Structural and Optical Properties of Pure and Sulfur-Doped Silicate–Phosphate Glass
Abstract
1. Introduction
2. Results and Discussion
2.1. Compositional Analysis of the Glass
2.2. Density and Molar Volume
2.2.1. Density
2.2.2. Optical Basicity
2.3. Structural Studies
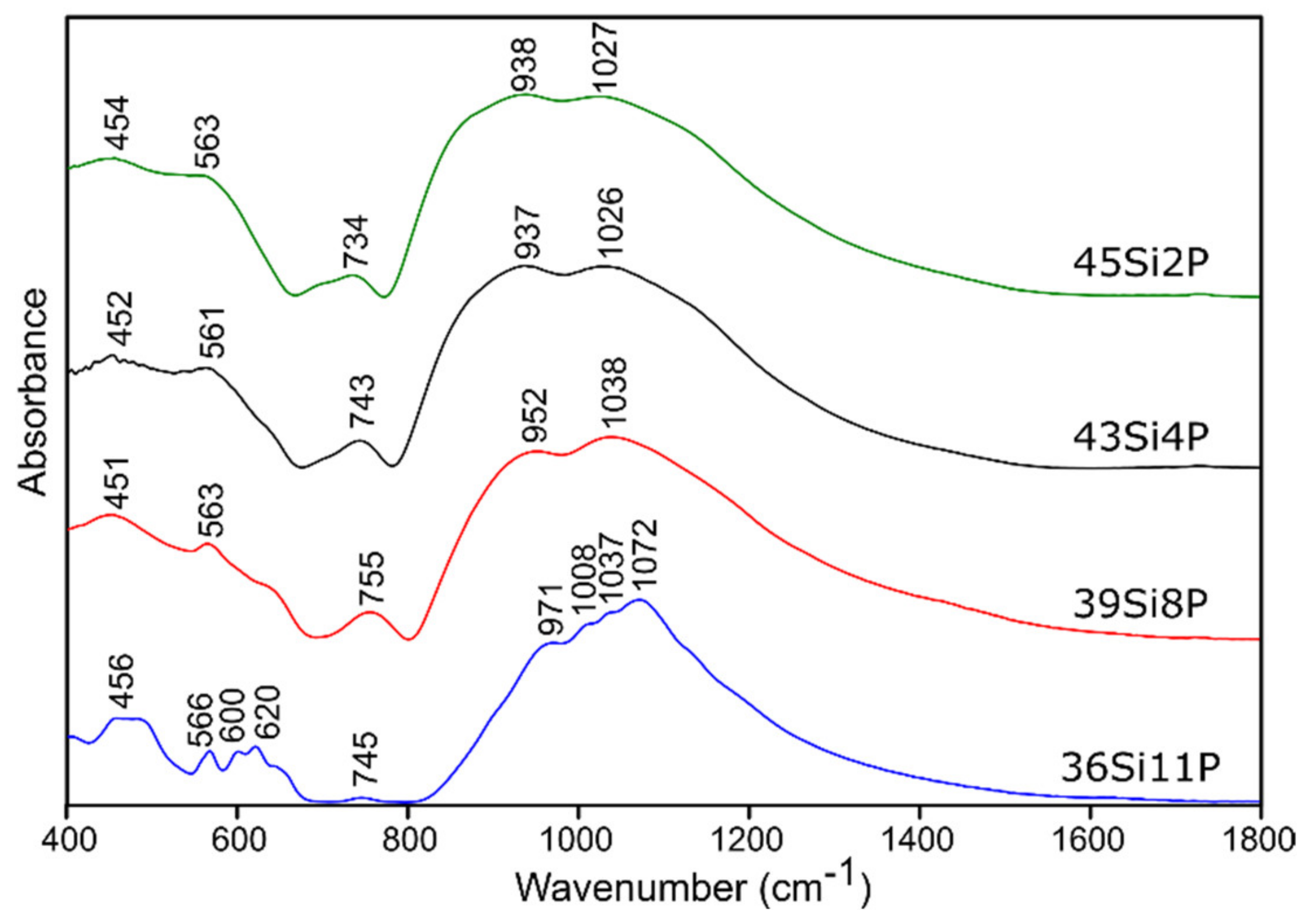
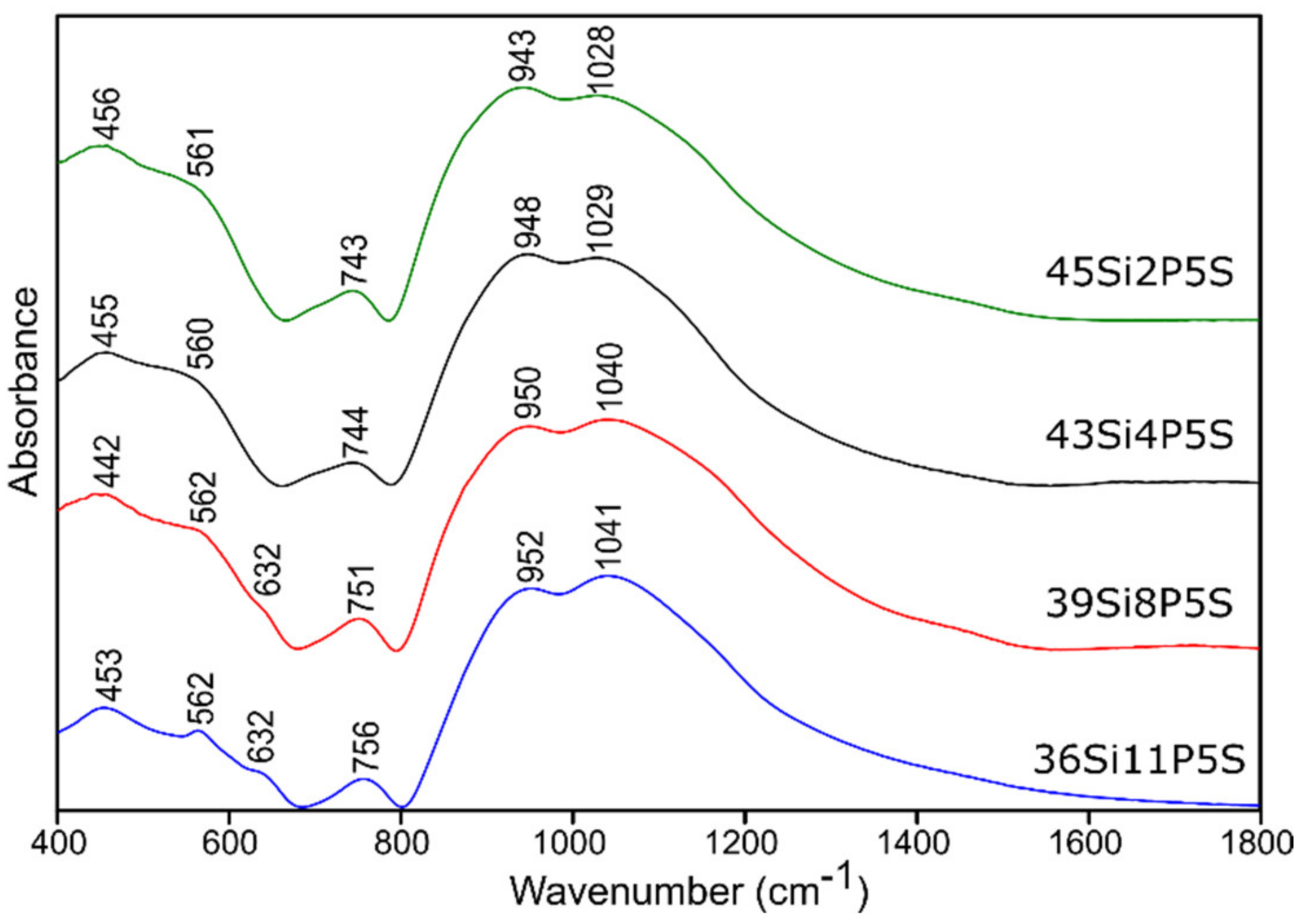
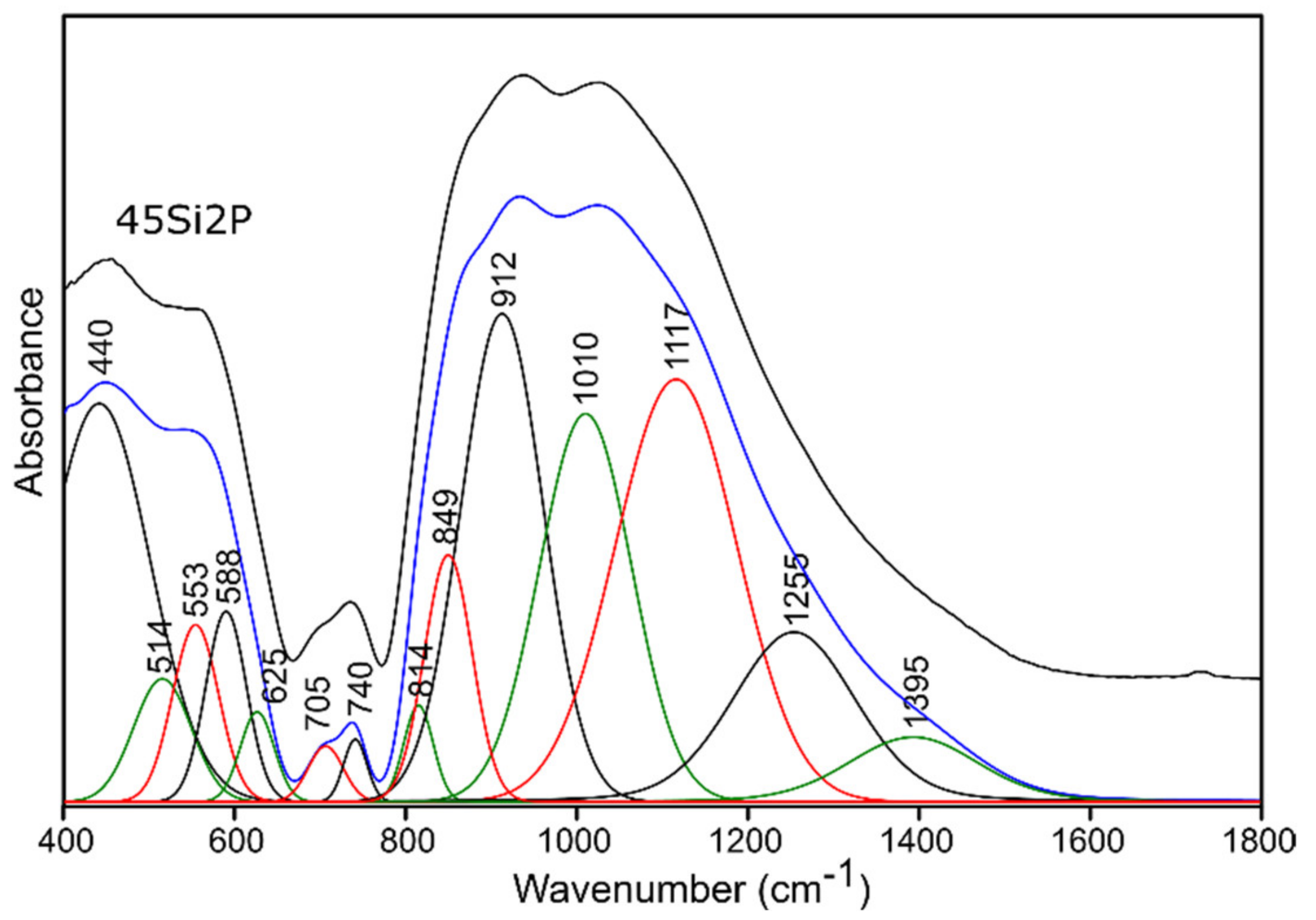
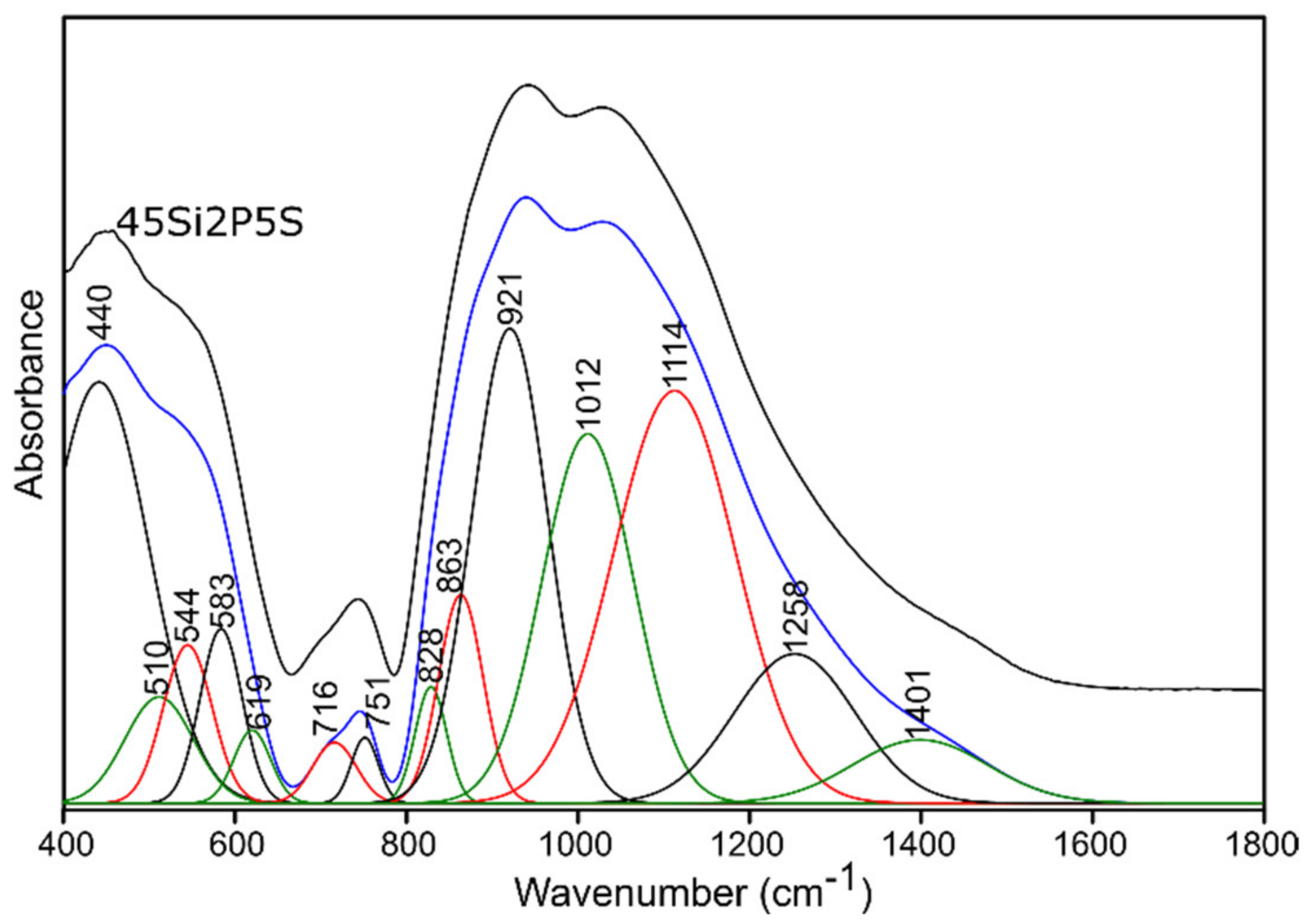
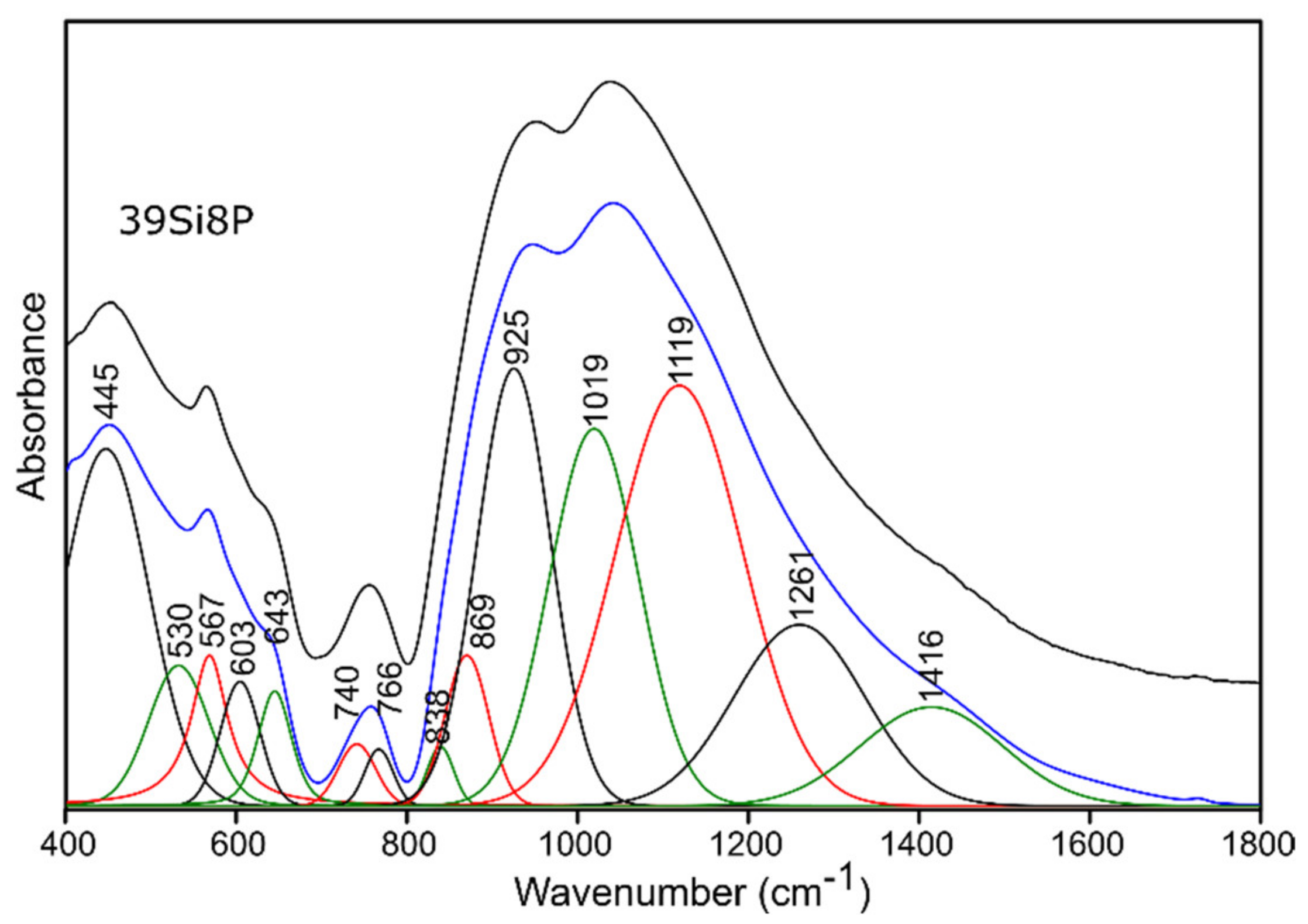
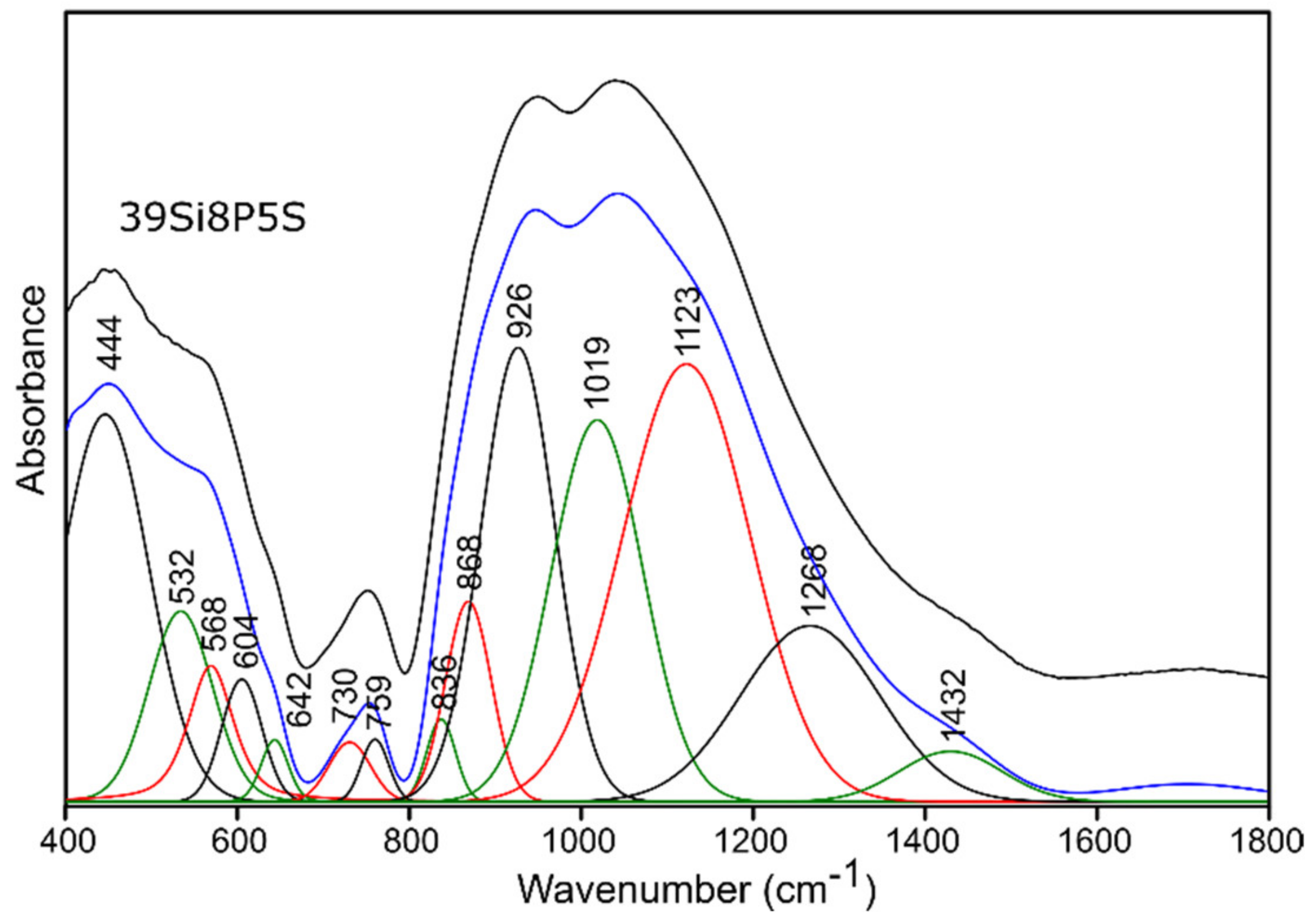
2.3.1. Infrared Spectroscopy Results
Pure Glass
Sulfur-Doped Glass
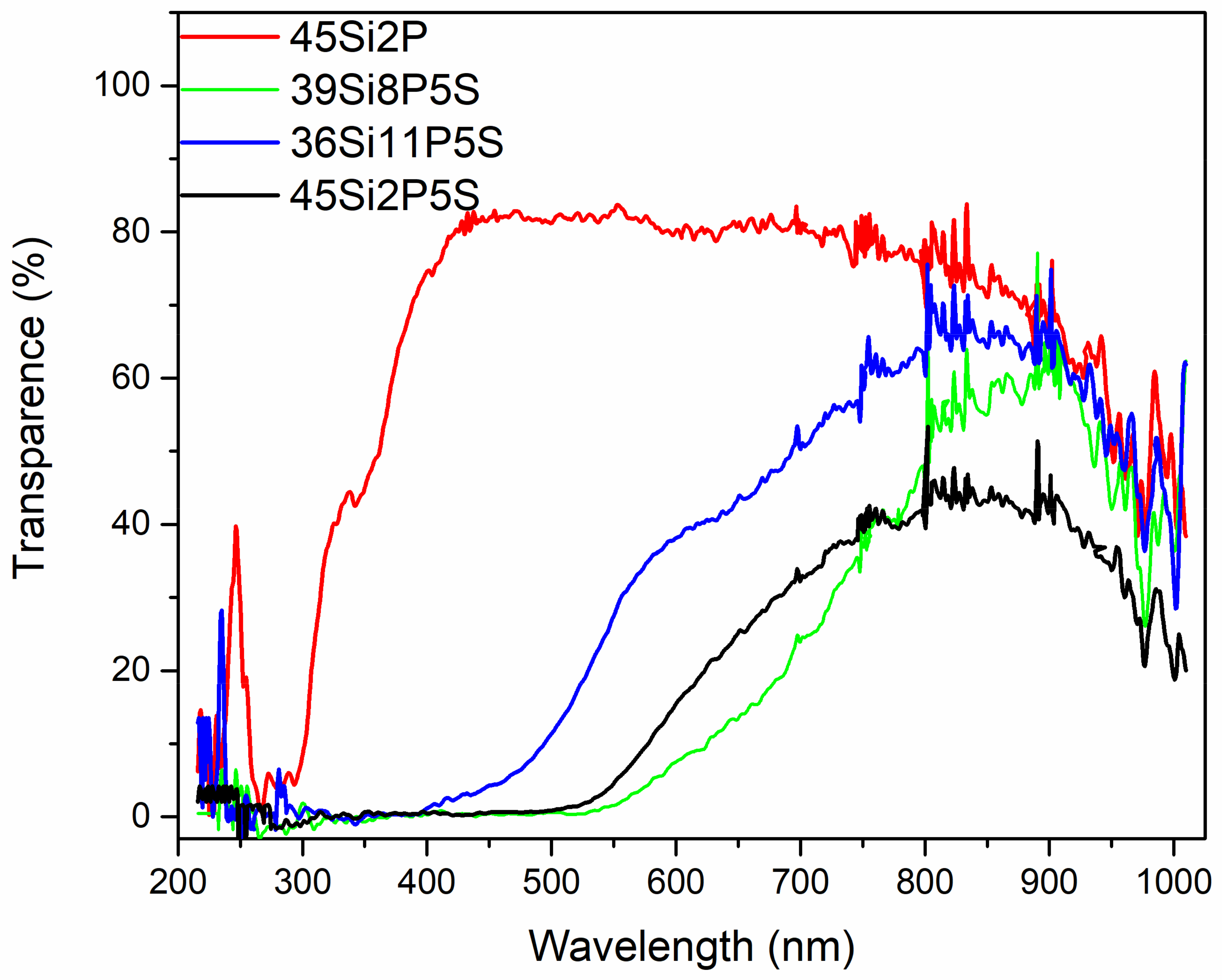
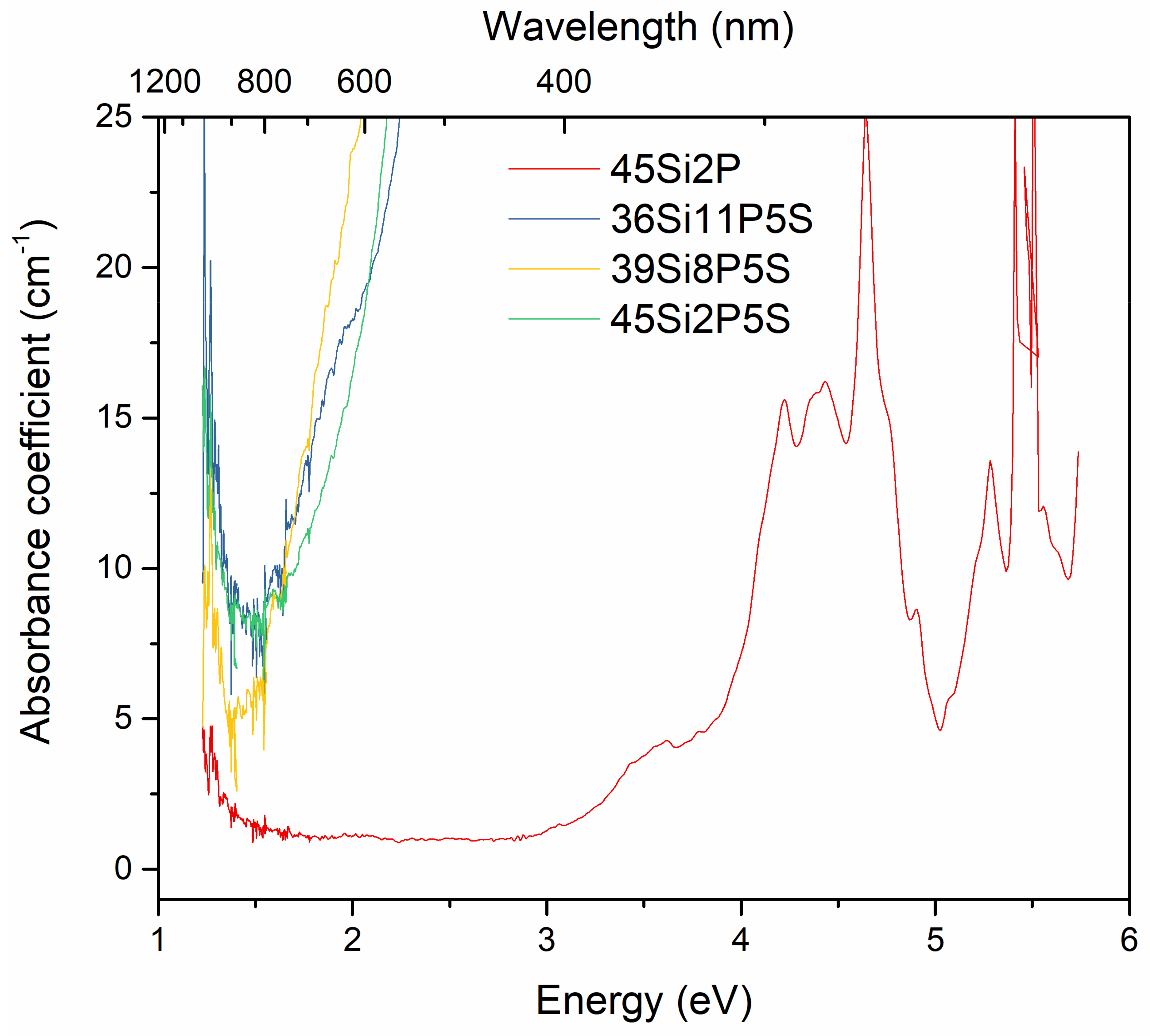
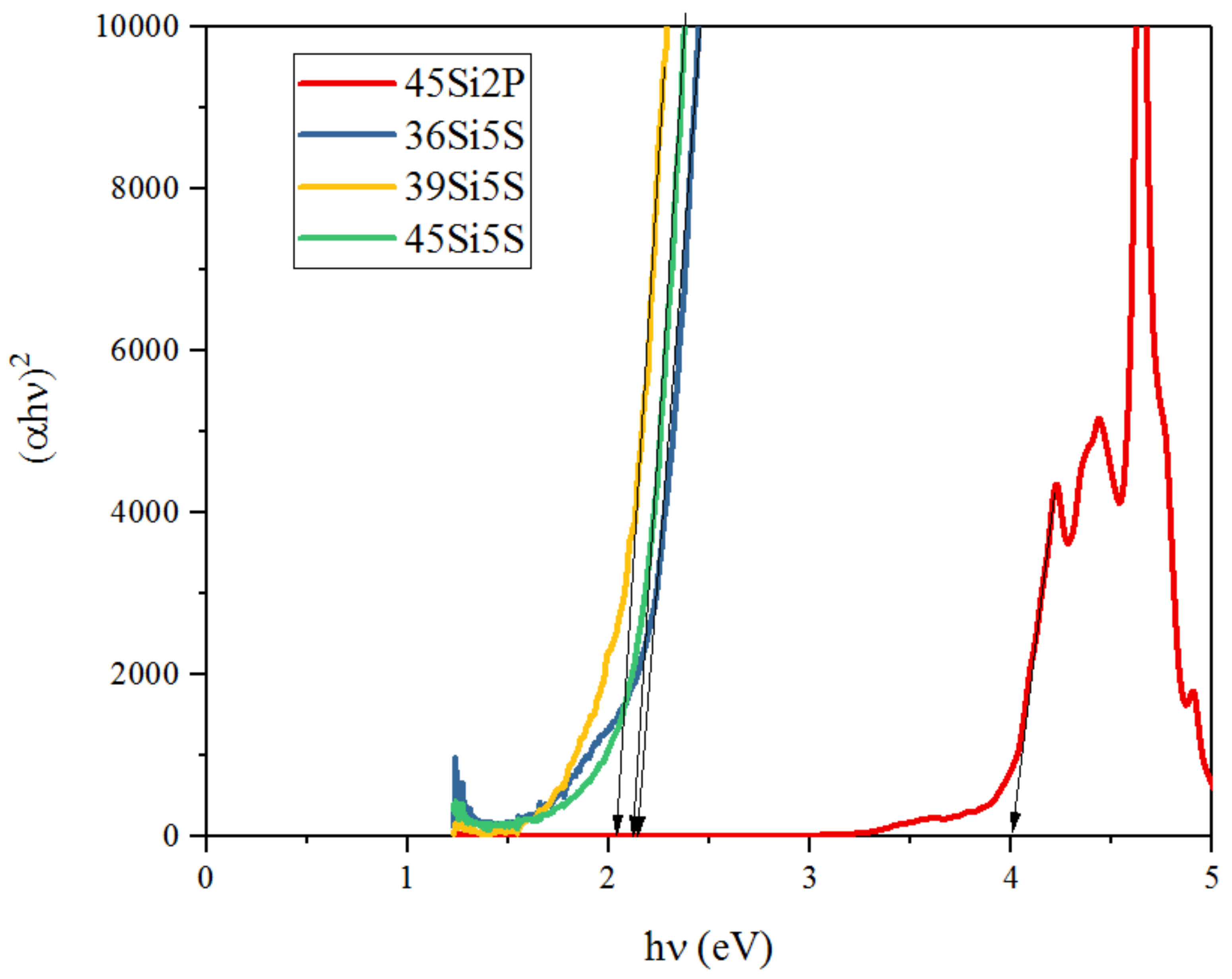
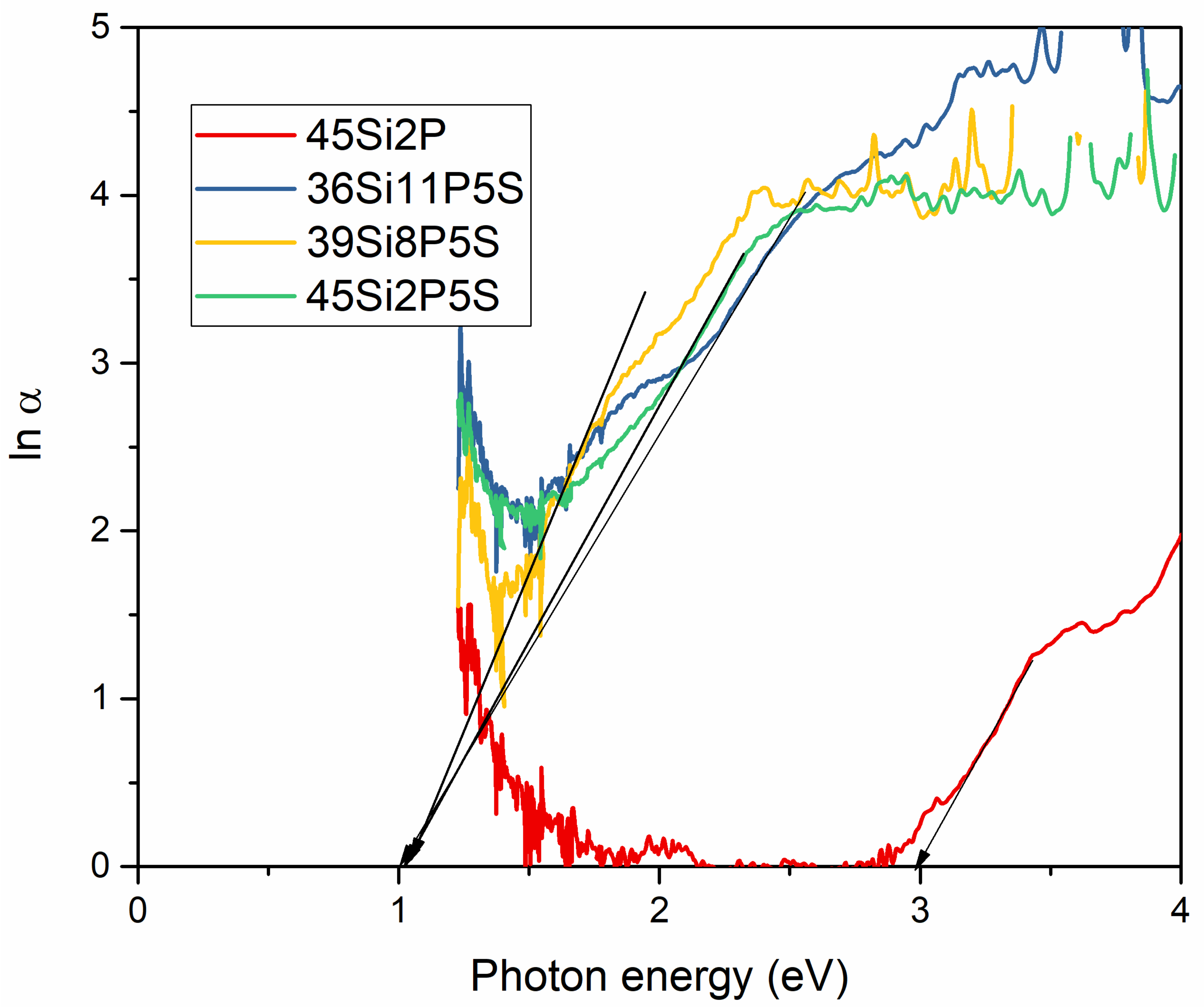
2.4. Optical Studies
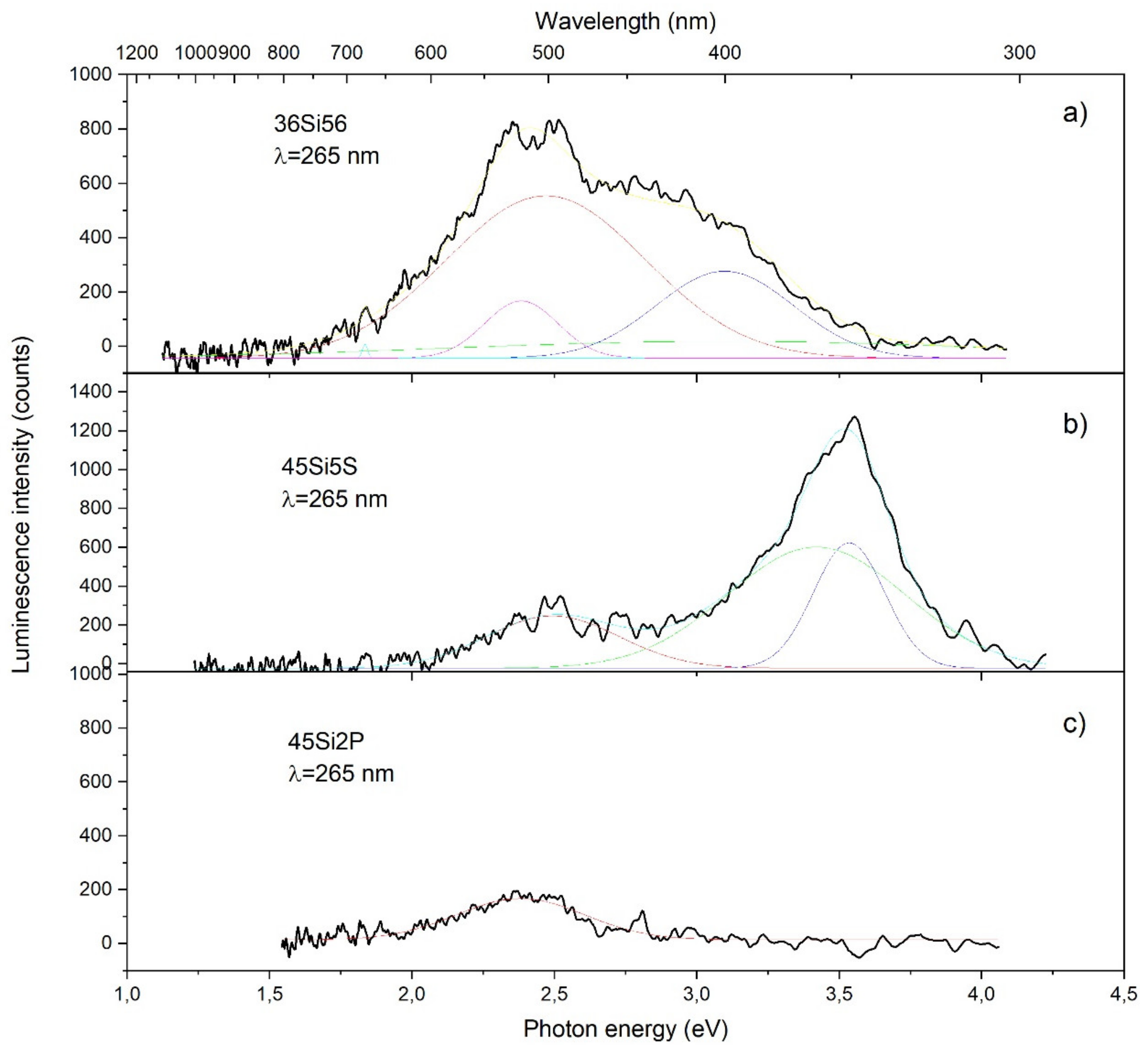
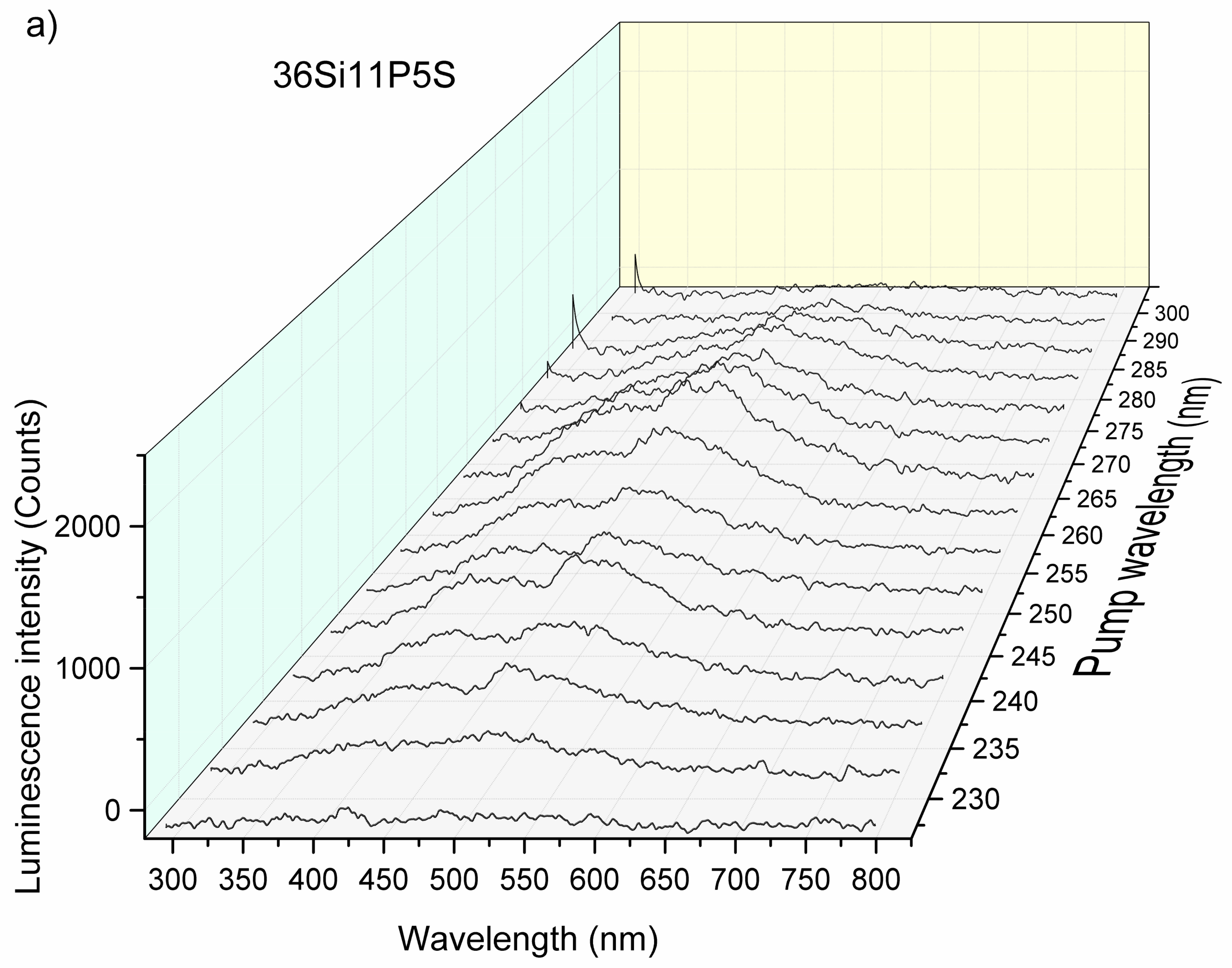
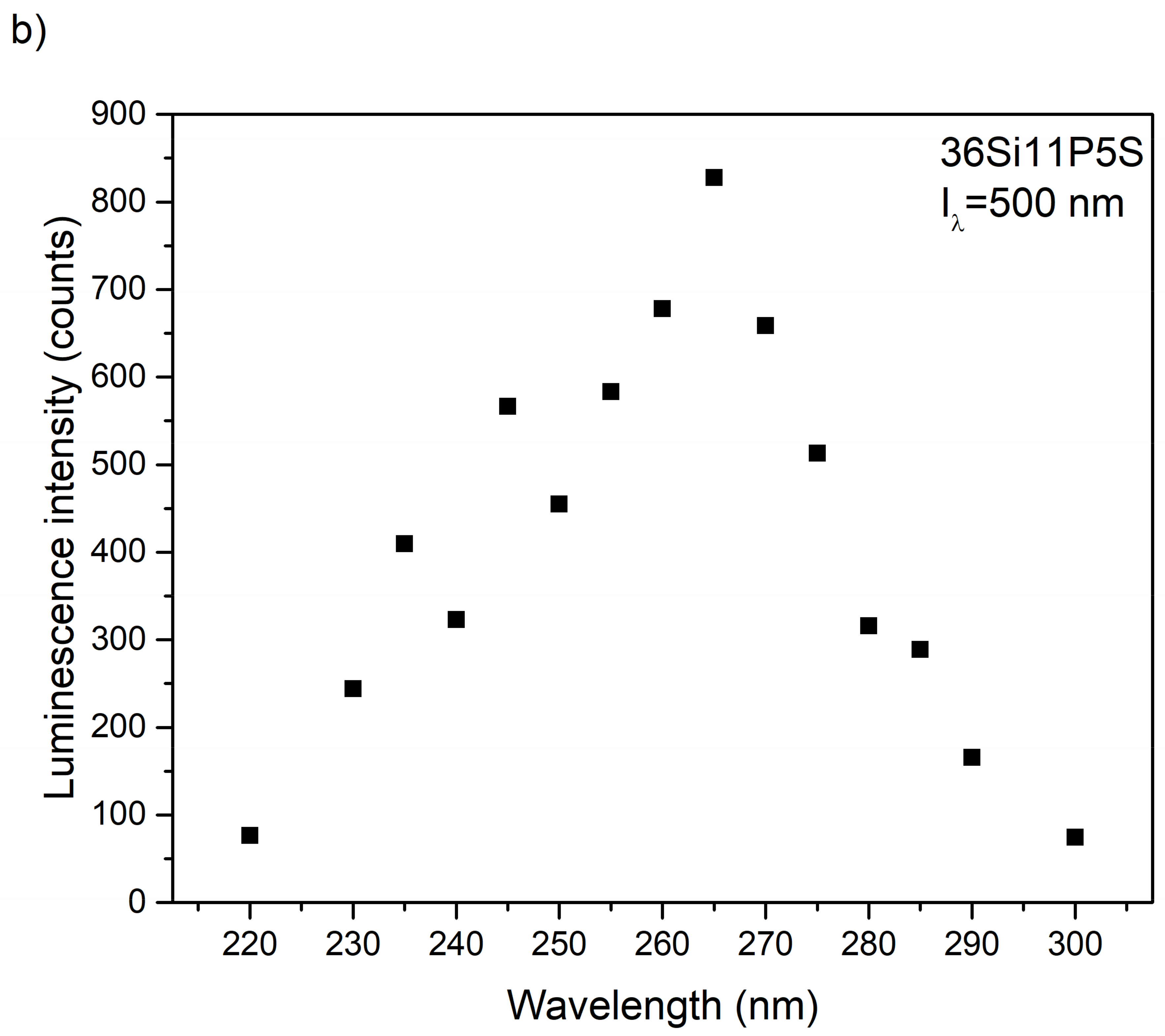
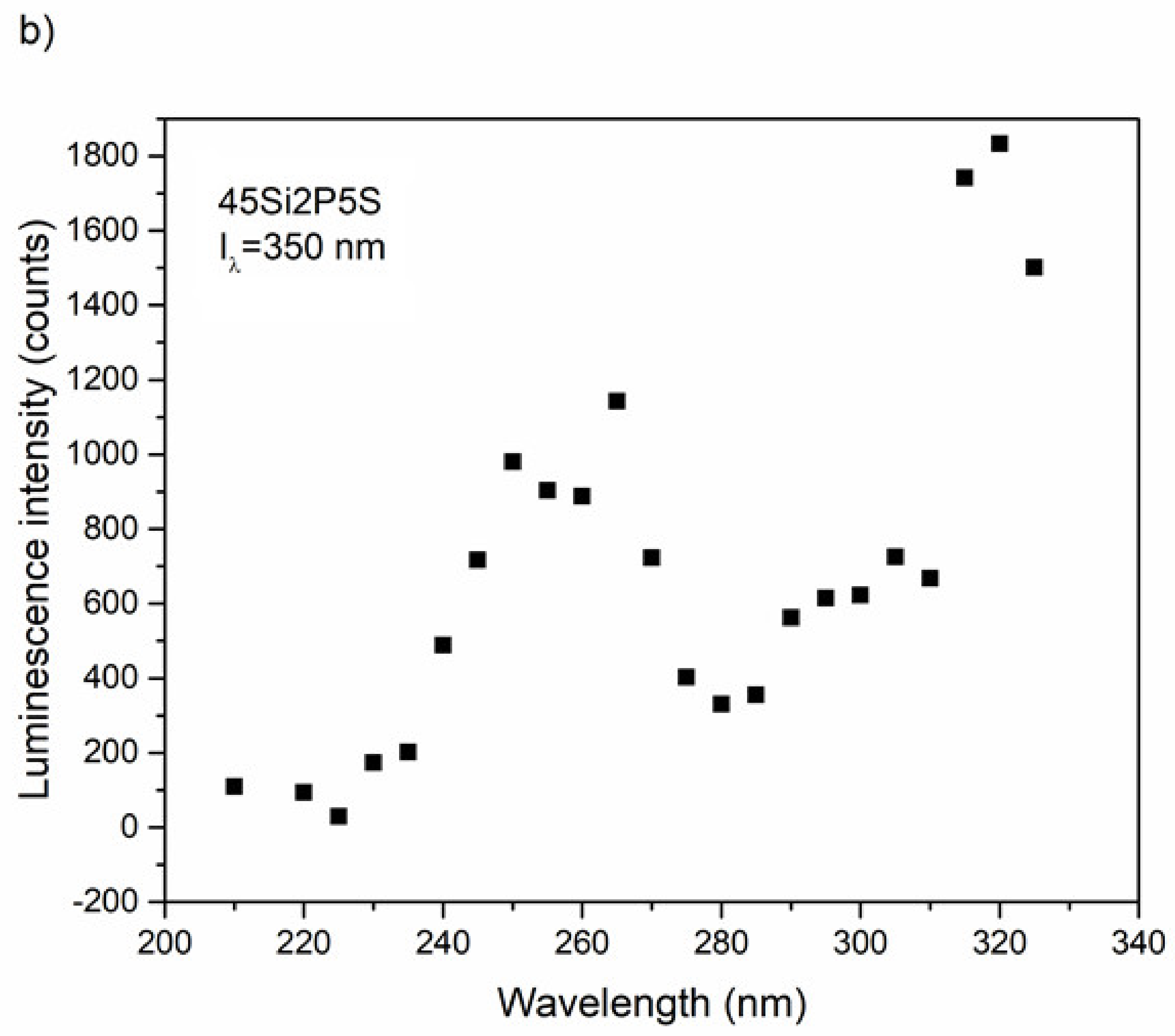
2.5. Photoluminescence Properties
3. Materials and Methods
3.1. Glass Synthesis and Compositional Analysis
3.2. Structural Characterization of the Glass
3.2.1. Density and Molar Volume
- xMO—mole fraction of the given glass component in the form of the oxide (MO);
- MMO—molar mass of the given glass component in the form of the oxide (MO);
- dr—experimentally determined true density of a given glass (g·cm−1).
3.2.2. Infrared Spectroscopy
3.3. Optical and Luminescence Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fleet, M.E. XANES spectroscopy of sulfur in earth materials. Can. Mineral. 2005, 43, 1811–1838. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry; Oxford University Press: Oxford, UK, 1986. [Google Scholar]
- Tsujimura, T.; Xue, X.; Kanzaki, M.; Walter, M.J. Sulfur speciation and network structural changes in sodium silicate glasses: Constraints from NMR and Raman spectroscopy. Geochim. Cosmochim. Acta. 2004, 68, 5081–5101. [Google Scholar] [CrossRef]
- Paris, E.; Giuli, G.; Carroll, M.R.; Davoli, I. The valence and speciation of sulfur in glasses by X-ray absorption spectroscopy. Can. Mineral. 2001, 39, 331–339. [Google Scholar] [CrossRef]
- Jugo, P.J.; Wilke, M.; Botcharnikov, R.E. Sulfur K-edge XANES analysis of natural and synthetic basaltic glasses: Implications for S speciation and S content as function of oxygen fugacity. Geochim. Cosmochim. Acta 2010, 74, 5926–5938. [Google Scholar] [CrossRef]
- McKeown, D.A.; Muller, I.S.; Gan, H.; Pegg, I.L.; Stolte, W.C. Determination of sulfur environments in borosilicate waste glasses using X-ray absorption near-edge spectroscopy. J. Non-Cryst. Solids 2004, 333, 74–84. [Google Scholar] [CrossRef]
- Hirashima, H.; Yoshida, T.; Brückner, R. Redox equilibria and constitution of polyvalent ions in oxide melts and glasses, Glastechn. Ber. Glass Sci. Technol. 1988, 68, 283–292. [Google Scholar]
- Bingham, P.A.; Connelly, A.J.; Hand, R.J.; Hyatt, N.C.; Northrup, P.A.; Alonso Mori, R.; Glatzer, P.; Kavčič, M.; Žitnik, M.; Bučar, K.; et al. A multi-spectroscopic investigation of sulfur speciation in silicate glasses and slags. Glass Technol. Eur. J. Glass Sci. Technol. A 2010, 51, 63–80. [Google Scholar]
- Angell, C.A. Sulfate and sulfate-chloride glasses. J. Am. Ceram. Soc. 1965, 48, 540. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. An Introduction to Nuclear Waste Immobilization; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Ghosh, K.; DasMohapatra, G.K.; Soodbiswas, N. Glass formation in K2SO4-CaO-P2O5 system. Phys. Chem. Glasses 2003, 44, 313–318. [Google Scholar]
- Malugani, J.P.; Mercier, R.; Fahys, B.; Robert, G. Ionic conductivity of and Raman spectroscopy investigation in binary oxosalts (1 − x)AgPO3·xAg2SO4 glasses. J. Solid State Chem. 1982, 45, 309–316. [Google Scholar] [CrossRef]
- Reibstein, S.; Da, N.; Simon, J.P.; Spiecker, E.; Wondraczek, L. Phase separation and crystal precipitation in supercooled sulphophosphate ionic melts. Phys. Chem. Glas. Eur. J. Glas. Sci. Technol. Part B 2012, 53, 61–67. [Google Scholar]
- Stefanovskii, S.V.; Aleksandrov, A.I. EPR spectra and structure of sodium sulfate borate glasses. Zhurnal Prikladnoi Spektroskopii 1991, 54, 150–154. [Google Scholar] [CrossRef]
- Wincott, P.L.; Vaughan, D.J. Spectroscopic Studies of Sulfides. Rev. Mineral. Geochem. 2006, 61, 181–229. [Google Scholar] [CrossRef]
- Lane, M.D. Mid-infrared emission spectroscopy of sulfate and sulfate-bearing minerals. Am. Mineral. 2007, 92, 1–18. [Google Scholar] [CrossRef]
- Jaroudi, O.E.; Picquenard, E.; Demortier, A.; Lelieur, J.-P.; Corset, J. Polysulfide anions II: Structure and vibrational spectra of the S42- and S52- anions. Influence of the cations on bond length, valence, and torsion angle. Inorg. Chem. 2000, 39, 2593–2603. [Google Scholar] [CrossRef]
- Santagneli, S.H.; Schneider, J.; Skripachev, I.; Ribeiro, S.J.; Messaddeq, Y. Preparation and characterization of new glassy system As2P2S8-Ga2S3. J. Phys. Chem. B 2008, 112, 4943–4947. [Google Scholar] [CrossRef]
- Kim, Y.; Saienga, J.; Martin, S.W. Glass formation in and structural investigation of Li2S+GeS2+GeO2 composition using Raman and IR spectroscopy. J. Non-Cryst. Solids 2005, 351, 3716–3724. [Google Scholar] [CrossRef]
- Bischoff, C.; Schuller, K.; Dunlap, N.; Martin, S.W. IR, Raman, and NMR Studies of the Short-Range Structures of 0.5Na2S + 0.5[xGeS2 + (1–x)PS5/2] Mixed Glass-Former Glasses. J. Phys. Chem. B 2014, 118, 1943–1953. [Google Scholar] [CrossRef]
- Le, Q.H.; Palenta, T.; Benzine, O.; Griebenow, K.; Limbach, R.; Kamitsos, E.I.; Wondraczek, L. Formation, structure and properties of fluoro-sulfo-phosphate poly-anionic glasses. J. Non-Cryst. Solids 2017, 477, 58–72. [Google Scholar] [CrossRef]
- Kmiec, S.; Joyce, A.; Martin, S.W. Glass formation and structural analysis of Na4P2S7-xOx, 0 ≤ x ≤ 7 sodium oxy-thiophosphate glasses. J. Non-Cryst. Solids 2018, 498, 177–189. [Google Scholar] [CrossRef]
- Thieme, A.; Möncke, D.; Limbach, R.; Fuhrmann, S.; Kamitsos, E.I.; Wondraczek, L. Structure and properties of alkali and silver sulfophosphate glasses. J. Non-Cryst. Solids 2015, 410, 142–150. [Google Scholar] [CrossRef]
- Goel, A.; McCloy, J.S.; Fox, K.M.; Leslie, C.J.; Riley, B.J.; Rodriguez, C.P.; Schweiger, M.J. Structural analysis of some sodium and alumina rich high-level nuclear waste glasses. J. Non-Cryst. Solids 2012, 358, 674–679. [Google Scholar] [CrossRef]
- Ehrt, D. Photoluminescence in glasses and glass ceramics. IOP Conf. Ser. Mater. Sci. Eng. 2009, 2, 012001. [Google Scholar] [CrossRef]
- Gerasimova, V.I.; Rybaltovskii, A.O.; Chernov, P.V.; Spasskii, D.A. Color Centers in Sulfur-Doped Silica Glasses: Spectroscopic Manifestations of an SO2 Interstitial Molecule. Glas. Phys. Chem. 2003, 29, 232–236. [Google Scholar] [CrossRef]
- Gerasimova, V.I.; Zavorotny, Y.S.; Rybaltovskii, A.O.; Chernov, P.V.; Sazhin, O.D.; Khrapko, R.R.; Frolov, A.A. Color Centers in Sulfur-Doped Silica Glasses: Spectroscopic Manifestations of an S2+ Interstitial Molecular Ion. Glass Phys. Chem. 2002, 28, 5–10. [Google Scholar] [CrossRef]
- Gerasimova, V.I.; Rybaltovskii, A.O.; Chernov, P.V.; Zimmerer, G. The Influence of Silica Glass Matrix on the Spectra of Interstitial Molecules S2. Glass Phys. Chem. 2002, 28, 59–65. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, P.; Yang, D.; Wang, Z. Synthesis, photoluminescence properties and sensing applications of luminescent sulfur nanodots. Chem. Commun. 2020, 56, 10982. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Liu, C.; Deng, Z.; Zhao, X.; Zhou, X. Size-dependent photoluminescence of PbS QDs embedded in silicate glasses. Opt. Mater. Express 2017, 7, 2194. [Google Scholar] [CrossRef]
- Han, N.; Liu, C.; Zhang, J.; Zhao, X.; Heo, J.; Jiang, Y. Infrared photoluminescence from lead sulfide quantum dots in glasses enriched in sulfur. J. Non-Cryst. Solids 2014, 391, 39–42. [Google Scholar] [CrossRef]
- Lezal, D.; Pedlíıková, J.; Zavadil, J.; Kostka, P.; Poulain, M. Preparation and characterization of sulfide, selenide and telluride glasses. J. Non-Cryst. Solids 2003, 326–327, 47–52. [Google Scholar] [CrossRef]
- Seki, M.; Hachiya, K.; Yoshida, K. Photoluminescence and states in the bandgap of germanium sulfide glasses. J. Non-Cryst. Solids 2003, 315, 107–113. [Google Scholar] [CrossRef]
- Stoch, L.; Stoch, Z.; Wacławska, I. Silicate Glass Fertilizer. Patent PL 185 229 B1, 30 April 2003. (In Polish). [Google Scholar]
- Wacławska, I.; Szumera, M. Reactivity of silicate-phosphate glasses in soil environment. J. Alloys Compd. 2009, 468, 246–253. [Google Scholar] [CrossRef]
- Sułowska, J.; Wacławska, I.; Olejniczak, Z. Effect of glass composition on the interactions between structural elements in Cu-containing silicate-phosphate glasses. J. Therm. Anal. Calorim. 2014, 116, 51–59. [Google Scholar] [CrossRef][Green Version]
- Nowotny, W. Szkła Barwne; Arkady: Warsaw, Poland, 1958; pp. 142–149. (In Polish) [Google Scholar]
- Manara, D.; Grandjean, A.; Pinet, O.; Dussossoy, J.L.; Neuville, D.R. Sulfur behaviour in silicate glasses and melts: Implications for sulphate incorporation in nuclear waste glasses as a function of alkali cation and V2O5 content. J. Non-Cryst. Solids 2007, 353, 12–23. [Google Scholar] [CrossRef]
- Hassaan, M.Y.; El-Desoky, M.M.; Moustafa, M.G.; Iida, L.Y.; Kubuki, S.; Nishida, T. Role of Sulfur as a Reducing Agent for the Transition Metals Incorporated into Lithium Silicate Glass. Croat. Chem. Acta 2015, 88, 505–510. [Google Scholar] [CrossRef][Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Baker, L.L.; Malcolm, J. Rutherford, Sulfur diffusion in rhyolite melts. Contrib. Mineral. Petrol. 1996, 123, 335–344. [Google Scholar] [CrossRef]
- Hassaan, M.Y.; Salem, S.M.; Moustafa, M.G. Study of nanostructure and ionic conductivity of Li1.3Nb0.3V1.7(PO4)3 glass ceramics used as cathode material for solid batteries. J. Non-Cryst. Solids 2014, 391, 6–11. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Wally, N.K.; Sheha, E.; Kamal, M. Impact of sodium oxide, sulfide, and fluoride-doped vanadium phosphate glasses on the thermoelectric power and electrical properties: Structure analysis and conduction mechanism. J. Mater. Sci. Mater. Electron. 2021, 32, 3699–3712. [Google Scholar] [CrossRef]
- Xu, X.; Youngman, R.E.; Kapoor, S.; Goel, A. Structural drivers controlling sulfur solubility in alkali aluminoborosilicate glasses. J. Am. Ceram. Soc. 2021, 1–20. [Google Scholar] [CrossRef]
- Kjeldsen, J.; Smedskjaer, M.M.; Mauro, J.C.; Youngman, R.E.; Huang, L.; Yue, Y. Mixed alkaline earth effect in sodium aluminosilicate glasses. J. Non-Cryst. Solids 2013, 369, 61–68. [Google Scholar] [CrossRef]
- Shareefuddin, M.; Ramadevudu, G.; Rao, S.; Narasimha Chary, M. Physical, Optical, and Spectroscopic Studies on MgO-BaO-B2O3 Glasses. Int. Sch. Res. Not. 2013, 2013, 419183. [Google Scholar] [CrossRef][Green Version]
- Sastry, S.S.; Rao, B.R.V. Structural and optical properties of vanadium doped alkaline earth lead zinc phosphate glasses. Indian J. Pure Appl. Phys. 2014, 52, 491–498. [Google Scholar]
- Dimitrov, V.; Komatsu, T. An interpretation of optical properties of oxides and oxide glasses in terms of the electronic ion polarizability and average single bond strength. J. Univ. Chem. Technol. Metall. 2010, 45, 219–250. [Google Scholar]
- Yousef, E.S.; Elokr, M.M.; AbouDeif, Y.N. Optical, elastic properties and DTA of TNZP host tellurite glasses doped with Er3+ ions. J. Mol. Struct. 2016, 1108, 257–262. [Google Scholar] [CrossRef]
- Sitarz, M. Influence of modifying cations on the structure and texture of silicate–phosphate glasses. J. Mol. Struct. 2008, 887, 237–248. [Google Scholar] [CrossRef]
- Sitarz, M. Structure of simple silicate glasses in the light of Middle Infrared spectroscopy studies. J. Non-Cryst. Solids 2011, 357, 1603. [Google Scholar] [CrossRef]
- Handke, M.; Sitarz, M.; Rokita, M.; Galuskin, E.W. Vibrational spectra of phosphosilicate biomaterials. J. Mol. Struct. 2003, 39, 651–653. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Sinegovskaya, L.M.; Gusarova, N.K. Vibrations of the S–S bond in elemental sulfur and organic polysulfides: A structural guide. J. Sulphur Chem. 2009, 30, 518–554. [Google Scholar] [CrossRef]
- Miladi, L.; Oueslati, A.; Guidara, K. Phase transition, conduction mechanism and modulus study of KMgPO4 compound. RSC Adv. 2016, 6, 83280. [Google Scholar] [CrossRef]
- Lebecq, I.; Désanglois, F.; Leriche, A.; Follet-Houttemane, C. Compositional dependence on the in vitro bioactivity of invert or conventional bioglasses in the Si-Ca-Na-P system. J. Biomed. Mater. Res. A 2007, 83, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.N.; Condrate, R.A., Sr. The Vibrational Spectra of Glasses in the Na2O-SiO2-P2O5 System with a 1:1 SiO2:P2O5 Molar Ratio. Phys. Chem. Glasses 1985, 26, 68–73. [Google Scholar]
- Serra, J.; González, P.; Liste, S.; Serra, C.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänem, H.O.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Cryst. Solids 2003, 332, 20. [Google Scholar] [CrossRef]
- McKeown, D.A.; Bell, M.I.; Kim, C.C. Raman spectroscopy of silicate rings: Benitoite and the three-membered ring. Phys. Rev. B 1993, 48, 22. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Gao, H.; Tan, T.; Wang, D. Effect of composition on the release kinetics of phosphate controlled release glasses in aqueous medium. J. Control. Release 2004, 96, 21–28. [Google Scholar] [CrossRef]
- Villegas, M.A.; Navarro, J.M.F. Characterization of B2O3-SiO2 glasses prepared via sol-gel. J. Mater. Sci. 1998, 23, 2464. [Google Scholar] [CrossRef]
- Ahsan, M.R.; Mortuza, M.G. Infrared spectra of xCaO(1-x-z)SiO2zP2O5 glasses. J. Non-Cryst. Solids 2005, 351, 2333–2340. [Google Scholar] [CrossRef]
- Szumera, M.; Wacławska, I. Spectroscopic and thermal studies of silicate-phosphate glasses. J. Therm. Anal. Calorim. 2007, 8, 151–156. [Google Scholar] [CrossRef]
- Varshneya, A.K. Fundamentals of Inorganic Glasses; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Sułowska, J.; Jeleń, P.; Olejniczak, Z.; Szumera, M. Sulfur speciation and network structural changes in silicate-phosphate glasses. J. Non-Cryst. Solids 2021, 557, 1–8. [Google Scholar] [CrossRef]
- Kęcki, Z. Podstawy Spektroskopii Molekularnej; PWN: Warsaw, Poland, 1992. (In Polish) [Google Scholar]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Wu, X.L. Photoluminescence and cathodoluminescence studies of stoichiometric and oxygen-deficient ZnO films. Appl. Phys. Lett. 2001, 78, 2285. [Google Scholar] [CrossRef]
- Skuja, L.; Hosono, H.; Hirano, M. Laser-induced color centers in silica. In Laser-Induced Damage in Optical Materials: 2000; Society of Photo-Optical Instrumentation Engineers (SPIE): Bellingham, WA, USA, 2001; Volume 4347, pp. 155–168. [Google Scholar] [CrossRef]
- Garcia-Guinea, L.; Correcher, V.; Sanchez-Muñoz, L.; Finch, A.A.; Hole, D.E.; Townsend, P.D. On the luminescence emission band at 340 nm of stressed tectosilicate lattices. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2007, 580, 648–651. [Google Scholar] [CrossRef]
- Fredholm, Y.C.; Karpukhina, N.; Law, R.V.; Hill, R.G. Strontium containing bioactive glasses: Glass structure and physical properties. J. Non-Cryst. Solids 2010, 356, 2548. [Google Scholar] [CrossRef]
- Kasymdzhanov, M.A.; Khabibullaev, P.K.; Kurbanov, S.; Kurbanov, E. UV-laser coloration and bleaching of unirradiated and gamma-irradiated silica glasses. In Laser-Induced Damage in Optical Materials: 2000; Society of Photo-Optical Instrumentation Engineers (SPIE): Bellingham, WA, USA, 2001; Volume 4347. [Google Scholar] [CrossRef]
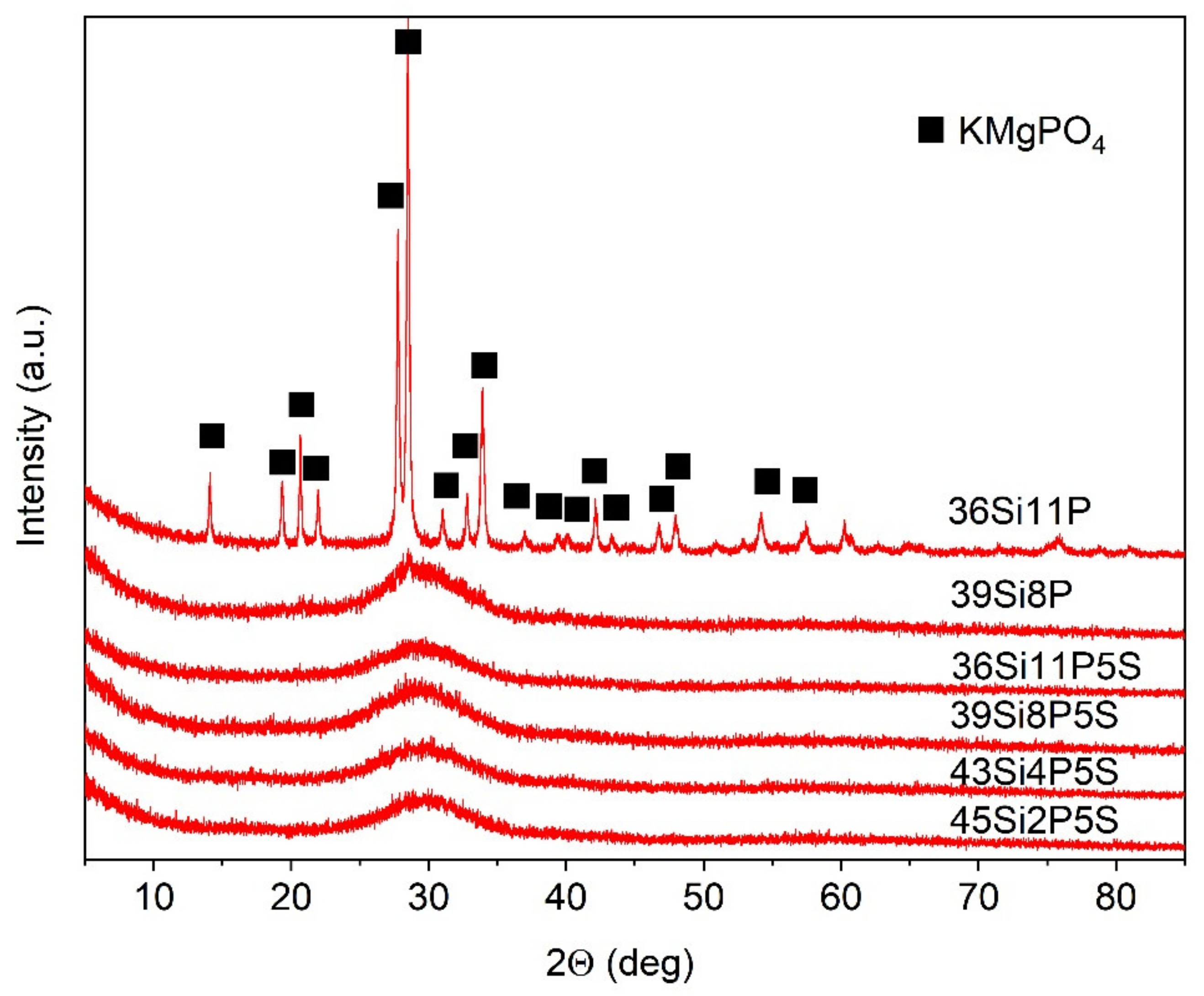




| Comp. | 45Si2P | 45Si2P5S | 43Si4P | 43Si4P5S | 39Si8P | 39Si8P5S | 36Si11P | 36Si11P5S |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 43.576 (45) | 48.040 (45) | 41.463 (43) | 46.311 (43) | 37.606 (39) | 43.675 (39) | 33.543 (36) | 38.918 (36) |
| P2O5 | 2.313 (2) | 1.238 (2) | 4.624 (4) | 0.796 (4) | 9.041 (8) | 3.802 (8) | 12.380 (11) | 7.034 (11) |
| K2O | 19.407 (20) | 16.385 (20) | 19.239 (20) | 15.279 (20) | 22.070 (20) | 16.964 (20) | 24.922 (20) | 17.433 (20) |
| MgO | 32.929 (33) | 30.489 (28) | 32.782 (33) | 30.807 (28) | 29.353 (33) | 30.631 (28) | 26.362 (33) | 31.035 (28) |
| SO3 | 0.063 | 1.721 (5) | 0.061 | 4.541 (5) | 0.036 | 2.993 (5) | 0.078 | 3.004 (5) |
| Al2O3 | 0.648 | 0.858 | 0.724 | 0.872 | 0.802 | 0.685 | 1.121 | 1.088 |
| Na2O | 0.839 | 1.028 | 0.826 | 1.176 | 0.763 | 1.041 | 0.686 | 1.191 |
| CaO | 0.131 | 0.149 | 0.179 | 0.133 | 0.145 | 0.124 | 0.733 | 0.167 |
| TiO2 | 0.016 | 0.022 | 0.021 | 0.024 | 0.036 | 0.030 | 0.000 | 0.033 |
| Fe2O3 | 0.014 | 0.020 | 0.017 | 0.018 | 0.019 | 0.020 | 0.032 | 0.020 |
| CuO | 0.006 | 0.004 | 0.000 | 0.005 | 0.011 | 0.000 | 0.017 | 0.005 |
| ZnO | 0.004 | 0.002 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.002 |
| Rb2O | 0.070 | 0.012 | 0.020 | 0.010 | 0.025 | 0.011 | 0.040 | 0.013 |
| SrO | 0.003 | 0.002 | 0.002 | 0.002 | 0.003 | 0.000 | 0.005 | 0.003 |
| ZrO2 | 0.006 | 0.004 | 0.003 | 0.003 | 0.038 | 0.000 | 0.006 | 0.026 |
| Cr2O3 | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| NiO | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Sm2O3 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| PtO2 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 |
| Cl | 0.015 | 0.026 | 0.027 | 0.024 | 0.045 | 0.023 | 0.075 | 0.028 |
| Glass Name | dr ± SD (g/cm3) | Vmol (cm3/mol) | Λth | |
|---|---|---|---|---|
| Pure glass | 45Si2P | 2.5918 ± 0.0008 | 23.8145 | 0.6510 |
| 43Si4P | 2.5482 ± 0.0009 | 24.9547 | 0.6314 | |
| 39Si8P | 2.5084 ± 0.0017 | 27.4620 | 0.6096 | |
| 36Si11P | 2.5639 ± 0.0010 | 28.4816 | 0.5991 | |
| Sulfur doped glass | 45Si2P5S | 2.5682 ± 0.0010 | 23.5923 | 0.6229 |
| 43Si4P5S | 2.5433 ± 0.0008 | 23.7021 | 0.6051 | |
| 39Si8P5S | 2.4919 ± 0.0028 | 25.3021 | 0.6020 | |
| 36Si11P5S | 2.5381 ± 0.0009 | 25.9264 | 0.5859 |
| Wavenumber Range/cm−1 | Glass Name | Assignment | |||
|---|---|---|---|---|---|
| 45Si2P | 39Si8P | 45Si2P5S | 39Si8P5S | ||
| Peak Position/cm−1 | |||||
| 400–650 | 440 | 445 | 440 | 444 | (O-Si-O) bending vibrations [50,51,52] (O-P-O) symmetric bending vibrations [55] for sulfur-doped glass samples: no evidence of bands from polysulfides (S-S stretching vibrations) [53,54] |
| 514 | 530 | 510 | 532 | (Si-O-Si) bending vibrations [56] | |
| 553 | 567 | 544 | 568 | (O-P-O) asymmetric bending vibrations [55] | |
| 588 | 603 | 583 | 604 | (O-P-O) asymmetric bending vibrations [56] for sulfur-doped glass samples: no evidence of bands from disulfides (S-S stretching) [54,55] | |
| 625 | 643 | 619 | 642 | (O-P-O) bending vibrations [57,58] for sulfur-doped glass samples: no evidence of bands from (SO42−) asymmetric bending vibrations [53] | |
| 650–760 | 705 | 740 | 716 | 730 | (Si-O-Si), (Si-O-P) symmetric stretching vibrations [50,51,52] |
| 740 | 766 | 751 | 759 | (Si-O-Si), (Si-O-P) symmetric stretching vibrations [50,51,52], (Si-O 3-4BO) asymmetric stretching vibrations [56] | |
| 800–1600 | 814 | 838 | 828 | 836 | (Si-O-Si) bending vibrations [58,59] |
| 849 | 869 | 863 | 868 | (Si-O-2NBO) stretching vibrations [56,60] | |
| 912 | 925 | 921 | 926 | (Si-O-2NBO) symmetric stretching vibrations [56,60] | |
| 1010 | 1019 | 1012 | 1019 | (Si-O-1NBO) asymmetric stretching vibrations [56,60] (PO43-) symmetric stretching vibrations [55] | |
| 1117 | 1119 | 1114 | 1123 | (Si-O-Si) stretching vibrations [50] (O-P-O) symmetric stretching vibrations [61] for sulfur doped glass samples: no evidence of bands from (SO42−) asymmetric stretching vibrations [53] | |
| 1255 | 1261 | 1258 | 1268 | Si-O-Si stretching vibrations [58,62] O-P-O asymmetric stretching vibrations [61] | |
| 1395 | 1416 | 1401 | 1432 | P=O stretching vibrations [57,63] | |
| n | 45Si2P | 36Si11P5S | 39Si8P5S | 45Si2P5S |
|---|---|---|---|---|
| 2 | 4 eV | 2.14 eV | 2.03 eV | 2.09 eV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułowska, J.; Madej, D.; Pokrzywka, B.; Szumera, M.; Kruk, A. Structural and Optical Properties of Pure and Sulfur-Doped Silicate–Phosphate Glass. Molecules 2021, 26, 3263. https://doi.org/10.3390/molecules26113263
Sułowska J, Madej D, Pokrzywka B, Szumera M, Kruk A. Structural and Optical Properties of Pure and Sulfur-Doped Silicate–Phosphate Glass. Molecules. 2021; 26(11):3263. https://doi.org/10.3390/molecules26113263
Chicago/Turabian StyleSułowska, Justyna, Dominika Madej, Bartłomiej Pokrzywka, Magdalena Szumera, and Andrzej Kruk. 2021. "Structural and Optical Properties of Pure and Sulfur-Doped Silicate–Phosphate Glass" Molecules 26, no. 11: 3263. https://doi.org/10.3390/molecules26113263
APA StyleSułowska, J., Madej, D., Pokrzywka, B., Szumera, M., & Kruk, A. (2021). Structural and Optical Properties of Pure and Sulfur-Doped Silicate–Phosphate Glass. Molecules, 26(11), 3263. https://doi.org/10.3390/molecules26113263







