The Peptide Functionalized Inorganic Nanoparticles for Cancer-Related Bioanalytical and Biomedical Applications
Abstract
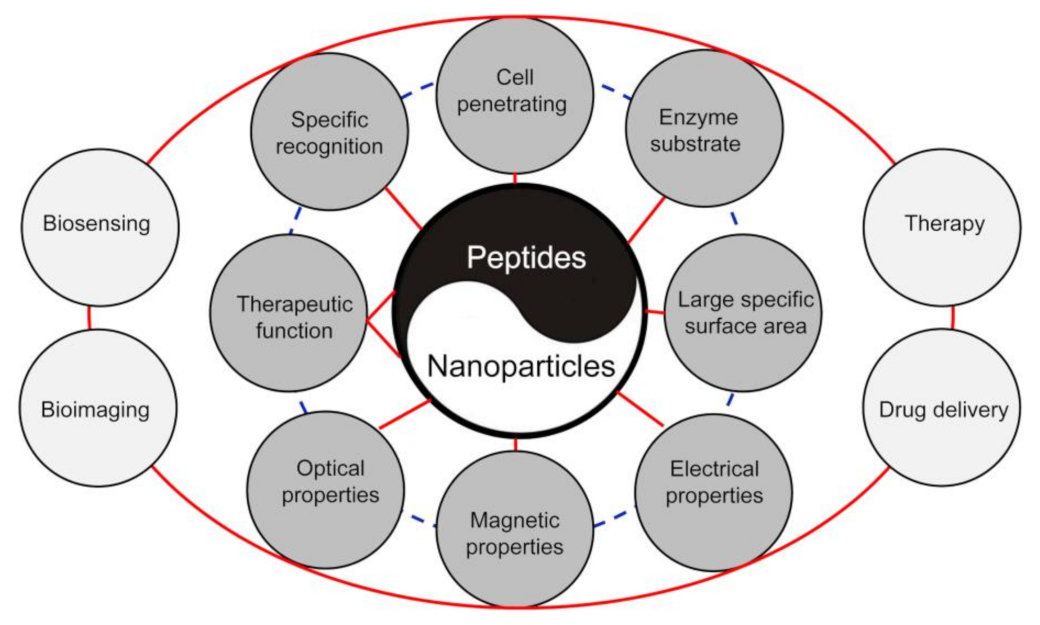
:1. Introduction
2. Synthesis of Peptide Functionalized Nanoparticles
2.1. Ligand Exchange
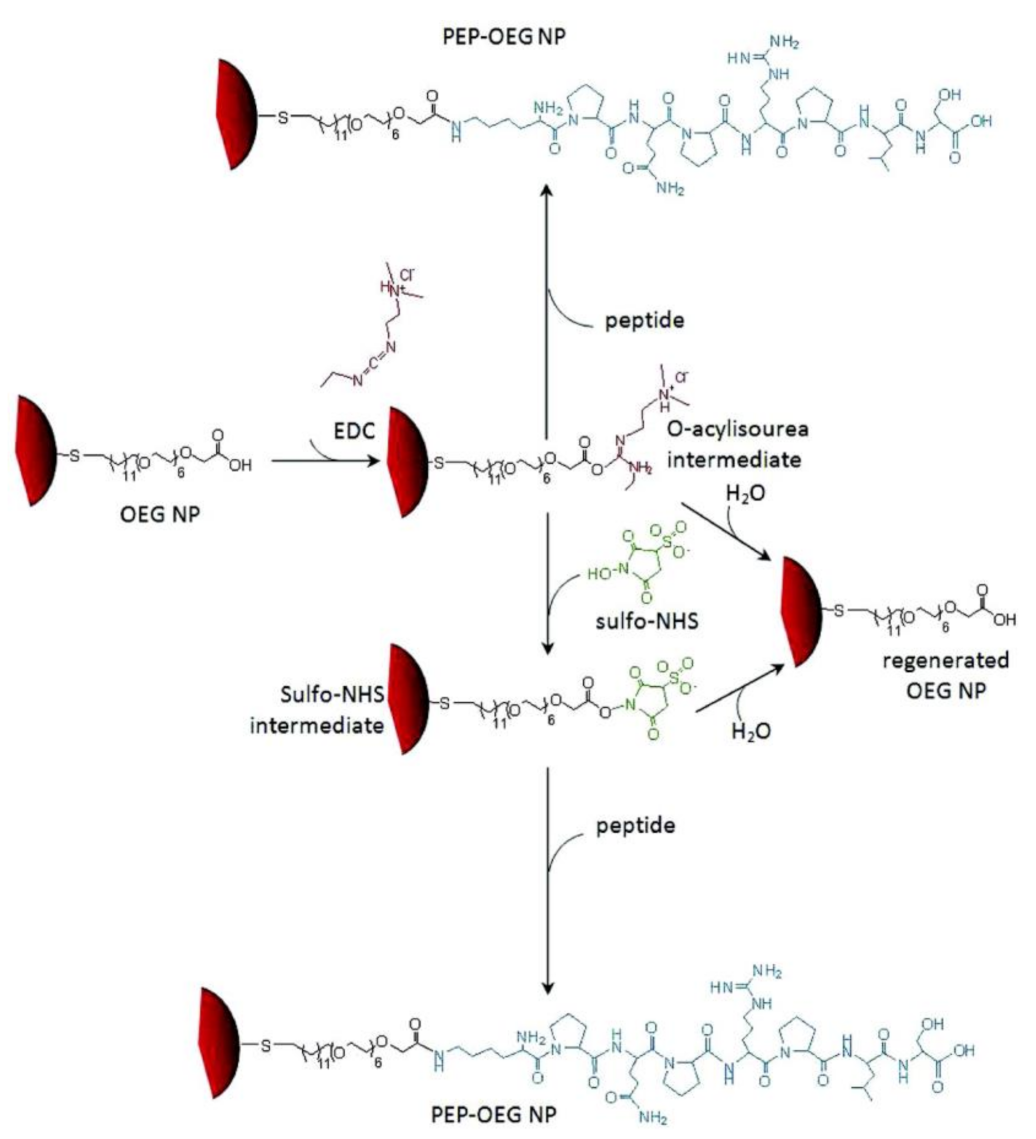
2.2. Chemical Conjugation
2.3. Chemical Reduction
3. Biosensing Platforms Based on Peptide Functionalized Nanoparticles
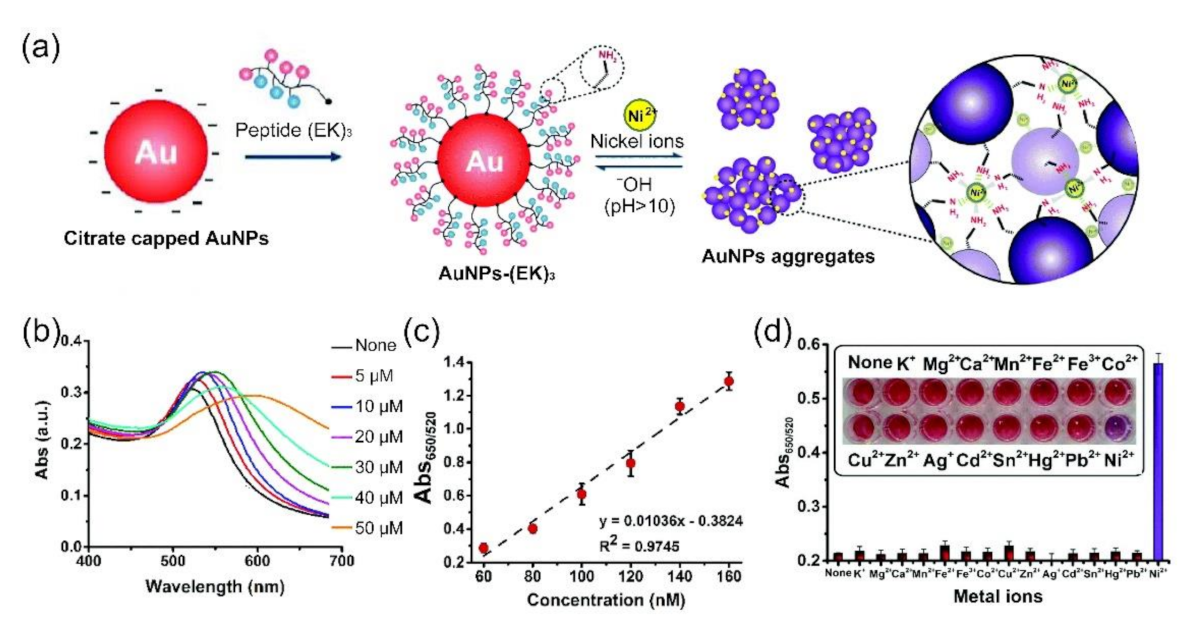
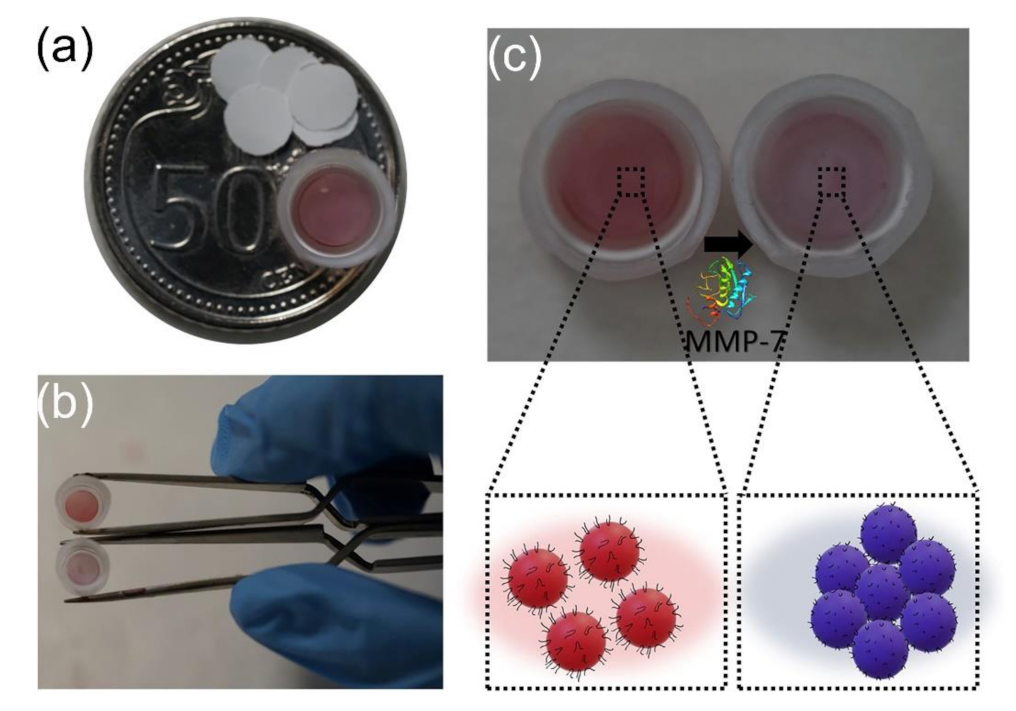
3.1. Colorimetric Assays Based on Peptide Functionalized AuNPs
3.2. Fluorescence Assays Based on Peptide Functionalized NPs
4. Employing Peptide Functionalized Nanoparticle as Positive Tumor-Targeting Nanomedicines
4.1. Enhancing Cellular Internalization and Targeting Cancer Cells
4.2. Cancer Therapy
4.2.1. Antiangiogenic Therapy
4.2.2. Photothermal Therapy
4.2.3. Radiotherapy
4.2.4. As Tumor Microenvironment Responsive Nanoprobes with Precision Tumor-Targeting
4.2.5. Employing Peptide Functionalized NPs as Positive Tumor-Targeting Drug Carries
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wild, C.; Weiderpass, E.; Stewart, B. World Cancer Report: Cancer Research for Cancer Prevention; Wild, C., Weiderpass, E., Stewart, B., Eds.; International Agency for Research on Cancer: Lyon, France, 2020; ISBN 978-92-832-0447-3. [Google Scholar]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef]
- Wang, Z.X.; Ma, L.N. Gold nanoparticle probes. Coordin. Chem. Rev. 2009, 253, 1607–1618. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.B.; Chen, X.Y. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouiller, A.J.; Hebie, S.; El Bahhaj, F.; Napporn, T.W.; Bertrand, P. Chemistry for oncotheranostic gold nanoparticles. Eur. J. Med. Chem. 2015, 99, 92–112. [Google Scholar] [CrossRef] [PubMed]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.M.; Ma, P.A.; Hou, Z.Y.; Cheng, Z.Y.; Li, C.X.; Lin, J. Current advances in lanthanide ion (Ln(3+))-based upconversion nanomaterials for drug delivery. Chem. Soc. Rev. 2015, 44, 1416–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.J.; Su, Q.Q.; Feng, W.; Li, F.Y. Anti-Stokes shift luminescent materials for bio-applications. Chem. Soc. Rev. 2017, 46, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Multifunctional gold-based nanocomposites for theranostics. Biomaterials 2016, 108, 13–34. [Google Scholar] [CrossRef]
- Zong, J.Y.; Cobb, S.L.; Cameron, N.R. Peptide-functionalized gold nanoparticles: Versatile biomaterials for diagnostic and therapeutic applications. Biomater. Sci. 2017, 5, 872–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.H.; Li, J.H. Plasmon-Based Colorimetric Nanosensors for Ultrasensitive Molecular Diagnostics. ACS Sens. 2017, 2, 857–875. [Google Scholar] [CrossRef]
- Zhang, P.C.; Cui, Y.G.; Anderson, C.F.; Zhang, C.L.; Li, Y.P.; Wang, R.F.; Cui, H.G. Peptide-based nanoprobes for molecular imaging and disease diagnostics. Chem. Soc. Rev. 2018, 47, 3490–3529. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Boer, J.C.; Selomulya, C.; Plebanski, M. Amino Acid Functionalized Inorganic Nanoparticles as Cutting-Edge Therapeutic and Diagnostic Agents. Bioconjugate Chem. 2018, 29, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and protein nanoparticle conjugates: Versatile platforms for biomedical applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, C.Y.; Xu, C.; Wang, X.L.; Liu, C.; Waterhouse, G.I.N.; Wang, Y.L.; Yin, H.Z. Ultrasmall Au nanoclusters for biomedical and biosensing applications: A mini-review. Talanta 2019, 200, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.D.; Hu, C.L.; Liu, Y.; Chen, F.; Zheng, Z.; Wang, X.L. Enzyme-/Redox-Responsive Mesoporous Silica Nanoparticles Based on Functionalized Dopamine as Nanocarriers for Cancer Therapy. ACS Omega 2019, 4, 6097–6105. [Google Scholar] [CrossRef] [Green Version]
- Mohebbi, S.; Moghadam, T.T.; Nikkhah, M.; Behmanesh, M. RGD-HK Peptide-Functionalized Gold Nanorods Emerge as Targeted Biocompatible Nanocarriers for Biomedical Applications. Nanoscale Res. Lett. 2019, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Liang, J.Y.; Yun, S.L.J.; Liang, K.; Yang, D.Y.; Gu, Z. Recent advances in improving tumor-targeted delivery of imaging nanoprobes. Biomater. Sci. 2020, 8, 4129–4146. [Google Scholar] [CrossRef]
- Pigliacelli, C.; Sanchez-Fernandez, R.; Garcia, M.D.; Peinador, C.; Pazos, E. Self-assembled peptide-inorganic nanoparticle superstructures: From component design to applications. Chem. Commun. 2020, 56, 8000–8014. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, Q.W.; Knoll, W.G.; Liedberg, B.; Wang, Y. Rational Design of Functional Peptide-Gold Hybrid Nanomaterials for Molecular Interactions. Adv. Mater. 2020, 32, 2000866. [Google Scholar] [CrossRef]
- Sun, D.X.; Zhou, S.; Gao, W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano 2020, 14, 12281–12290. [Google Scholar] [CrossRef]
- Chung, Y.J.; Kim, J.; Park, C.B. Photonic Carbon Dots as an Emerging Nanoagent for Biomedical and Healthcare Applications. ACS Nano 2020, 14, 6470–6497. [Google Scholar] [CrossRef]
- Liu, J.J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Lai, W.F.; Wong, W.T.; Rogach, A.L. Development of Copper Nanoclusters for In Vitro and In Vivo Theranostic Applications. Adv. Mater. 2020, 32, 1906872. [Google Scholar] [CrossRef]
- Quintana, C.; Cifuentes, M.P.; Humphrey, M.G. Transition metal complex/gold nanoparticle hybrid materials. Chem. Soc. Rev. 2020, 49, 2316–2341. [Google Scholar] [CrossRef]
- Nel, A.E. Transformational Impact of Nanomedicine: Reconciling Outcome with Promise. Nano Lett. 2020, 20, 5601–5603. [Google Scholar] [CrossRef]
- Shahbazi, M.A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Xiao, C.; Yong, T.Y.; Li, Z.F.; Gan, L.; Yang, X.L. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem. Soc. Rev. 2020, 49, 2273–2290. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Wu, T.T.; Li, M.Q.; Tao, Y. Recent advances in nanomaterials for colorimetric cancer detection. J. Mater. Chem. B. 2021, 9, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.Y.; Sena-Torralba, A.; Alvarez-Diduk, R.; Muthoosamy, K.; Merkoci, A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS Nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef]
- Kandell, R.M.; Waggoner, L.E.; Kwon, E.J. Nanomedicine for Acute Brain Injuries: Insight from Decades of Cancer Nanomedicine. Mol. Pharm. 2021, 18, 522–538. [Google Scholar] [CrossRef]
- Maeda, H. The 35th Anniversary of the Discovery of EPR Effect: A New Wave of Nanomedicines for Tumor-Targeted Drug Delivery—Personal Remarks and Future Prospects. J. Pers. Med. 2021, 11, 229. [Google Scholar] [CrossRef]
- Li, X.D.; Sun, Y.H.; Ma, L.N.; Liu, G.F.; Wang, Z.X. The Renal Clearable Magnetic Resonance Imaging Contrast Agents: State of the Art and Recent Advances. Molecules 2020, 25, 5072. [Google Scholar] [CrossRef]
- Desale, K.; Kuche, K.; Jain, S. Cell-penetrating peptides (CPPs): An overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef] [PubMed]
- Pierschbacher, M.D.; Ruoslahti, E. Cell Attachment Activity of Fibronectin Can Be Duplicated by Small Synthetic Fragments of the Molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.J.; Schwaiger, M.; Weinmuller, M.; Rader, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef]
- Morshed, R.A.; Muroski, M.E.; Dai, Q.; Wegscheid, M.L.; Auffinger, B.; Yu, D.; Han, Y.; Zhang, L.J.; Wu, M.J.; Cheng, Y.; et al. Cell-Penetrating Peptide-Modified Gold Nanoparticles for the Delivery of Doxorubicin to Brain Metastatic Breast Cancer. Mol. Pharm. 2016, 13, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, Z.H.; Prasad, P.N.; Knecht, M.R.; Swihart, M.T. Peptide-mediated synthesis of gold nanoparticles: Effects of peptide sequence and nature of binding on physicochemical properties. Nanoscale 2014, 6, 3165–3172. [Google Scholar] [CrossRef]
- Wang, W.; Anderson, C.F.; Wang, Z.Y.; Wu, W.; Cui, H.G.; Liu, C.J. Peptide-templated noble metal catalysts: Syntheses and applications. Chem. Sci. 2017, 8, 3310–3324. [Google Scholar] [CrossRef] [Green Version]
- Levy, R.; Thanh, N.T.K.; Doty, R.C.; Hussain, I.; Nichols, R.J.; Schiffrin, D.J.; Brust, M.; Fernig, D.G. Rational and combinatorial design of peptide capping Ligands for gold nanoparticles. J. Am. Chem. Soc. 2004, 126, 10076–10084. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Levy, R.; Fernig, D.G.; Brust, M. The peptide route to multifunctional gold nanoparticles. Bioconjugate Chem. 2005, 16, 497–500. [Google Scholar] [CrossRef]
- Krpetic, Z.; Nativo, P.; Porta, F.; Brust, M. A Multidentate Peptide for Stabilization and Facile Bioconjugation of Gold Nanoparticles. Bioconjugate Chem. 2009, 20, 619–624. [Google Scholar] [CrossRef]
- Krpetic, Z.; Saleemi, S.; Prior, I.A.; See, V.; Qureshi, R.; Brust, M. Negotiation of Intracellular Membrane Barriers by TAT-Modified Gold Nanoparticles. ACS Nano 2011, 5, 5195–5201. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yao, M.H.; Du, M.S.; Jin, R.M.; Zhao, D.H.; Ma, J.; Ma, Z.Y.; Zhao, Y.D.; Liu, B. A near-infrared light-controlled system for reversible presentation of bioactive ligands using polypeptide-engineered functionalized gold nanorods. Chem. Commun. 2015, 51, 2569–2572. [Google Scholar] [CrossRef]
- Fernandes, R.; Smyth, N.R.; Muskens, O.L.; Nitti, S.; Heuer-Jungemann, A.; Ardern-Jones, M.R.; Kanaras, A.G. Interactions of Skin with Gold Nanoparticles of Different Surface Charge, Shape, and Functionality. Small 2015, 11, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, E.; Chen, Q.B.; Davidson, A.M.; Paramelle, D.; Sullivan, M.B.; Volk, M.; Levy, R. Computational and Experimental Investigation of the Structure of Peptide Monolayers on Gold Nanoparticles. Langmuir 2017, 33, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, P.; Sukthankar, P.; Changstrom, J.; Holland, C.S.; Barry, S.; Hunter, W.B.; Sorensen, C.M.; Tomich, J.M. Synthesis and Characterization of Multifunctional Branched Amphiphilic Peptide Bilayer Conjugated Gold Nanoparticles. ACS Omega 2018, 3, 11071–11083. [Google Scholar] [CrossRef]
- Monti, S.; Barcaro, G.; Sementa, L.; Carravetta, V.; Agren, H. Dynamics and self-assembly of bio-functionalized gold nanoparticles in solution: Reactive molecular dynamics simulations. Nano Res. 2018, 11, 1757–1767. [Google Scholar] [CrossRef]
- Samieegohar, M.; Sha, F.; Clayborne, A.Z.; Wei, T. ReaxFF MD Simulations of Peptide-Grafted Gold Nanoparticles. Langmuir 2019, 35, 5029–5036. [Google Scholar] [CrossRef]
- Luo, J.; Cheng, Y.; Gong, Z.W.; Wu, K.; Zhou, Y.; Chen, H.X.; Gauthier, M.; Cheng, Y.Z.; Liang, J.; Zou, T. Self-Assembled Peptide Functionalized Gold Nanopolyhedrons with Excellent Chiral Optical Properties. Langmuir 2020, 36, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Kong, F.P.; Gao, X.N.; Jiang, L.L.; Li, X.F.; Gao, W.; Xu, K.H.; Tang, B. Avoiding Thiol Compound Interference: A Nanoplatform Based on High-Fidelity Au-Se Bonds for Biological Applications. Angew. Chem. Int. Ed. 2018, 57, 5306–5309. [Google Scholar] [CrossRef]
- Luan, M.M.; Shi, M.W.; Pan, W.; Li, N.; Tang, B. A gold-selenium-bonded nanoprobe for real-time in situ imaging of the upstream and downstream relationship between uPA and MMP-9 in cancer cells. Chem. Commun. 2019, 55, 5817–5820. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Liu, X.H.; Wan, X.Y.; Li, J.; Li, Y.H.; Lu, F.; Li, N.; Tang, B. Rapid Preparation of Au-Se-Peptide Nanoprobe Based on a Freezing Method for Bioimaging. Anal. Chem. 2019, 91, 15982–15987. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Y.; He, X.X.; Zhang, J.P.; Zhang, H.M.; Wang, Z.X. Employing Tryptone as a General Phase Transfer Agent to Produce Renal Clearable Nanodots for Bioimaging. Small 2015, 11, 3676–3685. [Google Scholar] [CrossRef]
- Chen, H.D.; Li, X.D.; Liu, F.Y.; Zhang, H.M.; Wang, Z.X. Renal Clearable Peptide Functionalized NaGdF4 Nanodots for High-Efficiency Tracking Orthotopic Colorectal Tumor in Mouse. Mol. Pharm. 2017, 14, 3134–3141. [Google Scholar] [CrossRef]
- Bartczak, D.; Kanaras, A.G. Preparation of Peptide-Functionalized Gold Nanoparticles Using One Pot EDC/Sulfo-NHS Coupling. Langmuir 2011, 27, 10119–10123. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Heuer-Jungemann, A.; Fernandes, A.R.; Kanaras, A.G.; Baptista, P.V. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int. J. Nanomed. 2016, 11, 2633–2639. [Google Scholar] [CrossRef] [Green Version]
- Nicolardi, S.; van der Burgt, Y.E.M.; Codee, J.D.C.; Wuhrer, M.; Hokke, C.H.; Chiodo, F. Structural Characterization of Biofunctionalized Gold Nanoparticles by Ultrahigh-Resolution Mass Spectrometry. ACS Nano 2017, 11, 8257–8264. [Google Scholar] [CrossRef]
- Wilder, L.M.; Fies, W.A.; Rabin, C.; Webb, L.J.; Crooke, R.M. Conjugation of an alpha-Helical Peptide to the Surface of Gold Nanoparticles. Langmuir 2019, 35, 3363–3371. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Chen, H.; Wang, Z.; Yang, W.; Zhang, H. CXC Chemokine Receptor 4 Antagonist Functionalized Renal Clearable Manganese-Doped Iron Oxide Nanoparticles for Active-Tumor-Targeting Magnetic Resonance Imaging-Guided Bio-Photothermal Therapy. ACS Appl. Bio Mater. 2019, 2, 3613–3621. [Google Scholar] [CrossRef]
- Zhang, W.T.; Liu, X.F.; Zhao, X.N.; Zhang, X.F. Controllable delivery of peptides by superparamagnetic Fe3O4/slica nanoparticle vehicles. Mater. Lett. 2017, 201, 177–180. [Google Scholar] [CrossRef]
- Li, X.X.; Liu, L.; Fu, Y.; Chen, H.D.; Abualrejal, M.M.A.; Zhang, H.; Wang, Z.X.; Zhang, H.M. Peptide-enhanced tumor accumulation of upconversion nanoparticles for sensitive upconversion luminescence/magnetic resonance dual-mode bioimaging of colorectal tumors. Acta Biomater. 2020, 104, 167–175. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.T.; Zhang, H.; Chen, H.D.; Abualrejal, M.M.A.; Song, D.Q.; Wang, Z.X. Six-in-one peptide functionalized upconversion@polydopamine nanoparticle-based ratiometric fluorescence sensing platform for real-time evaluating anticancer efficacy through monitoring caspase-3 activity. Sens. Actuator B Chem. 2021, 333, 129554. [Google Scholar] [CrossRef]
- Si, S.; Mandal, T.K. Tryptophan-based peptides to synthesize gold and silver nanoparticles: A mechanistic and kinetic study. Chem. Eur. J. 2007, 13, 3160–3168. [Google Scholar] [CrossRef] [PubMed]
- Graf, P.; Mantion, A.; Foelske, A.; Shkilnyy, A.; Masic, A.; Thunemann, A.E.; Taubert, A. Peptide-Coated Silver Nanoparticles: Synthesis, Surface Chemistry, and pH-Triggered, Reversible Assembly into Particle Assemblies. Chem. Eur. J. 2009, 15, 5831–5844. [Google Scholar] [CrossRef] [PubMed]
- Upert, G.; Bouillere, F.; Wennemers, H. Oligoprolines as Scaffolds for the Formation of Silver Nanoparticles in Defined Sizes: Correlating Molecular and Nanoscopic Dimensions. Angew. Chem. Int. Ed. 2012, 51, 4231–4234. [Google Scholar] [CrossRef]
- Fanelli, R.; Milli, L.; Cornia, A.; Moretto, A.; Castellucci, N.; Zanna, N.; Malachin, G.; Tavano, R.; Tomasini, C. Chiral Gold Nanoparticles Decorated with Pseudopeptides. Eur. J. Org. Chem. 2015, 2015, 6243–6248. [Google Scholar] [CrossRef]
- Papst, S.; Brimble, M.A.; Tilley, R.D.; Williams, D.E. One-Pot Synthesis of Functionalized Noble Metal Nanoparticles Using a Rationally Designed Phosphopeptide. Part. Part. Syst. Char. 2014, 31, 971–975. [Google Scholar] [CrossRef]
- Corra, S.; Lewandowska, U.; Benetti, E.M.; Wennemers, H. Size-Controlled Formation of Noble-Metal Nanoparticles in Aqueous Solution with a Thiol-Free Tripeptide. Angew. Chem. Int. Ed. 2016, 55, 8542–8545. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Kotal, A.; Mandal, T.K. One-dimensional assembly of peptide-functionalized gold nanoparticles: An approach toward mercury ion sensing. J. Phys. Chem. C. 2007, 111, 1248–1255. [Google Scholar] [CrossRef]
- Li, X.K.; Wang, J.N.; Sun, L.L.; Wang, Z.X. Gold nanoparticle-based colorimetric assay for selective detection of aluminium cation on living cellular surfaces. Chem. Commun. 2010, 46, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.R.; Li, X.K.; Liu, X.; Wang, J.N.; Wang, Z.X. Designing bifunctionalized gold nanoparticle for colorimetric detection of Pb2+ under physiological condition. Biosens. Bioelectron. 2012, 31, 505–509. [Google Scholar] [CrossRef]
- Chen, H.X.; Zhang, J.J.; Liu, X.J.; Gao, Y.M.; Ye, Z.H.; Li, G.X. Colorimetric copper(II) ion sensor based on the conformational change of peptide immobilized onto the surface of gold nanoparticles. Anal. Methods 2014, 6, 2580–2585. [Google Scholar] [CrossRef]
- Yu, Y.M.; Hong, Y.; Gao, P.; Nazeeruddin, M.K. Glutathione Modified Gold Nanoparticles for Sensitive Colorimetric Detection of Pb2+ Ions in Rainwater Polluted by Leaking Perovskite Solar Cells. Anal. Chem. 2016, 88, 12316–12322. [Google Scholar] [CrossRef]
- Li, X.Y.; Wu, Z.T.; Zhou, X.D.; Hu, J.M. Colorimetric response of peptide modified gold nanoparticles: An original assay for ultrasensitive silver detection. Biosens. Bioelectron. 2017, 92, 496–501. [Google Scholar] [CrossRef]
- Shinde, S.; Kim, D.Y.; Saratale, R.G.; Syed, A.; Ameen, F.; Ghodake, G. A Spectral Probe for Detection of Aluminum (III) Ions Using Surface Functionalized Gold Nanoparticles. Nanomaterials 2017, 7, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnsubsakul, A.; Oaew, S.; Surareungchai, W. Zwitterionic peptide-capped gold nanoparticles for colorimetric detection of Ni2+. Nanoscale 2018, 10, 5466–5473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.H.; Liu, Y.Z.; Luo, M.; Xu, Q.F.; Ji, X.H.; He, Z.K. Peptide-Capped Gold Nanoparticle for Colorimetric Immunoassay of Conjugated Abscisic Acid. ACS Appl. Mater. Inter. 2012, 4, 5010–5015. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.M.; Wang, X.Y.; Li, C.; Li, X.X.; Yin, Y.M.; Li, G.X. Colorimetric assay for protein detection based on “nano-pumpkin” induced aggregation of peptide-decorated gold nanoparticles. Biosens. Bioelectron. 2015, 71, 348–352. [Google Scholar] [CrossRef]
- Feng, T.T.; Gao, S.Q.; Wang, K. Colorimetric Sensing of Prostate Specific Membrane Antigen Based on Gold Nanoparticles. Acta Chim. Sin. 2019, 77, 422–426. [Google Scholar] [CrossRef]
- Retout, M.; Valkenier, H.; Triffaux, E.; Doneux, T.; Bartik, K.; Bruylants, G. Rapid and Selective Detection of Proteins by Dual Trapping Using Gold Nanoparticles Functionalized with Peptide Aptamers. ACS Sens. 2016, 1, 929–933. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.H.; Wang, Y.; Chen, P.; McCadden, A.; Palaniappan, A.; Zhang, J.L.; Liedberg, B. Peptide Functionalized Gold Nanoparticles with Optimized Particle Size and Concentration for Colorimetric Assay Development: Detection of Cardiac Troponin I. ACS Sens. 2016, 1, 1416–1422. [Google Scholar] [CrossRef]
- Wang, Z.X.; Levy, R.; Fernig, D.G.; Brust, M. Kinase-catalyzed modification of gold nanoparticles: A new approach to colorimetric kinase activity screening. J. Am. Chem. Soc. 2006, 128, 2214–2215. [Google Scholar] [CrossRef]
- Gupta, S.; Andresen, H.; Ghadiali, J.E.; Stevens, M.M. Kinase-Actuated Immunoaggregation of Peptide-Conjugated Gold Nanoparticles. Small 2010, 6, 1509–1513. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Q.W.; Luo, J.X.; Zhao, Y. Sensitive colorimetric detection of lysozyme in human serum using peptide-capped gold nanoparticles. Anal. Methods 2012, 4, 3874–3878. [Google Scholar] [CrossRef]
- Chen, P.; Selegard, R.; Aili, D.; Liedberg, B. Peptide functionalized gold nanoparticles for colorimetric detection of matrilysin (MMP-7) activity. Nanoscale 2013, 5, 8973–8976. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Wang, Y.; Chen, P.; Wang, Y.S.; Mang, J.L.; Aili, D.; Liedberg, B. Biofunctionalized Gold Nanoparticles for Colorimetric Sensing of Botulinum Neurotoxin A Light Chain. Anal. Chem. 2014, 86, 2345–2352. [Google Scholar] [CrossRef]
- Chandrawati, R.; Stevens, M.M. Controlled assembly of peptide-functionalized gold nanoparticles for label-free detection of blood coagulation Factor XIII activity. Chem. Commun. 2014, 50, 5431–5434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.O.; Kim, E.J.; Lim, B.; Kim, T.W.; Kim, Y.P. Rapid Detection of Protein Phosphatase Activity Using Zn(II)Coordinated Gold Nanosensors Based on His-Tagged Phosphopeptides. Anal. Chem. 2015, 87, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Shen, H.X.; Liu, C.H.; Li, Z.P. Phosphorylation-regulated crosslinking of gold nanoparticles: A new strategy for colorimetric detection of protein kinase activity. Analyst 2015, 140, 5685–5691. [Google Scholar] [CrossRef]
- Xia, N.; Wang, X.; Wang, X.J.; Zhou, B.B. Gold Nanoparticle-Based Colorimetric and Electrochemical Methods for Dipeptidyl Peptidase-IV Activity Assay and Inhibitor Screening. Materials 2016, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Aldewachi, H.S.; Woodroofe, N.; Turega, S.; Gardiner, P.H.E. Optimization of gold nanoparticle-based real-time colorimetric assay of dipeptidyl peptidase IV activity. Talanta 2017, 169, 13–19. [Google Scholar] [CrossRef]
- Mao, X.X.; Li, Y.F.; Han, P.; Wang, X.H.; Yang, S.Q.; Zhang, F.; Gong, X.Q.; Cao, Y. One-pot and one-step colorimetric detection of aminopeptidase N activity based on gold nanoparticles-based supramolecular structure. Sens. Actuator B Chem. 2018, 267, 336–341. [Google Scholar] [CrossRef]
- Aldewachi, H.; Woodroofe, N.; Gardiner, P. Study of the Stability of Functionalized Gold Nanoparticles for the Colorimetric Detection of Dipeptidyl Peptidase IV. Appl. Sci. 2018, 8, 2589. [Google Scholar] [CrossRef] [Green Version]
- Loynachan, C.N.; Soleimany, A.P.; Dudani, J.S.; Lin, Y.Y.; Najer, A.; Bekdemir, A.; Chen, Q.; Bhatia, S.N.; Stevens, M.M. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 2019, 14, 883–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, G.; Palaniappan, A.; Liedberg, B. Protease functional assay on membrane. Sens. Actuator B Chem. 2020, 305, 127442. [Google Scholar] [CrossRef]
- Gao, L.; Liu, M.Q.; Ma, G.F.; Wang, Y.L.; Zhao, L.N.; Yuan, Q.; Gao, F.P.; Liu, R.; Zhai, J.; Chai, Z.F.; et al. Peptide-Conjugated Gold Nanoprobe: Intrinsic Nanozyme-Linked Immunsorbant Assay of Integrin Expression Level on Cell Membrane. ACS Nano 2015, 9, 10979–10990. [Google Scholar] [CrossRef]
- Feng, J.Y.; Huang, P.C.; Shi, S.Z.; Deng, K.Y.; Wu, F.Y. Colorimetric detection of glutathione in cells based on peroxidase-like activity of gold nanoclusters: A promising powerful tool for identifying cancer cells. Anal. Chim. Acta. 2017, 967, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Geng, J.; Miyoshi, D.; Ren, J.S.; Sugimoto, N.; Qu, X.G. A rapid and sensitive “add-mix-measure” assay for multiple proteinases based on one gold nanoparticle-peptide-fluorophore conjugate. Biosens. Bioelectron. 2010, 26, 743–747. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.H.; Zhang, J.L.; Aili, D.; Liedberg, B. Time-resolved botulinum neurotoxin A activity monitored using peptide-functionalized Au nanoparticle energy transfer sensors. Chem. Sci. 2014, 5, 2651–2656. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.K.; Wang, L.; Liu, Q.; Su, X.G. A fluorescence sensor for protein kinase activity detection based on gold nanoparticles/copper nanoclusters system. Sens. Actuator B Chem. 2018, 256, 691–698. [Google Scholar] [CrossRef]
- Xu, S.M.; Zhang, F.M.; Xu, L.B.; Liu, X.; Ma, P.Y.; Sun, Y.; Wang, X.H.; Song, D.Q. A fluorescence resonance energy transfer biosensor based on carbon dots and gold nanoparticles for the detection of trypsin. Sens. Actuator B Chem. 2018, 273, 1015–1021. [Google Scholar] [CrossRef]
- Zhang, D.D.; Meng, Y.R.; Zhang, C.Y. Peptide-templated gold nanoparticle nanosensor for simultaneous detection of multiple posttranslational modification enzymes. Chem. Commun. 2020, 56, 213–216. [Google Scholar] [CrossRef]
- Wang, Y.H.; Shen, P.; Li, C.Y.; Wang, Y.Y.; Liu, Z.H. Upconversion Fluorescence Resonance Energy Transfer Based Biosensor for Ultrasensitive Detection of Matrix Metalloproteinase-2 in Blood. Anal. Chem. 2012, 84, 1466–1473. [Google Scholar] [CrossRef]
- Vuojola, J.; Riuttamaki, T.; Kulta, E.; Arppe, R.; Soukka, T. Fluorescence-quenching-based homogeneous caspase-3 activity assay using photon upconversion. Anal. Chim. Acta. 2012, 725, 67–73. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, T.; Wei, W.; Li, Z.; Wu, D.; Wang, L.; Guo, J.; He, X.W.; Ma, N. Compact, Programmable, and Stable Biofunctionalized Upconversion Nanoparticles Prepared through Peptide-Mediated Phase Transfer for High-Sensitive Protease Sensing and in Vivo Apoptosis Imaging. ACS Appl. Mater. Interfaces 2015, 7, 11849–11856. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Wang, X.Y.; Wang, K.; Guo, Z.J. An ultrasensitive fluorescent nanosensor for trypsin based on upconversion nanoparticles. Talanta 2017, 174, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Fang, A.J.; Zhang, Y.Y.; Yao, S.Z. Silver triangular nanoplates as an high efficiently FRET donor-acceptor of upconversion nanoparticles for ultrasensitive “Turn on-off” protamine and trypsin sensor. Talanta 2017, 174, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.N.; Li, Z.; Zhao, J.Y.; Chen, M.; Ma, N. Rational Engineering a Multichannel Upconversion Sensor for Multiplex Detection of Matrix Metalloproteinase Activities. ACS Sens. 2018, 3, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Chan, M.H.; Chen, C.W.; Liu, R.S.; Hsiao, M.; Tsai, D.P. Near-Infrared-Activated Fluorescence Resonance Energy Transfer-Based Nanocomposite to Sense MMP2-Overexpressing Oral Cancer Cells. ACS Omega 2018, 3, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, H.; Song, D.Q.; Wang, Z.X. An upconversion nanoparticle-based fluorescence resonance energy transfer system for effectively sensing caspase-3 activity. Analyst 2018, 143, 761–767. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Wang, Z.X.; Song, D.Q. Peptide-functionalized upconversion nanoparticles-based FRET sensing platform for Caspase-9 activity detection in vitro and in vivo. Biosens. Bioelectron. 2019, 141, 111403. [Google Scholar] [CrossRef]
- Jung, S.; Chen, X.Y. Quantum Dot-Dye Conjugates for Biosensing, Imaging, and Therapy. Adv. Healthc. Mater. 2018, 7, 1800252. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Jian, M.H.; Li, X.T.; Wei, J.; Meng, X.Y.; Wang, Z.X. Biosensors and bioassays for determination of matrix metalloproteinases: State of the art and recent advances. J. Mater. Chem. B 2020, 8, 3261–3291. [Google Scholar] [CrossRef]
- Dos Santos, M.C.; Algar, W.R.; Medintz, I.L.; Hildebrandt, N. Quantum dots for Forster Resonance Energy Transfer FRET. Trac-Trends Anal. Chem. 2020, 125, 115819. [Google Scholar] [CrossRef]
- Tong, X.; Shi, S.Y.; Tong, C.Y.; Iftikhar, A.; Long, R.Q.; Zhu, Y.F. Quantum/carbon dots-based fluorescent assays for enzyme activity. Trac-Trends Anal. Chem. 2020, 131, 116008. [Google Scholar] [CrossRef]
- Chern, M.; Toufanian, R.; Dennis, A.M. Quantum dot to quantum dot Forster resonance energy transfer: Engineering materials for visual color change sensing. Analyst 2020, 145, 5754–5767. [Google Scholar] [CrossRef]
- Arndt, N.; Tran, H.D.N.; Zhang, R.; Xu, Z.P.; Ta, H.T. Different Approaches to Develop Nanosensors for Diagnosis of Diseases. Adv. Sci. 2020, 7, 2001476. [Google Scholar] [CrossRef]
- Shi, L.F.; De Paoli, V.; Rosenzweig, N.; Rosenzweig, Z. Synthesis and application of quantum dots FRET-based protease sensors. J. Am. Chem. Soc. 2006, 128, 10378–10379. [Google Scholar] [CrossRef]
- Medintz, I.L.; Clapp, A.R.; Brunel, F.M.; Tiefenbrunn, T.; Uyeda, H.T.; Chang, E.L.; Deschamps, J.R.; Dawson, P.E.; Mattoussi, H. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat. Mater. 2006, 5, 581–589. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chen, N.T.; Sun, S.P.; Chang, J.C.; Wang, Y.C.; Yang, C.S.; Lo, L.W. The Protease-Mediated Nucleus Shuttles of Subnanometer Gold Quantum Dots for Real-Time Monitoring of Apoptotic Cell Death. J. Am. Chem. Soc. 2010, 132, 8309–8315. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.T.; Wang, M.; Cao, M.; Zhang, J.; Zhang, K.Z.; Zhou, P.; Liu, Z.X.; Liu, Y.; Guo, Z.R.; Lu, X. Functionalized Au@Ag-Au nanoparticles as an optical and SERS dual probe for lateral flow sensing. Anal. Bioanal. Chem. 2018, 410, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Kyaw, Y.M.E.; Tan, E.K.M.; Bekale, L.; Kang, M.W.C.; Kim, S.S.Y.; Tan, I.; Lam, K.P.; Kah, J.C.Y. Quantitative and Label-Free Detection of Protein Kinase A Activity Based on Surface-Enhanced Raman Spectroscopy with Gold Nanostars. Anal. Chem. 2018, 90, 6071–6080. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Liu, Y.; Wang, T.H.; Li, J.H. Highly Sensitive Electrogenerated Chemiluminescence Biosensor in Profiling Protein Kinase Activity and Inhibition Using Gold Nanoparticle as Signal Transduction Probes. Anal. Chem. 2010, 82, 9566–9572. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, B.; Charoudeh, H.N.; Shadjou, N.; Mohammad-Rezaei, R.; Omidi, Y.; Velaei, K.; Aliyari, Z.; Rashidi, M.R. Ultrasensitive caspase-3 activity detection using an electrochemical biosensor engineered by gold nanoparticle functionalized MCM-41: Its application during stem cell differentiation. Sens. Actuator B Chem. 2016, 231, 561–575. [Google Scholar] [CrossRef]
- Parnsubsakul, A.; Safitri, R.E.; Rijiravanich, P.; Surareungchai, W. Electrochemical assay of proteolytically active prostate specific antigen based on anodic stripping voltammetry of silver enhanced gold nanoparticle labels. J. Electroanal. Chem. 2017, 785, 125–130. [Google Scholar] [CrossRef]
- Huang, X.Y.; Liang, Y.R.; Ruan, L.G.; Ren, J.C. Chemiluminescent detection of cell apoptosis enzyme by gold nanoparticle-based resonance energy transfer assay. Anal. Bioanal. Chem. 2014, 406, 5677–5684. [Google Scholar] [CrossRef]
- Hong, Y.; Ku, M.; Lee, E.; Suh, J.S.; Huh, Y.M.; Yoon, D.S.; Yang, J. Localized surface plasmon resonance based nanobiosensor for biomarker detection of invasive cancer cells. J. Biomed. Opt. 2014, 19, 051202. [Google Scholar] [CrossRef]
- Dong, Z.M.; Jin, X.; Zhao, G.C. Amplified QCM biosensor for type IV collagenase based on collagenase-cleavage of gold nanoparticles functionalized peptide. Biosens. Bioelectron. 2018, 106, 111–116. [Google Scholar] [CrossRef]
- Cooper, B.M.; Iegre, J.; O’ Donovan, D.H.; Halvarsson, M.O.; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: Peptide-drug conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef]
- Wang, J.; Dai, J.; Yang, X.; Yu, X.Y.; Emory, S.R.; Yong, X.Q.; Xu, J.H.; Mei, L.; Xie, J.B.; Han, N.; et al. Intracellular targeted delivery of quantum dots with extraordinary performance enabled by a novel nanomaterial design. Nanoscale 2019, 11, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S.; Lebl, M.; Krchnak, V. The “one-bead-one-compound“ combinatorial library method. Chem. Rev. 1997, 97, 411–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wang, W.Z.; Bu, X.L.; Wei, Z.W.; Geng, L.L.; Wu, Y.; Dong, C.Y.; Li, L.Q.; Zhang, D.; Yang, S.; et al. Microarray Based Screening of Peptide Nano Probes for HER2 Positive Tumor. Anal. Chem. 2015, 87, 8367–8372. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, M.; Hallbrink, M.; Prochiantz, A.; Langel, U. Cell-penetrating peptides. Trends Pharmacol. Sci. 2000, 21, 99–103. [Google Scholar] [CrossRef]
- Josephson, L.; Tung, C.H.; Moore, A.; Weissleder, R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-tat peptide conjugates. Bioconjugate Chem. 1999, 10, 186–191. [Google Scholar] [CrossRef]
- Wunderbaldinger, P.; Josephson, L.; Weissleder, R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjugate Chem. 2002, 13, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Kircher, M.F.; Josephson, L.; Weissleder, R. Differential conjugation of tat peptide to superparamagnetic nanoparticles and its effect on cellular uptake. Bioconjugate Chem. 2002, 13, 840–844. [Google Scholar] [CrossRef]
- De la Fuente, J.M.; Berry, C.C. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjugate Chem. 2005, 16, 1176–1180. [Google Scholar] [CrossRef]
- Ruan, G.; Agrawal, A.; Marcus, A.I.; Nie, S. Imaging and tracking of tat peptide-conjugated quantum dots in living cells: New insights into nanoparticle uptake, intracellular transport, and vesicle shedding. J. Am. Chem. Soc. 2007, 129, 14759–14766. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Liu, D.J.; Wang, Z.X. Functional gold nanoparticle-peptide complexes as cell-targeting agents. Langmuir 2008, 24, 10293–10297. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Wang, J.E.; Wang, Z.X. Recognition and transmembrane delivery of bioconjugated Fe2O3@Au nanoparticles with living cells. Nanoscale 2010, 2, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yang, Q.Y.; Xiao, L.H. Tempo-spatially resolved cellular dynamics of human immunodeficiency virus transacting activator of transcription (Tat) peptide-modified nanocargos in living cells. Nanoscale 2014, 6, 10207–10215. [Google Scholar] [CrossRef]
- Kostiv, U.; Kotelnikov, I.; Proks, V.; Slouf, M.; Kucka, J.; Engstova, H.; Jezek, P.; Horak, D. RGDS- and TAT-Conjugated Upconversion of NaYF4:Yb3+/Er3+ & SiO2 Nanoparticles: In Vitro Human Epithelioid Cervix Carcinoma Cellular Uptake, Imaging, and Targeting. ACS Appl. Mater. Inter. 2016, 8, 20422–20431. [Google Scholar] [CrossRef]
- Dalal, C.; Jana, N.R. Multivalency Effect of TAT-Peptide-Functionalized Nanoparticle in Cellular Endocytosis and Subcellular Trafficking. J. Phys. Chem. B 2017, 121, 2942–2951. [Google Scholar] [CrossRef]
- Yong, X.Q.; Yang, X.; Emory, S.R.; Wang, J.; Dai, J.; Yu, X.Y.; Mei, L.; Xie, J.B.; Ruan, G. A potent, minimally invasive and simple strategy of enhancing intracellular targeted delivery of Tat peptide-conjugated quantum dots: Organic solvent-based permeation enhancer. Biomater. Sci. 2018, 6, 3085–3095. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhao, X.; Yan, X.P. Cell-Penetrating Peptide-Functionalized Persistent Luminescence Nanoparticles for Tracking J774A.1 Macrophages Homing to Inflamed Tissues. ACS Appl. Mater. Inter. 2019, 11, 19894–19901. [Google Scholar] [CrossRef]
- Shukla, R.; Hill, E.; Shi, X.Y.; Kim, J.; Muniz, M.C.; Sun, K.; Baker, J.R. Tumor microvasculature targeting with dendrimer-entrapped gold nanoparticles. Soft Matter 2008, 4, 2160–2163. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhang, Z.P.; Luo, M.; Yu, X.; Han, Y.; Wei, H.P.; Cui, Z.Q.; Zhang, X.E. Multifunctional ferritin cage nanostructures for fluorescence and MR imaging of tumor cells. Nanoscale 2012, 4, 188–193. [Google Scholar] [CrossRef]
- Lu, Y.M.; Zhong, Y.L.; Wang, J.; Su, Y.Y.; Peng, F.; Zhou, Y.F.; Jiang, X.X.; He, Y. Aqueous synthesized near-infrared-emitting quantum dots for RGD-based in vivo active tumour targeting. Nanotechnology 2013, 24, 135101. [Google Scholar] [CrossRef]
- Cheng, K.; Kothapalli, S.R.; Liu, H.G.; Koh, A.L.; Jokerst, J.V.; Jiang, H.; Yang, M.; Li, J.B.; Levi, J.; Wu, J.C.; et al. Construction and Validation of Nano Gold Tripods for Molecular Imaging of Living Subjects. J. Am. Chem. Soc. 2014, 136, 3560–3571. [Google Scholar] [CrossRef]
- Yan, J.; He, W.X.; Li, N.; Yu, M.; Du, Y.P.; Lei, B.; Ma, P.X. Simultaneously targeted imaging cytoplasm and nucleus in living cell by biomolecules capped ultra-small GdOF nanocrystals. Biomaterials 2015, 59, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Xue, J.P.; Xu, B.G.; Shen, D.W.; Sudlow, G.P.; Achilefu, S. Tunable Ultrasmall Visible-to-Extended Near-Infrared Emitting Silver Sulfide Quantum Dots for Integrin-Targeted Cancer Imaging. ACS Nano 2015, 9, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Wang, H.; Liu, H.; Wen, S.H.; Peng, C.; Shen, M.W.; Zhang, G.X.; Shi, X.Y. Multifunctional Dendrimer-Entrapped Gold Nanoparticles Modified with RGD Peptide for Targeted Computed Tomography/Magnetic Resonance Dug-Modal Imaging of Tumors. Anal. Chem. 2015, 87, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, Y.; Xu, Y.H.; Li, J.C.; Zhang, Z.X.; Wang, H.; Shen, M.W.; Shi, X.Y.; Zhang, G.X. Conjugation of Iron Oxide Nanoparticles with RGD-Modified Dendrimers for Targeted Tumor MR Imaging. ACS Appl. Mater. Inter. 2015, 7, 5420–5428. [Google Scholar] [CrossRef]
- Meng, F.Y.; Han, K.; Wang, B.D.; Liu, T.; Liu, G.X.; Li, Y.R.; Miao, P. Nanoarchitectured Electrochemical Cytosensor for Selective Detection of Cancer Cells. Chemistryselect 2016, 1, 1515–1517. [Google Scholar] [CrossRef]
- Meidell, K.Z.; Robinson, R.; Vieira-de-Abreu, A.; Gormley, A.J.; Ghandehari, H.; Grainger, D.W.; Campbell, R.A. RGDfK-functionalized gold nanorods bind only to activated platelets. J. Biomed. Mater. Res. A 2017, 105, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Boucher, M.; Geffroy, F.; Preveral, S.; Bellanger, L.; Selingue, E.; Adryanczyk-Perrier, G.; Pean, M.; Lefevre, C.T.; Pignol, D.; Ginet, N.; et al. Genetically tailored magnetosomes used as MRI probe for molecular imaging of brain tumor. Biomaterials 2017, 121, 167–178. [Google Scholar] [CrossRef]
- Su, G.X.; Jiang, H.Q.; Xu, B.H.; Yu, Y.Y.; Chen, X.Q. Effects of Protein Corona on Active and Passive Targeting of Cyclic RGD Peptide-Functionalized PEGylation Nanoparticles. Mol. Pharmaceutics 2018, 15, 5019–5030. [Google Scholar] [CrossRef]
- Simitzi, C.; Harimech, P.; Spanou, S.; Lanara, C.; Heuer-Jungemann, A.; Manousaki, A.; Fotakis, C.; Ranella, A.; Kanaras, A.G.; Stratakis, E. Cells on hierarchically-structured platforms hosting functionalized nanoparticles. Biomater. Sci. 2018, 6, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.H.; Li, X.L.; Zhu, C.L.; Lin, X.C.; Xie, Z.H. Integration of fluorescence/photoacoustic imaging and targeted chemo/photothermal therapy with Ag2Se@BSA-RGD nanodots. New J. Chem. 2020, 44, 4850–4857. [Google Scholar] [CrossRef]
- Gao, H.Y.; Chu, C.C.; Cheng, Y.; Zhang, Y.; Pang, X.; Li, D.F.; Wang, X.Y.; Ren, E.; Xie, F.F.; Bai, Y.; et al. In Situ Formation of Nanotheranostics to Overcome the Blood-Brain Barrier and Enhance Treatment of Orthotopic Glioma. ACS Appl. Mater. Inter. 2020, 12, 26880–26892. [Google Scholar] [CrossRef] [PubMed]
- Bunschoten, A.; Chin, P.T.K.; Buckle, T.; van der Linden, M.; Barendregt, A.; Verheijen, M.A.; van Leeuwen, F.W.B. Receptor-Targeted Luminescent Silver Bionanoparticles. Eur. J. Inorg. Chem. 2016, 3030–3035. [Google Scholar] [CrossRef]
- Willmore, A.M.A.; Simon-Gracia, L.; Toome, K.; Paiste, P.; Kotamraju, V.R.; Molder, T.; Sugahara, K.N.; Ruoslahti, E.; Braun, G.B.; Teesalu, T. Targeted silver nanoparticles for ratiometric cell phenotyping. Nanoscale 2016, 8, 9096–9101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, D.P.N.; Rodriguez, R.D.; Kurth, T.; Bray, L.J.; Binner, M.; Jungnickel, C.; Gur, F.N.; Poser, S.W.; Schmidt, T.L.; Zahn, D.R.T.; et al. Enhanced targeting of invasive glioblastoma cells by peptide-functionalized gold nanorods in hydrogel-based 3D cultures. Acta Biomater. 2017, 58, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Toome, K.; Willmore, A.M.A.; Paiste, P.; Tobi, A.; Sugahara, K.N.; Kirsimae, K.; Ruoslahti, E.; Braun, G.B.; Teesalu, T. Ratiometric in vivo auditioning of targeted silver nanoparticles. Nanoscale 2017, 9, 10094–10100. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Chen, H.D.; Yu, S.N.; Li, X.D.; Wang, Z.X. CXCR4 Peptide Conjugated Au-Fe2O3 Nanoparticles for Tumor-targeting Magnetic Resonance Imaging. Chem. Res. Chin. Univ. 2018, 34, 584–589. [Google Scholar] [CrossRef]
- Goncalves, D.P.N.; Park, D.M.; Schmidt, T.L.; Werner, C. Modular peptide-functionalized gold nanorods for effective glioblastoma multicellular tumor spheroid targeting. Biomater. Sci. 2018, 6, 1140–1146. [Google Scholar] [CrossRef]
- Lingasamy, P.; Tobi, A.; Haugas, M.; Hunt, H.; Paiste, P.; Asser, T.; Ratsep, T.; Kotamraju, V.R.; Bjerkvig, R.; Teesalu, T. Bi-specific tenascin-C and fibronectin targeted peptide for solid tumor delivery. Biomaterials 2019, 219, 119373. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Li, Y.J.; Zhu, J.Y.; Sun, N.; Song, N.N.; Xing, Y.; Huang, H.; Zhao, J.H. Chlorotoxin peptide-functionalized polyethylenimine-entrapped gold nanoparticles for glioma SPECT/CT imaging and radionuclide therapy. J. Nanobiotechnol. 2019, 17, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.Y.; Lin, C.T.; Kuo, S.Y.; Chang, D.K.; Wu, H.C. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res. 2007, 67, 10958–10965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
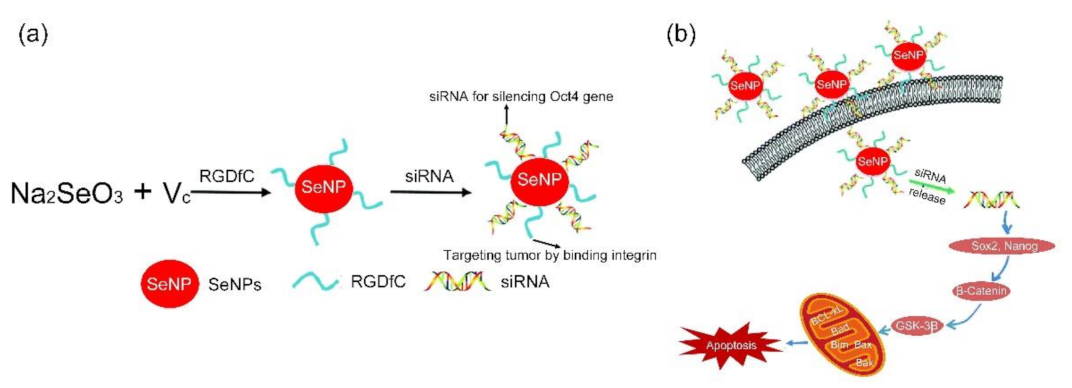
- Fu, X.Y.; Yang, Y.H.; Li, X.L.; Lai, H.Q.; Huang, Y.Y.; He, L.Z.; Zheng, W.J.; Chen, T.F. RGD peptide-conjugated selenium nanoparticles: Antiangiogenesis by suppressing VEGF-VEGFR2-ERK/AKT pathway. Nanomed. Nanotechnol. 2016, 12, 1627–1639. [Google Scholar] [CrossRef]
- Hu, H.; You, Y.Y.; He, L.Z.; Chen, T.F. The rational design of NAMI-A-loaded mesoporous silica nanoparticles as antiangiogenic nanosystems. J. Mater. Chem. B 2015, 3, 6338–6346. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.M.; Ji, X.Y.; Xu, H.; Zhang, L.; Jiang, A.R.; Song, B.; Su, Y.Y.; He, Y. Photostable and Biocompatible Fluorescent Silicon Nanoparticles-Based Theranostic Probes for Simultaneous Imaging and Treatment of Ocular Neovascularization. Anal. Chem. 2018, 90, 8188–8195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Tong, L.; Wang, J.G.; Luo, J.M.; Tang, J.; Zhong, L.J.; Xiao, Q.; Niu, W.B.; Li, J.H.; Zhu, J.Q.; et al. cRGD-functionalized nanoparticles for combination therapy of anti-endothelium dependent vessels and anti-vasculogenic mimicry to inhibit the proliferation of ovarian cancer. Acta Biomater. 2019, 94, 495–504. [Google Scholar] [CrossRef]
- Li, Q.R.; Zhou, R.H.; Sun, Y.; Xiao, D.X.; Liu, M.T.; Zhao, D.; Peng, S.L.; Chen, Y.; Lin, Y.F. Synthesis and Antitumor Application of Antiangiogenetic Gold Nanoclusters. ACS Appl. Mater. Inter. 2021, 13, 11708–11720. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, M.; Wang, P. Recent Advances in Small Copper Sulfide Nanoparticles for Molecular Imaging and Tumor Therapy. Mol. Pharm. 2019, 16, 3322–3332. [Google Scholar] [CrossRef]
- Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Fales, A.M.; Vo-Dinh, T. TAT Peptide-Functionalized Gold Nanostars: Enhanced Intracellular Delivery and Efficient NIR Photothermal Therapy Using Ultralow Irradiance. J. Am. Chem. Soc. 2012, 134, 11358–11361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, U.Y.; Youn, Y.S.; Park, J.; Lee, E.S. Y-Shaped Ligand-Driven Gold Nanoparticles for Highly Efficient Tumoral Uptake and Photothermal Ablation. ACS Nano 2014, 8, 12858–12865. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Wu, Y.; Tang, Y.; Xiao, H.P.; Chen, K.C.; Han, T.G.; Fang, N.; Wu, R.H.; El-Sayed, M.A. Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins. Proc. Natl Acad. Sci. USA 2017, 114, E5655–E5663. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.B.; Tang, J.; Zheng, R.; Guo, G.N.; Dong, A.G.; Wang, Y.J.; Yang, W.L. Nuclear-Targeted Multifunctional Magnetic Nanoparticles for Photothermal Therapy. Adv. Healthc. Mater. 2017, 6, 1601289. [Google Scholar] [CrossRef]
- Li, N.; Sun, Q.Q.; Yu, Z.Z.; Gao, X.N.; Pan, W.; Wan, X.Y.; Tang, B. Nuclear-Targeted Photothermal Therapy Prevents Cancer Recurrence with Near-Infrared Triggered Copper Sulfide Nanoparticles. ACS Nano 2018, 12, 5197–5206. [Google Scholar] [CrossRef]
- Arriortua, O.K.; Insausti, M.; Lezama, L.; de Muro, I.G.; Garaio, E.; de la Fuente, J.M.; Fratila, R.M.; Morales, M.P.; Costa, R.; Eceiza, M.; et al. RGD-Functionalized Fe3O4 nanoparticles for magnetic hyperthermia. Colloid Surf. B 2018, 165, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Zong, J.Y.; Cobb, S.L.; Cameron, N.R. Short elastin- like peptide- functionalized gold nanoparticles that are temperature responsive under near- physiological conditions. J. Mater. Chem. B 2018, 6, 6667–6674. [Google Scholar] [CrossRef]
- Liao, Y.T.; Liu, C.H.; Chin, Y.; Chen, S.Y.; Liu, S.H.; Hsu, Y.C.; Wu, K.C.W. Biocompatible and multifunctional gold nanorods for effective photothermal therapy of oral squamous cell carcinoma. J. Mater. Chem. B 2019, 7, 4451–4460. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Y.L.; Wu, M.; Zan, W.; Yang, Q. LyP-1 peptide-functionalized gold nanoprisms for SERRS imaging and tumor growth suppressing by PTT induced-hyperthermia. Chin. Chem. Lett. 2019, 30, 1335–1340. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Q.; Wang, H.Y.; Zha, Y.C.; Zheng, P.L.; Yang, T.; Ma, D.; Qiu, L.; Xu, X.M.; Hu, Y.; et al. Mitochondria-targeted magnetic gold nanoheterostructure for multi-modal imaging guided photothermal and photodynamic therapy of triple-negative breast cancer. Chem. Eng. J. 2021, 403, 126364. [Google Scholar] [CrossRef]
- Qiu, L.P.; Valente, M.; Dolen, Y.; Jager, E.; ter Beest, M.; Zheng, L.Y.; Figdor, C.G.; Verdoes, M. Endolysosomal-Escape Nanovaccines through Adjuvant-Induced Tumor Antigen Assembly for Enhanced Effector CD8(+) T Cell Activation. Small 2018, 14, 1703539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruje, C.; Yang, C.; Uertz, J.; van Prooijen, M.; Chithrani, B.D. Optimization of PEG coated nanoscale gold particles for enhanced radiation therapy. RSC Adv. 2015, 5, 101525–101532. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, Z.R.; Li, B.X.; Meng, J.; Shi, Z.L.; Li, P.; Fu, S. RGD-conjugated mesoporous silica-encapsulated gold nanorods enhance the sensitization of triple-negative breast cancer to megavoltage radiation therapy. Int. J. Nanomed. 2016, 11, 5595–5610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilug, L.E.; Leonte, R.A.; Barbinta Patrascu, M.E.; Ion, A.C.; Tuta, C.S.; Raicu, A.; Manda, G.; Niculae, D. In vitro binding kinetics study of gold nanoparticles functionalized with Ga-68-DOTA conjugated peptides. J. Radioanal. Nucl. Chem. 2017, 311, 1485–1493. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S.; Guleria, A.; Kumar, C.; Kunwar, A.; Nair, K.V.V.; Sarma, H.D.; Dash, A. Clinical scale synthesis of intrinsically radiolabeled and cyclic RGD peptide functionalized Au-198 nanoparticles for targeted cancer therapy. Nucl. Med. Biol. 2019, 72–73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, M.; Preveral, S.; Hoog, C.; Herault, J.; Perrier, G.A.; Lefevre, C.T.; Michel, H.; Pignol, D.; Doyen, J.; Pourcher, T.; et al. RGD-functionalized magnetosomes are efficient tumor radioenhancers for X-rays and protons. Nanomed. Nanotechnol. 2020, 23, 102084. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Z.F.; Wang, Y.; Chen, W.H.; Luo, G.F.; Cheng, S.X.; Zhuo, R.X.; Zhang, X.Z. Multifunctional Envelope-Type Mesoporous Silica Nanoparticles for Tumor-Triggered Targeting Drug Delivery. J. Am. Chem. Soc. 2013, 135, 5068–5073. [Google Scholar] [CrossRef]
- Black, K.C.L.; Akers, W.J.; Sudlow, G.; Xu, B.G.; Laforest, R.; Achilefu, S. Dual-radiolabeled nanoparticle SPECT probes for bioimaging. Nanoscale 2015, 7, 440–444. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Yang, C.X.; Chen, L.G.; Yan, X.P. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat. Commun. 2017, 8, 14998. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.C.; Hou, Y.; Zeng, J.F.; Liu, C.Y.; Zhang, P.S.; Jing, L.H.; Shangguan, D.; Gao, M.Y. Dual-Ratiometric Target-Triggered Fluorescent Probe for Simultaneous Quantitative Visualization of Tumor Microenvironment Protease Activity and pH &ITin Vivo&IT. J. Am. Chem. Soc. 2018, 140, 211–218. [Google Scholar] [CrossRef]
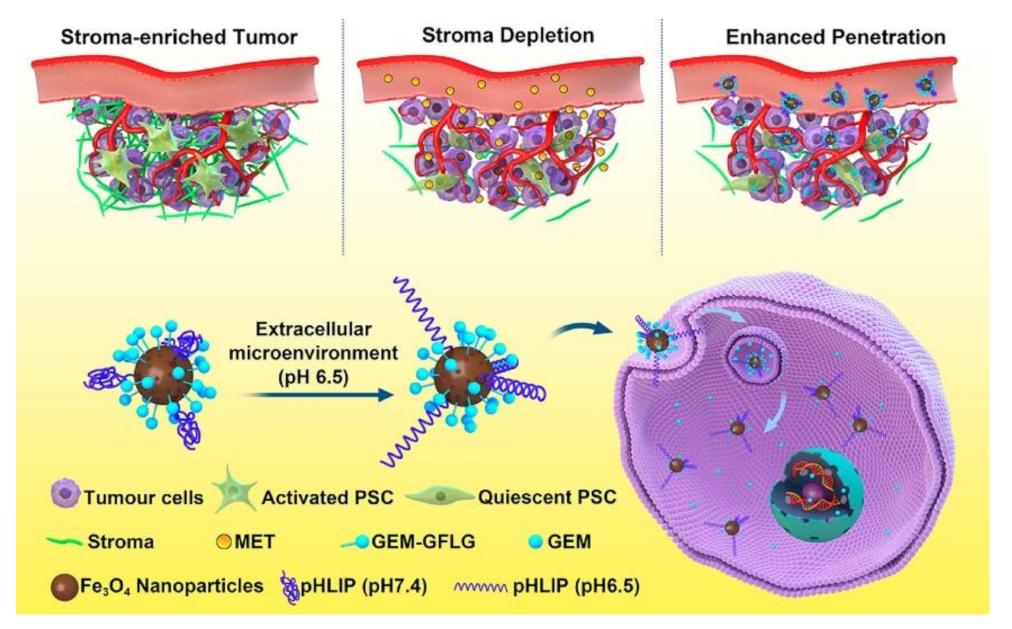
- Han, H.J.; Hou, Y.; Chen, X.H.; Zhang, P.S.; Kang, M.X.; Jin, Q.; Ji, J.; Gao, M.Y. Metformin-Induced Stromal Depletion to Enhance the Penetration of Gemcitabine-Loaded Magnetic Nanoparticles for Pancreatic Cancer Targeted Therapy. J. Am. Chem. Soc. 2020, 142, 4944–4954. [Google Scholar] [CrossRef]
- Zha, S.; Chau, H.F.; Chau, W.Y.; Chan, L.S.; Lin, J.; Lo, K.W.; Cho, W.C.S.; Yip, Y.L.; Tsao, S.W.; Farrell, P.J.; et al. Dual-Targeting Peptide-Guided Approach for Precision Delivery and Cancer Monitoring by Using a Safe Upconversion Nanoplatform. Adv. Sci. 2021, 8, 2002919. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wu, J.; Wang, Y.X.; Sun, S.; Yuan, Y.; Tao, X.F.; Lv, R.C. Peptide functionalized upconversion/NIR II luminescent nanoparticles for targeted imaging and therapy of oral squamous cell carcinoma. Biomater. Sci. 2021, 9, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Hong, H.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Xiao, Y.L.; Yang, Y.A.; Zhang, Y.; Nickles, R.; et al. cRGD-functionalized, DOX-conjugated, and Cu-64-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials 2011, 32, 4151–4160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Lee, T.S.; Ryu, J.; Hong, S.; Kang, M.; Im, K.; Kang, J.H.; Lim, S.M.; Park, S.; Song, R. RGD Peptide-Conjugated Multimodal NaGdF4:Yb3+/Er3+ Nanophosphors for Upconversion Luminescence, MR, and PET Imaging of Tumor Angiogenesis. J. Nucl. Med. 2013, 54, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cedrowska, E.; Pruszynski, M.; Majkowska-Pilip, A.; Meczynska-Wielgosz, S.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized TiO2 nanoparticles labelled with Ac-225 for targeted alpha radionuclide therapy. J. Nanopart. Res. 2018, 20, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.M.; He, Q.J.; Liu, J.N.; Chen, Y.; Ma, M.; Zhang, L.L.; Shi, J.L. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef]
- Luo, G.F.; Chen, W.H.; Liu, Y.; Zhang, J.; Cheng, S.X.; Zhuo, R.X.; Zhang, X.Z. Charge-reversal plug gate nanovalves on peptide-functionalized mesoporous silica nanoparticles for targeted drug delivery. J. Mater. Chem. B 2013, 1, 5723–5732. [Google Scholar] [CrossRef]
- Kinnari, P.J.; Hyvonen, M.L.K.; Makila, E.M.; Kaasalainen, M.H.; Rivinoja, A.; Salonen, J.J.; Hirvonen, J.T.; Laakkonen, P.M.; Santos, H.A. Tumour homing peptide-functionalized porous silicon nanovectors for cancer therapy. Biomaterials 2013, 34, 9134–9141. [Google Scholar] [CrossRef]
- Sun, J.; Kim, D.H.; Guo, Y.; Teng, Z.G.; Li, Y.J.; Zheng, L.F.; Zhang, Z.L.; Larson, A.C.; Lu, G.M. A c(RGDfE) conjugated multi-functional nanomedicine delivery system for targeted pancreatic cancer therapy. J. Mater. Chem. B 2015, 3, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.P.; Shah, B.R.; Zhang, Y.X.; Yang, L.T.; Lee, K.B. Real-Time Monitoring of ATP-Responsive Drug Release Using Mesoporous-Silica-Coated Multicolor Upconversion Nanoparticles. ACS Nano 2015, 9, 5234–5245. [Google Scholar] [CrossRef]
- Chen, G.C.; Xie, Y.S.; Peltier, R.; Lei, H.P.; Wang, P.; Chen, J.; Hu, Y.; Wang, F.; Yao, X.; Sun, H.Y. Peptide-Decorated Gold Nanoparticles as Functional Nano-Capping Agent of Mesoporous Silica Container for Targeting Drug Delivery. ACS Appl. Mater. Inter. 2016, 8, 11204–11209. [Google Scholar] [CrossRef] [PubMed]
- Noh, G.; Youn, Y.S.; Lee, E.S. Preparation of iron oxide nanoparticles functionalized with Y-shaped ligands for brain tumor targeting. J. Mater. Chem. B 2016, 4, 6074–6080. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Luo, G.F.; Lei, Q.; Cao, F.Y.; Fan, J.X.; Qiu, W.X.; Jia, H.Z.; Hong, S.; Fang, F.; Zeng, X.; et al. Rational design of multifunctional magnetic mesoporous silica nanoparticle for tumor-targeted magnetic resonance imaging and precise therapy. Biomaterials 2016, 76, 87–101. [Google Scholar] [CrossRef]
- Zhang, X.M.; Ai, F.J.; Sun, T.Y.; Wang, F.; Zhu, G.Y. Multimodal Upconversion Nanoplatform with a Mitochondria-Targeted Property for Improved Photodynamic Therapy of Cancer Cells. Inorg. Chem. 2016, 55, 3872–3880. [Google Scholar] [CrossRef]
- Han, L.; Tang, C.; Yin, C.H. pH-Responsive Core-Shell Structured Nanoparticles for Triple-Stage Targeted Delivery of Doxorubicin to Tumors. ACS Appl. Mater. Inter. 2016, 8, 23498–23508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Liao, Y.T.; Liu, C.H.; Yu, J.S.; Alamri, H.R.; Alothman, Z.A.; Hossain, M.S.A.; Yamauchi, Y.; Wu, K.C.W. Trifunctional Fe3O4/CaP/Alginate Core-Shell-Corona Nanoparticles for Magnetically Guided, pH-Responsive, and Chemically Targeted Chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 2366–2374. [Google Scholar] [CrossRef]
- Wu, J.L.; He, X.Y.; Liu, B.Y.; Gong, M.Q.; Zhuo, R.X.; Cheng, S.X. Fusion peptide functionalized hybrid nanoparticles for synergistic drug delivery to reverse cancer drug resistance. J. Mater. Chem. B 2017, 5, 4697–4704. [Google Scholar] [CrossRef]
- Chan, M.S.; Liu, L.S.; Leung, H.M.; Lo, P.K. Cancer-Cell-Specific Mitochondria-Targeted Drug Delivery by Dual-Ligand-Functionalized Nanodiamonds Circumvent Drug Resistance. ACS Appl. Mater. Inter. 2017, 9, 11780–11789. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.N.; Li, C.M.; Wang, W.P.; Li, H.J.; Sun, Z.; Song, C.Z.; Li, B.Y.; Duan, S.F.; Hu, Y.R. A 54 peptide-mediated functionalized gold nanocages for targeted delivery of DOX as a combinational photothermal-chemotherapy for liver cancer. Int. J. Nanomed. 2017, 12, 5163–5176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Luo, Y.P.; Huang, L.W.; Yu, B.Y.; Tian, J.W. A peptide-decorated and curcumin-loaded mesoporous silica nanomedicine for effectively overcoming multidrug resistance in cancer cells. RSC Adv. 2017, 7, 16401–16409. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Chen, Y.; Hua, L.; Zhao, M.Q.; Xu, T.T.; Wang, C.B.; Li, Y.H.; Zhu, B. Functionalized selenium nanoparticles for targeted delivery of doxorubicin to improve non-small-cell lung cancer therapy. Int. J. Nanomed. 2018, 13, 6929–6939. [Google Scholar] [CrossRef] [Green Version]
- Dalmau-Mena, I.; del Pino, P.; Pelaz, B.; Cuesta-Geijo, M.A.; Galindo, I.; Moros, M.; de la Fuente, J.M.; Alonso, C. Nanoparticles engineered to bind cellular motors for efficient delivery. J. Nanobiotechnol. 2018, 16, 33. [Google Scholar] [CrossRef]
- Ai, F.J.; Wang, N.; Zhang, X.M.; Sun, T.Y.; Zhu, Q.; Kong, W.; Wang, F.; Zhu, G.Y. An upconversion nanoplatform with extracellular pH-driven tumor-targeting ability for improved photodynamic therapy. Nanoscale 2018, 10, 4432–4441. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.Y.; Chan, L.; Zhang, D.; Huang, C.Q.; Mei, C.M.; Gao, P.; Huang, Y.Y.; Liang, J.Y.; He, L.Z.; Shi, C.Z.; et al. Precise delivery of a multifunctional nanosystem for MRI-guided cancer therapy and monitoring of tumor response by functional diffusion-weighted MRI. J. Mater. Chem. B 2019, 7, 2926–2937. [Google Scholar] [CrossRef]
- Ruan, L.P.; Chen, W.; Wang, R.N.; Lu, J.; Zink, J.I. Magnetically Stimulated Drug Release Using Nanoparticles Capped by Self-Assembling Peptides. ACS Appl. Mater. Inter. 2019, 11, 43835–43842. [Google Scholar] [CrossRef]
- Hou, L.; Chen, D.D.; Wang, R.T.; Wang, R.B.; Zhang, H.J.; Zhang, Z.Z.; Nie, Z.H.; Lu, S.Y. Transformable Honeycomb-Like Nanoassemblies of Carbon Dots for Regulated Multisite Delivery and Enhanced Antitumor Chemoimmunotherapy. Angew. Chem. Int. Ed. 2021, 60, 6581–6592. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.C.; Rossi, J.J. RNA-Based Therapeutics: Current Progress and Future Prospects. Chem. Biol. 2012, 19, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.J.; Solanki, A.; Memoli, K.A.; Kamei, K.; Kim, H.; Drahl, M.A.; Williams, L.J.; Tseng, H.R.; Lee, K. Selective Inhibition of Human Brain Tumor Cells through Multifunctional Quantum-Dot-Based siRNA Delivery. Angew. Chem. Int. Ed. 2010, 49, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiseh, O.; Kievit, F.M.; Fang, C.; Mu, N.; Jana, S.; Leung, M.C.; Mok, H.; Ellenbogen, R.G.; Park, J.O.; Zhang, M.Q. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials 2010, 31, 8032–8042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.M.; Wang, Y.Y.; Zhao, M.X.; Tan, C.P.; Li, Y.Q.; Le, X.Y.; Ji, L.N.; Mao, Z.W. Multifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time tracking. Biomaterials 2012, 33, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.J.; Wang, M.Q.; Ma, Y.J.; Xia, W.L.; Gu, H.C. A mesoporous silica nanoparticle—PEI—Fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials 2013, 34, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Gu, H.C.; Zhang, D.S.Z.; Li, F.; Liu, T.Y.; Xia, W.L. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of siRNA via multimodal mesoporous silica-based nanocarrier. Biomaterials 2014, 35, 10058–10069. [Google Scholar] [CrossRef]
- Wang, F.H.; Zhang, W.J.; Shen, Y.Y.; Huang, Q.; Zhou, D.J.; Guo, S.R. Efficient RNA delivery by integrin-targeted glutathione responsive polyethyleneimine capped gold nanorods. Acta Biomater. 2015, 23, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Lin, Z.F.; Li, Y.H.; Zhao, M.Q.; Wang, C.B.; Guo, M.; Zhang, B.; Zhu, B. Targeted delivery of siRNA using RGDfC-conjugated functionalized selenium nanoparticles for anticancer therapy. J. Mater. Chem. B 2017, 5, 6941–6952. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, T.T.; Wang, C.B.; Li, Y.H.; Lin, Z.F.; Zhao, M.Q.; Zhu, B. Novel functionalized nanoparticles for tumor-targeting co-delivery of doxorubicin and siRNA to enhance cancer therapy. Int. J. Nanomed. 2018, 13, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Tang, G.Y.; Wang, C.B.; Zhong, J.Y.; Chen, Y.; Hua, L.; Li, Y.H.; Liu, H.S.; Zhu, B. Functionalized selenium nanoparticles for targeted siRNA delivery silence Derlin1 and promote antitumor efficacy against cervical cancer. Drug Deliv. 2020, 27, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.B.; Xia, Y.; Huo, S.C.; Shou, D.W.; Mei, Q.; Tang, W.J.; Li, Y.H.; Liu, H.S.; Zhou, Y.J.; Zhu, B. Silencing of MEF2D by siRNA Loaded Selenium Nanoparticles for Ovarian Cancer Therapy. Int. J. Nanomed. 2020, 15, 9759–9770. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tang, G.Y.; Guo, M.; Xu, T.T.; Chen, H.Y.; Lin, Z.F.; Li, Y.H.; Chen, Y.; Zhu, B.; Liu, H.S.; et al. Silencing KLK12 expression via RGDfC-decorated selenium nanoparticles for the treatment of colorectal cancer in vitro and in vivo. Mat. Sci. Eng. C Mater. 2020, 110, 110594. [Google Scholar] [CrossRef] [PubMed]
- Ben Djemaa, S.; David, S.; Herve-Aubert, K.; Falanga, A.; Galdiero, S.; Allard-Vannier, E.; Chourpa, I.; Munnier, E. Formulation and in vitro evaluation of a siRNA delivery nanosystem decorated with gH625 peptide for triple negative breast cancer theranosis. Eur. J. Pharm. Biopharm. 2018, 131, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ben Djemaa, S.; Herve-Aubert, K.; Lajoie, L.; Falanga, A.; Galdiero, S.; Nedellec, S.; Souce, M.; Munnier, E.; Chourpa, I.; David, S.; et al. gH625 Cell-Penetrating Peptide Promotes the Endosomal Escape of Nanovectorized siRNA in a Triple-Negative Breast Cancer Cell Line. Biomacromolecules 2019, 20, 3076–3086. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.M.; Chan, M.S.; Liu, L.S.; Wong, S.W.; Lo, T.W.; Lau, C.H.; Tin, C.; Lo, P.K. Dual-Function, Cationic, Peptide-Coated Nanodiamond Systems: Facilitating Nuclear-Targeting Delivery for Enhanced Gene Therapy Applications. ACS Sustain. Chem. Eng. 2018, 6, 9671–9681. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, G.B.; He, Y.L.; Zhang, X.B.; Liu, Y.; Ju, H.X. A DNA-Azobenzene Nanopump Fueled by Upconversion Luminescence for Controllable Intracellular Drug Release. Angew. Chem. Int. Ed. 2019, 58, 18207–18211. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Q.; Yuan, S.R.; Ma, Z.; Ji, P.; Ma, X.N.; Wu, Z.H.; Qi, X.L. Genetic recombination of poly(l-lysine) functionalized apoferritin nanocages that resemble viral capsid nanometer-sized platforms for gene therapy. Biomater. Sci. 2020, 8, 1759–1770. [Google Scholar] [CrossRef]
- Veiseh, O.; Kievit, F.M.; Liu, V.; Fang, C.; Stephen, Z.R.; Ellenbogen, R.G.; Zhang, M.Q. In Vivo Safety Evaluation of Polyarginine Coated Magnetic Nanovectors. Mol. Pharm. 2013, 10, 4099–4106. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, K.; Banerjee, S.L.; Kundu, P.P.; Madras, G.; Chatterjee, K. Biofunctionalized surface-modified silver nanoparticles for gene delivery. J. Mater. Chem. B 2015, 3, 5266–5276. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Wang, X.R.; Liu, T.; Zhang, D.S.Z.; Wang, Y.F.; Gu, H.C.; Di, W. Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy. Int. J. Nanomed. 2015, 10, 2579–2594. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.D.; Qiu, J.R.; Shi, X.Y. Multifunctional PEI-entrapped gold nanoparticles enable efficient delivery of therapeutic siRNA into glioblastoma cells. J. Control. Release 2017, 259, E83–E84. [Google Scholar] [CrossRef]
- Li, N.; Yang, H.J.; Yu, Z.Z.; Li, Y.L.; Pan, W.; Wang, H.Y.; Tang, B. Nuclear-targeted siRNA delivery for long-term gene silencing. Chem. Sci. 2017, 8, 2816–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhao, L.J.; Wang, S.M.; Yang, J.; Lu, G.H.; Luo, N.N.; Gao, X.Y.; Ma, G.H.; Xie, H.Y.; Wei, W. Construction of a Biomimetic Magnetosome and Its Application as a SiRNA Carrier for High-Performance Anticancer Therapy. Adv. Funct. Mater. 2018, 28, 1703326. [Google Scholar] [CrossRef]
- Hematyar, M.; Soleimani, M.; Es-haghi, A.; Mokarram, A.R. Synergistic co-delivery of doxorubicin and melittin using functionalized magnetic nanoparticles for cancer treatment: Loading and in vitro release study by LC-MS/MS. Artif. Cell Nanomed. B. 2018, 46, S1226–S1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.L.; Guo, S.W.; Wu, L.N.; Chen, P.W.; Wang, L.Y.; Ying, L.; Ju, H.X. Near-infrared boosted ROS responsive siRNA delivery and cancer therapy with sequentially peeled upconversion nano-onions. Biomaterials 2019, 225, 119501. [Google Scholar] [CrossRef]
- Bian, Z.Y.; Yan, J.; Wang, S.M.; Li, Y.J.; Guo, Y.; Ma, B.H.; Guo, H.; Lei, Z.J.; Yin, C.; Zhou, Y.; et al. Awakening p53 in vivo by D-peptides-functionalized ultra-small nanoparticles: Overcoming biological barriers to D-peptide drug delivery. Theranostics 2018, 8, 5320–5335. [Google Scholar] [CrossRef] [PubMed]
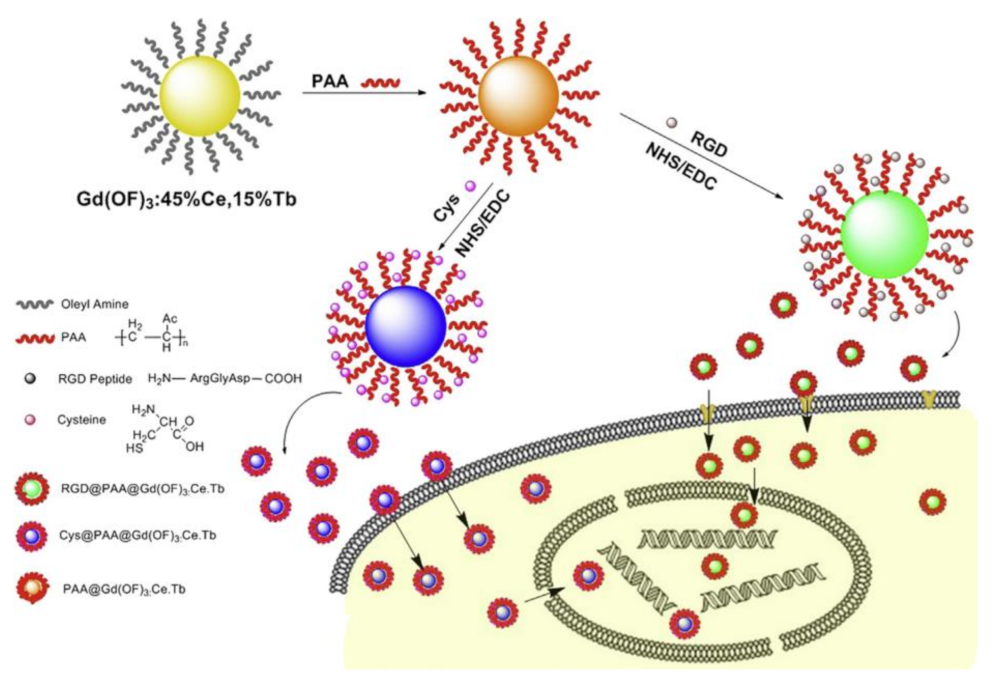



| Nanoparticle | Peptide Sequence | Functionalization Strategy | Analyte | Detection Principle | Linear Range/ Detection Limit | Refs |
|---|---|---|---|---|---|---|
| AuNPs | NH2-L-Aib-Y-OMe | Chemical reduction | Hg2+ | colorimetric assay | 4 to 10 ppm/4 ppm | [72] |
| AuNPs | CALNN | Ligand exchange | Al3+ | colorimetric assay | 0.5 to 6 mM/0.2 mM | [73] |
| AuNPs | CALNN/GSH | Ligand exchange | Pb2+ | colorimetric assay | 500 nM to 15 mM/500 nM | [74] |
| AuNPs | GIRLRLEEIEYELKRISGGGC | Ligand exchange | Cu2+ | colorimetric assay | 10 to 150 mM/1 mM | [75] |
| AuNPs | GSH | Ligand exchange | Pb2+ | colorimetric assay | 30 nM to 2 mM/13 nM | [76] |
| AuNPs | RFPRGGDD | Ligand exchange | Ag+ | colorimetric assay | 10 nM to 1 mM/7.4 nM | [77] |
| AuNPs | EKEKEKPPPPC | Ligand exchange | Ni2+ | colorimetric assay | 60 to 160 nM/34 nM | [79] |
| AuNPs | CALNNGK(Abscisic Acid)G | Ligand exchange | Abscisic acid glucose ester | colorimetric assay | 5 nM to 10 mM/2.2 nM | [80] |
| AuNPs | WHSDMEWWYLLGGGGGC | Ligand exchange | Vascular endothelial growth factor receptor 1 | colorimetric assay | 0.2 to 10 nM/0.2 nM | [81] |
| AuNPs | KKHHHHHHKK | Ligand exchange | Prostate specific membrane antigen | colorimetric assay | 2 to 10 nM/0.5 nM | [82] |
| AuNPs | Peptide-p53 and peptide-p14 | Ligand exchange | Mdm2 | colorimetric assay | 30 to 50 nM/20 nM | [83] |
| AuNPs | H6GLRRAS(P)LG | Chemical conjugation | protein phosphatase 2A | colorimetric assay | -/- | [91] |
| AuNPs | GPDC or VP-ethylene diamine-DC | Ligand exchange | Dipeptidyl peptidase IV | colorimetric assay | 0 to 12 U L−1/1.2 U L−1 or 0 to 30 U L−1/1.5 U L−1 | [94] |
| AuNPs | FGGFELLC | Ligand exchange | Aminopeptidase N | colorimetric assay | 5 to 15 mg mL−1/0.42 mg mL−1 | [95] |
| AuNPs | NAADLEKAIEALEKHLEAKGPCDAAQLEKQLEQAFEAFERAG | Ligand exchange | MMP-7 | colorimetric assay | 5 to 25 mg mL−1/3.3 mg mL−1 | [98] |
| AuNPs | CCYKKKKQAGDV | Ligand exchange | Integrin GPIIb/IIIa | colorimetric assay | 31.25 to 375 ng mL L−1/31.25 ng mL L−1 | [99] |
| AuNCs | GSH | Chemical reduction | Cancer cell | colorimetric assay | -/- | [100] |
| AuNPs | FITC-KGRRPED(Ac)K-biotin and biotin-K(Cy5)HRHPRY(P)G | Ligand exchange | histone deacetylase and protein tyrosine phosphatase 1B | FRET | 1 nM to 1 mM/28 pM and 0.015 to 0.3 nM/0.8 pM | [105] |
| UCNPs and carbon NPs | GHHYYGPLGVRGC | Chemical conjugation | MMP-2 | FRET | 10 to 500 pg mL−1/10 pg mL−1 | [106] |
| UCNPs | (H)6YGKAGK-TAMRA | Ligand exchange | Trypsin | FRET | 0.5−500 nM/0.05 nM | [108] |
| UCNPs and AuNPs | DDDDARC | Chemical conjugation and ligand exchange | Trypsin | FRET | 12 to 208 ng mL−1/4.15 ng mL−1 | [109] |
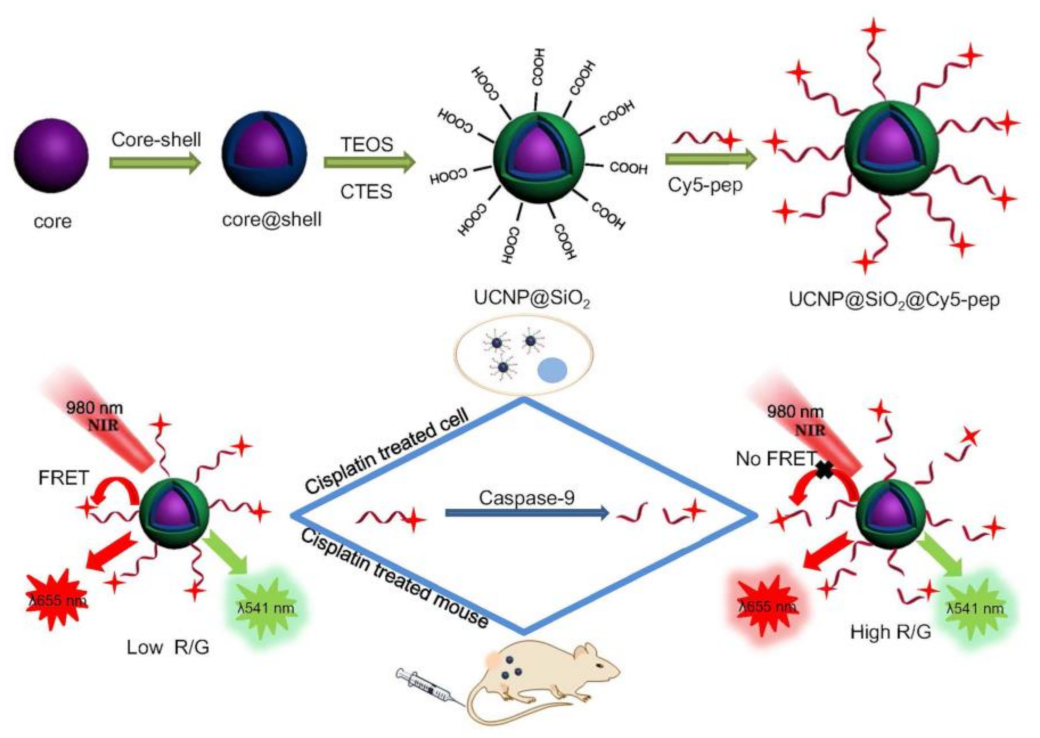
| UCNPs | CGRGGLEHDGGRK-Cy5 | Chemical conjugation | Caspase-9 | FRET | 0.5–100 U mL−1/0.068 U mL−1 | [114] |
| CdSe/ZnS QDs | Rhodamine-RGDC | Ligand exchange | Collagenase | FRET | 0 to 5 mg mL−1/- | [121] |
| Gold QDs | NES-linker-DEVD-linker-NLS | Chemical conjugation | Caspase-3 | Fluorescence assay | -/- | [123] |
| Gold nanostars | LRRASLG | Chemical conjugation and ligand exchange | Protein kinase A | Surface-enhanced Raman spectroscopy | 5 mU mL−1 to 5 kU mL−1/5 mU mL−1 | [125] |
| AuNPs | 3-mercaptopropionic acid-HSSKLQ-K (biotin) | Ligand exchange | Proteolytically active prostate specific antigen | Electrochemical sensor | 0.1 to 100 ng mL−1/27 pg mL−1 | [128] |
| AuNPs | RRRRRAGGPAC | Ligand exchange | Type IV collagenase | Quartz crystal microbalance biosensor | 10 to 60 ng mL−1/0.96 ng mL−1 | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jian, M.; Sun, Y.; Zhu, Q.; Wang, Z. The Peptide Functionalized Inorganic Nanoparticles for Cancer-Related Bioanalytical and Biomedical Applications. Molecules 2021, 26, 3228. https://doi.org/10.3390/molecules26113228
Li X, Jian M, Sun Y, Zhu Q, Wang Z. The Peptide Functionalized Inorganic Nanoparticles for Cancer-Related Bioanalytical and Biomedical Applications. Molecules. 2021; 26(11):3228. https://doi.org/10.3390/molecules26113228
Chicago/Turabian StyleLi, Xiaotong, Minghong Jian, Yanhong Sun, Qunyan Zhu, and Zhenxin Wang. 2021. "The Peptide Functionalized Inorganic Nanoparticles for Cancer-Related Bioanalytical and Biomedical Applications" Molecules 26, no. 11: 3228. https://doi.org/10.3390/molecules26113228
APA StyleLi, X., Jian, M., Sun, Y., Zhu, Q., & Wang, Z. (2021). The Peptide Functionalized Inorganic Nanoparticles for Cancer-Related Bioanalytical and Biomedical Applications. Molecules, 26(11), 3228. https://doi.org/10.3390/molecules26113228







