Amino Acids as the Potential Co-Former for Co-Crystal Development: A Review
Abstract
:1. Introduction
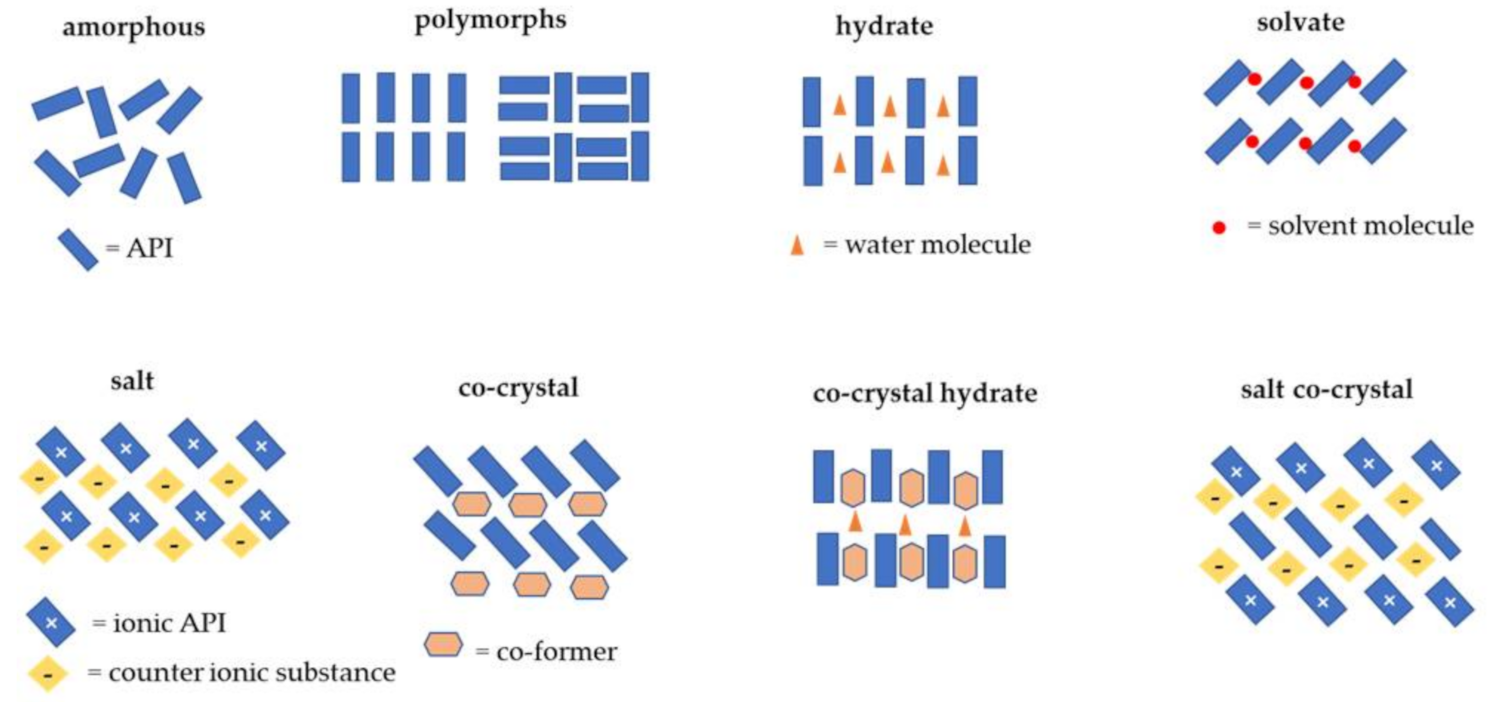
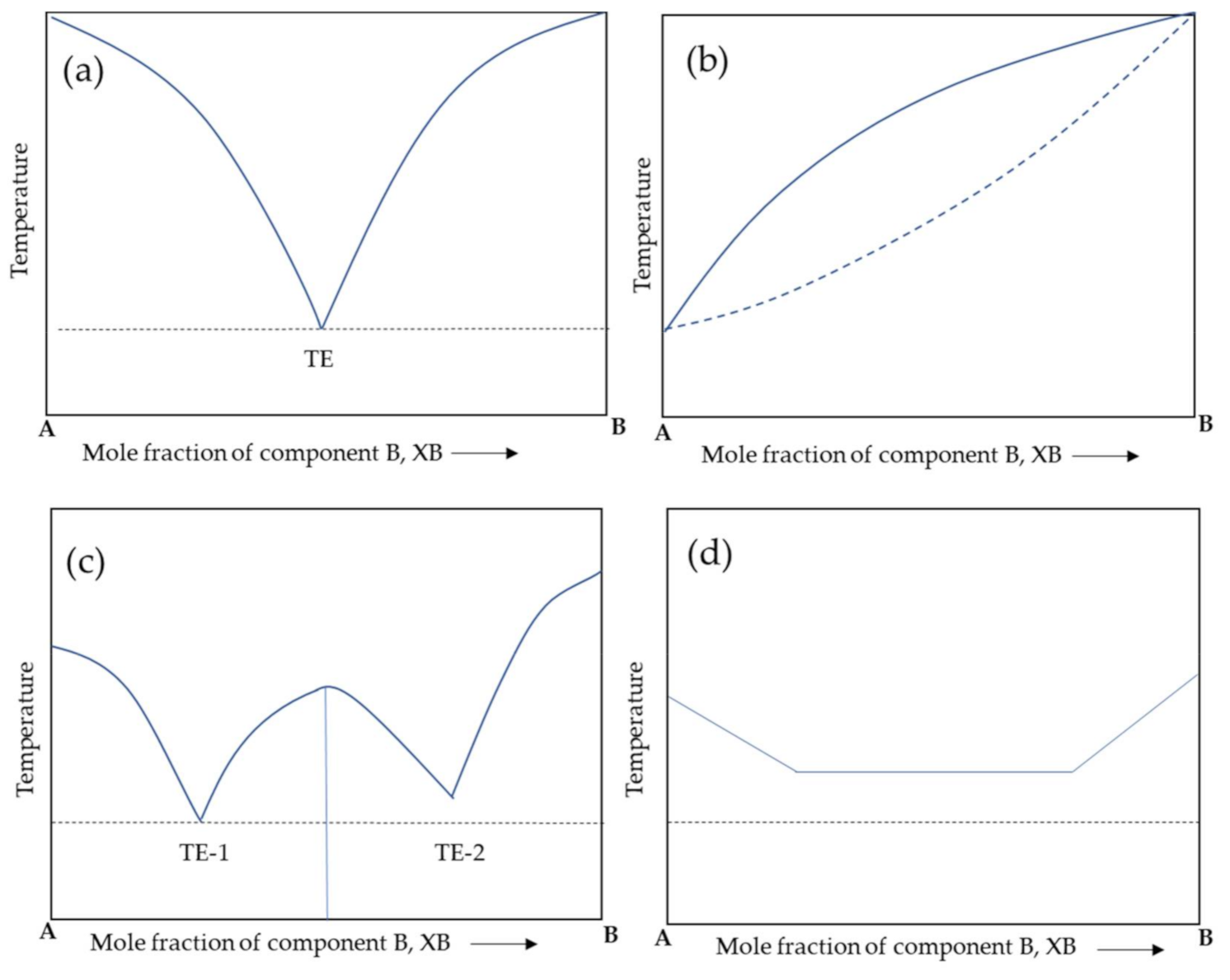
2. Co-Crystals
3. Amino Acids
4. Amino Acids as Co-Formers
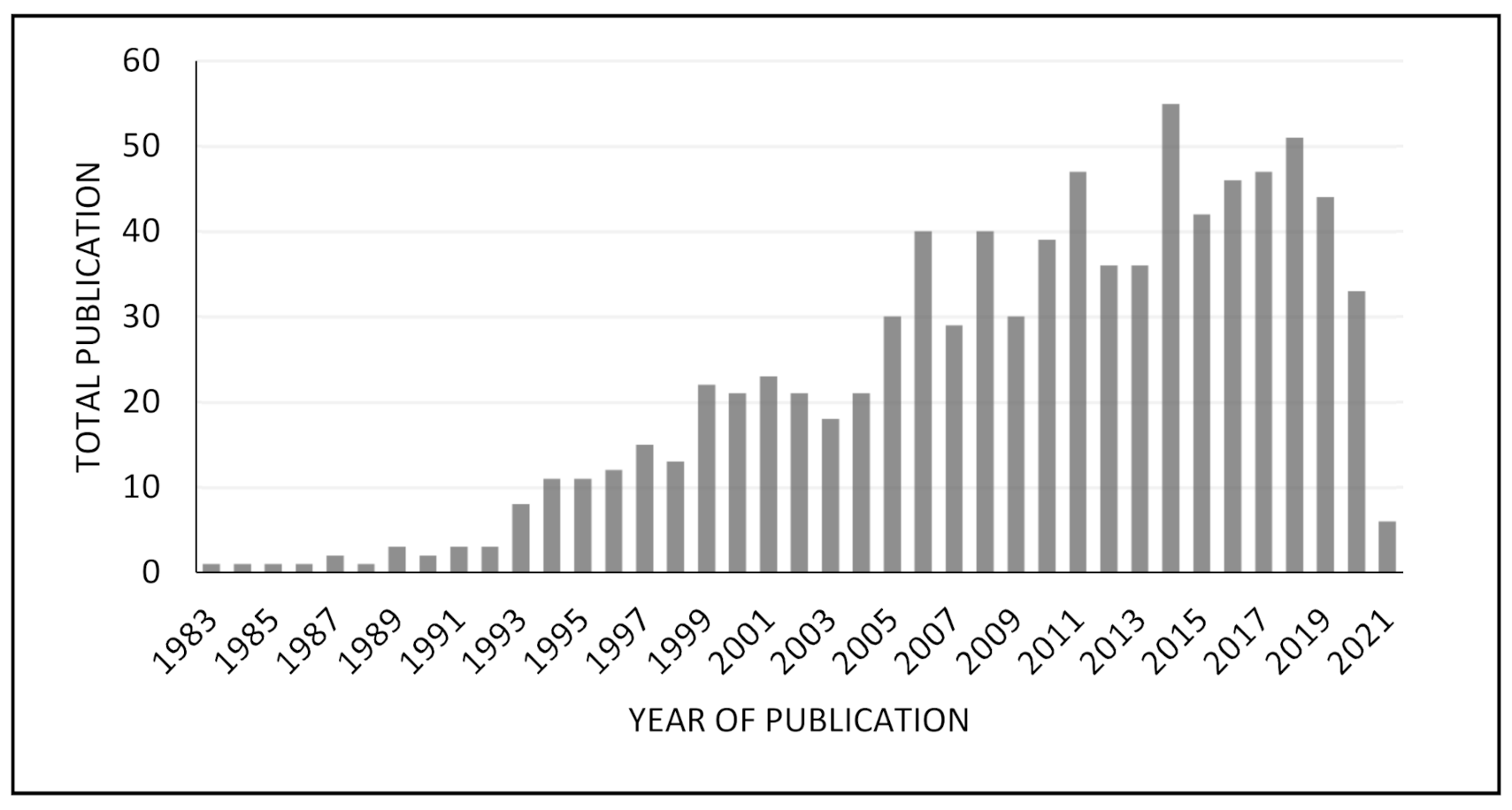
4.1. Screening for Amino Acids Used as Co-Formers
4.2. l-Proline as the Most Fruitful Amino Acid Co-Former
4.2.1. The Structure of l-Proline-Based Co-Crystals
4.2.2. Modification of the Physicochemical Properties of l-Proline-Based Co-Crystal
5. Brief Review
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredients |
| BCS | Biopharmaceutics Classification System |
| NG | Neat Grinding |
| LAG | Liquid-Assisted Grinding |
| SE | Solvent Evaporation |
| GAS | Gas Anti-Solvent Precipitation |
| GRAS | Generally Recognized as Safe |
| FDA | Food and Drug Administration |
| DDC | Drug-Drug Co-crystal |
| PXRD | Powder X-Ray Diffraction |
| SXRD | Single X-Ray Diffraction |
| IR | Infra-Red |
| ss-NMR | Solid State-Nuclear Magnetic Resonance |
| DSC | Differential Scanning Calorimetry |
| DTA | Differential Thermal Analysis |
| TGA | Thermogravimetric Analysis |
| SEM | Scanning Electron Microscopy |
| NSAID | Non-Steroidal Anti-Inflammatory Drugs |
| NADES | Natural Deep Eutectic Solvents |
| CSD | Cambridge Structural Database |
| CAS | Chemical Abstracts Service |
| IDR | Intrinsic Dissolution Rate |
| RH | Relative Humidity |
References
- Aitipamula, S.; Tan, B.H. Pharmaceutical co-crystals: Crystal engineering and applications. In Multi-Component Crystals: Synthesis, Concepts, Function; Tiekink, E.R., Schpector, J.Z., Eds.; De Gruyter: Berlin, Germany, 2018; pp. 1–27. [Google Scholar]
- Chaudhari, S.; Nikam, S.A.; Khatri, N.; Wakde, S. Co-Crystals: A Review. J. Drug Deliv. Ther. 2018, 8, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Nanda, A. Approaches to Design of Pharmaceutical Cocrystals: A Review. Mol. Cryst. Liq. Cryst. 2019, 667, 54–77. [Google Scholar] [CrossRef]
- Elkholy, N.C.; Sultan, A.A.; Elosaily, G.H.; El Maghraby, G.M. Acetone assisted co-processing of meloxicam with amino acids for enhanced dissolution rate. Pharm. Dev. Technol. 2020, 25, 882–891. [Google Scholar] [CrossRef]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New solid phase modification approaches for formulation of API. Pharmaceutics 2018, 10, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Rekdal, M.; Pai, A.; Choudhari, R.; Sathyanarayana, M.B. Applications of Co-Crystals in Pharmaceutical Drugs. Syst. Rev. Pharm. 2018, 9, 55–57. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arafa, M.F.; El-Gizawy, S.A.; Osman, M.A.; El Maghraby, G.M. Xylitol as a potential co-crystal co-former for enhancing dissolution rate of felodipine: Preparation and evaluation of sublingual tablets. Pharm. Dev. Technol. 2018, 23, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Lamprou, D.A.; Douroumis, D. Engineering and manufacturing of pharmaceutical co-crystals: A review of solvent-free manufacturing technologies. CrystEngComm 2016, 52, 8772–8786. [Google Scholar] [CrossRef] [Green Version]
- Bruni, G.; Monteforte, F.; Maggi, L.; Friuli, V.; Ferrara, C.; Mustarelli, P.; Girella, A.; Berbenni, V.; Capsoni, D.; Milanese, C.; et al. Probenecid and benzamide: Cocrystal prepared by a green method and its physico-chemical and pharmaceutical characterization. J. Therm. Anal. Calorim. 2020, 140, 1859–1869. [Google Scholar] [CrossRef]
- Ong, T.T.; Kavuru, P.; Nguyen, T.; Cantwell, R.; Zaworotko, M.J. 2:1 Cocrystals of Homochiral and Achiral Amino Acid Zwitterions with Li+ Salts: Water-Stable Zeolitic and Diamondoid Metal-Organic Materials. J. Am. Chem. Soc. 2011, 133, 9224–9227. [Google Scholar] [CrossRef]
- An, J.H.; Lim, C.; Ryu, H.C.; Kim, J.S.; Kim, H.M.; Kiyonga, A.N.; Park, M.; Suh, Y.G.; Park, G.H.; Jung, K. Structural Characterication of Febuxostat/L-Pyroglutamic Acid Cocrystal Using Solid-State 13C-NMR and Investigational Study of Its Water Solubility. Crystals 2017, 7, 365. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Han, J.; Pang, M.; Song, H.; Ma, Y.; Meng, J. Achiral Benzoic Acid Derivatives as Chiral Cocrystal Building Blocks in Supramolecular Chemistry: Adducts with Organic Amine. Cryst. Growth Des. 2006, 6, 1186–1193. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Panikkattu, S.; Chopade, P.D.; Desper, J. Competing hydrogen-bond and halogen-bond donors in crystal engineering. CrystEngComm 2013, 15, 3125–3136. [Google Scholar]
- Braga, D.; Shemchuk, O.; Grepioni, F. Organic-inorganic Ionic Co-crystals: A New Class of Multipurpose Compounds. CrystEngComm 2018, 20, 2212–2220. [Google Scholar] [CrossRef]
- Shemchuk, O.; Braga, D.; Grepioni, F. Ionic Cocrystals of Levodopa and Its Biological Precursors L-Tyrosine and L-Phenylalanine with LiCl. Cryst. Growth Des. 2019, 19, 6560–6565. [Google Scholar] [CrossRef]
- Shemchuk, O.; Tsenkova, B.K.; Braga, D.; Duarte, T.; André, V.; Grepioni, F. Ionic Co-Crystal Formation as a Path Towards Chiral Resolution in the Solid State. Chem. Eur. J. 2018, 24, 12564–12573. [Google Scholar] [CrossRef]
- Shemchuk, O.; Spoletti, E.; Braga, D.; Grepioni, F. Solvent Effect on the Preparation of Ionic Cocrystals of dl-Amino Acids with Lithium Chloride: Conglomerate versus Racemate Formation. Cryst. Growth Des. 2021, in press. [Google Scholar] [CrossRef]
- Liu, M.; Hong, C.; Yao, Y.; Shen, H.; Ji, G.; Li, G.; Xie, Y. Development of a pharmaceutical cocrystal with solution crystallization technology: Preparation, characterization, and evaluation of myricetin-proline cocrystal. Eur. J. Pharm. Biopharm. 2016, 107, 151–159. [Google Scholar]
- Othman, M.F.; Jamburi, N.; Anuar, N.; Rahim, S.A.; Rohalim, N.H. Ibuprofen-Amino Acids Co-Crystal Screening Via Co-Grinding Method. MATEC Web Conf. 2016, 69, 3002. [Google Scholar] [CrossRef]
- Tilborg, A.; Leyssens, T.; Norberg, B.; Wouters, J. Structural Study of Prolinium/Fumaric Acid Zwitterionic Cocrystals: Focus on Hydrogen-Bonding Pattern Involving Zwitterionic (Ionic) Heterosynthons. Cryst. Growth Des. 2013, 13, 2373–2389. [Google Scholar] [CrossRef]
- Nugrahani, I.; Kumalasari, R.A.; Auli, W.N.; Horikawa, A.; Uekusa, H. Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study. Pharmaceutics 2020, 12, 690. [Google Scholar] [CrossRef]
- Wang, X.; Du, S.; Zhang, R.; Jia, X.; Yang, T.; Zhang, X. Drug-Drug Cocrystals: Opportunities and Challenges. Asian J. Pharm. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Perry, M.L.; Almarsson, O.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicine. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef]
- Varshosaz, J.; Talari, R.; Mostafavi, S.A.; Nokhodchi, A. Dissolution enhancement of gliclazide using in situ micronization by solvent change methods. Powder Technol. 2008, 187, 222–230. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersion. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Elder, D.P.; Holm, R.; De-Diego, H.L. Use of pharmaceutical salts and cocrystals to address the issue of poor solubility. Int. J. Pharm. 2013, 453, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.R.; Nekkanti, V. Improved delivery of poorly soluble compounds using nanoparticle technology: A review. Drug Deliv. Transl. Res. 2016, 6, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Fukte, S.; Wagh, M.P.; Rawat, S. Coformer selection: An important tool in cocrystal formation. Int. J. Pharm. Sci. 2014, 6, 9–14. [Google Scholar]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Shaikh, R.; Singh, R.; Walker, G.M.; Croker, D.M. Pharmaceutical Cocrystal Drug Products: An Outlook on Product Development. Trends Pharmacol. Sci. 2018, 39, 1033–1048. [Google Scholar] [CrossRef]
- Grifasi, F.; Chierotti, M.R.; Gaglioti, K.; Gobetto, R.; Maini, L.; Braga, D.; Dichiarante, E.; Curzi, M. Using salt cocrystals to improve the solubility of niclosamide. Cryst. Growth Des. 2015, 15, 1939–1948. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Zhang, S.; Zhou, X. Synthesis, crystal structure, and solubility analysis of a famotidine cocrystal. Crystals 2019, 9, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Han, Y.; Xie, Y.F.; Gong, N.B.; Guo, F. Carbamazepine cocrystals with several aromatic carboxylic acids in different stoichiometries: Structures and solid-state characterization. J. Mol. Struct. 2018, 1168, 145–152. [Google Scholar] [CrossRef]
- Sokal, A.; Pindelska, E.; Szeleszczuk, L.; Kolodziejski, W. Pharmaceutical properties of two ethenzamide-gentisic acid cocrystal polymorphs: Drug release profiles, spectroscopic studies and theoretical calculations. Int. J. Pharm. 2017, 522, 80–89. [Google Scholar] [CrossRef]
- Rai, S.K.; Allu, S.; Nangia, A.K. Salts and Cocrystal of Etodolac: Advantage of Solubility, Dissolution, and Permeability. Cryst. Growth Des. 2020, 20, 4512–4522. [Google Scholar] [CrossRef]
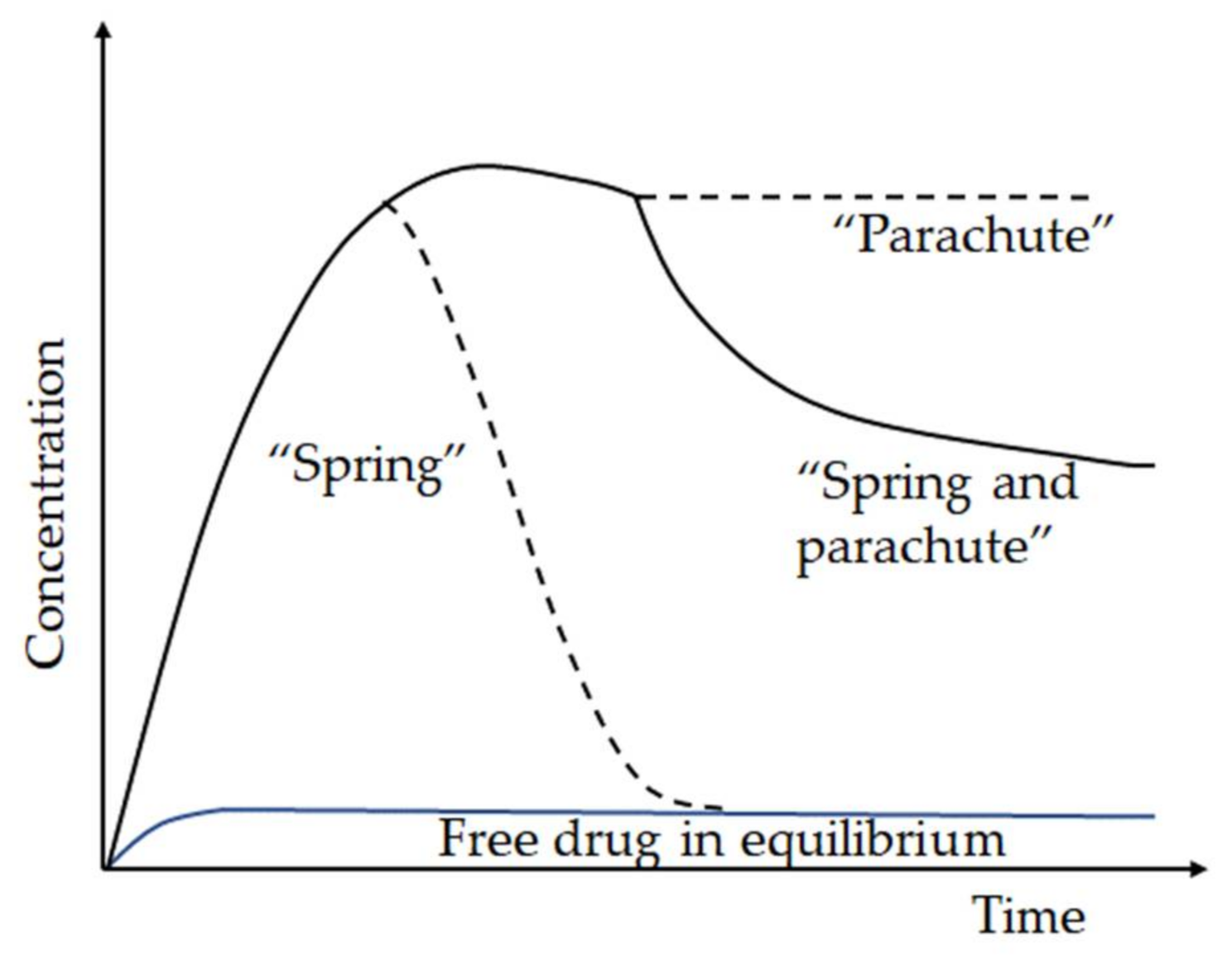
- Wei, Y.; Zhang, L.; Wang, N.; Shen, P.; Dou, H.; Ma, K.; Gao, Y.; Zhang, J.; Qian, S. Mechanistic Study on Complexation-Induced Spring and Hover Dissolution Behavior of Ibuprofen-Nicotinamide Cocrystal. Cryst. Growth Des. 2018, 18, 7343–7355. [Google Scholar] [CrossRef]
- Zaini, E.; Fitriani, L.; Sari, R.Y.; Rosaini, H.; Horikawa, A.; Uekusa, H. Multicomponent Crystal of Mefenamic Acid and N-Methyl-D-Glucamine: Crystal Structures and Dissolution Study. J. Pharm. Sci. 2019, 108, 2341–2348. [Google Scholar] [CrossRef]
- Panzade, P.; Shendarkar, G.; Shaikh, S.; Rathi, P.B. Pharmaceutical Cocrystal of Piroxicam: Design, Formulation and Evaluation. Adv. Pharm. Bull. 2017, 7, 399–408. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, Z.; Dang, L.; Wei, H. Solid-liquid phase equilibrium and ternary phase diagrams of ethenzamide-saccharin cocrystals in different solvent. Fluid. Phase Equilib. 2016, 419, 24–30. [Google Scholar] [CrossRef]
- Kerr, H.E.; Softley, L.K.; Suresh, K.; Hodgkinson, P.; Evans, I.R. Structure and Physicochemical Characterization of a Naproxen-Picolinamide Cocrystal. Acta Crystallogr. Sect. C Struct. Chem 2017, C73, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Bartos, C.; Ambrus, R.; Katona, G.; Sovány, T.; Gáspár, R.; Márki, A.; Ducza, E.; Ivanov, A.; Tömösi, F.; Janáky, T.; et al. Transformation of meloxicam containing nanosuspension into surfactant-free solid compositions to increase the product stability and drug bioavailability for rapid analgesia. Drug Des. Dev. Ther. 2019, 13, 4007–4020. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Y.; Bu, F.Z.; Li, Y.T.; Wu, Z.Y.; Yan, W. A Sulfathiazole-Amantadine Hydrochloride Cocrystal: The First Codrug Simultaneously Comprising Antiviral and Antibacterial Component. Cyst. Growth Des. 2020, 20, 3236–3246. [Google Scholar] [CrossRef]
- Khatioda, R.; Bora, P.; Sarma, B. Trimorphic Ethenzamide Cocrystal: In Vitro Solubility and Membrane Efflux Studie. Cryst. Growth Des. 2018, 18, 4637–4645. [Google Scholar] [CrossRef]
- Allu, S.; Bolla, G.; Tothadi, S.; Nangia, A. Novel Pharmaceutical Cocrystals and Salts of Bumetanide. Cryst. Growth Des. 2020, 20, 793–803. [Google Scholar] [CrossRef]
- Yu, X.Z.; Wang, L.Y.; Liu, F.; Li, Y.T.; Wu, Z.Y.; Yan, C.W. Sustained-Release Dual-Drug Ternary Salt Cocrystal of Piperazine Ferulate with Pyrazinamide: Synthesis, Structure, and Hirshfeld Surface Analysis. Cryst. Growth Des. 2020, 20, 2064–2073. [Google Scholar] [CrossRef]
- Zheng, K.; Li, A.; Wu, W.; Qian, S.; Liu, B.; Pang, Q. Preparation, characterization, in vitro and in vivo evaluation of metronidazole–gallic acid cocrystal: A combined experimental and theoretical investigation. J. Mol. Struct. 2019, 1197, 727–735. [Google Scholar] [CrossRef]
- Lu, Q.; Dun, J.; Chen, J.M.; Liu, S.; Sun, C.C. Improving solid-state properties of berberine chloride through forming a salt cocrystal with citric acid. Int. J. Pharm. 2019, 554, 14–20. [Google Scholar] [CrossRef]
- Nugraha, Y.P.; Uekusa, H. Suppressed hydration in metoclopramide hydrochloride by salt cocrystallisation. CrystEngComm 2018, 20, 2653–2662. [Google Scholar] [CrossRef]
- Guo, W.; Du, S.; Lin, Y.; Lu, B.; Yang, C.; Zeng, Y. Structural and computational insights into the enhanced solubility of dipfluzine by complexation: Salt and salt-cocrystal. New J. Chem. 2018, 42, 15068–15078. [Google Scholar] [CrossRef]
- Akram, M.; Asif, H.M.; Uzair, M.; Akhtar, N.; Madni, A.; Shah, S.M.A.; Hasan, Z.U.; Ullah, A. Amino acids: A review article. J. Med. Plants Res. 2011, 5, 3997–4000. [Google Scholar]
- McKee, T.; Mckee, J. Amino Acids, Peptides, and Proteins. In Biochemistry: The Molecular Basis of Life, 5th ed.; Noe, J., Kramer, J., Eds.; Oxford University Press: Oxford, MA, USA, 2011; pp. 1–59. [Google Scholar]
- Grosch, W. Amino Acids, Peptides, Proteins. In Food Chemistry, 4th ed.; Belitz, H.D., Grosch, W., Schieberle, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 8–92. [Google Scholar]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Discovery and Chemistry of Amino Acids. In Amino Acids: Biochemistry and Nutrition; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–32. [Google Scholar]
- Bowden, N.A.; Sanders, J.P.M.; Bruins, M.E. Solubility of the Proteinogenic α-Amino Acids in Water, Ethanol, and Ethanol−Water Mixture. J. Chem. Eng. Data 2018, 63, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Acqua, S.D. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elik, A.; Demirbas, A.; Altunay, N. Developing a new and simple natural deep eutectic solvent based ultrasonic-assisted microextraction procedure for determination and preconcentration of As and Se from rice samples. Anal. Methods 2019, 11, 3429–3438. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. C R Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Radošević, K.; Čanak, I.; Panič, M.; Markov, K.; Bubalo, M.; Frece, J.; Srček, V.G.; Redovnikovič, I. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. Springer Plus 2016, 5, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.; Tomašević, M.; Ganić, K.K.; Redovnikovič, I. Natural Deep Eutectic Solvents as Beneficial Extractants for Enhancement of Plant Extracts Bioactivity. LWT Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Sut, S.; Faggian, M.; Baldan, V.; Poloniato, G.; Castagliuolo, I.; Grabnar, I.; Perissutti, B.; Brun, P.; Maggi, F.; Voinovich, D.; et al. Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules 2017, 22, 1921. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Dou, L.; Guo, L.; Li, P.; Liu, E. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Newman, A.; Reutzel-edens, S.M.; Zogra, G. Coamorphous Active Pharmaceutical Ingredient e Small Molecule Mixtures: Considerations in the Choice of Coformers for Enhancing Dissolution and Oral Bioavailability. J. Pharm. Sci. 2018, 107, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.T.; Löbmann, K.; Rades, T.; Grohganz, H. Improving Co-Amorphous Drug Formulations by the Addition of the Highly Water Soluble Amino Acid, Proline. Pharmaceutics 2014, 6, 416–435. [Google Scholar] [CrossRef]
- Laitinen, R.; Löbmann, K.; Grohganz, H.; Strachan, C.; Rades, T. Amino acids as co-amorphous excipients for simvastatin and glibenclamide: Physical properties and stability. Mol. Pharm. 2014, 11, 2381–2389. [Google Scholar] [CrossRef]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Löbmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs—Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. Biopharm. 2013, 85, 873–881. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.Y.; Liu, F.; Li, Y.T.; Wu, Z.Y.; Yan, W. Simultaneously enhancing the in vitro/in vivo performances of acetazolamide using proline as a zwitterionic coformer for cocrystallization. CrystEngComm 2019, 21, 3064–3073. [Google Scholar] [CrossRef]
- Kasten, G.; Grohganz, H.; Rades, T.; Löbmann, K. Development of a screening method for co-amorphous formulations of drugs and amino acid. Eur. J. Pharm. Sci. 2016, 95, 28–35. [Google Scholar] [CrossRef]
- Mishra, J.; Löbmann, K.; Grohganz, H.; Rades, T. Influence of preparation technique on co-amorphization of carvedilol with acidic amino acid. Int. J. Pharm. 2018, 552, 407–413. [Google Scholar] [CrossRef]
- Kasten, G.; Löbmann, K.; Grohganz, H.; Rades, T. Co-former selection for co-amorphous drug-amino acid formulations. Int. J. Pharm. 2019, 557, 366–373. [Google Scholar] [CrossRef]
- Tilborg, A.; Norberg, B.; Wouters, J. Pharmaceutical salts and cocrystals involving amino acids: A brief structural overview of the state-of-art. Eur. J. Med. Chem. 2014, 74, 411–426. [Google Scholar] [CrossRef]
- Shah, K.; Borhade, S.; Londhe, V. Utilization of co-crystallization for solubility enhancement of a poorly soluble antiretroviral drug–ritonavi. Int. J. Pharm. Pharm. Sci. 2014, 6, 556–558. [Google Scholar]
- Tumanova, N.; Tumanov, N.; Robeyns, K.; Filinchuk, Y.; Wouters, J.; Leyssens, T. Structural insight into cocrystallization with zwitterionic co-formers: Cocrystal of S-naproxen. CrystEngComm 2014, 16, 8185–8196. [Google Scholar] [CrossRef]
- National Center of Biotechnology Information (NCBI). Bethesda (MD): National Library of Medicine (US). Available online: https://pubmed.ncbi.nlm.nih.gov/?term=amino+acid%3B+cocrystal (accessed on 11 January 2021).
- Shete, A.; Murthy, S.; Korpale, S.; Yadav, A.; Sajane, S.; Sakhare, S.; Doijad, R. Cocrystals of itraconazole with amino acids: Screening, synthesis, solid state characterization, in vitro drug release and antifungal activity. J. Drug Deliv. Sci. Technol. 2015, 28, 46–55. [Google Scholar] [CrossRef]
- Rambabu, D.; Satyanarayana, J.; Saraswatula, V.G.; Ravikumar, N.; Kamalakaran, S.A.; Gopikrishna, G.; Kumar, K.A. Novel cocrystals/molecular salts of mesalamine to be used as improved anti-inflammatory drug. WIPO Pat. Appl. 2012, A1, 090224. [Google Scholar]
- Wróblewska, A.; Śniechowska, J.; Kaźmierski, S.; Wielgus, E.; Bujacz, G.D.; Mlostoń, G.; Chworos, A.; Suwara, J.; Potrzebowslo, M.J. Application of 1-Hydroxy-4, 5-Dimethyl-Imidazole 3-Oxide as Coformer in Formation of Pharmaceutical Cocrystals. Pharmaceutics 2020, 12, 359. [Google Scholar] [CrossRef] [Green Version]
- Tumanova, N.; Tumanov, N.; Fischer, F.; Morelle, F.; Ban, V.; Robeyns, K.; Filinchuk, Y.; Wouters, J.; Emmerling, F.; Lessens, T. Exploring polymorphism and stoichiometric diversity in naproxen/proline cocrystal. CrystEngComm 2018, 20, 7308–7321. [Google Scholar] [CrossRef]
- Nugrahani, I.; Utami, D.; Permana, B.; Ibrahim, S. Development of the NSAID-L-Proline Amino Acid Zwitterionic Cocrystals. J. Appl. Pharm. Sci. 2018, 8, 57–63. [Google Scholar]
- Losev, E.; Boldyreva, E. The effect of amino acid backbone length on molecular packing: Crystalline tartrates of glycine, β-alanine, γ-aminobutyric acid (GABA) and DL-α-aminobutyric acid (AABA). Acta Crystallogr. Sect. C Struct. Chem. 2018, C74, 177–185. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [Green Version]
- Nugrahani, I.; Komara, S.W.; Horikawa, A.; Uekusa, H. Composing Novel Diclofenac Potassium and L-Proline Salt Cocrystal As A Strategy to Increase Solubility And Dissolution. J. Pharm. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Tumanova, N.; Tumanov, N.; Robeyns, K.; Fischer, F.; Fusaro, L.; Morelle, F.; Ban, V.; Hautier, G.; Filinchuk, Y.; Wouters, J.; et al. Opening Pandora’s Box: Chirality, Polymorphism, and Stoichiometric Diversity in Flurbiprofen/Proline Cocrystals. Cryst. Growth Des. 2018, 18, 954–961. [Google Scholar] [CrossRef]
- Tilborg, A.; Springuel, G.; Norberg, B.; Wouters, J.; Leyssens, T. On the influence of using a zwitterionic coformer for cocrystallization: Structural focus on naproxen–proline cocrystal. Cryst. Eng Comm. 2013, 15, 3341–3350. [Google Scholar] [CrossRef]
- Silva, J.L.A.; Santos, P.P.; André, V.; Galego, F. New cocrystals of Flurbiprofen and Proline: Structural effect of enantiomorphism. Acta Crystallogr. Sect. A Found. Adv. 2016, A72, s356. [Google Scholar]
- De Maere d’Aertrycke, J.B.; Robeyns, K.; Willocq, J.; Leyssens, T. Cocrystallization as a tool to solve deliquescence issues: The case of L-lactic acids. J. Cryst. Growth 2017, 472, 3–10. [Google Scholar] [CrossRef]
- Wang, L.Y.; Yu, Y.M.; Jiang, F.B.; Li, Y.T.; Wu, Z.Y.; Yan, W. The first zwitterionic cocrystal of indomethacin with amino acid showing optimized physicochemical properties as well as accelerated absorption and slowed elimination in vivo. New J. Chem. 2020, 44, 3930–3939. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M. Ternary systems of naproxen with hydroxypropyl-β-cyclodextrin and amino acids. Int. J. Pharm. 2003, 260, 293–302. [Google Scholar] [CrossRef]
- Nugrahani, I.; Utami, D.; Ibrahim, S.; Nugraha, Y.P.; Uekusa, H. Zwitterionic cocrystal of diclofenac and L-proline: Structure determination, solubility, kinetics of cocrystallization, and stability study. Eur. J. Pharm. Sci. 2018, 117, 168–176. [Google Scholar] [CrossRef]
- Hirano, A.; Kameda, T.; Arakawa, T.; Shiraki, K. Arginine-assisted solubilization system for drug substances: Solubility experiment and simulation. J. Phys. Chem. B 2010, 114, 13455–13462. [Google Scholar] [CrossRef]
- Vayá, I.; Jiménez, M.; Miranda, M.A. Stereodifferentiation in the fluorescence of naproxen-arginine salts in the solid state. Tetrahedron Asymmetry 2005, 16, 2167–2171. [Google Scholar] [CrossRef]
- Bartolomei, M.; Bertocchi, P.; Antoniella, E.; Rodomonte, A. Physico-chemical characterization and intrinsic dissolution studies of a new hydrate form of diclofenac sodium: Comparison with anhydrous form. J. Pharm. Biomed. Anal. 2006, 40, 1105–1113. [Google Scholar] [CrossRef]
- Teng, R.; Wang, L.; Chen, M.; Fang, W.; Gao, Z.; Chai, Y.; Zhao, P.; Bao, Y. Amino acid based pharmaceutical cocrystals and hydrate cocrystals of the chlorothiazide: Structural studies and physicochemical properties. J. Mol. Struct. 2020, 1217, 128432. [Google Scholar] [CrossRef]
- Stolk, P.; Souverein, P.; Wilting, I.; Leufkens, H.G.; Klein, D.F.; Rapoport, S.I.; Heerdink, E. Is aspirin useful in patients on lithium? A pharmacoepidemiological study related to bipolar disorder. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.J.; Kim, S.H.; Duggirala, N.K.; Jin, J.; Wojtas, L.; Ehrhart, J.; Giunta, B.; Tan, J.; Zaworotko, M.J.; Shytle, R.D. Improving Lithium Therapeutics by Crystal Engineering of Novel Ionic Cocrystals. Mol. Pharm. 2013, 10, 4728–4738. [Google Scholar] [CrossRef]
- Habib, A.; Sawmiller, D.; Li, S.; Xiang, Y.; Rongo, D.; Tian, J.; Hou, H.; Zeng, J.; Smith, A.; Fan, S.; et al. LISPRO mitigates β-amyloid and associated pathologies in Alzheimer’s mice. Cell Death Dis. 2017, 8, e2880. [Google Scholar] [CrossRef] [Green Version]
- Habib, A.; Shytle, D.; Sawmiller, D.; Koilraj, S.; Munna, S.A.; Rongo, D.; Hou, H.; Borlongan, C.V.; Currier, G.; Tan, J. Comparing the effect of the novel ionic cocrystal of lithium salicylate proline (LISPRO) with lithium carbonate and lithium salicylate on memory and behavior in female APPswe/PS1dE9 Alzheimer’s mice. J. Neurosci. Res. 2019, 97, 1066–1080. [Google Scholar] [CrossRef]
- Shimpi, M.R.; Childs, S.L.; Boström, D.; Velaga, S.P. New cocrystals of ezetimibe with l-proline and imidazole. CrystEngComm 2014, 16, 8984–8993. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- He, H.; Huang, Y.; Zhang, Q.; Wang, J.; Mei, X. Zwitterionic Cocrystals of Flavonoids and Proline: Solid-State Characterization, Pharmaceutical Properties, and Pharmacokinetic Performance. Cryst. Growth Des. 2016, 16, 2348–2356. [Google Scholar] [CrossRef]
- Ren, S.; Liu, M.; Hong, C.; Li, G.; Sun, J.; Wang, J.; Zang, L.; Xie, Y. The effects of pH, surfactant, ion concentration, coformer, and molecular arrangement on the solubility behavior of myricetin cocrystals. Acta Pharm. Sin. B 2019, 9, 59–73. [Google Scholar] [CrossRef]
- Báthori, N.B.; Kilinkissa, O.E.Y. Are gamma amino acids promising tools of crystal engineering?—Multicomponent crystals of baclofen. CrystEngComm 2015, 17, 8264–8272. [Google Scholar] [CrossRef]
- Nugrahani, I.; Auli, W.N. Diclofenac-proline nano-co-crystal development, characterization, in vitro dissolution and diffusion study. Heliyon 2020, 6, e04864. [Google Scholar] [CrossRef]
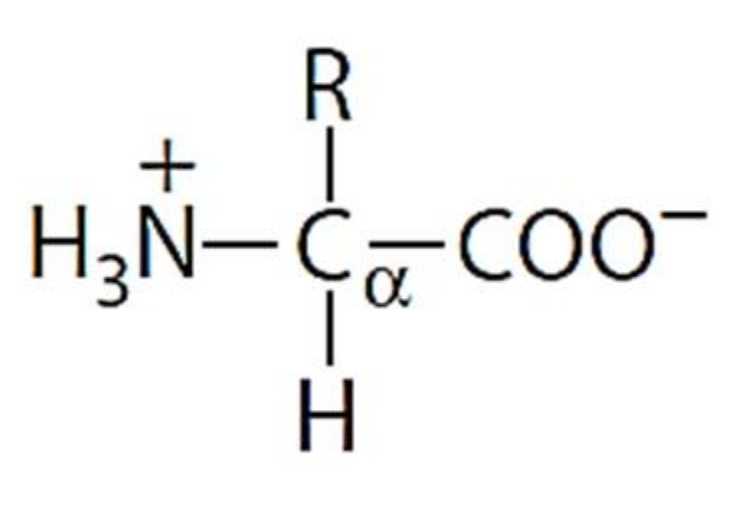



| Non-Polar Amino Acids | Polar Amino Acids | Electrically Charged Amino Acids | |
|---|---|---|---|
| Acidic | Basic | ||
Glycine (GLY)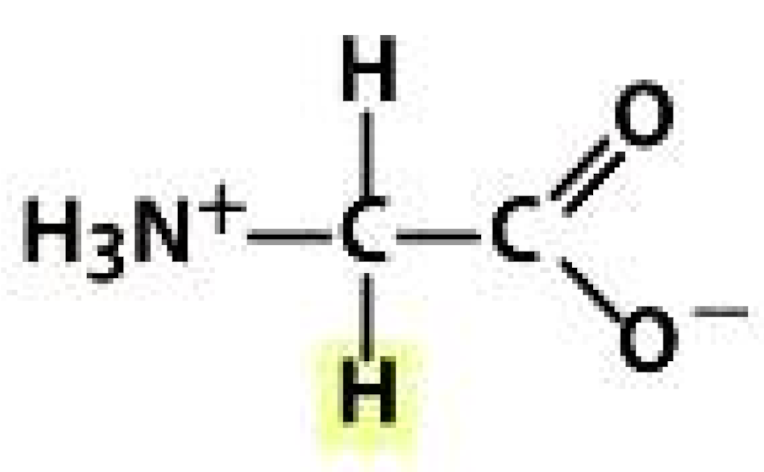 | Serine (SER) | Aspartic acid (ASP)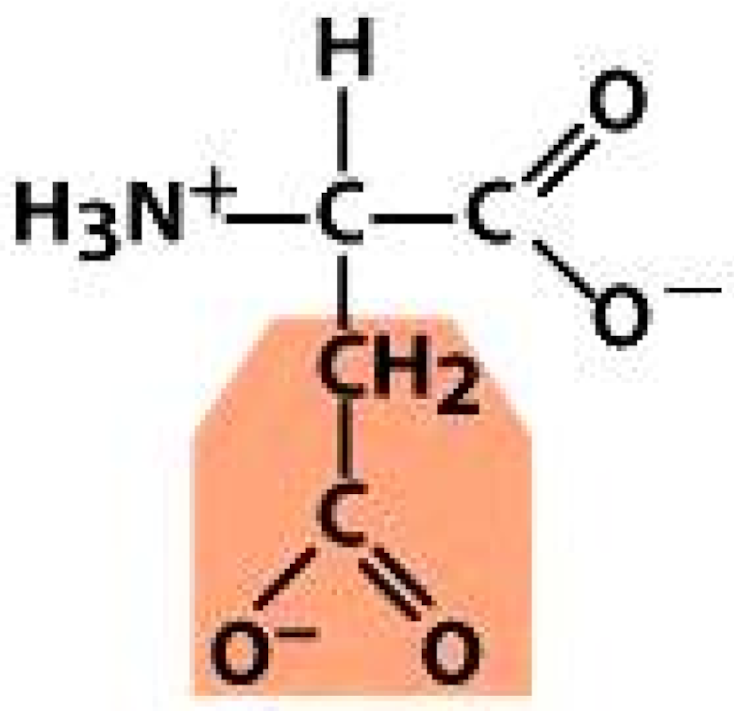 | Histidine (HIS)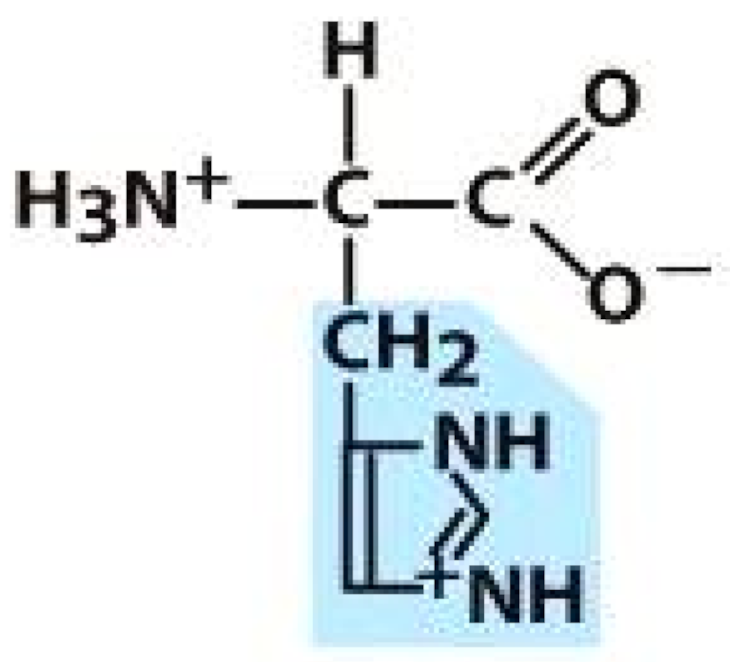 |
Alanine (ALA) | Threonine (THR)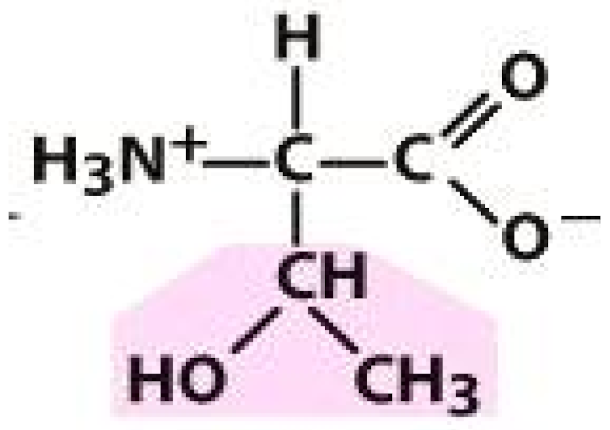 | Glutamic acid (GLU)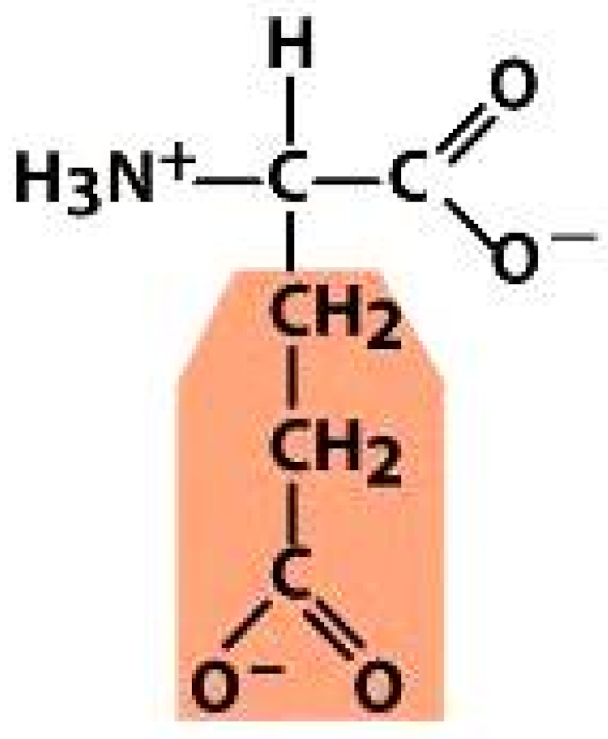 | Arginine (ARG)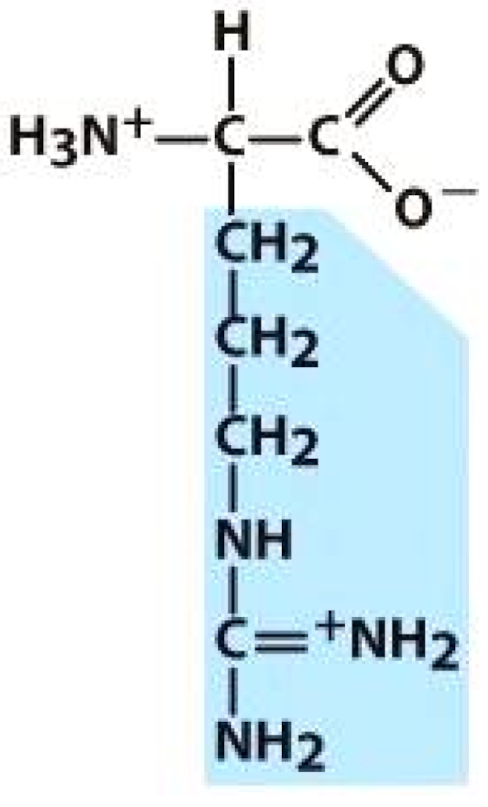 |
Valine (VAL)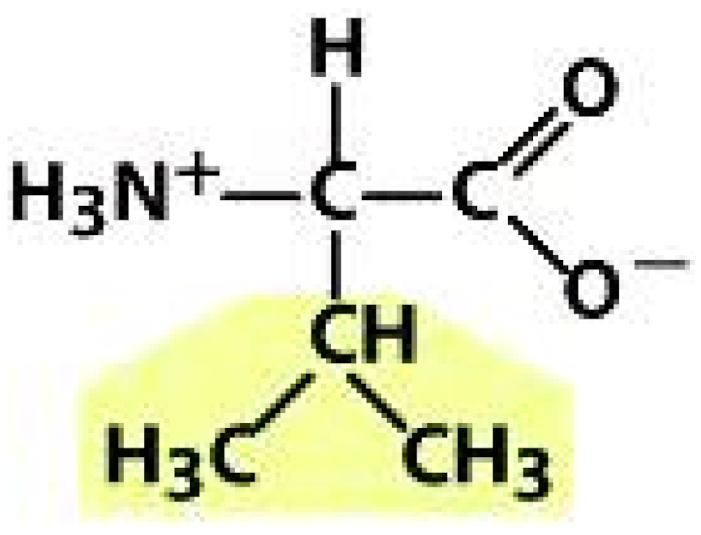 | Cysteine (CYS)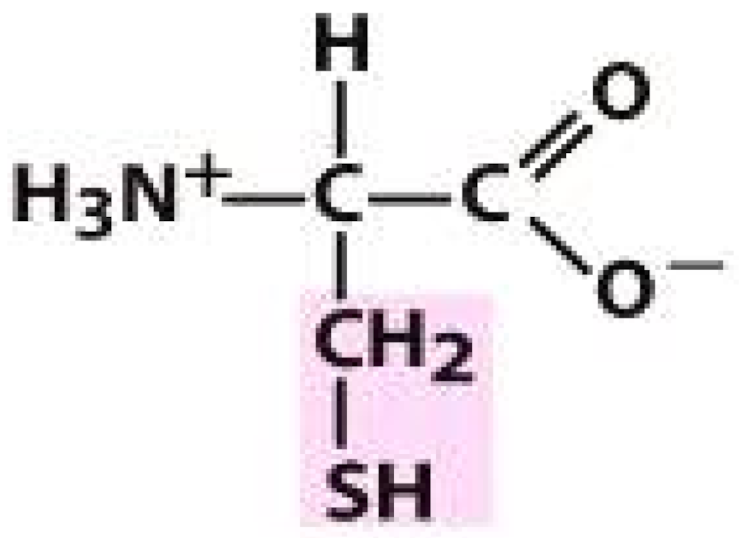 | Lysine (LYS)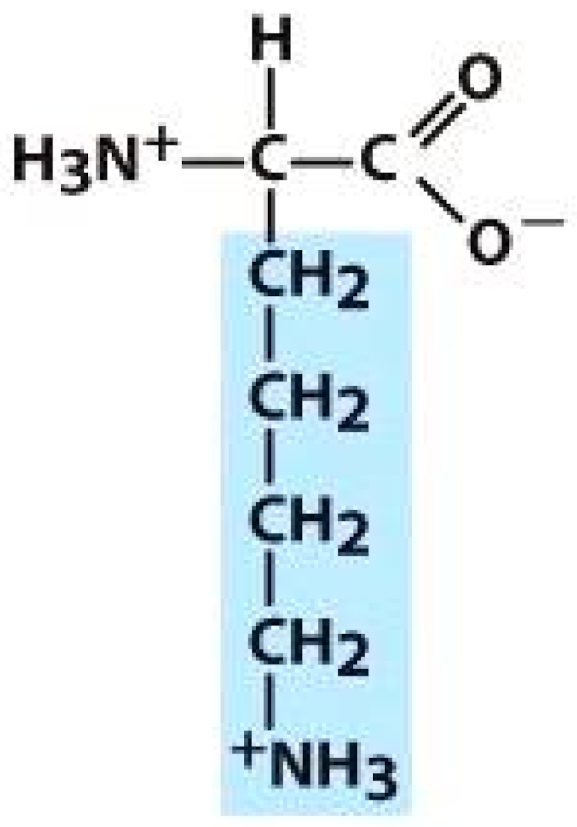 | |
Leucine (LEU)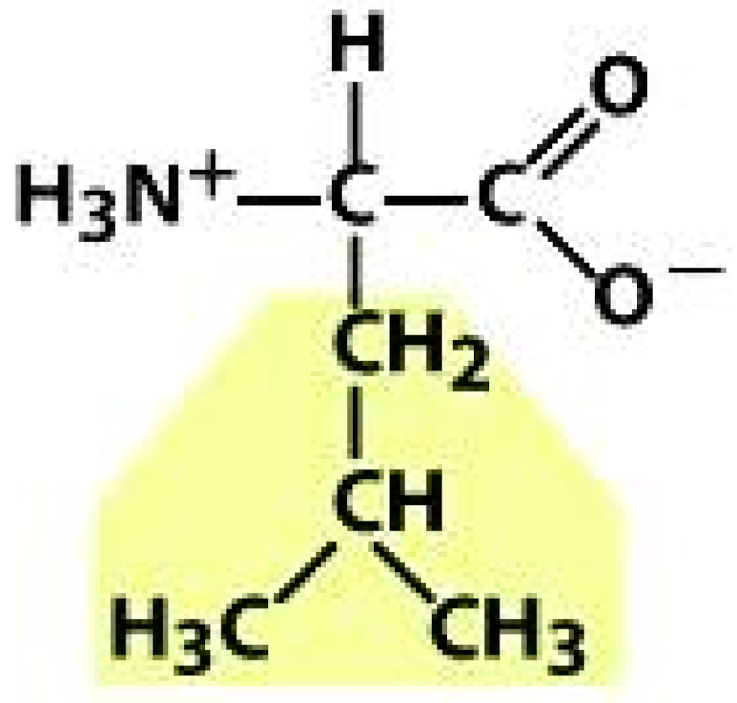 | Tyrosine (TYR) | ||
Isoleucine (ILE) | Asparagine (ASN) | ||
Proline (PRO)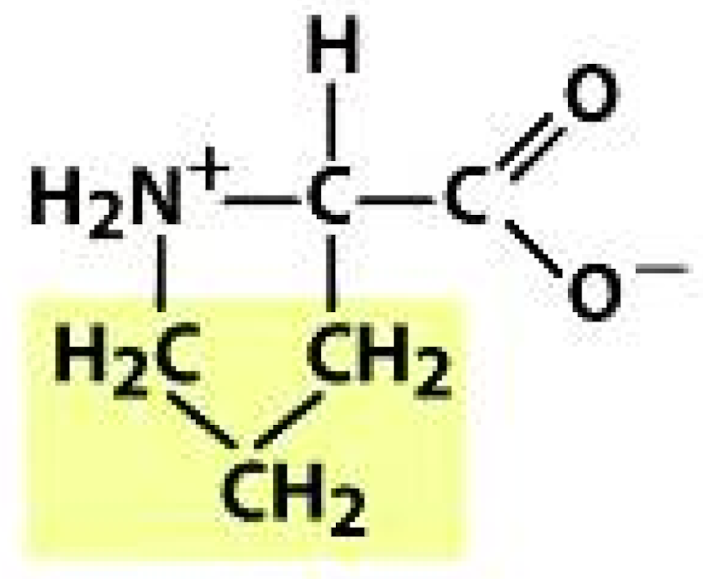 | Glutamine (GLN)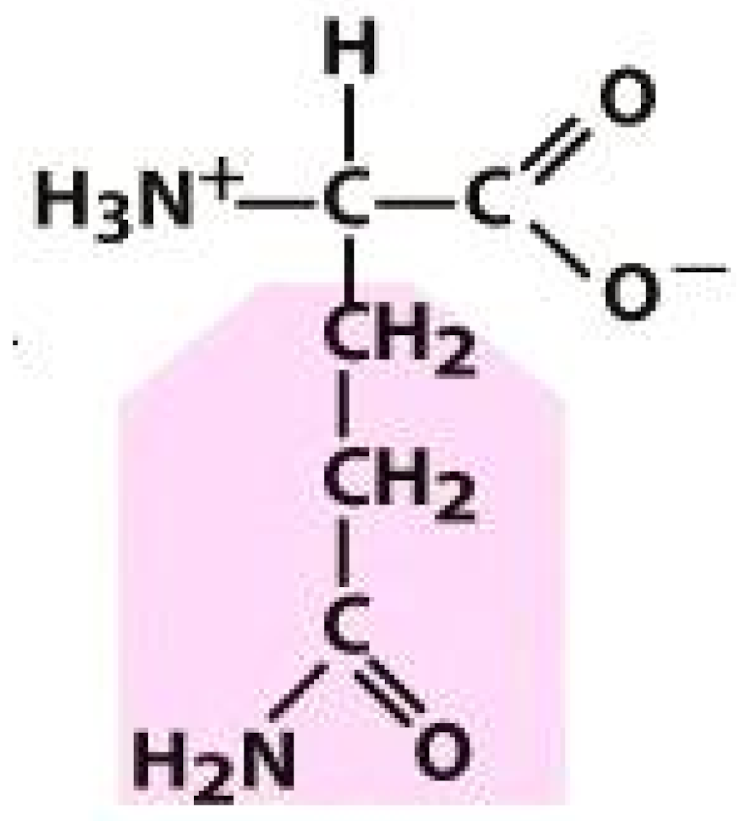 | ||
Phenylalanine (PHE)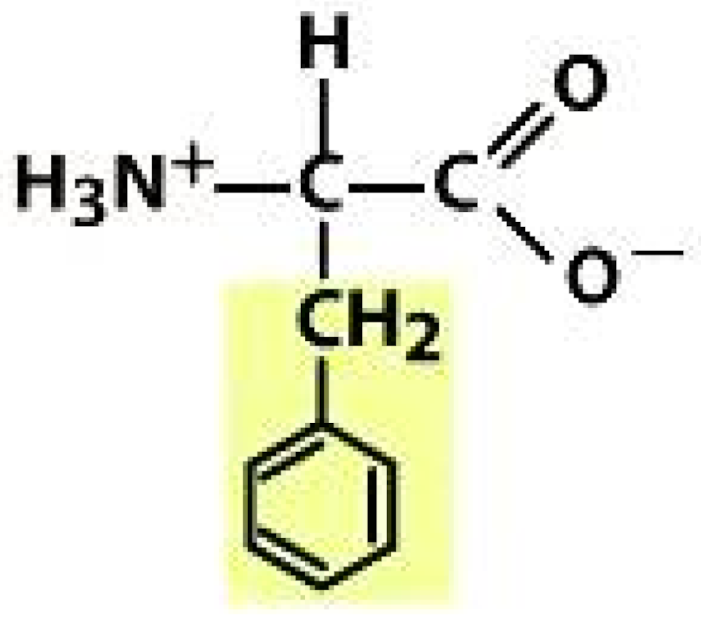 | |||
Tryptophan (TRP)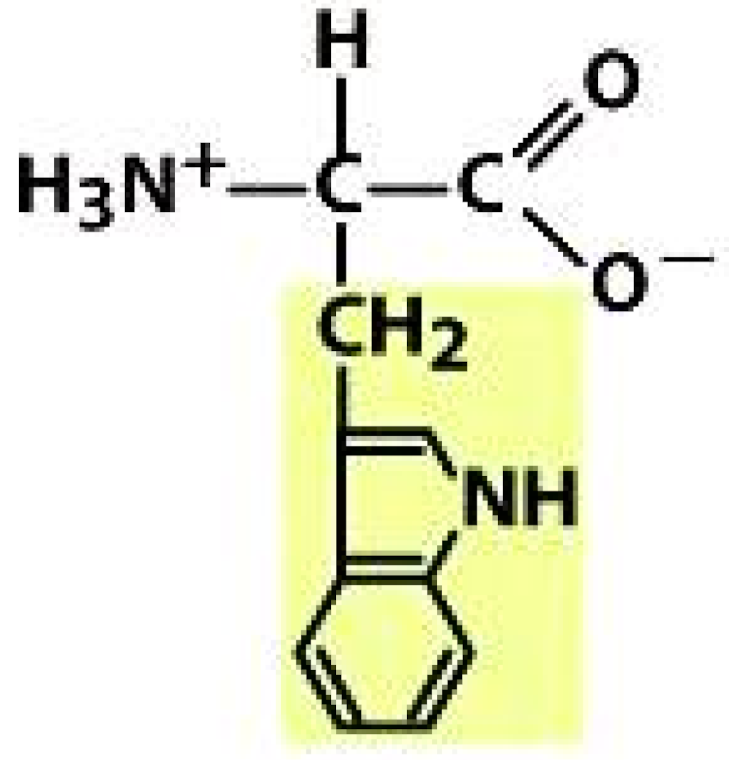 | |||
Methionine (MET)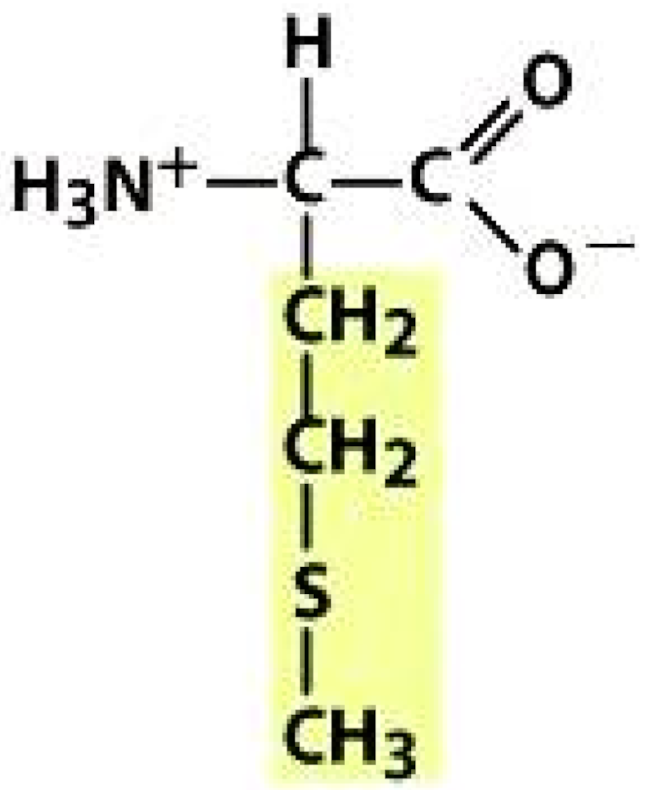 | |||
| No | API-Amino Acid Co-Crystal | Molar Ratio | Preparation Method | Solubility | Dissolution | Pharmacokinetics Effect | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Febuxostat-l-Pyroglutamic acid (FB-PG) | 2:1 | LAG 1 | Co-crystal exhibits higher solubility than pure febuxostat in various medium | - | - | [12] |
| 2 | Myricetin-Proline | 1:2 | SE 2 | Co-crystal exhibits higher solubility than pure myricetin and the physical mixture | Co-crystal exhibits a higher dissolution rate and increases the concentration of myricetin dissolved than pure myricetin and the physical mixture | Co-crystal exhibits faster absorption rate and higher Cmax, AUC value, and the relative bioavailability than pure myricetin | [19,104] |
| 3 | Acetazolamide-l-Proline (ACA-PRO) | 1:1 | SE 2 | Co-crystal exhibits higher solubility than pure acetazolamide and the physical mixture in the three buffers tested. The solubility enhances with increasing pH values | The IDR values of the co-crystal in the three buffers are around three to four times over pure acetazolamide. The time that the co-crystal reached the maximum concentrations decreases with increasing pH values | Co-crystal exhibits higher Cmax and AUC value. Co-crystal showed a faster onset time and a longer duration of action than pure acetazolamide | [68] |
| 4 | Ritonavir-D-Alanine (RTN-DAL) | 1:5 | LAG 1 | Co-crystal exhibits higher solubility than pure ritonavir, but not higher than ritonavir-succinic acid and ritonavir-adipic acid | Co-crystal exhibit marginal increase than pure ritonavir | - | [75] |
| 5 | Itraconazole-Aspartic acid | 1:1 | NG 3 | Co-crystal significantly improves the solubility of itraconazole in simulated gastric fluid | Co-crystal showed enhancement in the dissolution rate than pure itraconazole | - | [78] |
| 6 | Itraconazole-Glycine | ||||||
| 7 | Itraconazole-Proline | ||||||
| 8 | Itraconazole-Serine | ||||||
| 9 | Diclofenac sodium-l-Proline Tetrahydrate (NDPT) | 1:1 | SE 2 | Co-crystal exhibits higher solubility than pure diclofenac sodium | Co-crystal exhibits a higher dissolution rate and increases the percentage of drug release than pure diclofenac sodium | - | [22] |
| 10 | Diclofenac sodium-l-Proline Monohydrate (NDPM) | ||||||
| 11 | Indomethacin-l-Proline (IND-PL) | 3:1 | LAG 1 | Co-crystal exhibits higher solubility than pure indomethacin and the physical mixture in the three buffers tested. The solubility enhances with increasing pH values | The IDR values of the co-crystal in the three buffers are around two times over pure indomethacin. The IDR values become higher with increasing pH values | Co-crystal showed a faster onset time and a longer duration of action than pure indomethacin. Co-crystal has comparably high bioavailability | [90] |
| 12 | Diclofenac-l-Proline (DFA-PRO) | 1:1 | LAG 1 | Co-crystal exhibits higher solubility than pure diclofenac | - | - | [92] |
| 13 | Diclofenac Acid-Proline nano-co-crystal | 1:1 | Fast evaporation | - | The result indicates a 1.32-fold increase in nano co-crystal in pH 1.2 buffer, 1.14-fold in pH 6.8 buffer, and 2.46-fold in pH 7.4 buffer | - | [106] |
| 14 | Diclofenac potassium-l-Proline (DKPH) | 1:1 | SE 2 | Co-crystal exhibits higher solubility than pure diclofenac potassium and the physical mixture | The IDR values of the co-crystal in two buffers are around three times over pure diclofenac potassium and the physical mixture | - | [85] |
| 15 | Chlorothiazide-dl-Proline (CTZ-DL-PRO) | 1:1 | LAG 1 | - | Co-crystal showed a slight improvement in dissolution property | - | [96] |
| 16 | Ezetimibe-Proline (EZT-PRO) | 1:1 | SE 2 | Co-crystal exhibits higher solubility than pure ezetimibe | Co-crystal exhibits a higher dissolution rate and increases the concentration of ezetimibe dissolved than pure ezetimibe | - | [101] |
| 17 | Kaempferol-l-Proline (Kae-l-Pro) | 1:2 | SE 2 | Co-crystal exhibits higher solubility than pure kaempferol | Co-crystal exhibits a higher dissolution rate than pure kaempferol | Co-crystal exhibits higher Cmax and AUC value. Co-crystal showed a faster onset time than pure kaempferol and the physical mixture | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugrahani, I.; Jessica, M.A. Amino Acids as the Potential Co-Former for Co-Crystal Development: A Review. Molecules 2021, 26, 3279. https://doi.org/10.3390/molecules26113279
Nugrahani I, Jessica MA. Amino Acids as the Potential Co-Former for Co-Crystal Development: A Review. Molecules. 2021; 26(11):3279. https://doi.org/10.3390/molecules26113279
Chicago/Turabian StyleNugrahani, Ilma, and Maria Anabella Jessica. 2021. "Amino Acids as the Potential Co-Former for Co-Crystal Development: A Review" Molecules 26, no. 11: 3279. https://doi.org/10.3390/molecules26113279