Molecular Dynamics of Cobalt Protoporphyrin Antagonism of the Cancer Suppressor REV-ERBβ
Abstract
:1. Introduction
2. Results
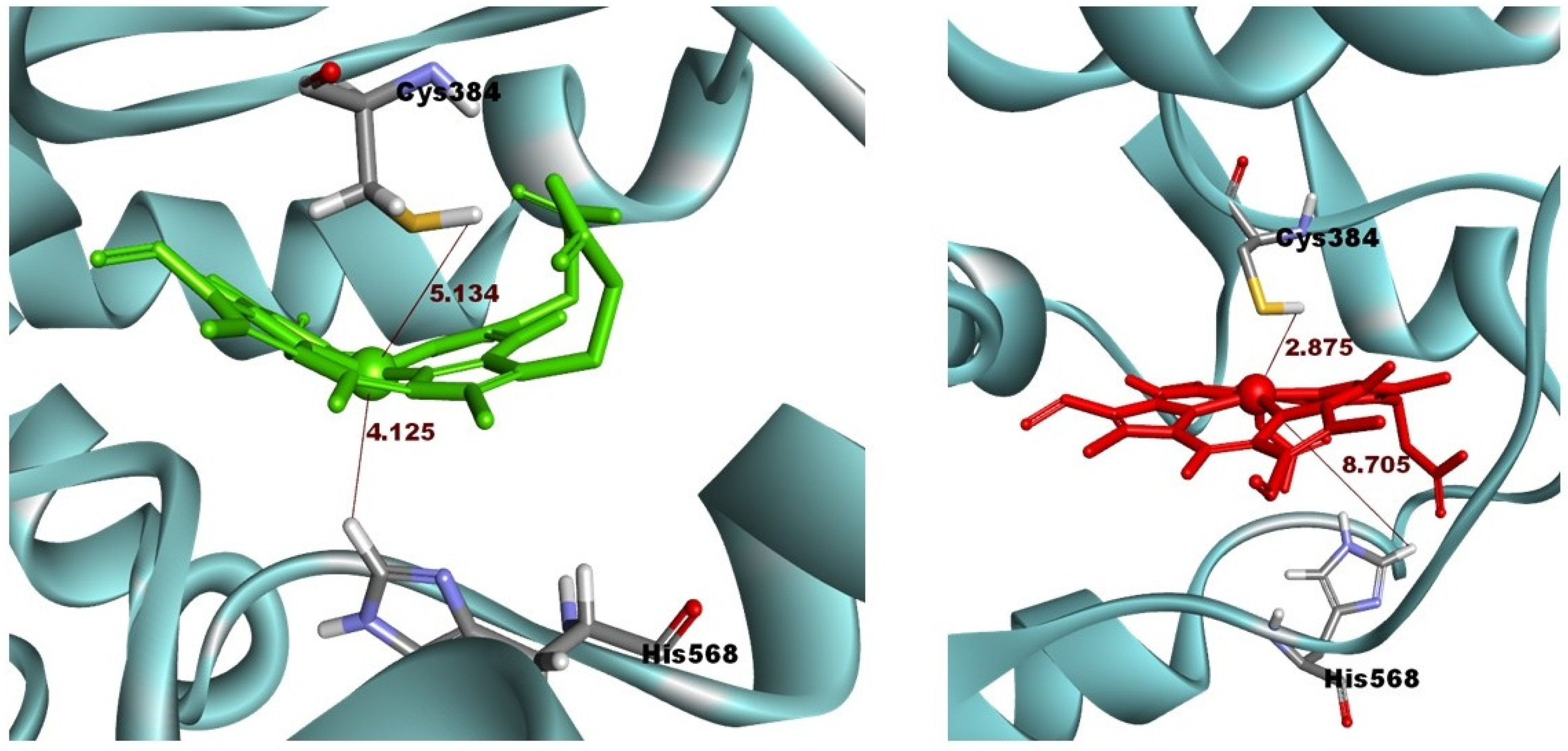
2.1. Initial Pose of the Ligand–Protein Complex
2.2. The Binding Free Energy of Agonist and Antagonist Ligands against REV-ERBβ Ligand-Binding Domain (LBD)
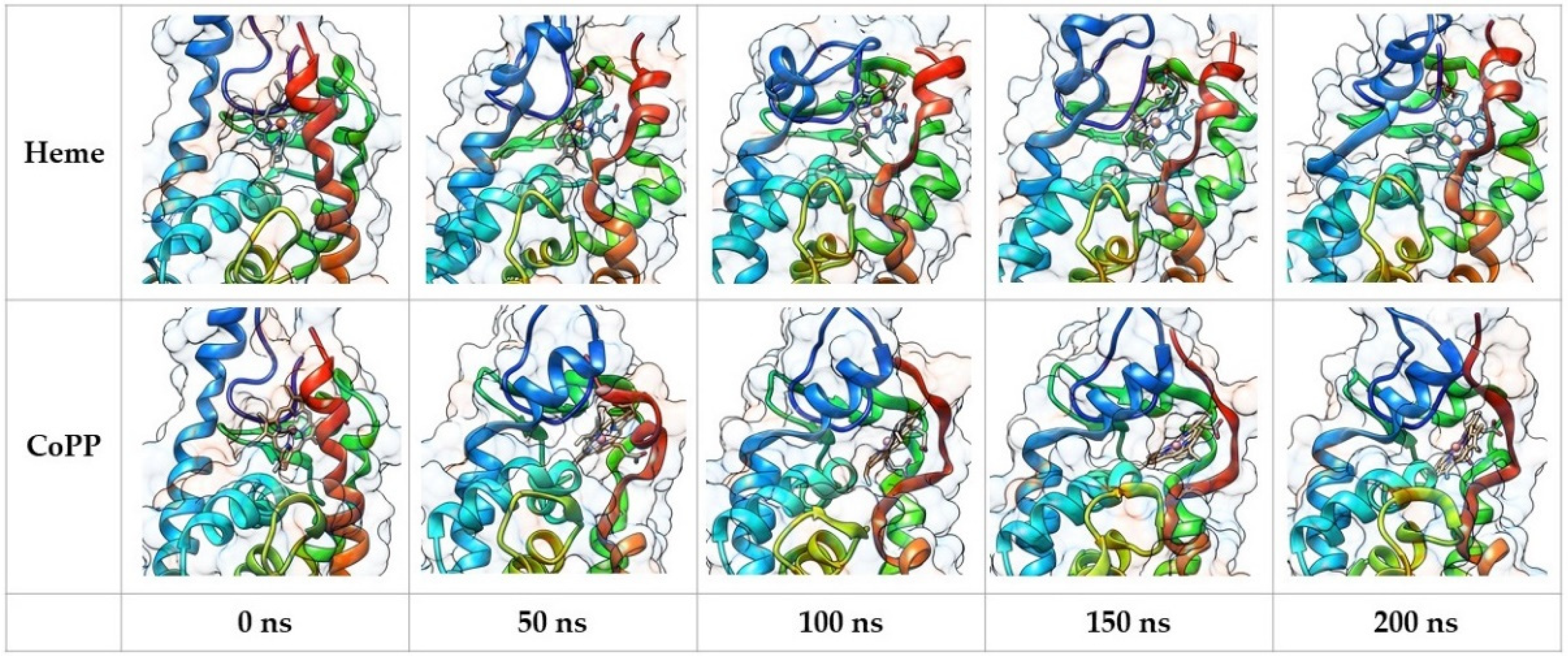
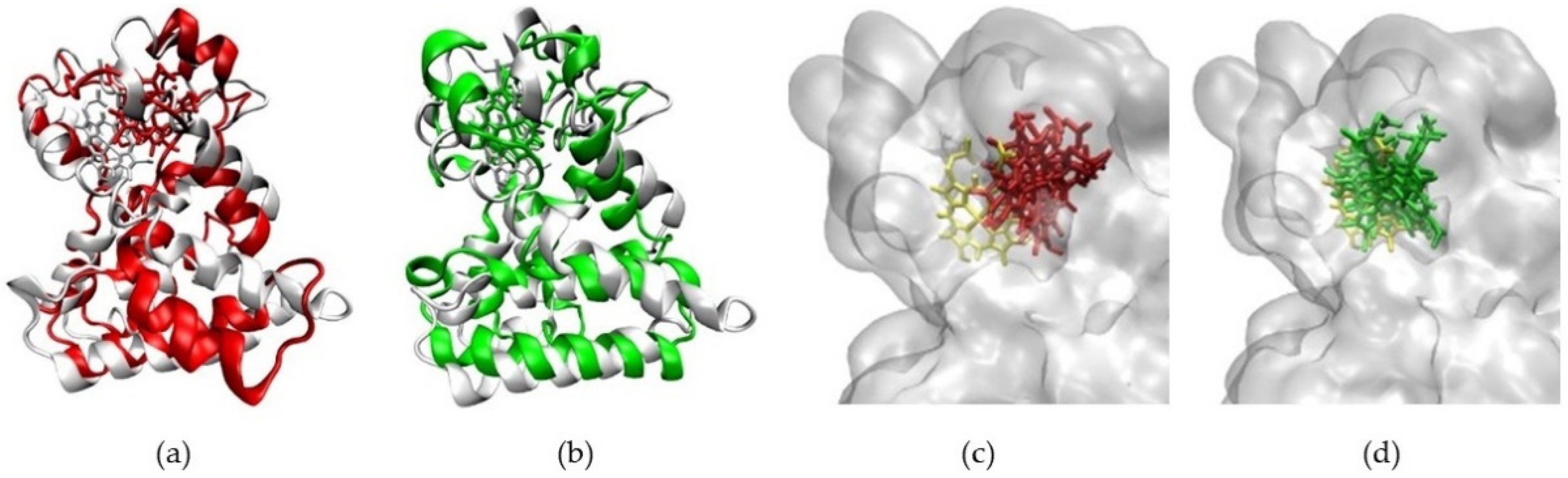
2.3. Structural Analysis from Molecular Dynamics Simulation
2.4. Dynamic Behavior of REV-ERBβ in the Antagonist Form
2.5. Dynamic Behavior of REV-ERBβ in the Agonist Form
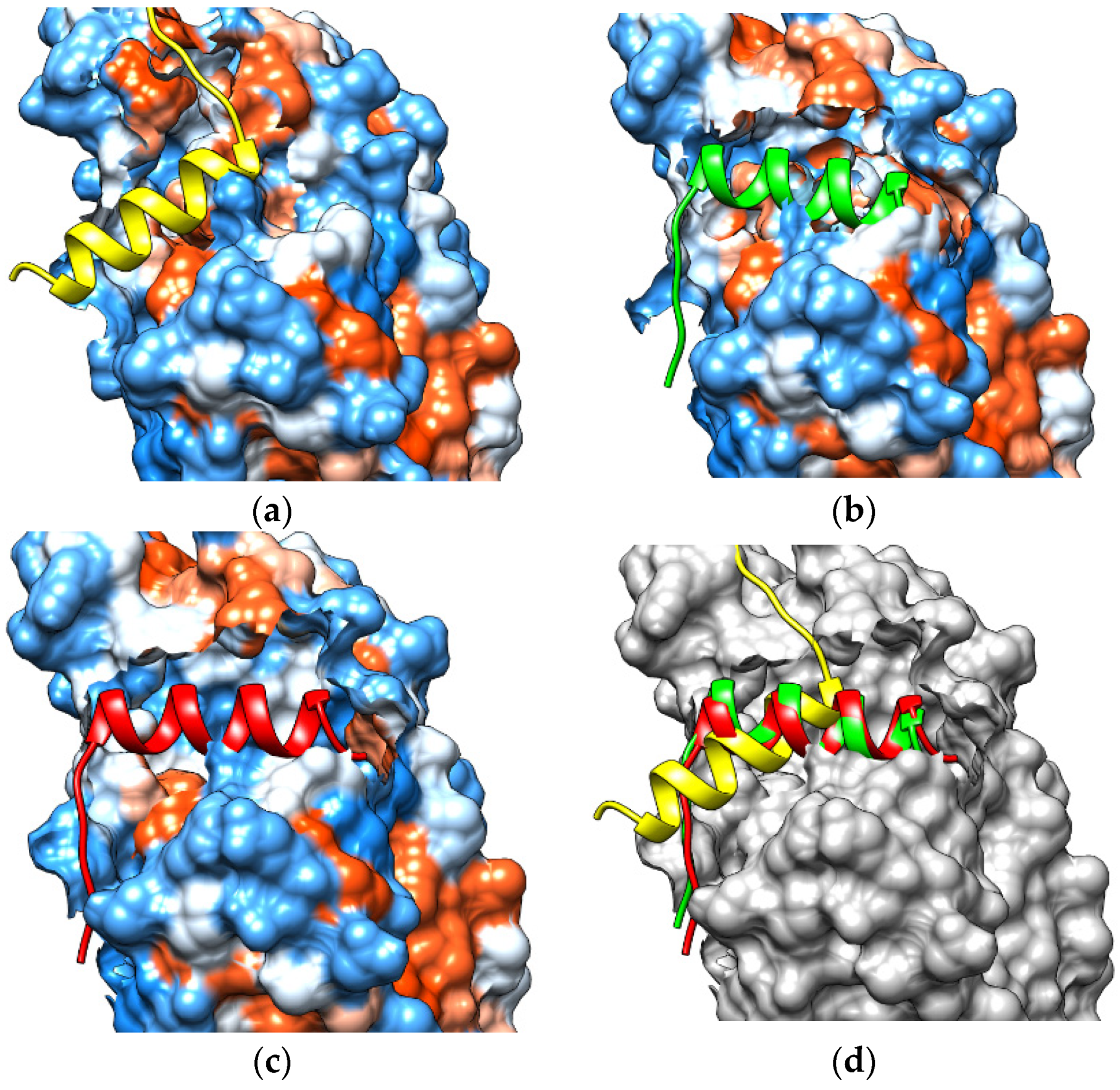
2.6. Recruitment of NCoR by Protoporphyrin IX
3. Discussion
4. Materials and Methods
4.1. Macromolecule Preparation
4.2. Ligand Preparation
4.3. Molecular Docking Study
4.4. Molecular Dynamics Simulation
4.5. Protein-Peptide Docking
4.6. MM/PBSA Calculation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malatesti, N.; Munitic, I.; Jurak, I. Porphyrin-based cationic amphiphilic photosensitisers as potential anticancer, antimicrobial and immunosuppressive agents. Biophys. Rev. 2017, 9, 149–168. [Google Scholar] [CrossRef] [Green Version]
- Cai, W. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 29, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.W.-B. Porphyrin-Based Agents and Their Applications in Cancer Imaging and Therapy. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2013. Available online: http://hdl.handle.net/1807/35884 (accessed on 3 April 2021).
- Sawicki, K.T.; Chang, H.C.; Ardehali, H. Role of heme in cardiovascular physiology and disease. J. Am. Heart Assoc. 2015, 4, e001138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, F.J.; Gelboin, H.V. Human cytochromes p450: Evolution and cDNA-directed expression. Environ. Health Perspect. 1992, 98, 81–85. [Google Scholar] [CrossRef]
- Danielson, P.B. The Cytochrome P450 Superfamily: Biochemistry, Evolution and Drug Metabolism in Humans. Curr. Drug Metab. 2005, 3, 561–597. [Google Scholar] [CrossRef]
- Correia, M.A.; Sinclair, P.R.; De Matteis, F. Cytochrome P450 Regulation: The Interplay Between Its Heme and Apoprotein Moieties in Synthesis, Assembly, Repair, and Disposal. Drug. Metab. Rev. 2011, 43, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as diagnostic and therapeutic agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Azhar Khan, M.; Tariq, M. Emerging applications of porphyrins and metalloporphyrins in biomedicine and diagnostic magnetic resonance imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.D.; De Silva, J.A.E.; Fonseca, S.M.; Arranja, C.T.; Urbano, A.M.; Sobral, A.J.F.N. Photophysical Characterization and in Vitro Phototoxicity Evaluation of 5,10,15,20-Tetra(quinolin-2-yl)porphyrin as a Potential Sensitizer for Photodynamic Therapy. Molecules 2016, 21, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Liu, T.W.B.; Chen, J.; Green, D.; Jaffray, D.; Wilson, B.C.; Wang, F.; Zheng, G. Transforming a Targeted Porphyrin Theranostic Agent into a PET Imaging Probe for Cancer. Theranostics 2012, 1, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, B.F.O.; Pereira, N.A.M.; Valente, A.J.M.; Pinho e Melo, T.M.; Pineiro, M. A review on (hydro)porphyrin-loaded polymer micelles: Interesting and valuable platforms for enhanced cancer nanotheranostics. Pharmaceutics 2019, 11, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kojetin, D.; Burris, T.P. Anti-proliferative actions of a synthetic REV-ERBα/β agonist in breast cancer cells. Biochem. Pharmacol. 2015, 96, 315–322. [Google Scholar] [CrossRef]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Yin, H.; Li, W.; Lee, J.; Yechoor, V.K.; Ma, K. The Nuclear Receptor and Clock Repressor Rev-erbα Suppresses Myogenesis. Sci. Rep. 2019, 9, 4585. [Google Scholar] [CrossRef] [Green Version]
- Burris, T.P. Nuclear hormone receptors for heme: REV-ERBα and REV-ERBβ are ligand-regulated components of the mammalian clock. Mol. Endocrinol. 2008, 22, 1509–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.M.; Guo, C.; Zhang, J. Nuclear receptor corepressor complexes in cancer: Mechanism, function and regulation. Am. J. Clin. Exp. Urol. 2014, 2, 169–187. [Google Scholar]
- Kojetin, D.; Wang, Y.; Kamenecka, T.M.; Burris, T.P. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem. Biol. 2011, 6, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Pardee, K.I.; Xu, X.; Reinking, J.; Schuetz, A.; Dong, A.; Liu, S.; Zhang, R.; Tiefenbach, J.; Lajoie, G.; Plotnikov, A.N.; et al. The structural basis of gas-responsive transcription by the human nuclear hormone receptor REV-ERBβ. PLoS Biol. 2009, 7, e1000043. [Google Scholar] [CrossRef]
- Matta-Camacho, E.; Banerjee, S.; Hughes, T.S.; Solt, L.A.; Wang, Y.; Burris, T.P.; Kojetin, D.J. Structure of REV-ERBβ ligand-binding domain bound to a porphyrin antagonist. J. Biol. Chem. 2014, 289, 20054–20066. [Google Scholar] [CrossRef] [Green Version]
- Marden, M.C.; Kiger, L.; Poyart, C.; Rashid, A.K.; Kister, J.; Stetzkowski-Marden, F.; Caron, G.; Haque, M.; Moens, L. Modulation of the oxygen affinity of cobalt-porphyrin by globin. FEBS Lett. 2000, 472, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Puttaiah, B.; Veerapandian, V.; Kumar, S.U. Crystal structures of unsymmetrically mixed β-pyrrole substituted nickel(II)-meso-tetraphenylporphyrins. J. Chem. Sci. 2016, 128, 1047–1055. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Li, S.C.; Wang, L.; Zhu, D. Protein-protein binding site identification by enumerating the configurations. BMC Bioinform. 2012, 13, 158. [Google Scholar] [CrossRef] [Green Version]
- Frego, L. Conformational changes of the glucocorticoid receptor ligand binding domain induced by ligand and cofactor binding, and the location of cofactor binding sites determined by hydrogen/deuterium exchange mass spectrometry. Protein Sci. 2006, 15, 722–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgson, M.C.; Shen, H.C.; Hollenberg, A.N.; Balk, S.P. Structural basis for nuclear receptor corepressor recruitment by antagonist-liganded androgen receptor. Mol. Cancer Ther. 2008, 7, 3187–3194. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wickramasinghe, S.R.; Qian, X. Ion-specificity in protein binding and recovery for the responsive hydrophobic poly(vinylcaprolactam) ligand. RSC Adv. 2017, 7, 36351–36360. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.M.M.; Raymundo, M.A.V.; Chen, W.; Chang, C.E.A. Mechanism of the Association Pathways for a Pair of Fast and Slow Binding Ligands of HIV-1 Protease. Biochemistry 2017, 56, 1311–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Fusani, L.; Palmer, D.S.; Somers, D.O.; Wall, I.D. Exploring Ligand Stability in Protein Crystal Structures Using Binding Pose Metadynamics. J. Chem. Inf. Model. 2020, 60, 1528–1539. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Ems, T.; Lucia, K.S.; Huecker, M.R. Biochemistry, iron absorption. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Nicolai, A.; Li, M.; Kim, D.H.; Peterson, S.J.; Vanella, L.; Positano, V.; Gastaldelli, A.; Rezzani, R.; Rodella, L.F.; Drummond, G.; et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 2009, 53, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Vaissière, A.; Berger, S.; Harrus, D.; Dacquet, C.; Le Maire, A.; Boutin, J.A.; Ferry, G.; Royer, C.A. Molecular mechanisms of transcriptional control by Rev-erbα: An energetic foundation for reconciling structure and binding with biological function. Protein Sci. 2015, 24, 1129–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Eheim, A.L.; Klein, S.; Uschner, F.E.; Smith, A.C.; Brandon-Warner, E.; Ghosh, S.; Bonkovsky, H.L.; Trebicka, J.; Schrum, L.W. Novel role of nuclear receptor rev-erbα in hepatic stellate cell activation: Potential therapeutic target for liver injury. Hepatology 2014, 59, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Lengalova, A.; Martínek, V.; Martínková, M. Heme: Emergent roles of heme in signal transduction, functional regulation and as catalytic centres. Chem. Soc. Rev. 2019, 48, 5624–5657. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.; Morris, G.M.; Forli, S. Using AutoDock 4 and AutoDock Vina with AutoDockTools: A Tutorial; The Scripps Research Institute Molecular Graphics Laboratory: La Jolla, CA, USA, 2012. [Google Scholar]
- Kadioglu, O.; Saeed, M.E.M.; Valoti, M.; Frosini, M.; Sgaragli, G.; Efferth, T. Interactions of human P-glycoprotein transport substrates and inhibitors at the drug binding domain: Functional and molecular docking analyses. Biochem. Pharmacol. 2016, 104, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D. Discovery Studio Modeling Environment, Release 2017; DassaultSystèmes: San Diego, CA, USA, 2016; Available online: http//accelrys.com/products/collaborative-science/biovia-discoverystudio/visualizationdownload.php (accessed on 5 April 2021).
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Makarewicz, T.; Kaźmierkiewicz, R. Molecular dynamics simulation by GROMACS using GUI plugin for PyMOL. J. Chem. Inf. Model. 2013, 53, 1229–1234. [Google Scholar] [CrossRef]
- Aragones, J.L.; Noya, E.G.; Valeriani, C.; Vega, C. Free energy calculations for molecular solids using GROMACS. J. Chem. Phys. 2013, 139, 1–14. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; Van Der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Prall, M. VMD: A graphical tool for the modern chemists. J. Comput. Chem. 2001, 22, 132–134. [Google Scholar] [CrossRef]
- Petrov, D.; Zagrovic, B. Are Current Atomistic Force Fields Accurate Enough to Study Proteins in Crowded Environments? PLoS Comput. Biol. 2014, 10, 1–11. [Google Scholar] [CrossRef]
- Serafeim, A.P.; Salamanos, G.; Patapati, K.K.; Glykos, N.M. Sensitivity of Folding Molecular Dynamics Simulations to even Minor Force Field Changes. J. Chem. Inf. Model. 2016, 56, 2035–2041. [Google Scholar] [CrossRef]
- Sousa Da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, A.; Collier, T.; Birch, H.L.; de Leeuw, N.H. ForceGen: Atomic covalent bond value derivation for Gromacs. J. Mol. Model. 2018, 24, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gao, X.; Fang, J. Multiple Staggered Mesh Ewald: Boosting the Accuracy of the Smooth Particle Mesh Ewald Method. J. Chem. Theory Comput. 2016, 12, 5596–5608. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.R.; Sørensen, J.; Hensley, N.; Wong, C.; Zhu, C.; Perison, T.; Amaro, R.E. POVME 3.0: Software for Mapping Binding Pocket Flexibility. J. Chem. Theory Comput. 2017, 13, 4584–4592. [Google Scholar] [CrossRef]
- Phelan, C.A.; Gampe, R.T.; Lambert, M.H.; Parks, D.J.; Montana, V.; Bynum, J.; Broderick, T.M.; Hu, X.; Williams, S.P.; Nolte, R.T.; et al. Structure of Rev-erbα bound to N-CoR reveals a unique mechanism of nuclear receptor-co-repressor interaction. Nat. Struct. Mol. Biol. 2010, 17, 808–814. [Google Scholar] [CrossRef] [Green Version]
- Fakih, T.M. Dermaseptin-Based Antiviral Peptides to Prevent COVID-19 through In Silico Molecular Docking Studies against SARS-CoV-2 Spike Protein. Pharm. Sci. Res. 2020, 7, 65–70. [Google Scholar] [CrossRef]
- Aruleba, R.T.; Adekiya, T.A.; Oyinloye, B.E.; Kappo, A.P. Structural Studies of Predicted Ligand Binding Sites and Molecular Docking Analysis of Slc2a4 as a Therapeutic Target for the Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 386. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, S.K.; Chouhan, U. Computational Approaches for the Prediction of Antimicrobial Potential Peptides from Ocimum Tenuiflorum. Asian J. Pharm. Clin. Res. 2018, 11, 398–401. [Google Scholar] [CrossRef]
- Horowitz, B.; Sharf, R.; Avital-Shacham, M.; Pechkovsky, A.; Kleinberger, T. Structure- and modeling-based identification of the adenovirus E4orf4 binding site in the protein phosphatase 2A B55α subunit. J. Biol. Chem. 2013, 288, 13718–13727. [Google Scholar] [CrossRef] [Green Version]
- Alam, N.; Goldstein, O.; Xia, B.; Porter, K.A.; Kozakov, D.; Schueler-Furman, O. High-resolution global peptide-protein docking using fragments-based PIPER-FlexPepDock. PLoS Comput. Biol. 2017, 13, e1005905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, R.; Kumar, R.; Consortium, O.S.D.D.; Lynn, A. g_mmpbsa—A GROMACS tool for MM-PBSA and its optimization for high-throughput binding energy calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Molavi Tabrizi, A.; Goossens, S.; Mehdizadeh Rahimi, A.; Knepley, M.; Bardhan, J.P. Predicting solvation free energies and thermodynamics in polar solvents and mixtures using a solvation-layer interface condition. J. Chem. Phys. 2017, 146, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.D.; Wang, B.; Wei, G.W. Accurate, robust, and reliable calculations of Poisson–Boltzmann binding energies. J. Comput. Chem. 2017, 38, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, J.; Okimoto, N.; Morimoto, G.; Taiji, M. A new set of atomic radii for accurate estimation of solvation free energy by Poisson-Boltzmann solvent model. J. Comput. Chem. 2014, 35, 2132–2139. [Google Scholar] [CrossRef] [Green Version]

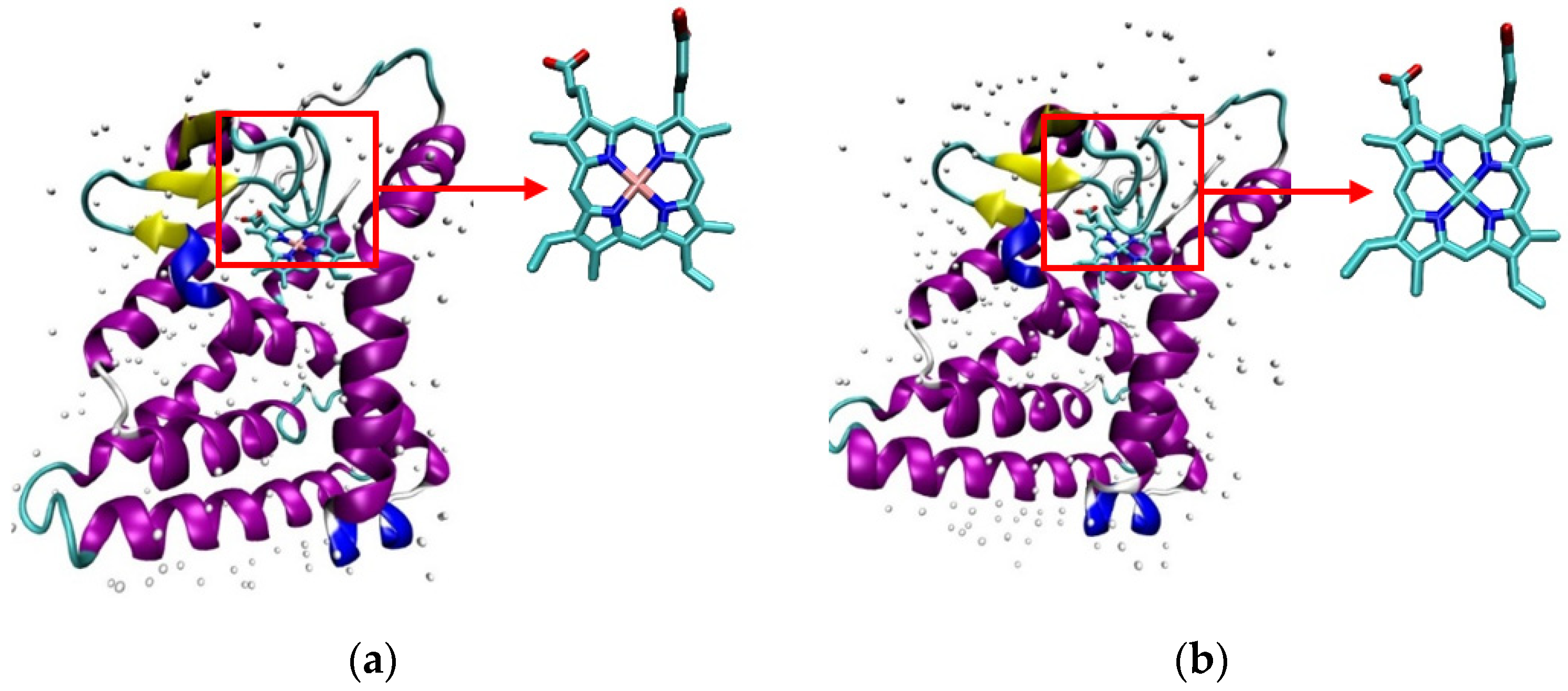
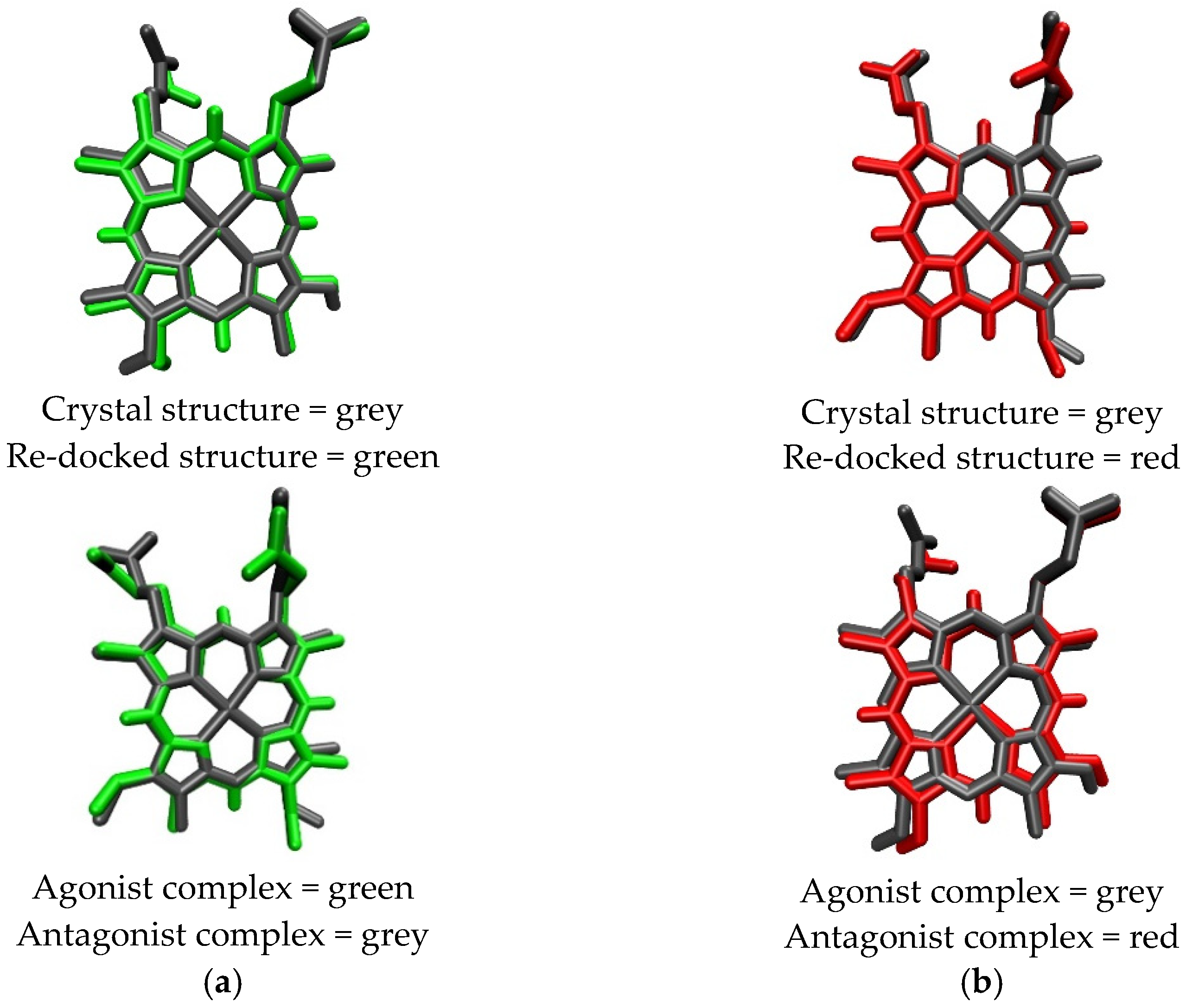
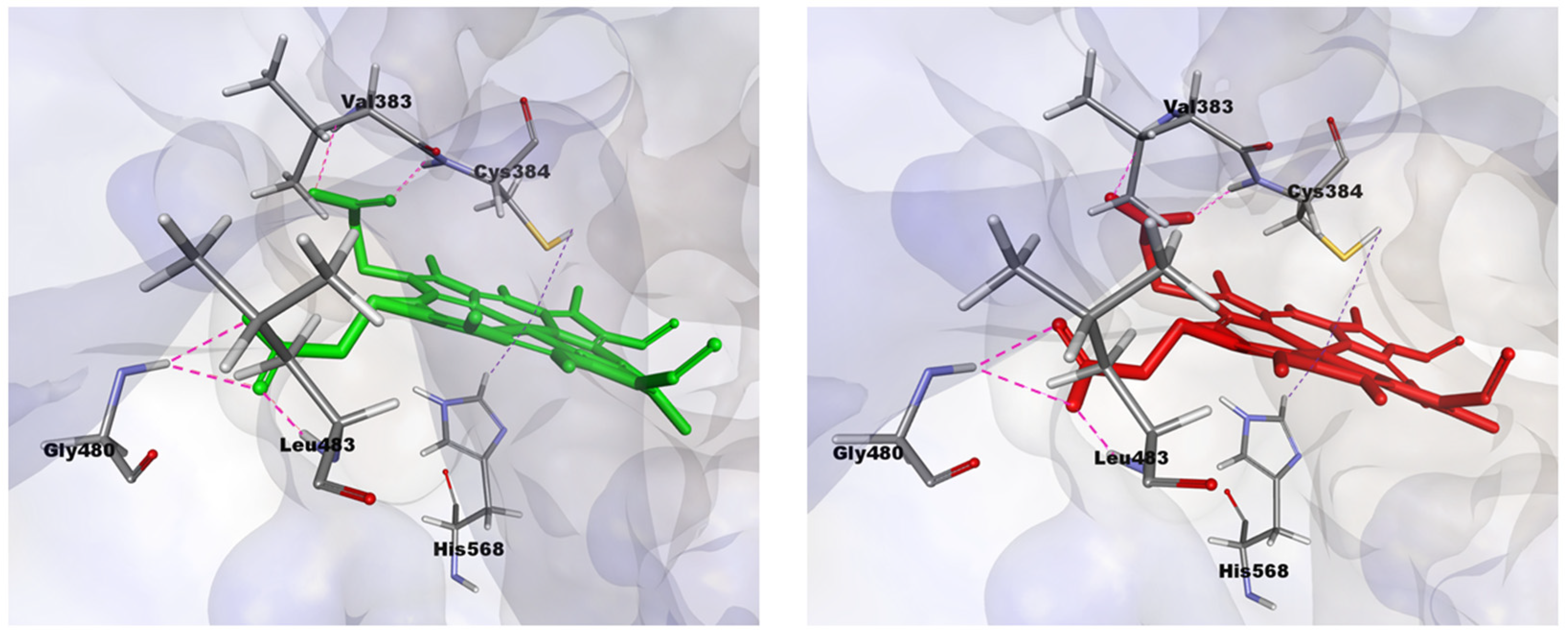
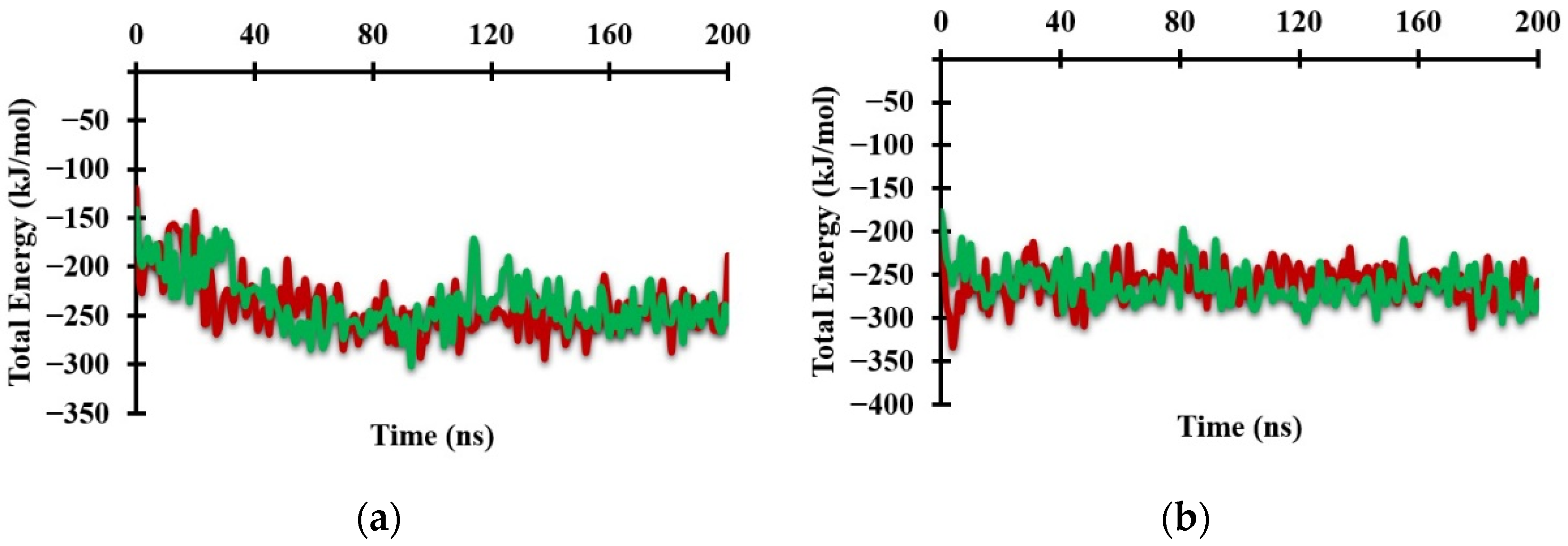



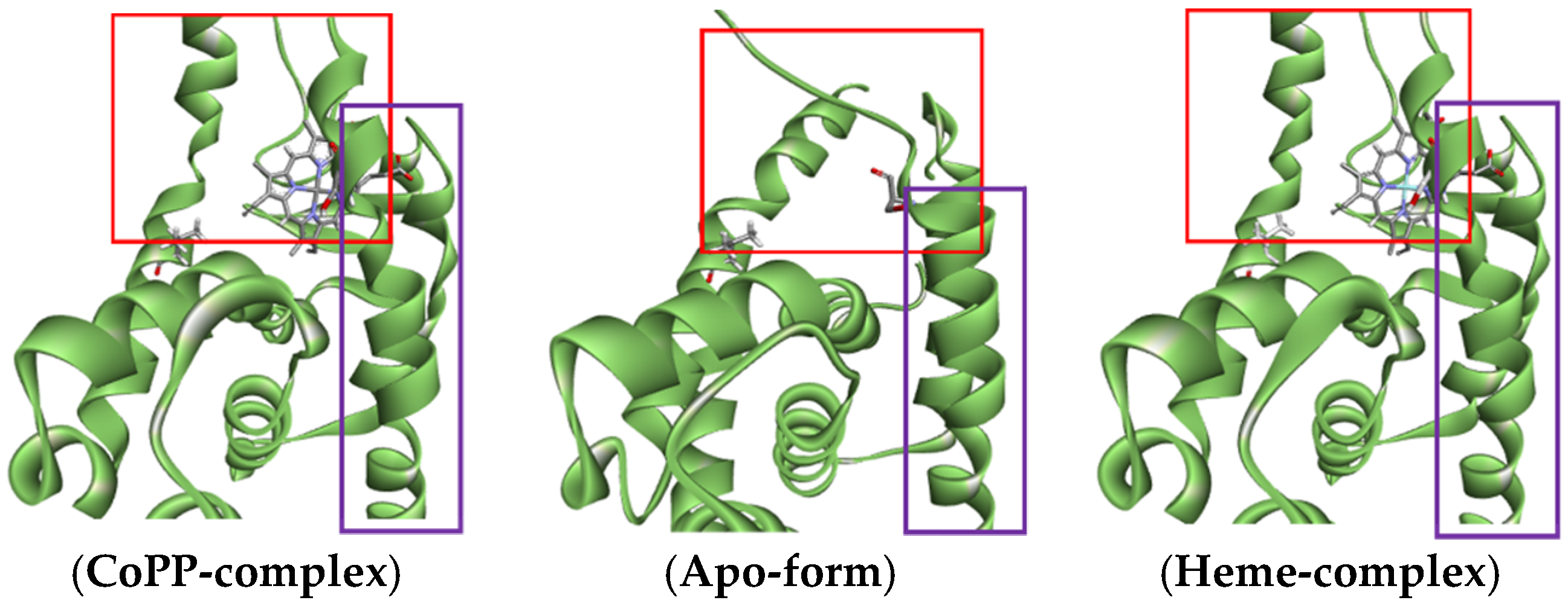
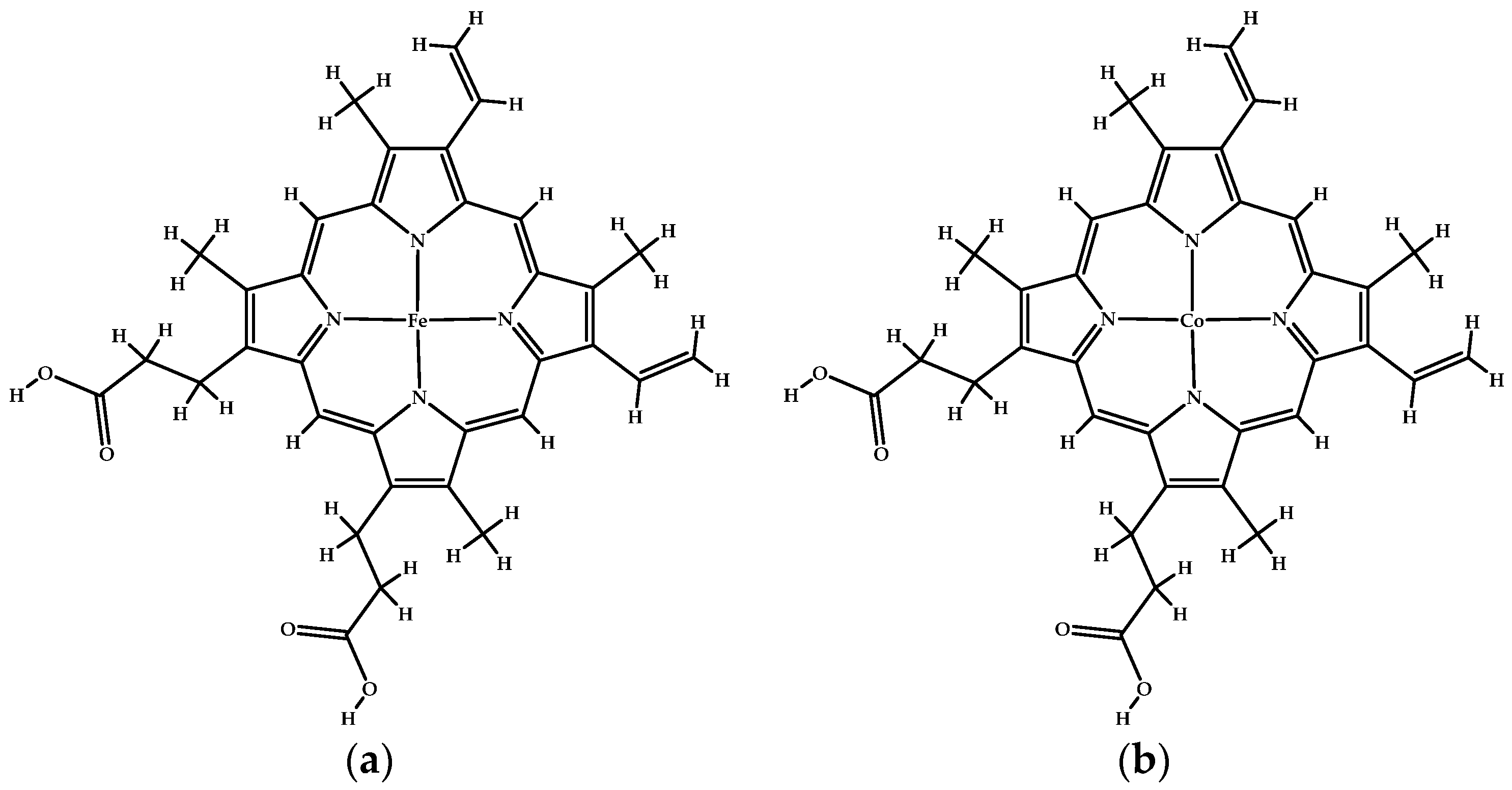
| Protoporphyrin Ligand | Re-Docking | Docking |
|---|---|---|
| Heme | RMSD = 0.3078 Å ∆G (agonist) = −71.10 kJ/mol | RMSD = 0.3703 Å ∆G (antagonist) = −65.91 kJ/mol |
| CoPP | RMSD = 0.3540 Å ∆G (antagonist) = −56.87 kJ/mol | RMSD = 0.7110 Å ∆G (agonist) = −56.95 kJ/mol |
| Complex REV-ERBβ | ∆Evdw (kJ/mol) | ∆Eele (kJ/mol) | ∆GPB (kJ/mol) | ∆GNP (kJ/mol) | ∆GBind (kJ/mol) |
|---|---|---|---|---|---|
| Heme-Agonist-REV-ERBβ | −309.61 | −208.23 | 284.17 | −28.72 | −262.38 |
| CoPP–Agonist-REV-ERBβ | −283.36 | −203.37 | 255.76 | −27.33 | −258.30 |
| Heme–Antagonist-REV-ERBβ | −300.41 | −170.95 | 261.51 | −27.24 | −237.10 |
| CoPP–Antagonist-REV-ERBβ | −307.76 | −162.81 | 257.03 | −27.26 | −240.79 |
| Amino Acid Residue | RMSD |
|---|---|
| Trp402, Val413, Lys439, Gly441, Leu482, Leu483, Ser499, Ser513, Gln529, Thr552, Arg562, His568, His578 | 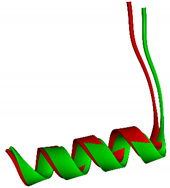 1.3757 Å Crystal structure = red Re-docked structure = green |
| Complex REV-ERBβ | Result | ||
|---|---|---|---|
| Score | ACE (kJ/mol) | ∆GBind (kJ/mol) | |
| Crystal Structure Agonist-REV-ERBβ | 11332 | −1903.89 | −400.43 |
| Heme−Agonist-REV-ERBβ | 9882 | −74.60 | −13.33 |
| CoPP−Agonist-REV-ERBβ | 10424 | 453.13 | 43.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakih, T.M.; Kurniawan, F.; Yusuf, M.; Mudasir, M.; Tjahjono, D.H. Molecular Dynamics of Cobalt Protoporphyrin Antagonism of the Cancer Suppressor REV-ERBβ. Molecules 2021, 26, 3251. https://doi.org/10.3390/molecules26113251
Fakih TM, Kurniawan F, Yusuf M, Mudasir M, Tjahjono DH. Molecular Dynamics of Cobalt Protoporphyrin Antagonism of the Cancer Suppressor REV-ERBβ. Molecules. 2021; 26(11):3251. https://doi.org/10.3390/molecules26113251
Chicago/Turabian StyleFakih, Taufik Muhammad, Fransiska Kurniawan, Muhammad Yusuf, Mudasir Mudasir, and Daryono Hadi Tjahjono. 2021. "Molecular Dynamics of Cobalt Protoporphyrin Antagonism of the Cancer Suppressor REV-ERBβ" Molecules 26, no. 11: 3251. https://doi.org/10.3390/molecules26113251
APA StyleFakih, T. M., Kurniawan, F., Yusuf, M., Mudasir, M., & Tjahjono, D. H. (2021). Molecular Dynamics of Cobalt Protoporphyrin Antagonism of the Cancer Suppressor REV-ERBβ. Molecules, 26(11), 3251. https://doi.org/10.3390/molecules26113251







