Visual Detection of Triethylamine and a Dual Input/Output Logic Gate Based on a Eu3+-Complex
Abstract
1. Introduction
2. Results and Discussion
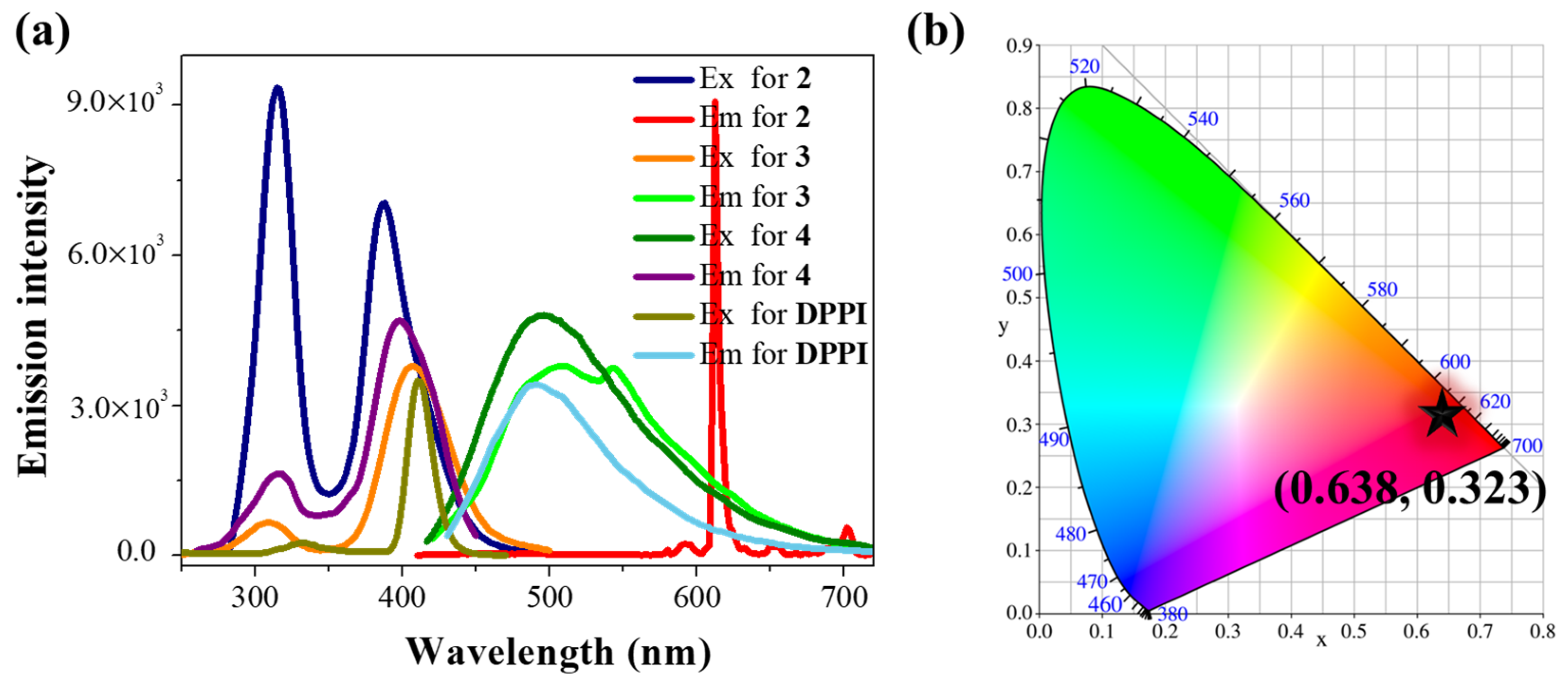
2.1. Synthesis and Photophysical Properties of the Ln-Complexes
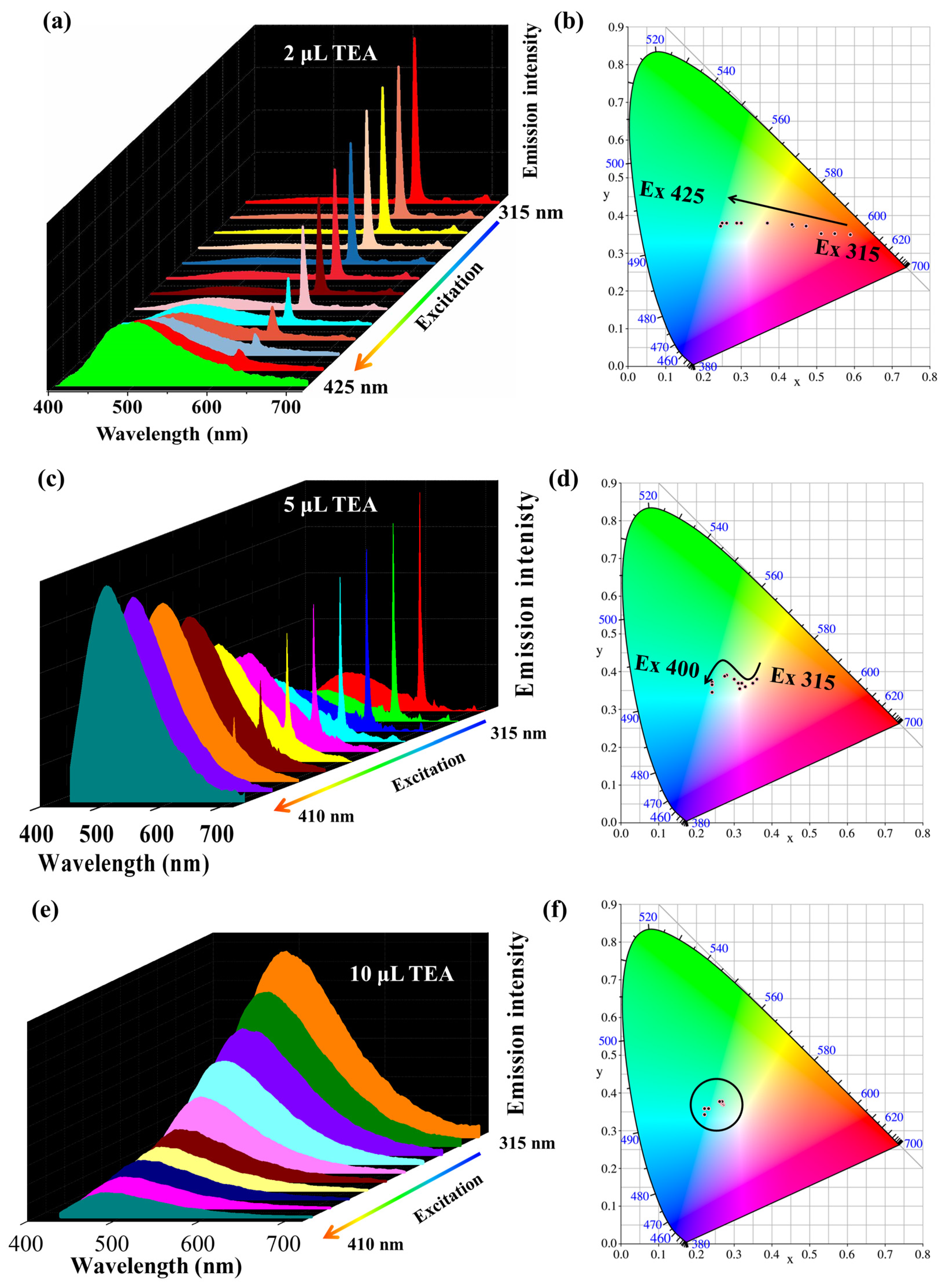
2.2. Adjustable Color Emission of Complex 2 with TEA Stimulate
2.3. Potential Application in IMPLICATION Logic Gate
3. Experimental Section
3.1. Synthesis of 1,10-Phenanthroline-5,6-Dione (PHD)
3.2. Synthesis of DPPI
3.3. Synthesis and Characterization of Ln(TTA)3(DPPI) (Ln = La, 1; Ln = Eu, 2; Ln = Tb, 3; Ln = Gd, 4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K. MOFs as proton conductors-challenges and opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef]
- Guo, M.Y.; Li, P.; Yang, S.L.; Bu, R.; Piao, X.Q.; Gao, E.Q. Distinct and Selective Amine- and Anion-Responsive Behaviors of an Electron-Deficient and Anion-Exchangeable Metal-Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 43958–43966. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Z.; Yao, D.; Li, H. Colorimetric sensor arrays for amines based on responsive lanthanide complex entrapment. J. Mater. Chem. C 2017, 5, 6805–6811. [Google Scholar] [CrossRef]
- Chu, B.; Song, B.; Ji, X.; Su, Y.; Wang, H.; He, Y. Fluorescent Silicon Nanorods-Based Ratiometric Sensors for Long-Term and Real-Time Measurements of Intracellular pH in Live Cells. Anal. Chem. 2017, 89, 12152–12159. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Mo, J.; Sun, S.; Yin, S.; Wang, H.; Pan, M. Acid-base Vapor Sensing Enabled by ESIPT-attributed Cd(II) Coordination Polymer with Switchable Luminescence. Chem. Res. Chin. Univ. 2020, 36, 755–759. [Google Scholar] [CrossRef]
- Du, B.B.; Zhu, Y.X.; Pan, M.; Yue, M.Q.; Hou, Y.J.; Wu, K.; Zhang, L.Y.; Chen, L.; Yin, S.Y.; Fan, Y.N.; et al. Direct white-light and a dual-channel barcode module from Pr(III)-MOF crystals. Chem. Commun. 2015, 51, 12533–12536. [Google Scholar] [CrossRef] [PubMed]
- Biju, S.; Xu, L.-J.; Sun, C.-Z.; Chen, Z.-N. White OLEDs based on a novel EuIII-tetrakis-β-diketonate doped into 4,4′-N,N′-dicarbazolebiphenyl as emitting material. J. Mater. Chem. C 2015, 3, 5775–5782. [Google Scholar] [CrossRef]
- Yin, S.-Y.; Fu, P.-Y.; Pan, M.; Guo, J.; Fan, Y.-N.; Su, C.-Y. Reverse photoluminescence responses of Ln(III) complexes to methanol vapor clarify the differentiated energy transfer pathway and potential for methanol detection and encryption. J. Mater. Chem. C 2020, 8, 16907–16914. [Google Scholar] [CrossRef]
- Okutani, K.; Nozaki, K.; Iwamura, M. Specific chiral sensing of amino acids using induced circularly polarized luminescence of bis(diimine)dicarboxylic acid europium(III) complexes. Inorg. Chem. 2014, 53, 5527–5537. [Google Scholar] [CrossRef]
- Wang, G.-D.; Li, Y.-Z.; Shi, W.-J.; Zhang, B.; Hou, L.; Wang, Y.-Y. A robust cluster-based Eu-MOF as multi-functional fluorescence sensor for detection of antibiotics and pesticides in water. Sens. Actuators B 2021, 331, 129377. [Google Scholar] [CrossRef]
- Ma, Y.-J.; Hu, J.-X.; Han, S.-D.; Pan, J.; Li, J.-H.; Wang, G.-M. Manipulating on/off single-molecule magnet behavior in a Dy(III)-based photochromic complex. J. Am. Chem. Soc. 2020, 142, 2682–2689. [Google Scholar] [CrossRef]
- Jeon, H.-G.; Kim, H.; Byeon, S.-H. Flexibly transparent luminescent organic-inorganic-polymer composite films: Intense full-color emissions at a single excitation wavelength. Chem. Eng. J. 2021, 405, 126675. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Wang, Y.; Wang, Y.; Li, H. Luminescent europium(III)-beta-diketonate complexes hosted in nanozeolite L as turn-on sensors for detecting basic molecules. Chem. Commun. 2014, 50, 13680–13682. [Google Scholar] [CrossRef] [PubMed]
- Lucas, F.; Quinton, C.; Fall, S.; Heiser, T.; Tondelier, D.; Geffroy, B.; Leclerc, N.; Rault-Berthelot, J.; Poriel, C. Universal host materials for red, green and blue high-efficiency single-layer phosphorescent organic light-emitting diodes. J. Mater. Chem. C 2020, 8, 16354–16367. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Chi, H.; Yang, S.; Niu, Q.; Wu, D.; Cao, W.; Li, T.; Ma, H.; Wei, Q. Self-Luminescent Lanthanide Metal–Organic Frameworks as Signal Probes in Electrochemiluminescence Immunoassay. J. Am. Chem. Soc. 2020, 143, 504–512. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Wu, J.; Huang, J. Vertical Organic Nanowire Arrays: Controlled Synthesis and Chemical Sensors. J. Am. Chem. Soc. 2009, 131, 3158–3159. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Li, J. Emission “Off-On” effect from europium complexes triggered by AcO anion: Synthesis, characterization and sensing performance. Spectrochim. Acta Part A 2014, 127, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shi, M.; Liu, Z.; Li, F.; Yi, T.; Huang, C. Luminescence Modulation of a Terbium Complex with Anions and Its Application as a Reagent. Eur. J. Inorg. Chem. 2006, 2006, 2277–2284. [Google Scholar] [CrossRef]
- Xu, J.; Corneillie, T.M.; Moore, E.G.; Law, G.-L.; Butlin, N.G.; Raymond, K.N. Octadentate Cages of Tb(III) 2-Hydroxyisophthalamides: A New Standard for Luminescent Lanthanide Labels. J. Am. Chem. Soc. 2011, 133, 19900–19910. [Google Scholar] [CrossRef]
- Sato, T.; Higuchi, M. A vapoluminescent Eu-based metallo-supramolecular polymer. Chem. Commun. 2012, 48, 4947–4949. [Google Scholar] [CrossRef]
- Chen, P.; Li, Q.; Grindy, S.; Holten-Andersen, N. White-Light-Emitting Lanthanide Metallogels with Tunable Luminescence and Reversible Stimuli-Responsive Properties. J. Am. Chem. Soc. 2015, 137, 11590–11593. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.H.; Xue, L.L.; Jiang, J.G. Two-Stage Cultivation of Dunaliella tertiolecta with Glycerol and Triethylamine for Lipid Accumulation: A Viable Way To Alleviate the Inhibitory Effect of Triethylamine on Biomass. Appl. Environ. Microbiol. 2019, 85, 02614–02618. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yao, M.; Mu, S.; Wang, Y. Oxygen Vacancies Enhance Triethylamine Sensing Properties of SnO2 Nanoparticles. ChemistrySelect 2019, 4, 11268–11274. [Google Scholar] [CrossRef]
- Lu, Y.; Yan, B. Lanthanide organic-inorganic hybrids based on functionalized metal-organic frameworks (MOFs) for a near-UV white LED. Chem. Commun. 2014, 50, 15443–15446. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, J.-P.; Zhou, Z.; Wang, R.; Wang, L.-Y.; Zang, S.-Q. Robust lanthanide metal–organic frameworks with “all-in-one” multifunction: Efficient gas adsorption and separation, tunable light emission and luminescence sensing. J. Mater. Chem. C 2021, 9, 3429–3439. [Google Scholar] [CrossRef]
- Zheng, H.-Q.; Guo, Y.-P.; Yin, M.-C.; Fan, Y.-T. Synthesis, characterization of a new photosensitive compound [Ru(bpy)2 (TPAD)](PF6)2 and its application for photocatalytic hydrogen production. Chem. Phys. Lett. 2016, 653, 17–23. [Google Scholar] [CrossRef]
- Zheng, H.; Deng, Y.-K.; Ye, M.-Y.; Xu, Q.-F.; Kong, X.-J.; Long, L.-S.; Zheng, L.-S. Lanthanide-Titanium Oxo Clusters as the Luminescence Sensor for Nitrobenzene Detection. Inorg. Chem. 2020, 59, 12404–12409. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pang, M.; Chen, H.; Fu, G.; Li, B.; Lü, X.; Wang, L. Efficient and high colour-purity green-light polymer light-emitting diodes (PLEDs) based on a PVK-supported Tb3+-containing metallopolymer. J. Mater. Chem. C 2017, 5, 9021–9027. [Google Scholar] [CrossRef]
- Ren, Y.; Feng, J. Poly(MMA-co-FMA) as a platform for tuning emission by clicking with luminescent lanthanide complexes. J. Mater. Chem. C 2018, 6, 10202–10206. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, Q.; Wang, C.; Fan, D.; Wang, Y. Full color-emitting (Y,Tb,Eu)NbO4 nanophosphors: Calcination-assisted hydrothermal synthesis, energy interaction, and application in deep UV chip-based WLEDs. J. Mater. Chem. C 2020, 8, 14548–14558. [Google Scholar] [CrossRef]
- Liao, W.-M.; Li, C.-J.; Wu, X.; Zhang, J.-H.; Wang, Z.; Wang, H.-P.; Fan, Y.-N.; Pan, M.; Su, C.-Y. Homometallic Ln(III)-complexes from an ILCT ligand with sensitized vis-NIR emission, excitation-dependent PL color tuning and white-light emission. J. Mater. Chem. C 2018, 6, 3254–3259. [Google Scholar] [CrossRef]
- Sun, S.-S.; Wang, Z.; Wu, X.W.; Zhang, J.-H.; Li, C.-J.; Yin, S.-Y.; Chen, L.; Pan, M.; Su, C.-Y. ESIPT-Modulated Emission of Lanthanide Complexes: Different Energy-Transfer Pathways and Multiple Responses. Chem. Eur. J. 2018, 24, 10091–10098. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Hu, W.-J.; Qin, Y.; Zhao, G.-L. Synthesis, Crystal Structure and Fluorescence Spectrum of a Europium Compound [Eu(NO3)3(H2O)4]·(C10H9N2)2·(NO3)2. Asian J. Chem. 2013, 25, 8418–8422. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Sengupta, S.K.; Pandey, O.P. Synthesis, Characterization and Photo-Physical Properties of Europium(III) and Terbium(III) Complexes with Thiosemicarbazones. Asian J. Chem. 2020, 32, 952–958. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, R.; Zhao, P.; Huang, W. Monochromic Red-Emitting Nonconjugated Copolymers Containing Double-Carrier-Trapping Phosphine Oxide Eu3+ Segments: Toward Bright and Efficient Electroluminescence. J. Phys. Chem. C 2011, 115, 15627–15638. [Google Scholar] [CrossRef]
- Dou, C.; Han, L.; Zhao, S.; Zhang, H.; Wang, Y. Multi-Stimuli-Responsive Fluorescence Switching of a Donor-Acceptor π-Conjugated Compound. J. Phys. Chem. Lett. 2011, 2, 666–670. [Google Scholar] [CrossRef]
- Yang, C.; Fu, L.M.; Wang, Y.; Zhang, J.P.; Wong, W.T.; Ai, X.C.; Qiao, Y.F.; Zou, B.S.; Gui, L.L. A highly luminescent europium complex showing visible-light-sensitized red emission: Direct observation of the singlet pathway. Angew. Chem. Int. Ed. 2004, 43, 5010–5013. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Wu, K.; Jiang, J.J.; Hsu, C.W.; Pan, M.; Lehn, J.M.; Su, C.Y. Pure white-light and yellow-to-blue emission tuning in single crystals of Dy(III) metal-organic frameworks. Chem. Commun. 2014, 50, 7702–7704. [Google Scholar] [CrossRef]
- Francke, R.; Little, R.D. Optimizing Electron Transfer Mediators Based on Arylimidazoles by Ring Fusion: Synthesis, Electrochemistry, and Computational Analysis of 2-Aryl-1-methylphenanthro[9,10-d]imidazoles. J. Am. Chem. Soc. 2013, 136, 427–435. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, J.; Wu, W.; Yu, X.; Liu, Y. Accessing the long-lived triplet excited states in bodipy-conjugated 2-(2-hydroxyphenyl) benzothiazole/benzoxazoles and applications as organic triplet photosensitizers for photooxidations. J. Org. Chem. 2012, 77, 6166–6178. [Google Scholar] [CrossRef]
- Chuang, W.T.; Hsieh, C.C.; Lai, C.H.; Lai, C.H.; Shih, C.W.; Chen, K.Y.; Hung, W.Y.; Hsu, Y.H.; Chou, P.T. Excited-state intramolecular proton transfer molecules bearing o-hydroxy analogues of green fluorescent protein chromophore. J. Org. Chem. 2011, 76, 8189–8202. [Google Scholar] [CrossRef]
- Goudappagouda; Asokan, K.; Nayak, R.; Krishnan, R.; Babu, S.S. Tuning phosphorescence features of triphenylamines by varying functional groups and intermolecular interactions. Dyes Pigments 2020, 173, 107931. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Beni, A.S.; Azari, M.; Zarandi, M.; Karami, M. Novel D-π—A type triphenylamine based chromogens for DSSC: Design, synthesis and performance studies. New J. Chem. 2016, 40, 8371–8381. [Google Scholar] [CrossRef]
- Perrier, A.; Jacquemin, D. Theoretical investigation of the photochromic properties of [2.2]paracyclophane-bridged imidazole dimers and bis(imidazole) dimers. Tetrahedron 2017, 73, 4936–4949. [Google Scholar] [CrossRef]
- Boxi, S.; Jana, D.; Parui, P.P.; Ghorai, B.K. Dibenzo[a,c]phenazine-Based Donor-Acceptor (D-A) Tetra Branched Molecules: Fine Tuning of Optical Properties. ChemistrySelect 2018, 3, 6953–6959. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, C.J.; Liu, W.; Liang, K.; Shi, Y.Z.; Tao, S.L.; Lee, C.S.; Ou, X.M.; Zhang, X.H. Avoiding Energy Loss on TADF Emitters: Controlling the Dual Conformations of D-A Structure Molecules Based on the Pseudoplanar Segments. Adv. Mater. 2017, 29, 170146. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Lv, S.L.; You, M.H.; Lin, M.J. Three-component D-A hybrid heterostructures with enhanced photochromic, photomodulated luminescence and selective anion-sensing properties. Dalton Trans. 2020, 49, 13083–13089. [Google Scholar] [CrossRef]
- Zhao, B.; Fang, Y.; Xu, Y.; Deng, Q.; Liu, T.; Kan, W.; Wang, L. Dual-responsive pH sensor based on a phenanthro[9,10-d]imidazole fluorophore modified by amino diacetate. Tetrahedron Lett. 2016, 57, 1825–1830. [Google Scholar] [CrossRef]
- Jang, H.; Shin, C.-H.; Jung, B.-J.; Kim, D.-h.; Shim, H.-K.; Do, Y. Synthesis and Characterization of Dinuclear Europium Complexes Showing Pure Red Electroluminescence. Eur. J. Inorg. Chem. 2006, 2006, 718–725. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.-N.; Liu, Y.-Y.; Wang, Y.-P.; Pan, M. Visual Detection of Triethylamine and a Dual Input/Output Logic Gate Based on a Eu3+-Complex. Molecules 2021, 26, 3244. https://doi.org/10.3390/molecules26113244
Li B-N, Liu Y-Y, Wang Y-P, Pan M. Visual Detection of Triethylamine and a Dual Input/Output Logic Gate Based on a Eu3+-Complex. Molecules. 2021; 26(11):3244. https://doi.org/10.3390/molecules26113244
Chicago/Turabian StyleLi, Bao-Ning, Yuan-Yuan Liu, Ya-Ping Wang, and Mei Pan. 2021. "Visual Detection of Triethylamine and a Dual Input/Output Logic Gate Based on a Eu3+-Complex" Molecules 26, no. 11: 3244. https://doi.org/10.3390/molecules26113244
APA StyleLi, B.-N., Liu, Y.-Y., Wang, Y.-P., & Pan, M. (2021). Visual Detection of Triethylamine and a Dual Input/Output Logic Gate Based on a Eu3+-Complex. Molecules, 26(11), 3244. https://doi.org/10.3390/molecules26113244





