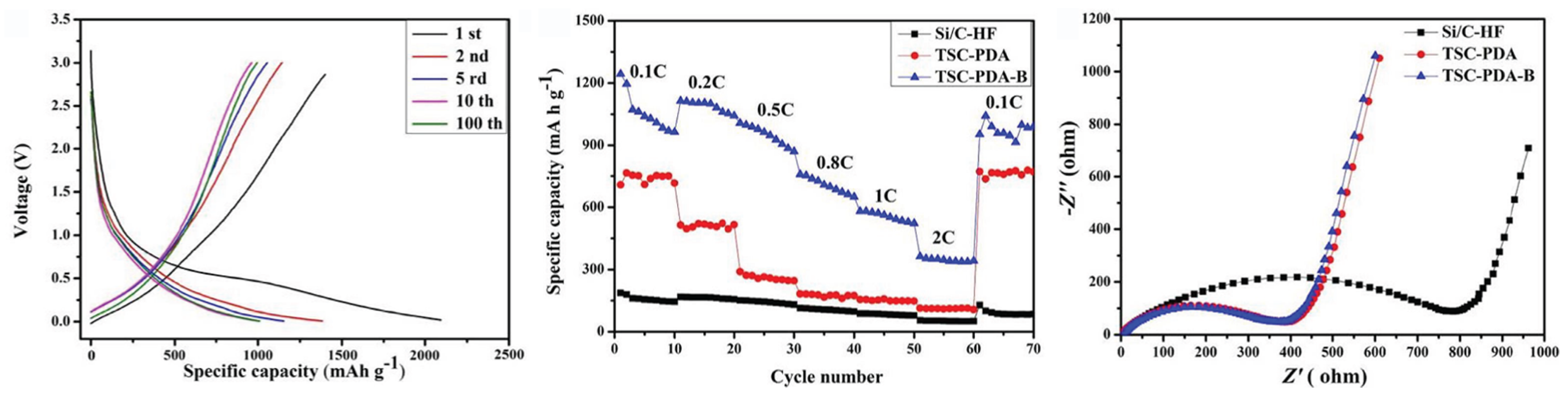
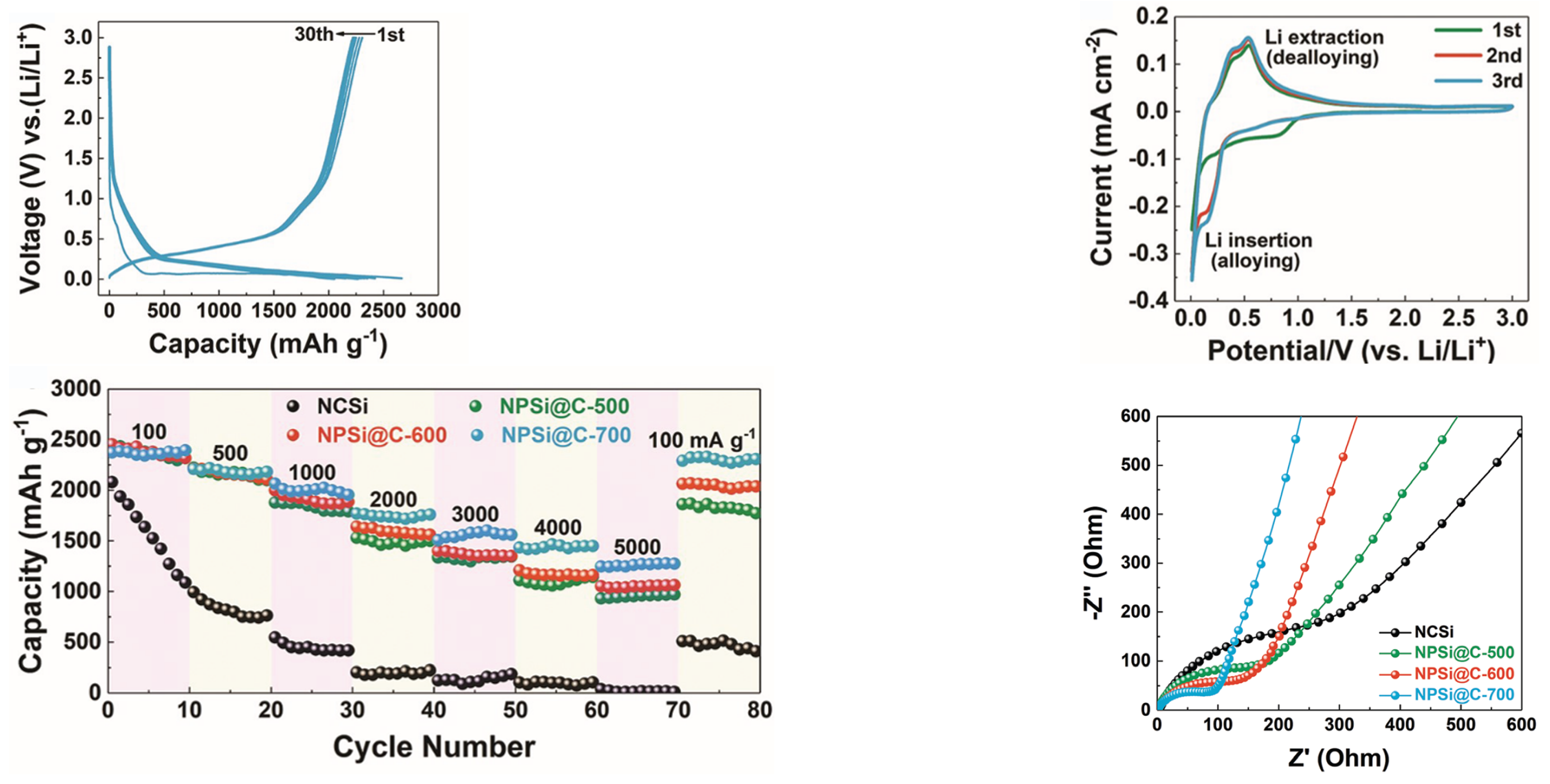

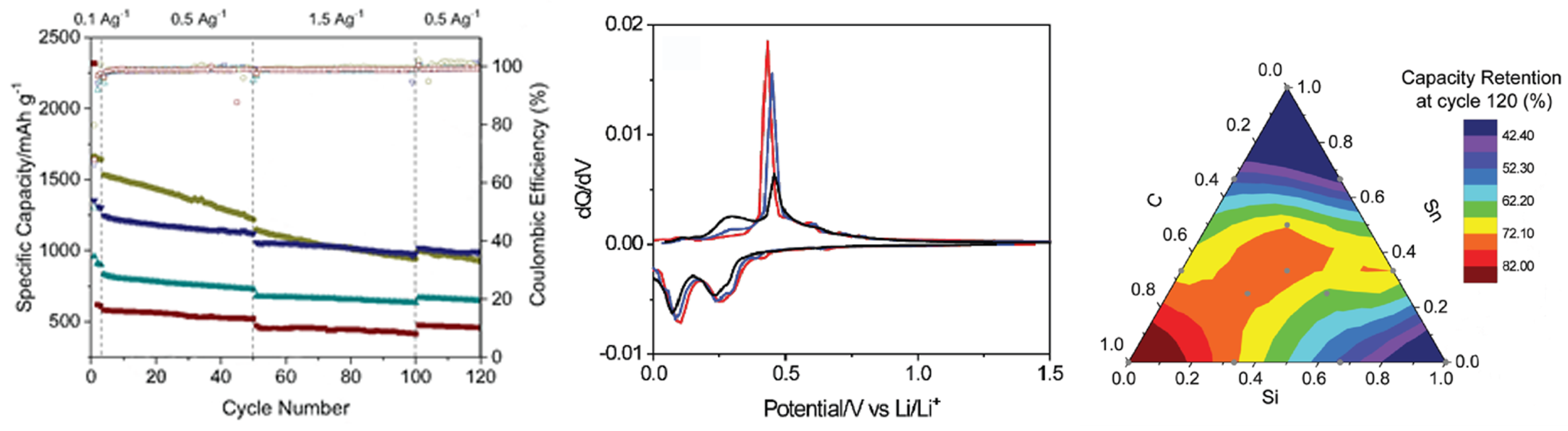
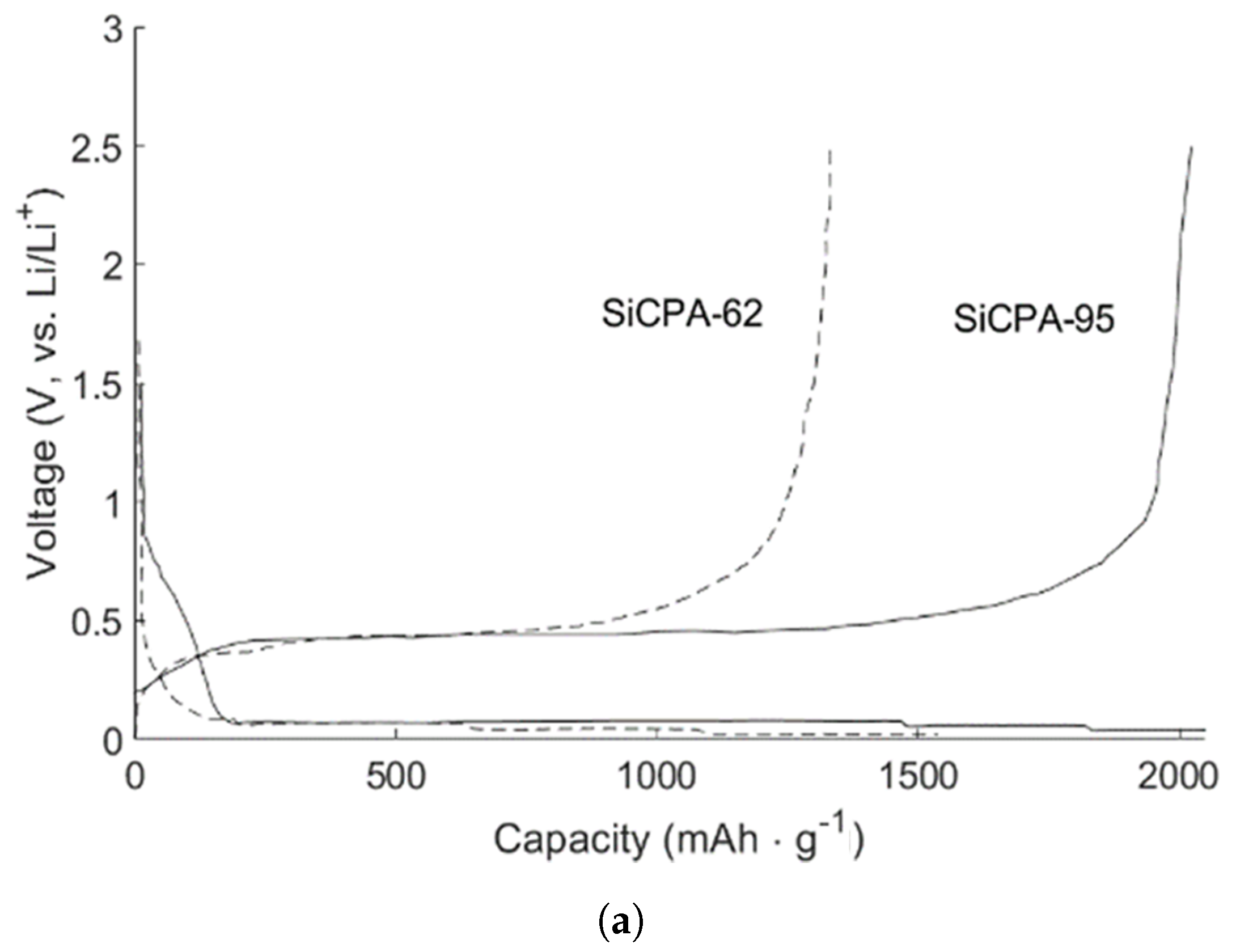
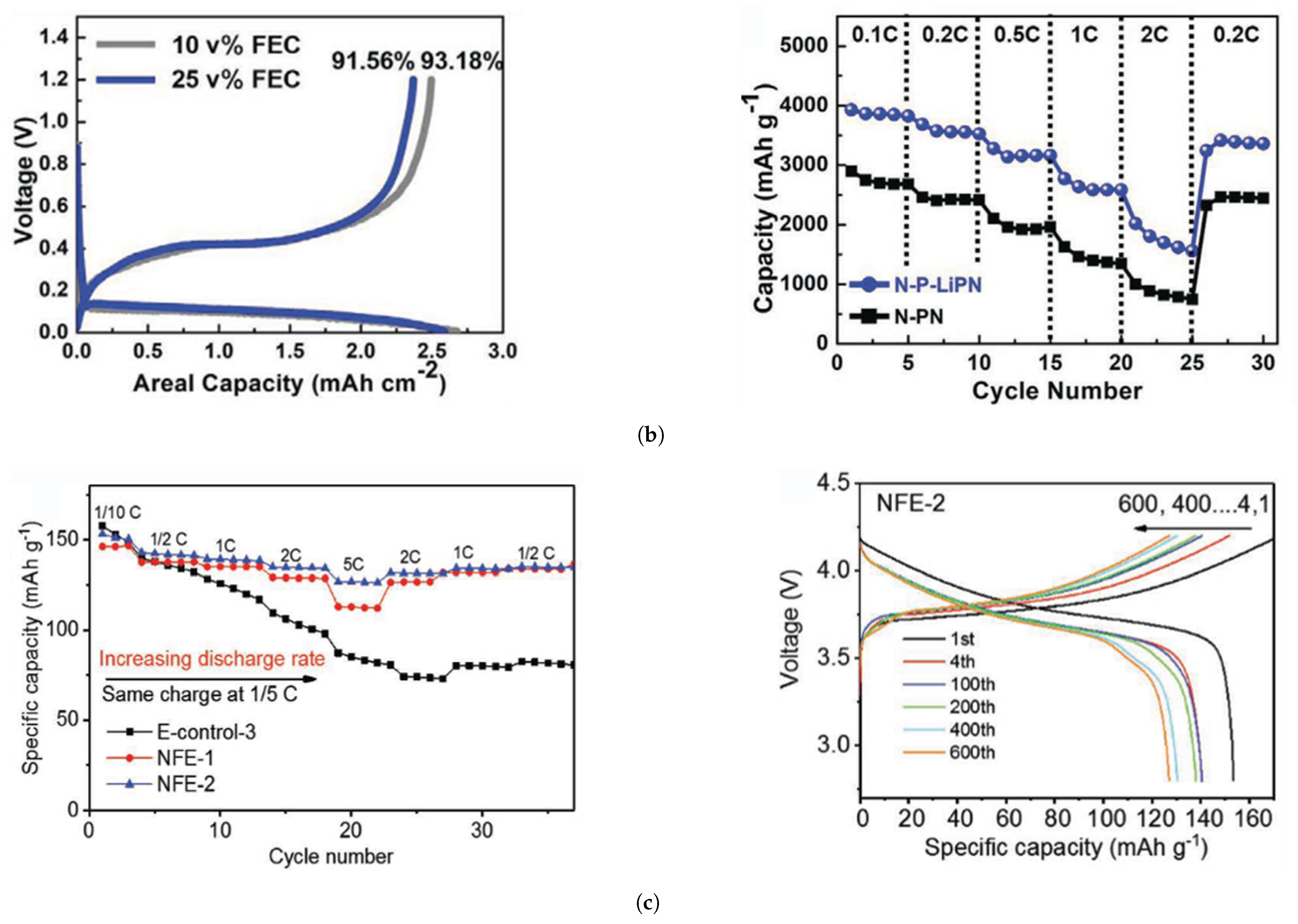
| Cathode Type | Formula (General) | Experimental Capacity (mAh·g) | Plateau Voltage (V vs. Li/Li) | Thermal Runaway (C) | Cycle Life (No. of Cycles) |
|---|---|---|---|---|---|
| Li Cobalt Oxide (LCO) | LiCoO | 150 | 4.3–3.8 | 150 | 500–1000 |
| Li Manganese Oxide (LMO) | LiMnO | 120–130 | 4.3–3.8 | 250 | 300–700 |
| Li Nickel-Manganese-Cobalt oxide (NMC) | LiNiMnCoO (x + y + z = 1) | 150 | 4.3–3.7 | 210 | 1000–2000 |
| Li Nickel-Cobalt-Aluminum oxide (NCA) | LiNiCoAlO (x + y+z = 1) | 175 | 4.3–3.5 | 150 | 500 |
| Li-Iron Phosphate (LFP) | LiFePO | 160–170 | 3.3 | 270 | >2000 |
| Cathode Type | Ratios (R) or Cell Designation (S) | Manufacturer | No. of Cells (Series, Parallel) | EV Model | Specific Energy (Wh/kg) | Energy (Usable) (kWh) | Range (km) |
|---|---|---|---|---|---|---|---|
| Li-Nickel- Cobalt- Aluminum oxide (NCA) | 18650 (S) 2170 (S) | Panasonic | 8256 (s96p86) 4416 (s96p46) | Tesla Model S, Tesla Model X, Tesla Model 3 | 162 168 | 102.4 (98.4) 80.5 (76) | 593, 487, 530 |
| Li-Manganese Oxide (LMO) | - | Yuasa | 80 | Citroen Zero (LEV50 battery) | 107 | 14.5 | 150 |
| Li-Cobalt Oxide (LCO) | - | LG Chem | 96 | Smart Fortwo e | 150–200 | 17.6 (17.2) | 127 |
| Lithium-Iron Phosphate (LFP) | - | Elektrofahrzeu- ge Stuttgart CATL BYD Blade | - - - 102 | Iridium E_Mo- bil Tesla Model 3 BYD Han EV | 90–120 125 - | 106 106 65 | 400 400 506 |
| Cathode Type | Ratios (R) or Cell Designation (S) | Manufacturer | No. of Cells (Series, Parallel) | EV Model | Specific Energy (Wh/kg) | Energy (Usable) (kWh) | Range (km) |
|---|---|---|---|---|---|---|---|
| 532 (R) | Nissan CATL Envision AESC | 288 216 (s108p2) 192 (s96p2) | Nissan Leaf e+ Peugeot e-208, Opel Corsa-e Nissan Leaf | - 140 130 | 62 50 (46) 39.5 (36) | 385 349, 336 270 | |
| 333 (R) | Samsung SDI | 264 (s88p3) | Volkswagen e-Golf | 103 | 35.8 (32) | 232 | |
| 721 (R) | LG Chem | 192 (s96p2) | Renault ZOE | 168 | 54.7 (52) | 232 | |
| Li-Nickel Manganese Cobalt oxide (NMC) | 622 (R) | Samsung SDI SK Innovation LG Chem | 96 (s96p1) 294 (s98p3) 168 (s84p2) 176 (s88p2) 294 (s98p3) 384 (s96p4) 396 (s198p2) 432 (s108p4) 288 (s96p3) | BMW i3 Kia e-Soul, Kia e-Niro Volkswagen e-UP, Seat Mii Electric, Skoda CITIGo-e Hyundai Ioniq-e Hyundai Kona-e Mercedes-Benz EQC Porsche Taycan Jaguar I-Pace Audi e-tron 55 Quattro Chevrolet bolt | 152 148 148 112.4 149 130 148 149 136 143 | 42.2 (37.9) 67.5 (64) 36.8 (32.3) 40.4 (38.3) 67.5 (64) 85 (80) 93.4 (83.7) 90 (84.7) 95 (86.5) 68 | 293 451, 454 260, 256, 265 310 447 417 333 470 402 417 |
| Anode Type | Application | Voltage | Capacity | Specific Energy | Cycle life |
|---|---|---|---|---|---|
| (V vs. Li/Li) | (mAh·g) | (Wh·kg) | |||
| Graphite (C) | Most commercially available batteries | 0.15–0.25 | 372 | 100–156 | 2000 |
| Lithium-Titanium Oxide LiTiO (LTO) | LFP batteries | 1.5 | 175 | 50–80 | 3000–7000 |
| Silicon | Nanowire (SiNW) Amprius Technologies: Airbus Zephyr S pseudo satellite HAPS Military vehicles | 0.4 | 4200 (Silicon) 3579 (SiNW) | 435 (Amprius) | >2000 (SiNW) |
| Anode Type | Cycle Voltage (V) | Initial Discharge Capacity (mAh· g) | ICE (%), No of Cycles for >99% |
|---|---|---|---|
| SiNP [105] | 0.01–1.5 | 2914.3 | ∼80 |
| SiONP [110] | - | 1755 | 50.4 |
| Si@C-CNT-Cu [111] | - | ∼2341 | ∼88, 10 |
| Si@C/CNTs@GS [105] | 0.01–1.5 | 2533.3 | 87.6 |
| SiO@TiO/CNF [110] | - | 1782 | 69.8, 11 |
| Si@SiO@C [109] | 0.05–3 | 2108 | 71 |
| NP-Si@C [107] | - | 2305.9 | 86.56 |
| C-SCP [108] | - | 3346 | 81 |
| Si@CMR [106] | 0.01–1.2 | 1834.2 | 71, 5 |
| Si@CRF [106] | 0.01–1.2 | ∼3100 | - |
| Si@CPDA [106] | 0.01–1.2 | ∼1834 | - |
| Si@CGLU [106] | 0.01–1.2 | ∼2800 | - |
| SnSiC [116] | - | 1499.5 | 86.7 |
| SiCPA-62 [117] | 0.005–2.5 | 1247 | 86 |
| SiCPA-95 [117] | 0.005–2.5 | 2350 | 87 |
| Si@N-P-LiPN [120] | 0.005–1.2 | 3614 | 93.18 |
| Si@Fe-PDA/PAA [119] | 0.01–2 | 4000 | - |
| Anode Type | Discharge Capacity (mAh· g), after x Cycles | Rate Performance |
|---|---|---|
| SiNP [105] | <500, 130 | - |
| SiONP [110] | <158, 200 | - |
| Si@C-CNT-Cu [111] | 1500, 900 | 2168, 1837, 1577, 1236, 942 (0.2, 0.5, 1, 2, 4 /A·g) |
| Si@C/CNTs@GS [105] | 1524.3, 130 | 1910, 1630, 1430, 1000, 1530 (0.2, 0.4, 0.8, 1.6, 0.1 /A·g) |
| SiO@TiO/CNF [110] | ∼760, 200 | 875, 696, 588, 502, 460, 384, 338, 713 (0.2, 0.4, 0.6, 0.8, 1, 2, 3, 0.2 /A·g) |
| Si@SiO@C [109] | 113, 200 | 1243, 1050, 870, 650, 520, 340, 960 (0.1, 0.2, 0.5, 0.8, 1, 2, 0.1 /A·g) |
| NP-Si@C [107] | 2126.2, 120 | ∼2180, 1990, 1750, 1530, 1490, 1271.3, 2287.3 (0.5, 1, 2, 3, 4, 5, 0.1 /A·g) |
| C-SCP [108] | 1050, 1000 | 2202.6, 1870.4, 1408.3, 873.7, 2323.3 (0.84, 2.4, 4.2, 8.4, 0.84 /A·g) |
| Si@CMR [106] | ∼1614.6, 200 | 2126.7, 1993.6, 1851.2, 1741.8, 1628.7, 1994.4 (0.2, 0.4, 0.6, 0.8, 1, 2, 0.2 /A·g) |
| Si@CRF [106] | 1064.5, 200 | ∼1700, 1570, 1450, 1380, 1320, 1050, 1500 (0.2, 0.4, 0.6, 0.8, 1, 2, 0.2 /A·g) |
| Si@CPDA [106] | 880.1, 200 | ∼1200, 1000, 875, 780, 730, 630, 1050 (0.2, 0.4, 0.6, 0.8, 1, 2, 0.2 /A·g) |
| Si@CGLU [106] | 700.1, 200 | ∼850, 817, 770, 740, 710, 620, 700 (0.2, 0.4, 0.6, 0.8, 1, 2, 0.2 /A·g) |
| SnSiC [116] | - | 1165, 965, 809, 822 (0.1, 0.5, 1.5, 0.5 /A·g) |
| SiCPA-62 [117] | ∼700, 250 | 1279.8, 1094.3, 922.1, 729.4 (0.2, 0.5, 1, 2 /A·g) |
| SiCPA-95 [117] | ∼1287, 50 | 1812.4, 1290.6, 920.7, 524.6 (0.2, 0.5, 1, 2 /A·g) |
| Si@N-P-LiPN [120] | ∼2159, 100 | 3859.7, 3533.1, 3199.0, 2620.9, 1598.9, 3417.3 (0.1C, 0.2C, 0.5C, 1C, 2C, 0.2C; 5 cycles step) |
| Si@Fe-PDA/PAA [119] | ∼2000, 200 | 2800, 2100, 1400, 350 (0.2C, 0.5C, 1C, 5C) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The Latest Trends in Electric Vehicles Batteries. Molecules 2021, 26, 3188. https://doi.org/10.3390/molecules26113188
Salgado RM, Danzi F, Oliveira JE, El-Azab A, Camanho PP, Braga MH. The Latest Trends in Electric Vehicles Batteries. Molecules. 2021; 26(11):3188. https://doi.org/10.3390/molecules26113188
Chicago/Turabian StyleSalgado, Rui Martim, Federico Danzi, Joana Espain Oliveira, Anter El-Azab, Pedro Ponces Camanho, and Maria Helena Braga. 2021. "The Latest Trends in Electric Vehicles Batteries" Molecules 26, no. 11: 3188. https://doi.org/10.3390/molecules26113188
APA StyleSalgado, R. M., Danzi, F., Oliveira, J. E., El-Azab, A., Camanho, P. P., & Braga, M. H. (2021). The Latest Trends in Electric Vehicles Batteries. Molecules, 26(11), 3188. https://doi.org/10.3390/molecules26113188









