Overview of the Structure–Dynamics–Function Relationships in Borohydrides for Use as Solid-State Electrolytes in Battery Applications
Abstract
:1. Introduction
1.1. Why Study Borohydrides for Solid-State Electrolytes? The Versatility of Boron–Hydrogen Chemistry
1.2. Key Technical Challenges for Borohydrides as Solid-State Electrolytes
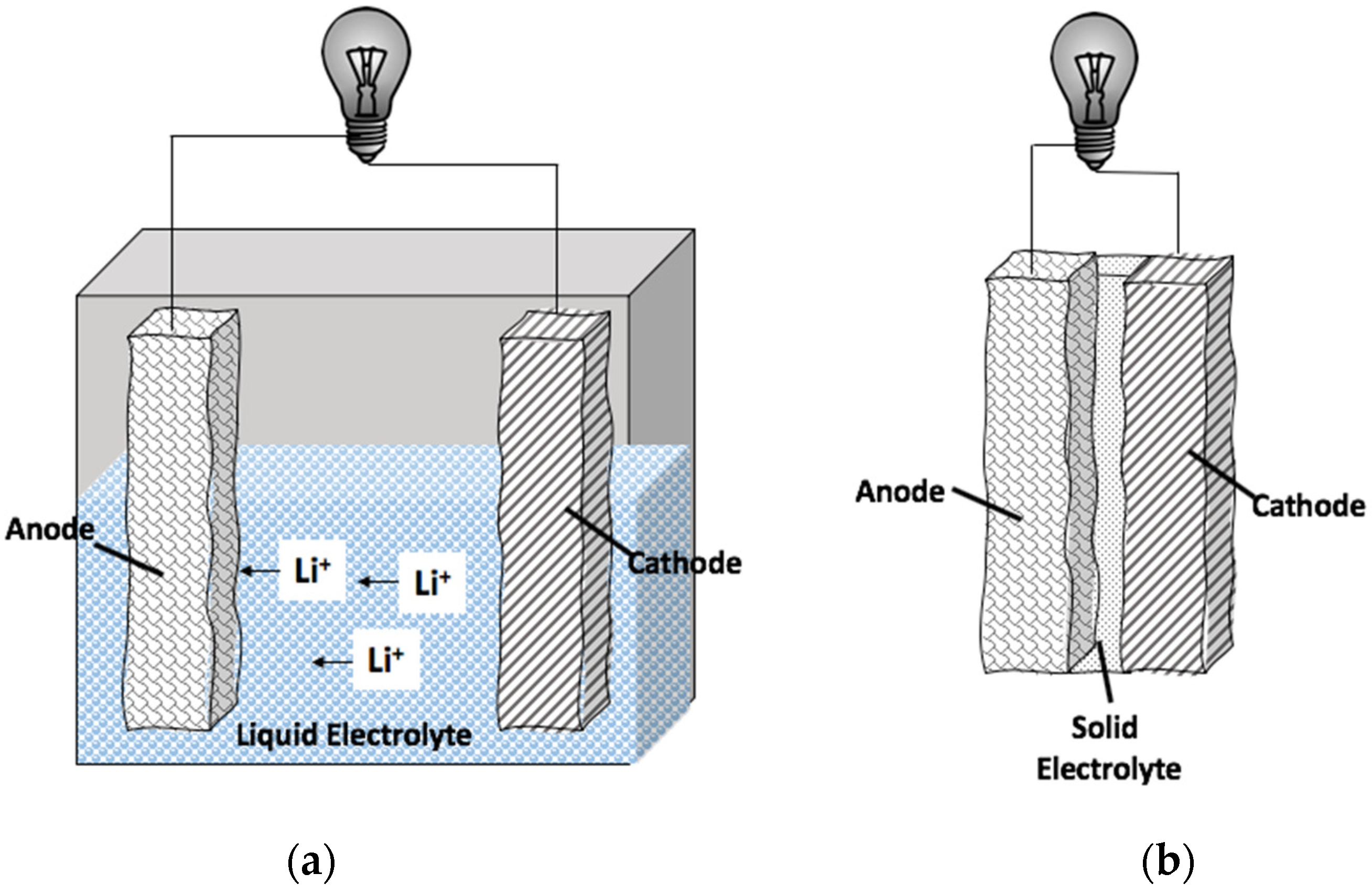
2. Borohydrides as Solid-State Electrolytes
2.1. Foundational Work with Borohydrides in the Electrolyte Layer of Batteries
2.2. A Survey of Ionic Conductivity in Borohydrides
2.3. Electrochemical Stability Window of Borohydrides
2.4. Useful Characterization Techniques to Gain Insights on Structure-Dynamic-Property Relationships
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Hassel, B.A. Niche Application Opportunities. Department of Energy Materials-Based Hydrogen Storage Summit: Defining Pathways for Onboard Automotive Applications. Available online: http://energy.gov/sites/prod/files/2015/02/f19/fcto_h2_storage_summit_van_hassel.pdf (accessed on 9 May 2021).
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Matsuo, M.; Orimo, S.-I. Lithium Fast-Ionic Conduction in Complex Hydrides: Review and Prospects. Adv. Energy Mater. 2011, 1, 161–172. [Google Scholar] [CrossRef]
- Cuan, J.; Zhou, Y.; Zhou, T.; Ling, S.; Rui, K.; Guo, Z.; Liu, H.; Yu, X. Borohydride-Scaffolded Li/Na/Mg Fast Ionic Conductors for Promising Solid-State Electrolytes. Adv. Mater. 2018, 31, e1803533. [Google Scholar] [CrossRef] [Green Version]
- Lipscomb, W.N. Boron Hydrides, Dover Edition (2012); W.A. Benjamin Inc.: New York, NY, USA, 1963. [Google Scholar]
- Lu, Z.; Ciucci, F. Metal Borohydrides as Electrolytes for Solid-State Li, Na, Mg, and Ca Batteries: A First-Principles Study. Chem. Mater. 2017, 29, 9308–9319. [Google Scholar] [CrossRef]
- Tutusaus, O.; Mohtadi, R.; Arthur, T.S.; Mizuno, F.; Nelson, E.G.; Sevryugina, Y.V. An Efficient Halogen-Free Electrolyte for Use in Rechargeable Magnesium Batteries. Angew. Chem. Int. Ed. 2015, 54, 7900–7904. [Google Scholar] [CrossRef]
- Kweon, K.E.; Varley, J.B.; Shea, P.; Adelstein, N.; Mehta, P.; Heo, T.W.; Udovic, T.J.; Stavila, V.; Wood, B.C. Structural, Chemical, and Dynamical Frustration: Origins of Superionic Conductivity in closo-Borate Solid Electrolytes. Chem. Mater. 2017, 29, 9142–9153. [Google Scholar] [CrossRef]
- Tang, W.S.; Unemoto, A.; Zhou, W.; Stavila, V.; Matsuo, M.; Wu, H.; Orimo, S.-I.; Udovic, T.J. Unparalleled lithium and sodium superionic conduction in solid electrolytes with large monovalent cage-like anions. Energy Environ. Sci. 2015, 8, 3637–3645. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Toyama, N.; Oguchi, H.; Sato, T.; Takagi, S.; Ikeshoji, T.; Orimo, S.-I. Fast Lithium-Ion Conduction in Atom-Deficient closo-Type Complex Hydride Solid Electrolytes. Chem. Mater. 2018, 30, 386–391. [Google Scholar] [CrossRef]
- Weeks, J.A.; Tinkey, S.C.; Ward, P.A.; Lascola, R.; Zidan, R.; Teprovich, J.A. Investigation of the Reversible Lithiation of an Oxide Free Aluminum Anode by a LiBH4 Solid State Electrolyte. Inorganics 2017, 5, 83. [Google Scholar] [CrossRef] [Green Version]
- Unemoto, A.; Ikeshoji, T.; Yasaku, S.; Matsuo, M.; Stavila, V.; Udovic, T.J.; Orimo, S.-I. Stable Interface Formation between TiS2 and LiBH4 in Bulk-Type All-Solid-State Lithium Batteries. Chem. Mater. 2015, 27, 5407–5416. [Google Scholar] [CrossRef]
- Xiang, M.; Zhang, Y.; Lin, H.; Zhu, Y.; Guo, X.; Chen, J.; Li, L. LiBH4-NaX (X=Cl, I) composites with enhanced lithium ionic conductivity. J. Alloys Compd. 2018, 764, 307–313. [Google Scholar] [CrossRef]
- Das, S.; Ngene, P.; Norby, P.; Vegge, T.; De Jongh, P.E.; Blanchard, D. All-Solid-State Lithium-Sulfur Battery Based on a Nanoconfined LiBH4Electrolyte. J. Electrochem. Soc. 2016, 163, A2029–A2034. [Google Scholar] [CrossRef] [Green Version]
- El Kharbachi, A.; Uesato, H.; Kawai, H.; Wenner, S.; Miyaoka, H.; Sørby, M.H.; Fjellvåg, H.; Ichikawa, T.; Hauback, B.C. MgH2–CoO: A conversion-type composite electrode for LiBH4-based all-solid-state lithium ion batteries. RSC Adv. 2018, 8, 23468–23474. [Google Scholar] [CrossRef] [Green Version]
- Tutusaus, O.; Mohtadi, R. Paving the Way towards Highly Stable and Practical Electrolytes for Rechargeable Magnesium Batteries. ChemElectroChem 2014, 2, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Mohtadi, R.; Matsui, M.; Arthur, T.S.; Hwang, S.-J. Magnesium Borohydride: From Hydrogen Storage to Magnesium Battery. Angew. Chem. Int. Ed. 2012, 51, 9780–9783. [Google Scholar] [CrossRef] [PubMed]
- Tuerxun, F.; Abulizi, Y.; Nuli, Y.; Su, S.; Yang, J.; Wang, J. High concentration magnesium borohydride/tetraglyme electrolyte for rechargeable magnesium batteries. J. Power Sources 2015, 276, 255–261. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Udovic, T.J.; Matsuo, M.; Unemoto, A.; Verdal, N.; Stavila, V.; Skripov, A.V.; Rush, J.J.; Takamura, H.; Orimo, S.-I. Sodium superionic conduction in Na2B12H12. Chem. Commun. 2014, 50, 3750–3752. [Google Scholar] [CrossRef] [PubMed]
- Makiura, R.; Yonemura, T.; Yamada, T.; Yamauchi, M.; Ikeda, R.; Kitagawa, H.; Kato, K.; Takata, M. Size-controlled stabilization of the superionic phase to room temperature in polymer-coated AgI nanoparticles. Nat. Mater. 2009, 8, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Maniadaki, A.E.; Lodziana, Z. Theoretical description of alkali metal closo-boranes—Towards the crystal structure of MgB12H12. Phys. Chem. Chem. Phys. 2018, 20, 30140–30149. [Google Scholar] [CrossRef]
- Lipscomb, W.N.; Pitochelli, A.R.; Hawthorne, M.F. PROBABLE STRUCTURE OF THE B10H10−2 ION. J. Am. Chem. Soc. 1959, 81, 5833–5834. [Google Scholar] [CrossRef]
- Lipscomb, W.N. Framework Rearrangement in Boranes and Carboranes. Science 1966, 153, 373–378. [Google Scholar] [CrossRef]
- Chen, Z.; King, R.B. Spherical Aromaticity: Recent Work on Fullerenes, Polyhedral Boranes, and Related Structures†. Chem. Rev. 2005, 105, 3613–3642. [Google Scholar] [CrossRef]
- Zimmermann, L.W.; Kleeberg, F.M.; Schleid, T. Synthesis and X-ray Crystallography of Two Lead(II) Decahydro-closo-Decaborate Hydrates. Z. Anorg. Allg. Chem. 2017, 643, 365–372. [Google Scholar] [CrossRef]
- Dobrott, R.D.; Lipscomb, W.N. Structure of Cu2B10H10. J. Chem. Phys. 1962, 37, 1779. [Google Scholar] [CrossRef]
- Wu, H.; Tang, W.S.; Stavila, V.; Zhou, W.; Rush, J.J.; Udovic, T.J. Structural Behavior of Li2B10H10. J. Phys. Chem. C 2015, 119, 6481–6487. [Google Scholar] [CrossRef]
- Bernard, R.; Cornu, D.; Perrin, M.; Scharff, J.-P.; Miele, P. Synthesis and X-ray structural characterisation of the tetramethylene oxonium derivative of the hydrodecaborate anion. A versatile route for derivative chemistry of [B10H10]2−. J. Organomet. Chem. 2004, 689, 2581–2585. [Google Scholar] [CrossRef]
- Panda, M. Synthesis and Characterization of Alkali Metal Borides and Closo-Hydroborates. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2006. [Google Scholar]
- Unemoto, A.; Wu, H.; Udovic, T.J.; Matsuo, M.; Ikeshoji, T.; Orimo, S.-I. Fast lithium-ionic conduction in a new complex hydride–sulphide crystalline phase. Chem. Commun. 2015, 52, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Nanda, J.; Wang, C.; Liu, P. Frontiers of solid-state batteries. MRS Bull. 2018, 43, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Li, Z.; Wen, Z.; Han, W.-Q. Li/Li7La3Zr2O12/LiFePO4 All-Solid-State Battery with Ultrathin Nanoscale Solid Electrolyte. J. Phys. Chem. C 2017, 121, 1431–1435. [Google Scholar] [CrossRef]
- Shoji, M.; Munakata, H.; Kanamura, K. Fabrication of All-Solid-State Lithium-Ion Cells Using Three-Dimensionally Structured Solid Electrolyte Li7La3Zr2O12 Pellets. Front. Energy Res. 2016, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Roedern, E.; Kühnel, R.-S.; Remhof, A.; Battaglia, C. Magnesium Ethylenediamine Borohydride as Solid-State Electrolyte for Magnesium Batteries. Sci. Rep. 2017, 7, srep46189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y.; Karahashi, T.; Orimo, S.-I. Halide-Stabilized LiBH4, a Room-Temperature Lithium Fast-Ion Conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar] [CrossRef]
- Didelot, E.; Černý, R. Ionic conduction in bimetallic borohydride borate, LiCa3(BH4)(BO3)2. Solid State Ionics 2017, 305, 16–22. [Google Scholar] [CrossRef]
- Yan, Y.; Kühnel, R.-S.; Remhof, A.; Duchêne, L.; Reyes, E.C.; Rentsch, D.; Łodziana, Z.; Battaglia, C. A Lithium Amide-Borohydride Solid-State Electrolyte with Lithium-Ion Conductivities Comparable to Liquid Electrolytes. Adv. Energy Mater. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Blake, A. Characterization of Boron Hydride Compounds for Potential use in Pressurized Water Nuclear Reactors. Master’s Thesis, Pittsburg State University, Pittsburg, KS, USA, 2015. [Google Scholar]
- Ceder, G.; Ong, S.P.; Wang, Y. Predictive modeling and design rules for solid electrolytes. MRS Bull. 2018, 43, 746–751. [Google Scholar] [CrossRef]
- Mueller, T.; Ceder, G. Effect of Particle Size on Hydrogen Release from Sodium Alanate Nanoparticles. ACS Nano 2010, 4, 5647–5656. [Google Scholar] [CrossRef]
- Gerhardt, R. Impedance and dielectric spectroscopy revisited: Distinguishing localized relaxation from long-range conductivity. J. Phys. Chem. Solids 1994, 55, 1491–1506. [Google Scholar] [CrossRef]
- Sullivan, E.M.; Gerhardt, R.A.; Wang, B.; Kalaitzidou, K. Effect of compounding method and processing conditions on the electrical response of exfoliated graphite nanoplatelet/polylactic acid nanocomposite films. J. Mater. Sci. 2016, 51, 2980–2990. [Google Scholar] [CrossRef]
- Jin, Y.; Xia, N.; Gerhardt, R.A. Enhanced dielectric properties of polymer matrix composites with BaTiO3 and MWCNT hybrid fillers using simple phase separation. Nano Energy 2016, 30, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.; Hagemann, H.; Yvon, K. Lithium boro-hydride LiBH4 II. Raman spectroscopy. J. Alloys Compd. 2002, 346, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Duchêne, L.; Lunghammer, S.; Burankova, T.; Liao, W.C.; Embs, J.P.; Copéret, C.; Wilkening, H.M.; Remhof, A.; Hagemann, H.; Battaglia, C. Borate Solid-State Electrolyte: Interplay of Disorder and Ion-Ion Interactions. Chem. Mater. 2019, 31, 3449–3460. [Google Scholar] [CrossRef]
- Verdal, N.; Hartman, M.R.; Jenkins, T.; Devries, D.J.; Rush, J.J.; Udovic, T.J. Re-orientational Dynamics of NaBH4 and KBH4. J. Phys. Chem. C 2010, 114, 10027–10033. [Google Scholar] [CrossRef]
- Verdal, N.; Udovic, T.J.; Rush, J.J.; Stavila, V.; Wu, H.; Zhou, W.; Jenkins, T. Low-temperature tunneling and rotational dynamics of the ammonium cations in (NH4)2B12H12. J. Chem. Phys. 2011, 135, 094501. [Google Scholar] [CrossRef]
- Scrosati, B. History of lithium batteries. J. Solid State Electrochem. 2011, 15, 1623–1630. [Google Scholar] [CrossRef]





| Material | Temperature (°C) | Solid-State Cationic Conductivity (S/cm) | Reference | |
|---|---|---|---|---|
| Traditional “Good” Ionic Conductors | Perovskites (e.g., LLZO, LLTO, LiTi(PO4)3) | RT | 10−3 to 10−5 | [6] |
| Untreated Borohydrides | LiBH4 | 107 | 2 × 10−3 | [16] |
| Mg(BH4)2 | 30 | 10−12 | [39] | |
| LiB10H10 | 60 | 3 × 10−2 | [10] | |
| NaB10H10 | RT | 3 × 10−2 | [10] | |
| Nanoconfined | LiBH4 | RT | 10−3 | [18] |
| Adducts | Mg(BH4)2—NH2 | 30 | 5 × 10−8 | [39] |
| Mg(BH4)2—NH2 | 70 | 6 × 10−5 | [39] | |
| Cationic Doped and Anion Additions | Li4(BH4)3I | RT | 10−2 | [40] |
| Na-doped LiBH4-BO3 | RT | 10−5 | [41] | |
| LiCa3(BH4)(BO3)2 | RT | 1 × 10−5 to 2.5 × 10−6 | [41] | |
| Anionic Additions | LiBH4-LiX (X = Cl, Br, I) | RT | 10−4 to 10−7 | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobbins, T.A. Overview of the Structure–Dynamics–Function Relationships in Borohydrides for Use as Solid-State Electrolytes in Battery Applications. Molecules 2021, 26, 3239. https://doi.org/10.3390/molecules26113239
Dobbins TA. Overview of the Structure–Dynamics–Function Relationships in Borohydrides for Use as Solid-State Electrolytes in Battery Applications. Molecules. 2021; 26(11):3239. https://doi.org/10.3390/molecules26113239
Chicago/Turabian StyleDobbins, Tabbetha A. 2021. "Overview of the Structure–Dynamics–Function Relationships in Borohydrides for Use as Solid-State Electrolytes in Battery Applications" Molecules 26, no. 11: 3239. https://doi.org/10.3390/molecules26113239






