Fish and Shellfish-Derived Anti-Inflammatory Protein Products: Properties and Mechanisms
Abstract
1. Introduction
2. Chronic Inflammation
3. Fish and Shellfish-Derived Anti-Inflammatory Protein Products: Properties and Mechanisms
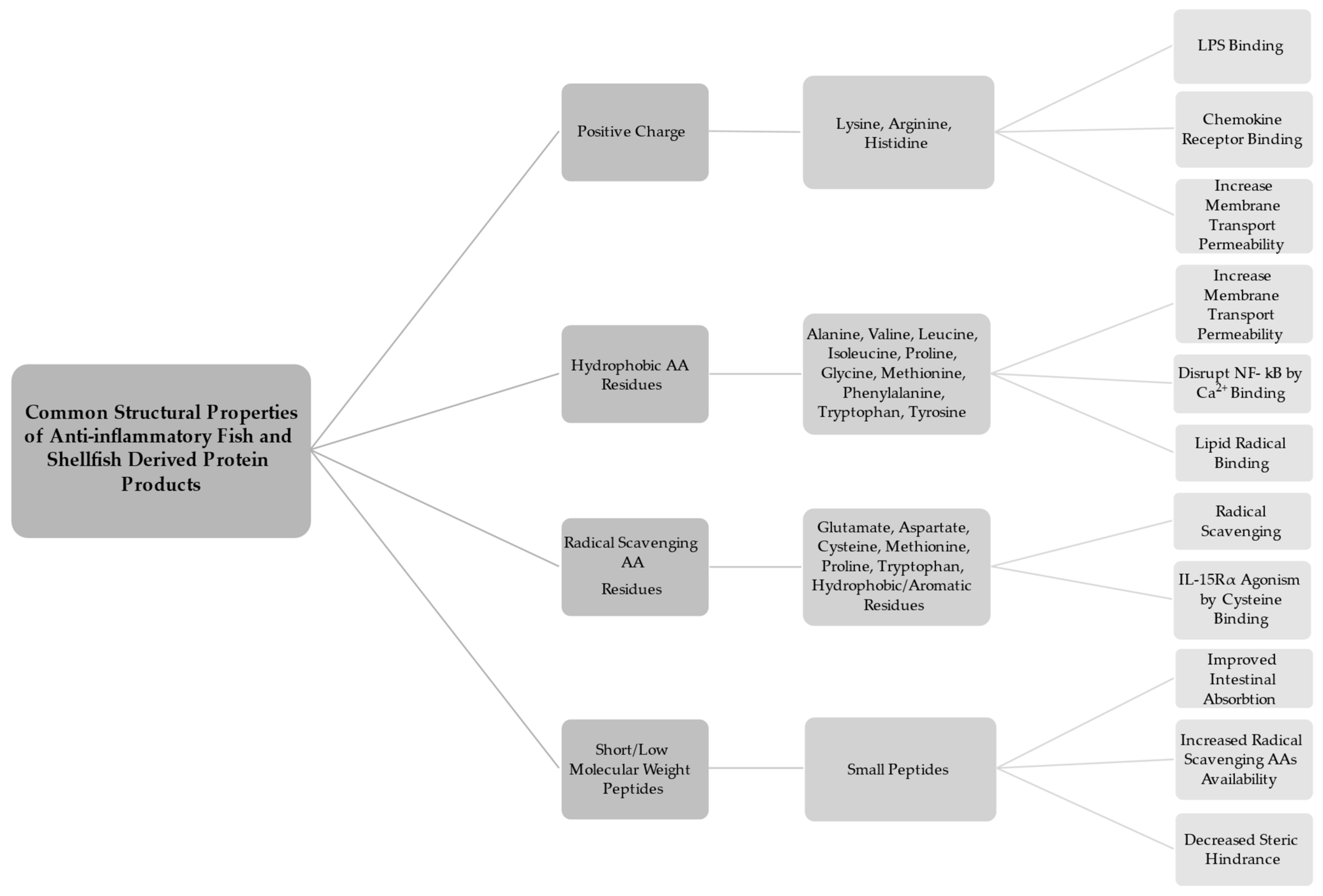
3.1. Properties
3.2. Mechanisms
| Protein Product | Source | Preparation Method | Model(s) | Mechanism(s) | Reference |
|---|---|---|---|---|---|
| Peptides: IVPAS, FDKPVSPLL | Herring Milt | From Hydrolysate Enzyme(s): mix (confidential) | Cellular: LPS-stimulated J774 mouse macrophage cells | Inhibition of iNOS activity, oxygen radical scavenging. | [43] |
| Peptide: EGLLGDVF | Green mussel | From Hydrolysate Enzyme(s): Alcalase | Cellular: LPS-stimulated RAW 264.7 mouse macrophage cells | Downregulation of iNOS and COX-2 protein expression. | [56] |
| Peptide: LGLGAAVL | Marine crab leg muscle | From Hydrolysate Enzyme(s): Trypsin | Cellular: LPS-stimulated RAW 264.7 mouse macrophage cells | Suppression of COX-2 expression. | [24] |
| Hydrolysate | Threadfin bream muscle protein | Hydrolysate fraction: Treated with ultrasound 300W, and Microwave 100W Enzyme(s): Alcalase | Chemical: ABTS and DPPH radical scavenging assays Cellular: H2O2-stimulated RAW 264.7 mouse macrophage cells | Radical scavenging. Upregulation of SOD and CAT activity. Downregulation of TNF-α and IL-1β. | [51] |
| Collagen Peptide Fraction: <3 kDa collagen peptide | Milkfish scales | Collagen extraction and ultrafiltration | Chemical: Lox-1 activity and nitric oxide radical production assays | Decrease in ROS and RNS activity via inhibition of lipoxygenase and NO | [14] |
| Hydrolysate: high molecular weight (>5 kDa) fraction | Blue mussel | From Hydrolysate: Enzyme: Pepsin | Cellular: LPS-stimulated RAW 264.7 mouse macrophage cells | Inhibition of iNOS and COX-2 gene expression and reduction in TNF-α, IL-6, and IL-1β. Inhibition of of NF-κB translocation by preventing phosphorylation of IκBα and inhibition of PGE2 secretion. | [61] |
| Peptide: YA | Oyster | From Hydrolysate Enzyme(s): Protamex, and Neutrase | Chemical: COX-2 and 5-LOX activity assays Cellular: IL-1β and TNF-α mRNA expression in EtOH induced Chang liver cells | Inhibition of COX-2 and 5-LO, and a reduction in IL-1β, TNF-α | [70] |
| Peptides: KIWHHTF, VHYAGTVDY, HLDDALRGQE | Sturgeon back muscle | From Hydrolysate Enzyme(s): Pepsin | Cellular: LPS-stimulated RAW 264.7 mouse macrophage cells | Downregulation of JNK and p38 phosphoylation. Decrease in IL-1β and IL-6. Upregulation of SOD activity. | [46] |
| Hydrolysates | Anchovy (Engraulis encrasicolus) viscera | From Hydrolysate Enzyme(s): Protamex, Flavourzyme, and Alcalase | Cellular: LPS-stimulated RAW 264.7 mouse macrophage cells in vivo: female six-month-old B6.129P2-ApoE−/− mice | Inhibition of COX-2 and the nuclear translocation of NF-κB by preventing phosphorylation of IκBα. Modulation of iNOS, MnSOD and HO-1 expression. Downregulation of TNF-α, IL-1α, and IL-6, and modulation of SOD, catalase (CAT), Glutathione peroxidase (Gpx), and Heme oxygenase in mice aorta and heart tissue. | [15] |
| Hydrolysate Fraction: <1.5 kDa | Sturgeon Muscle | From Hydrolysate Enzyme(s): Pepsin | Chemical: ABTS and DPPH radical scavenging assays Cellular: LPS-stimulated RAW 264.7 mouse macrophage cells | Radical scavenging. Inhibition of IL-1β, IL-6, TNF-α, and phosphrylation of MAPKs/IκBα | [63] |
| Peptides: SNKGGGRPN, PGVATAPTH, LLGLGLPPA | Salmon Bones | From Hydrolysate Enzyme(s): Papain | Cellular: LPS-stimulated RAW 264.7 mouse macrophage cells | Inhibition of NO, COX-2, IL-6, iNOS, and TNF-α mRNA | [44] |
| Peptide: HKGQCC | Saltwater clams (Meretrix meretrix) | From Hydrolysate Enzyme(s): Trypsin | Chemical: Albumin denaturation assay Cellular: Human red blood cell (HRBC) stabilization assay Cellular: LPS-stimulated RAW 264.7 mouse macrophage cells in vivo: 6–7 month old wild type zebrafish | Inhibition of NO, TNF-α, IL-1β, and COX-2 in LPS-stimulated RAW 264.7 cells Inhibition of TNF-α, IL-1β, iNOS, and COX-2 mRNA in LPS-stimulated Zebrafish | [48] |
| Peptide: PAY | Salmon pectoral fin | From Hydrolysate Enzyme: Pepsin | Cellular: LPS-stimulated RAW 264.7 mouse macrophage cells | Inhibition of NO/iNOS, PGE2/COX-2 pathways. Reduced TNF-α, IL-6, and IL-1β expression. | [65] |
| Hydrolysates | Sandfish (Arctoscopus japonicus) muscle (MHA) and roe (RHC) | From Hydrolysate Enzymes: Collupulin MG (RHC), and Alcalase (MHA) | Chemical: DPPH radical scavenging assay | Radical scavenging. | [82] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino Acid |
| BAP | Bioactive Peptide |
| BPH | Bioactive Protein Hydrolysate |
| CAT | Catalase |
| COX-2 | Cyclooxygenase-2 |
| Gpx | Glutathione Peroxidase |
| HO-1 | Heme Oxygenase-1 |
| iNOS | Inducible Nitric Oxide Synthase |
| kDa | Kilodalton |
| LMW | Low-Molecular Weight |
| LPS | Lipopolysaccharide |
| NSAID | Non-Steroidal Anti-inflammatory Drug |
| PGE2 | Prostaglandin E2 |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SCI | Systemic Chronic Inflammation |
| SOD | Superoxide Dismutase |
| SPB | Seafood Processing Byproduct |
References
- Venkatraman, K.L.; Mehta, A. Health benefits and pharmacological effects of Porphyra species. Plant Foods Hum. Nutr. 2019, 74, 10–17. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food waste and byproducts: An opportunity to minimize malnutrition and hunger in developing countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Takayama, K.; Rentier, C.; Asari, T.; Nakamura, A.; Saga, Y.; Shimada, T.; Nirasawa, K.; Sasaki, E.; Muguruma, K.; Taguchi, A.; et al. Development of potent myostatin inhibitory peptides through hydrophobic residue-directed structural modification. ACS Med. Chem. Lett. 2017, 8, 751–756. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.-F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Y.-J.; Cheng, Y. Complementary and alternative therapies for inflammatory diseases. Evid. Based Complement. Altern. Med. 2016, 2016, 8324815. [Google Scholar] [CrossRef]
- Ruijters, E.; Haenen, G.; Willemsen, M.; Weseler, A.; Bast, A. Food-derived bioactives can protect the anti-inflammatory activity of cortisol with antioxidant-dependent and -independent mechanisms. Int. J. Mol. Sci. 2016, 17, 239. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Gündüz, H.; Göztürk, F.; Hamzaçebi, S.; Akpınar, M.D. The assessment of seafood processing waste. Aquat. Sci. Eng. 2018, 33, 1–5. [Google Scholar] [CrossRef]
- Petrova, I.; Tolstorebrov, I.; Eikevik, T.M. Production of fish protein hydrolysates step by step: Technological aspects, equipment used, major energy costs and methods of their minimizing. Int. Aquat. Res. 2018, 10, 223–241. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Liang, C.-H.; Wu, H.-T.; Pang, H.-Y.; Chen, C.; Wang, G.-H.; Chan, L.-P. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J. Food Sci. Technol. 2018, 55, 2310–2317. [Google Scholar] [CrossRef]
- Giannetto, A.; Esposito, E.; Lanza, M.; Oliva, S.; Riolo, K.; di Pietro, S.; Abbate, J.M.; Briguglio, G.; Cassata, G.; Cicero, L.; et al. Protein hydrolysates from anchovy (Engraulis encrasicolus) waste: In vitro and in vivo biological activities. Mar. Drugs 2020, 18, 86. [Google Scholar] [CrossRef]
- FAO. Fish and Their By-Products; FAO: Rome, Italy, 2016. [Google Scholar]
- Surasani, V.K.R. Acid and alkaline solubilization (PH Shift) process: A better approach for the utilization of fish processing waste and by-products. Environ. Sci. Pollut. Res. 2018, 25, 18345–18363. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish waste: From problem to valuable resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Mora, L.; Aristoy, M.-C.; Toldrá, F. Bioactive peptides. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 381–389. ISBN 9780128140451. [Google Scholar]
- Chalamaiah, M.; Ulug, S.K.; Hong, H.; Wu, J. Regulatory requirements of bioactive peptides (protein hydrolysates) from food proteins. J. Funct. Foods 2019, 58, 123–129. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Narayanasamy, A.; Balde, A.; Raghavender, P.; Shashanth, D.; Abraham, J.; Joshi, I.; Nazeer, R.A. Isolation of marine crab (Charybdis natator) leg muscle peptide and its anti-inflammatory effects on macrophage cells. Biocatal. Agric. Biotechnol. 2020, 25, 101577. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-derived bioactive peptides on inflammation and oxidative stress. Biomed. Res. Int. 2014, 2014, 608979. [Google Scholar] [CrossRef] [PubMed]
- Cal, R.; Davis, H.; Kerr, A.; Wall, A.; Molloy, B.; Chauhan, S.; Trajkovic, S.; Holyer, I.; Adelfio, A.; Khaldi, N. Preclinical evaluation of a food-derived functional ingredient to address skeletal muscle atrophy. Nutrients 2020, 12, 2274. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Daliri, E.; Oh, D.; Lee, B. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- La Manna, S.; di Natale, C.; Florio, D.; Marasco, D. Peptides as therapeutic agents for inflammatory-related diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Strategies for the Discovery and Identification of Food Protein-Derived Biologically Active Peptides. Trends Food Sci. Technol. 2017, 69, 289–305. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, L.; Zhang, L.; Qiao, Q.; Farooq, M.Z.; Xu, Q. The Potential of Food Protein-Derived Bioactive Peptides against Chronic Intestinal Inflammation. Mediat. Inflamm. 2020, 2020, 6817156. [Google Scholar] [CrossRef]
- Urakova, I.N.; Pozharitskaya, O.N.; Demchenko, D.V.; Shikov, A.N.; Makarov, V.G. The biological activities of fish peptides and methods of their isolation. Russ. J. Mar. Biol. 2012, 38, 417–422. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Jönsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 2013, 109, S1–S34. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.S.; Hasan, M.M.; Kurata, H. PreAIP: Computational prediction of anti-inflammatory peptides by integrating multiple complementary features. Front. Genet. 2019, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and pancreatic cancer: Focus on metabolism, cytokines, and immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Marcum, Z.A.; Hanlon, J.T. Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Ann. Longterm. Care 2010, 18, 24–27. [Google Scholar]
- Majumder, K.; Mine, Y.; Wu, J. The potential of food protein-derived anti-inflammatory peptides against various chronic inflammatory diseases. J. Sci. Food Agric. 2016, 96, 2303–2311. [Google Scholar] [CrossRef]
- Selamassakul, O.; Laohakunjit, N.; Kerdchoechuen, O.; Yang, L.; Maier, C.S. Bioactive peptides from brown rice protein hydrolyzed by bromelain: Relationship between biofunctional activities and flavor characteristics. J. Food Sci. 2020, 85, 707–717. [Google Scholar] [CrossRef]
- Durand, R.; Pellerin, G.; Thibodeau, J.; Fraboulet, E.; Marette, A.; Bazinet, L. Screening for metabolic syndrome application of a herring by-product hydrolysate after its separation by electrodialysis with ultrafiltration membrane and identification of novel anti-inflammatory peptides. Sep. Purif. Technol. 2020, 235, 116205. [Google Scholar] [CrossRef]
- Saisavoey, T.; Sangtanoo, P.; Reamtong, O.; Karnchanatat, A. Free radical scavenging and anti-inflammatory potential of a protein hydrolysate derived from salmon bones on RAW 264.7 macrophage cells. J. Sci. Food Agric. 2019, 99, 5112–5121. [Google Scholar] [CrossRef]
- Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Anti-inflammatory action of two novel peptides derived from peanut worms (Sipunculus nudus) in lipopolysaccharide-induced RAW264.7 macrophages. Food Funct. 2020, 11, 552–560. [Google Scholar] [CrossRef]
- Gao, R.; Shu, W.; Shen, Y.; Sun, Q.; Bai, F.; Wang, J.; Li, D.; Li, Y.; Jin, W.; Yuan, L. Sturgeon protein-derived peptides exert anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages via the MAPK pathway. J. Funct. Foods 2020, 72, 104044. [Google Scholar] [CrossRef]
- Vogel, H.J.; Schibli, D.J.; Jing, W.; Lohmeier-Vogel, E.M.; Epand, R.F.; Epand, R.M. Towards a structure-function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides. Biochem. Cell Biol. 2002, 80, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Joshi, I.; Mohideen, H.S.; Nazeer, R.A. A Meretrix meretrix visceral mass derived peptide inhibits lipopolysaccharide-stimulated responses in RAW264.7 cells and adult zebrafish model. Int. Immunopharmacol. 2021, 90, 107140. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, L.; Yu, Z.; Ma, S.; Du, Z.; Zhang, T.; Liu, J. Importance of terminal amino acid residues to the transport of oligopeptides across the caco-2 cell monolayer. J. Agric. Food Chem. 2017, 65, 7705–7712. [Google Scholar] [CrossRef]
- Yu, W.; Field, C.J.; Wu, J. Purification and identification of anti-inflammatory peptides from spent hen muscle proteins hydrolysate. Food Chem. 2018, 253, 101–107. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Impact of combined ultrasound-microwave treatment on structural and functional properties of golden threadfin bream (Nemipterus virgatus) myofibrillar proteins and hydrolysates. Ultrason. Sonochem. 2020, 65, 105063. [Google Scholar] [CrossRef]
- Joshi, I.; Sudhakar, S.; Nazeer, R.A. Anti-inflammatory properties of bioactive peptide derived from gastropod influenced by enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2016, 180, 1128–1140. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Calcium binding to amino acids and small glycine peptides in aqueous solution: Toward peptide design for better calcium bioavailability. J. Agric. Food Chem. 2016, 64, 4376–4389. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bradford, B.U.; Wheeler, M.D.; Stimpson, S.A.; Pink, H.M.; Brodie, T.A.; Schwab, J.H.; Thurman, R.G. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: Role for glycine-gated chloride channel. Infect. Immun. 2001, 69, 5883–5891. [Google Scholar] [CrossRef] [PubMed]
- Joshi, I.; Nazeer, R.A. EGLLGDVF: A novel peptide from green mussel Perna viridis foot exerts stability and anti-inflammatory effects on LPS-stimulated RAW264.7 cells. Protein Pept. Lett. 2020, 27, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Dadar, M.; Shahali, Y.; Chakraborty, S.; Prasad, M.; Tahoori, F.; Tiwari, R.; Dhama, K. Antiinflammatory peptides: Current knowledge and promising prospects. Inflamm. Res. 2019, 68, 125–145. [Google Scholar] [CrossRef]
- Andrade, V.S.; Rojas, D.B.; de Andrade, R.B.; Kim, T.D.H.; Vizuete, A.F.; Zanatta, Â.; Wajner, M.; Gonçalves, C.-A.S.; Wannmacher, C.M.D. A possible anti-inflammatory effect of proline in the brain cortex and cerebellum of rats. Mol. Neurobiol. 2017, 55, 4068–4077. [Google Scholar] [CrossRef]
- Subhan, F.; Kang, H.Y.; Lim, Y.; Ikram, M.; Baek, S.-Y.; Jin, S.; Jeong, Y.H.; Kwak, J.Y.; Yoon, S. Fish scale collagen peptides protect against CoCl 2 /TNF-α-induced cytotoxicity and inflammation via inhibition of ROS, MAPK, and NF-κB pathways in HaCaT cells. Oxid. Med. Cell. Longev. 2017, 2017, 9703609. [Google Scholar] [CrossRef]
- Kaul, S.; Sharma, S.S.; Mehta, I.K. Free radical scavenging potential of L-proline: Evidence from in vitro assays. Amino Acids 2008, 34, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Ahn, C.-B.; Je, J.-Y. Anti-inflammatory action of high molecular weight Mytilus edulis hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-ΚB and MAPK pathways. Food Chem. 2016, 202, 9–14. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, B.; Zheng, Q.; Zhu, G.; Cao, W.; Qin, X.; Zhang, C. Ameliorative effects of peptides from the oyster (Crassostrea hongkongensis) protein hydrolysates against UVB-induced skin photodamage in mice. Mar. Drugs 2020, 18, 288. [Google Scholar] [CrossRef]
- Gao, R.; Shu, W.; Shen, Y.; Sun, Q.; Jin, W.; Li, D.; Li, Y.; Yuan, L. Peptide fraction from sturgeon muscle by pepsin hydrolysis exerts anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages via MAPK and NF-ΚB pathways. Food Sci. Hum. Wellness 2021, 10, 103–111. [Google Scholar] [CrossRef]
- Xiang, X.-W.; Zhou, X.-L.; Wang, R.; Shu, C.-H.; Zhou, Y.-F.; Ying, X.-G.; Zheng, B. Protective effect of tuna bioactive peptide on dextran sulfate sodium-induced colitis in mice. Mar. Drugs 2021, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.-B.; Cho, Y.-S.; Je, J.-Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ngamsuk, S.; Hsu, J.-L.; Huang, T.-C.; Suwannaporn, P. Ultrasonication of milky stage rice milk with bioactive peptides from rice bran: Its bioactivities and absorption. Food Bioprocess Technol. 2020, 13, 462–474. [Google Scholar] [CrossRef]
- Bamdad, F.; Bark, S.; Kwon, C.H.; Suh, J.-W.; Sunwoo, H. Anti-inflammatory and antioxidant properties of peptides released from β-lactoglobulin by high hydrostatic pressure-assisted enzymatic hydrolysis. Molecules 2017, 22, 949. [Google Scholar] [CrossRef] [PubMed]
- Neelima, R.S.; Rajput, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Hsieh, C.-C.; de Lumen, B.O. Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808. [Google Scholar] [CrossRef]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.-J.; Shin, E.-J.; Woo, M.S.; Kim, J.-M.; Kim, J.H.; Lee, D.K.; Hahm, J.R.; Kim, H.J.; et al. Dipeptide YA is responsible for the positive effect of oyster hydrolysates on alcohol metabolism in single ethanol binge rodent models. Mar. Drugs 2020, 18, 512. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Sutikno, L.A.; Lee, S.B.; Jin, D.H.; Hong, Y.K.; Kim, Y.S.; Jin, H.J. Identification of the minimum region of flatfish myostatin propeptide (Pep45-65) for myostatin inhibition and its potential to enhance muscle growth and performance in animals. PLoS ONE 2019, 14, e0215298. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Nagahawatta, D.P.; Yang, H.-W.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.-J.; de Zoysa, M.; Whang, I.; Ryu, B. Octominin inhibits LPS-induced chemokine and pro-inflammatory cytokine secretion from RAW 264.7 macrophages via blocking TLRs/NF-ΚB signal transduction. Biomolecules 2020, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, T.; Hashemian, S.M. Computer-aided design of amino acid-based therapeutics: A review. Drug Des. Devel. Ther. 2018, 12, 1239–1254. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-inflammatory agents: Present and future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Sabio, G.; Davis, R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1β during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The role of mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) in inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, M.; Chen, W.; Zhao, T.; Wei, Y. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed. Pharmacother. 2019, 118, 109249. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Rodríguez-Iturbe, B. Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 582–593. [Google Scholar] [CrossRef]
- Jang, H.L.; Liceaga, A.M.; Yoon, K.Y. Isolation and characteristics of anti-inflammatory peptides from enzymatic hydrolysates of sandfish (Arctoscopus japonicus) protein. J. Aquat. Food Prod. Technol. 2017, 26, 234–244. [Google Scholar] [CrossRef]
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.-I.; Williams, M. Feeding 9 billion by 2050—Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemp, D.C.; Kwon, J.Y. Fish and Shellfish-Derived Anti-Inflammatory Protein Products: Properties and Mechanisms. Molecules 2021, 26, 3225. https://doi.org/10.3390/molecules26113225
Kemp DC, Kwon JY. Fish and Shellfish-Derived Anti-Inflammatory Protein Products: Properties and Mechanisms. Molecules. 2021; 26(11):3225. https://doi.org/10.3390/molecules26113225
Chicago/Turabian StyleKemp, David C., and Jung Yeon Kwon. 2021. "Fish and Shellfish-Derived Anti-Inflammatory Protein Products: Properties and Mechanisms" Molecules 26, no. 11: 3225. https://doi.org/10.3390/molecules26113225
APA StyleKemp, D. C., & Kwon, J. Y. (2021). Fish and Shellfish-Derived Anti-Inflammatory Protein Products: Properties and Mechanisms. Molecules, 26(11), 3225. https://doi.org/10.3390/molecules26113225






