Synthesis and Neurotropic Activity of New Heterocyclic Systems: Pyridofuro[3,2-d]pyrrolo[1,2-a]pyrimidines, Pyridofuro[3,2-d]pyrido[1,2-a]pyrimidines and Pyridofuro[3′,2′:4,5]pyrimido[1,2-a]azepines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. PASS Prediction
2.3. Biological Assay
2.4. Molecular Docking
2.4.1. Docking Studies for Prediction of the Mechanism of Anticonvulsant and Anxiolytic Activity (Docking to GABAA Receptor)
2.4.2. Docking to SERT Transporter and 5-HT1A Receptor
2.5. Drug Likeness
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Method for the Preparation of Compounds 2a–d,g,h
3.1.3. General Method for the Preparation of Compounds 3c,n
3.1.4. General Method for the Preparation of Compounds 4c,f,n
3.1.5. General Method for the Preparation of Compounds 5a–d, 6a–n and 7a–h
3.2. Biological Evaluation
3.2.1. Evaluation of the Anticonvulsant Activity of the Synthesized Compounds
3.2.2. Evaluation of the Psychotropic Properties of the Synthesized Compounds
3.2.3. Evaluation of Incoordination of Movements in the Rotating Rod Test
3.3. Docking Studies
3.4. Drug-Likeness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mkrtchyan, A.P.; Kazaryan, S.G.; Noravyan, A.S.; Dzhagatspanyan, I.A.; Nazaryan, I.M.; Akopyan, A.G. Synthesis and anticonvulsive activity of a series of new pyrano(thiopyrano,pyrido)[4′,3′:4,5]thieno[2,3-d]pyrimidines. Pharm. Chem. J. 1998, 32, 469–473. [Google Scholar] [CrossRef]
- Kapustina, M.V.; Amelkin, O.Y.; Kharizomenova, A.I.; Shvedov, V.I.; Filitis, L.N. Synthesis and antitubercular activity of benzothieno[2,3-d]pyrimidines. Pharm. Chem. J. 1991, 25, 475–477. [Google Scholar] [CrossRef]
- Hermecz, I.; Vasvari-Debreczy, L.; Horvath, A.; Balogh, M.; Kokosi, J.; Devos, C.; Rodriguez, L. Nitrogen bridgehead compounds. 66. Bronchodilator nitrogen bridgehead compounds with a pyrimidinone moiety. J. Med. Chem. 1987, 30, 1543–1549. [Google Scholar] [CrossRef]
- Ferrara, M.; Crescenzi, B.; Donghi, M.; Muraglia, E.; Nizi, E.; Pesci, S.; Summa, V.; Gardelli, C. Synthesis of a hexahydropyrimido[1,2-a]azepine-2-carboxamide derivative useful as an HIV integrase inhibitor. Tetrahedron Lett. 2007, 48, 8379–8382. [Google Scholar] [CrossRef]
- Zhong, Y.; Krska, S.W.; Zhou, H.; Reamer, R.A.; Lee, J.; Sun, Y.; Askin, D. Catalytic Asymmetric Synthesis of an HIV Integrase Inhibitor. Org. Lett. 2009, 11, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Sirakanyan, S.N.; Geronikaki, A.; Spinelli, D.; Paronikyan, R.G.; Dzhagatspanyan, I.A.; Nazaryan, I.M.; Akopyan, A.G.; Hovakimyan, A.A. Pyridofuropyrrolo[1,2-a]pyrimidines and pyridofuropyrimido[1,2-a]azepines: New chemical entities (NCE) with anticonvulsive and psychotropic properties. RSC Adv. 2016, 6, 49028–49038. [Google Scholar] [CrossRef]
- Rosowsky, A.; Papathansopoulos, N. Pyrimido[4,5-c]isoquinolines. 2. Synthesis and biological evaluation of some 6-alkyl-, 6-aralkyl-, and 6-aryl-1,3-diamino-7,8,9,10-tetrahydropyrimido[4,5-c]isoquinolines as potential folate antagonists. J. Med. Chem. 1974, 17, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Paronikyan, E.G.; Sirakanyan, S.N.; Noravyan, A.S. The Chemistry and Biological Activity of Nitrogen-Containing Heterocycles and Alkaloids; Kartsev, V.G., Tolstikov, G.A., Eds.; Iridium Press: Moscow, Russian, 2001; Volume 1, pp. 441–448. Available online: http://cbcconf.com/izd/azot-e.htm (accessed on 28 May 2021).
- Paronikyan, E.G.; Sirakanyan, S.N.; Lindeman, S.V.; Aleqsanyan, M.S.; Karapetyan, A.A.; Noravyan, A.S.; Struchkov, Y.T. Synthesis and structure of 3-oxopyrano[3,4-c]pyridines. Chem. Heterocycl. Compd. 1989, 25, 953–958. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Geronikaki, A.; Spinelli, D.; Hovakimyan, A.A.; Noravyan, A.S. Synthesis and structure of condensed triazolo- and tetrazolopyrimidines. Tetrahedron 2013, 69, 10637–10643. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Hovakimyan, A.A.; Noravyan, A.S. On the reaction of 2-[(4-cyano-5,6,7,8-tetrahydroisoquinolin-3-yl)oxy]acetamides with bases: 1-amino-6,7,8,9-tetrahydrofuro[2,3-c]isoquinoline-2-carboxamides and 3-amino-4-cyano-5,6,7,8-tetrahydroisoquinolines via a Smiles-type rearrangement. Tetrahedron 2015, 71, 3263–3272. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Hovakimyan, A.A. New Methods for the Synthesis of 3-Amino-6,7-dihydro-5H-cyclopenta[c]pyridine-4-carbonitriles and Cyclopenta[d]pyrazolo[3,4-b]-pyridines via a Smiles-type Rearrangement. J. Heterocycl. Chem. 2017, 54, 1199–1209. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Ghazaryan, S.G.; Hakobyan, E.K.; Hovakimyan, A.A. Synthesis and Transformations of Oxy Amides Derived from Cycloalka[c]- and Pyrano[3,4-c]pyridines. Russ. J. Org. Chem. 2020, 56, 1854–1858. [Google Scholar] [CrossRef]
- Lalezari, I.; Jabari-Sahbari, M.H. New polyheterocyclic series based on 4,5,6,7-tetrahydrothieno[2,3-c]-pyridine. J. Heterocycl. Chem. 1978, 15, 873–875. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Poroikov, V.V. Computer-aided drug design: From discovery of novel pharmaceutical agents to systems pharmacology. Biochem. Suppl. Ser. B Biomed. Chem. 2020, 14, 216–227. [Google Scholar] [CrossRef]
- Vogel, H.G.; Vogel, V.H. Psychotropic and Neurotropic activity. In Drug Discovery and Evalution: Pharmacological Assays; Vogel, H.E., Ed.; Springer: Berlin, Germany, 2008; Volume 58, pp. 569–874. Available online: https://scholar.google.com/scholar_lookup?title=Drug+Discovery+and+Evaluation (accessed on 28 May 2021).
- Loscher, W.; Schmidt, D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988, 2, 145–181. [Google Scholar] [CrossRef]
- Schwale, K.; Ebert, U. Animal models of neuropsychiatric deseases. In Animal models of Epilepsy. Methods and Innovations; Baraban, S.C., Ed.; Humana Press: Berlin, Germany, 2009; pp. 75–117. [Google Scholar] [CrossRef]
- Little, Y.M.; Conrad, E.A. Pentylenetetrazol seizure activity in mice as influenced by route of administration, acute adrenalectomy and reserpine. J. Pharmacol. Exp. Ther. 1960, 129, 454–461. [Google Scholar]
- Loscher, W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 2002, 50, 105–123. [Google Scholar] [CrossRef]
- Swinyard, E.A.; Woodhead, J.H.; White, H.S.; Franklin, M.R. Experimental Selection, Qualification and Evalution of Anticonvulsant, Antiepileptic Drugs; Lery, R.H., Dreyfuss, F.E., Mattson, R.M., Meddrum, B.S., Pentry, J.K., Eds.; Raven Press: New York, NY, USA, 1989; pp. 85–102. Available online: https://europepmc.org/article/med/4007184 (accessed on 28 May 2021).
- Dunham, N.W.; Miya, T.S.; Edwards, L.D. The pharmacological activity of a series of basic esters of mono- and dialkylmalonic acids. J. Am. Pharm. Assoc. Sci. Ed. 1957, 46, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exper. Ther. 1949, 96, 99–113. [Google Scholar]
- Mironov, A.H. Manual for Preclinical Studies of Drugs; The 1th Part; Medicine: Moscow, Russian, 2012; pp. 235–250. (In Russian) [Google Scholar]
- Mashkovskii, M.D. Lekarstvennie Sredstva [Medicinal Products]; Novaya Volna: Moscow, Russian, 2006. [Google Scholar]
- Sofia, R.D. Comparison of Two Methods for Measuring Drug-Induced Neurotoxicity. J. Pharm. Sci. 1969, 58, 900–901. [Google Scholar] [CrossRef] [PubMed]
- File, S.E. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav. Brain Res. 2001, 125, 151–157. [Google Scholar] [CrossRef]
- Pelow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1985, 24, 525–529. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Löscher, W. The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 2004, 5, 553–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammerer, M.; Rassner, M.P.; Freiman, T.M.; Feuerstein, T.J. Effects of antiepileptic drugs on GABA release from rat and human neocortical synaptosomes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 384, 47–57. [Google Scholar] [CrossRef]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABAA receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saier, M.H., Jr. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 2000, 64, 354–411. [Google Scholar] [CrossRef] [Green Version]
- Perrone, R.; Berardi, F.; Colabufo, N.A.; Lacivita, E.; Larizza, C. Design and synthesis of long-chain arylpiperazines with mixed affinity for serotonin transporter (SERT) and 5-HT1A receptor. J. Pharm. Pharmacol. 2005, 57, 1319–1327. [Google Scholar] [CrossRef]
- Blier, P. Pharmacology of rapid-onset antidepressant treatment strategies. J. Clin. Psychiatry 2001, 62 (Suppl. 15), 12–17. [Google Scholar]
- Singh, S.K.; Piscitelli, C.L.; Yamashita, A.; Gouaux, E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science 2008, 322, 1655–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuipers, W.; Link, R.; Standaar, P.J.; Stoit, A.R.; van Wijngaarden, I.; Leurs, R.; Ijzerman, A.P. Study of the interaction between aryloxypropanolamines and Asn386 in helix VII of the human 5-hydroxytryptamine1A receptor. Mol. Pharmacol. 1997, 51, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Wacker, D.; Fenalti, G.; Brown, M.A.; Katritch, V.; Abagyan, R.; Cherezov, V.; Stevens, R.C. Conserved Binding Mode of Human β2 Adrenergic Receptor Inverse Agonists and Antagonist Revealed by X-ray Crystallography. J. Am. Chem. Soc. 2010, 132, 11443–11445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandaru, S.; Tiwari, G.; Akka, J.; Marri, V.K.; Alvala, M.; Gutlapalli, V.R.; Nayarisseri, A.; Mundluru, H.P. Identification of high affinity bioactive Salbutamol conformer directed against mutated (Thr164Ile) beta 2 adrenergic receptor. Curr. Top. Med. Chem. 2015, 15, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Accelrys Discovery Studio® Visualizer 3.5.0.12158, (Copyright© 2005-12, Accelrys Software Inc.) BIOVIA, Dassault Systèmes, (Discovery Studio 2021 Client); Dassault Systèmes: San Diego, CA, USA, 2021.
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Wars, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
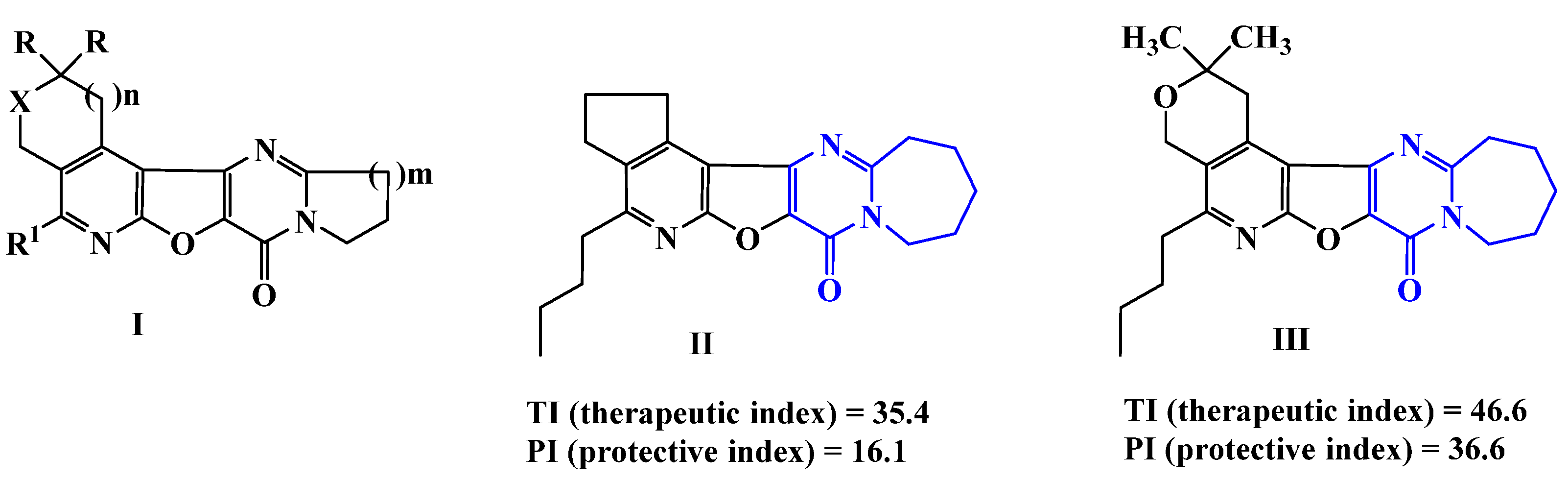
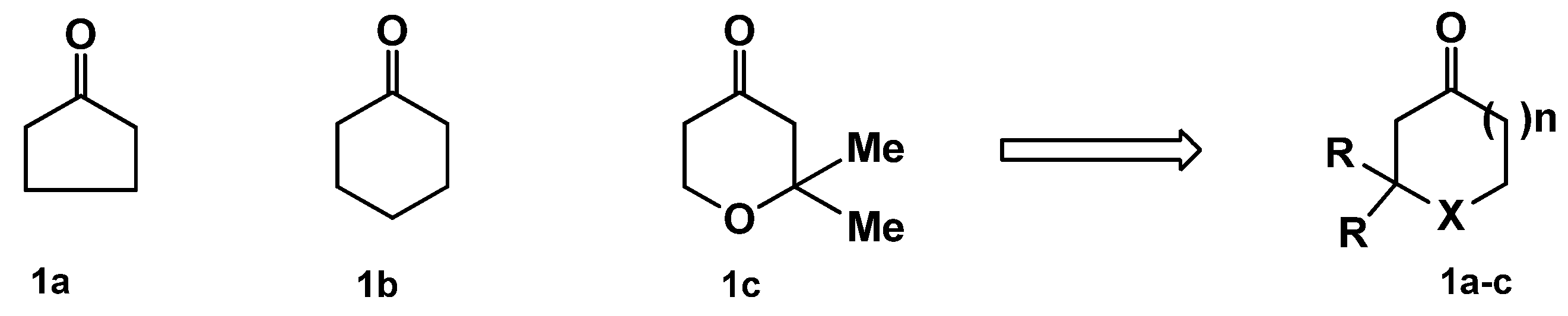









| Compound | X | R | n | R1 | Yield (%) |
|---|---|---|---|---|---|
| 5a | CH2 | H | 1 | Me | 68 |
| 5b | CH2 | H | 1 | Et | 70 |
| 5c | O | Me | 1 | i-Pr | 65 |
| 5d | O | Me | 1 | Ph | 69 |
| 6a | CH2 | H | 0 | i-Pr | 57 |
| 6b | CH2 | H | 0 | n-Bu | 53 |
| 6c | CH2 | H | 0 | C6H11 | 56 |
| 6d | CH2 | H | 1 | Me | 61 |
| 6e | CH2 | H | 1 | Et | 54 |
| 6f | CH2 | H | 1 | i-Pr | 58 |
| 6g | CH2 | H | 1 | i-Bu | 55 |
| 6h | O | Me | 1 | Me | 60 |
| 6i | O | Me | 1 | Et | 62 |
| 6j | O | Me | 1 | i-Pr | 57 |
| 6k | O | Me | 1 | n-Bu | 61 |
| 6l | O | Me | 1 | i-Bu | 53 |
| 6m | O | Me | 1 | Ph | 59 |
| 6n | O | Me | 1 | 2-furyl | 56 |
| 7a | CH2 | H | 0 | C6H11 | 68 |
| 7b | CH2 | H | 0 | 2-furyl | 70 |
| 7c | CH2 | H | 1 | Me | 66 |
| 7d | CH2 | H | 1 | Et | 65 |
| 7e | O | Me | 1 | i-Pr | 67 |
| 7f | O | Me | 1 | i-Bu | 64 |
| 7g | O | Me | 1 | Ph | 71 |
| 7h | O | Me | 1 | 2-furyl | 69 |
| No. | Pa | Pi | Activity | No | Pa | Pi | Activity |
|---|---|---|---|---|---|---|---|
| 5c | 0.275 | 0.043 | Anticonvulsant | 6k | 0.286 | 0.129 | Anticonvulsant |
| 5d | 0.423 | 0.055 | Anticonvulsant | 6l | 0.386 | 0.039 | Anticonvulsant |
| 6b | 0.281 | 0.121 | Anticonvulsant | 6m | 0.430 | 0.053 | Anticonvulsant |
| 6c | 0.276 | 0.030 | Anticonvulsant | 6n | 0.284 | 0.127 | Anticonvulsant |
| 6h | 0.364 | 0.081 | Anticonvulsant | 7e | 0.362 | 0.082 | Anticonvulsant |
| 6i | 0.267 | 0.142 | Anticonvulsant | 7f | 0.336 | 0.098 | Anticonvulsant |
| 6j | 0.362 | 0.082 | Anticonvulsant | 7g | 0.430 | 0.053 | Anticonvulsant |
| Compound | ED50 * mg/kg (by PTZ Antagonism) | TD50 * mg/kg | LD50 * mg/kg | TI | Latency of Convulsions Induced by TSC, min | |
|---|---|---|---|---|---|---|
| М ± м | I ** | |||||
| Control | – | – | – | 69 ± 2.0 | 1.0 | |
| 6b | 35.0 (23.0 ÷ 56.1) *** | >200 | 450 (375 ÷ 548) | 13 | 123 ± 15.2 | 1.78 |
| 6j | 35.0 (23.0 ÷ 56.7) | >200 | 580.0 (464 ÷ 725) | 17 | 90.0 ± 12.3 | 1.3 |
| 6k | 30.0 (17.6 ÷ 51.0) | >200 | 620 (504 ÷ 762.6) | 21 | 125 ± 10.8 | 1.8 |
| 6n | 41.0 (22.5 ÷ 74.6) | >200 | 930 (808.7 ÷ 1070) | 23 | 158 ± 13.5 | 2.3 |
| 7c | 42.0 (22.5 ÷ 75.1) | >200 | 890 (712 ÷ 1112.5) | 21 | 113 ± 10.1 | 1.64 |
| 7h | 30.0 (17.6 ÷ 51.0) | >200 | 680 (557 ÷ 829.6) | 23 | 116 ± 16.8 | 1.68 |
| Ethosuximide (200 mg/kg) | 155 (117.5 ÷ 204.5) | 520 (413÷655) | 1325 (1200 ÷ 1462) | 8.5 | 118 ± 14.0 | 1.7 |
| Diazepam (2 mg/kg) | 0.5 (0.4 ÷ 0.7) | 2.7 (1.4 ÷ 5.5) | 180 (128.5 ÷ 252.0) | 360 | 65 ± 3.5 | 0.9 |
| Compound | Amount (Absolute Data during 5 min) * | ||
|---|---|---|---|
| Horizontal Displacement | Vertical Displacement | Cells | |
| Control | 14.4 (11.5 ÷ 18.0) | 3.4 (2.8 ÷ 4.1) | 0.8 (0.4 ÷ 1.2) |
| 6b | 57.4 (44.6 ÷ 70.2) ** | 9.6 (7.3 ÷ 11.9) ** | 4.0 (2.8 ÷ 5.2) ** |
| 6j | 57.6 (30.9 ÷ 84.3) ** | 11.0 (7.4 ÷ 14.6) ** | 4.2 (3.0 ÷ 5.4)** |
| 6k | 49.6 (39.7 ÷ 62.0) ** | 9.0 (6.9 ÷ 11.1) ** | 2.4 (1.3 ÷ 3.5) ** |
| 6n | 64.6 (48.5 ÷ 80.7) ** | 7.0 (5.8 ÷ 8.4) ** | 5.0 (2.4 ÷ 7.4) ** |
| 7c | 51.0 (36.6 ÷ 65.4) ** | 8.5 (4.6 ÷ 12.4) ** | 3.2 (1.2 ÷ 5.2) ** |
| 7h | 55.0 (42.6 ÷ 67.4) ** | 12.0 (5.9 ÷ 18.1)** | 3.4 (2.1 ÷ 4.7) ** |
| Ethosuximide | 16.8 (13.4 ÷ 21.0) | 3.6 (3.0 ÷ 4.4) | 0.6 (0.5 ÷ 0.72) |
| Diazepam | 33.6 (27.7 ÷ 40.7) ** | 6.4 (5.4 ÷ 7.4) ** | 5.0 (4.0 ÷ 6.25) ** |
| Compound Dose 50 mg/kg | Time Spent in Closed Arms /s/* | Number of Entries into the Closed Arms * | Time Spent in the Center /s/* | Time Spent in the Open Arms /s/* |
|---|---|---|---|---|
| Control | 271.8 (246.2 ÷ 294.4) | 5.0 (3.5 ÷ 7.7) | 28.1 (23.0 ÷ 33.7) | – |
| 6j | 212 (184.3 ÷ 243.8) ** | 2.6 (0.8 ÷ 4.4) | 85.0 (51.6 ÷ 118.4) ** | – |
| 6k | 197 (168.3 ÷ 230.5) ** | 4.4 (1.9 ÷ 6.9) | 102.4 (75.2 ÷ 177.6) ** | – |
| 7h | 209.8 (182.4 ÷ 241) ** | 8.0 (5.5 ÷ 10.5) | 90.2 (50.5 ÷ 129.9) ** | – |
| 6n | 182.0 (155.6 ÷ 209) ** | 6.2 (4.0 ÷ 8.2) | 118.0 (75.1 ÷ 193.1) ** | 21.8 (18.3 ÷ 40) ** |
| 6b | 166 (123.7 ÷ 208.7) ** | 6.0 (4.2 ÷ 7.8) | 116 (81.7 ÷ 115.9) ** | 17.0 (5.9 ÷ 28.1) ** |
| 7c | 178.0 (130.9 ÷ 225) ** | 4.0 (2.2 ÷ 6.2) | 108.0 (80.1 ÷ 135.9) ** | 14.0 (3.3 ÷ 24.7) ** |
| Ethosuximide (200 mg/kg) | 247.2 (212.9 ÷ 277.5) | 8.1 (5.6 ÷ 10.6) | 52.8 (44.0 ÷ 63.4) ** | – |
| Diazepam (2 mg/kg) | 257.5 (226.2 ÷ 288.8) | 5.5 (4.58 ÷ 6.6) | 42.5 (34.8 ÷ 51.85) ** | 57 (47.5 ÷ 68.4) ** |
| Compound | Dose mg/kg | Time of Active Swimming (s), Latent Period First Immobilization * | Total Time of Immobilization (s) * | Total Time of Active Swimming (s) * |
|---|---|---|---|---|
| Control | – | 127.5 (106.3 ÷ 155.6) | 42.8 (29.4 ÷ 57.2) | 317.2 (302.8 ÷ 331.6) |
| 6b | 50 | 132.0 (111.4 ÷ 152.6) | 56.0 (28.6 ÷ 83.4) | 304.0 (265.9 ÷ 342.1) |
| 6j | 50 | 168.0 (140 ÷ 201.6) | 135.2 (87.4 ÷ 183.0) ** | 140.8 (84.3 ÷ 196.7) ** |
| 6k | 50 | 188.0 (163.5 ÷ 216.2) ** | 79.0 (59.5 ÷ 98.5) ** | 281.0 (226.8 ÷ 355.2) |
| 6n | 50 | 146.0 (120.0 ÷ 175.2) | 44.0 (35.9 ÷ 52.1) | 316.0 (293.4 ÷ 338.6) |
| 7c | 50 | 164.0 (138.3 ÷ 189.7) | 49.0 (36.3 ÷ 61.7) | 311.0 (275.5 ÷ 346.5) |
| 7h | 50 | 172.0 (139.4 ÷ 204.6) | 81.0 (65.1 ÷ 96.9) ** | 279.0 (263.2 ÷ 294.8) ** |
| Ethosuximide | 200 | 125 (102.4 ÷ 152.5) | 98 (75.3 ÷ 127.4) ** | 262 (199.9 ÷ 324.1) |
| Diazepam | 2 | 174 (144.0 ÷ 204.0) | 24 (20.0 ÷ 28.8) ** | 336 (282.6 ÷ 389.4) |
| Compound | Est. Binding Energy (kcal/mol) | I-H | Residues Involved in Hydrogen Bond Formation | Hydrophobic Interactions | Aromatic Interactions |
|---|---|---|---|---|---|
| 6b | −8.02 | 1 | Thr202 | Ala25, Tyr62, Leu99, Phe200 | Phe200 |
| 6j | −7.14 | 1 | Tyr202 | Tyr62, Leu99, Phe200 | Phe200 |
| 6k | −7.95 | 1 | Thr202 | Thr176, Phe200, Ala201 | - |
| 6n | −6.37 | 1 | Thr62 | Leu99, Phe200 | - |
| 7c | −6.25 | - | - | Ty157, Thr202, Tyr205 | - |
| 7h | −7.63 | 1 | Thr62 | Leu99, Phe200, Thr202 | - |
| Diazepam | −8.90 | 1 | Thr202 | Tyr62, Thr176, Phe200, Ala201, Tyr205 | Phe200 |
| No. | Est. Binding Energy (kcal/mol) | I-H | Residues Involved in Hydrogen Bond Formation | Residues Involved in Van der Waals Interactions | Residues Involved in pi-pi Interactions |
|---|---|---|---|---|---|
| 6b | −8.96 | 1 | Arg7 | Arg431, Gly432, Gly433, Lys436, Asp267 | Asp265, Ile434 |
| 6j | −7.93 | 1 | Arg7 | Asp267, Gly432, Gly433, Lys436 | Lys264 |
| 6k | −7.26 | - | - | Arg7, Arg431, Gly432, Gly433 | Asp265, Lys264 |
| 6n | −10.25 | 2 | Arg7, Gln266 | Asp267, Gly432, Gly433 | Arg7, Arg263, Lys264, Asp265, Ile434 |
| 7c | −8.72 | 1 | Arg7 | Asp267, Gly432, Gly433 | Asp265, Lys264 |
| 7h | −7.85 | 1 | Asp265 | Arg7, Gly432, Lys436 | Asp265 |
| No. | Est. Binding Energy (kcal/mol) | I-H | Residues Involved in Hydrogen Bond Formation | Residues Involved in Van der Waals Interactions | Residues Involved in pi-pi Interactions |
|---|---|---|---|---|---|
| 6b | −10.16 | 2 | Asn312, Tyr316 | Trp109, Asp113, Thr118, Ser204, Phe108, Trp286 | Val114, Val117 |
| 6j | −7.35 | 1 | Tyr308 | Asp113, Thr118, Ala200, Ser204, Trp286, Asn312 | Val114, Phe290 |
| 6k | −8.13 | 1 | Asn312 | Asp113, Ala200, Ser207, Phe108, Trp286, Tyr308 | Val114, Val117, Tyr199 |
| 6n | −11.21 | 3 | Asn293, Tyr308, Tyr316 | Trp109, Thr110, Asp113, Thr118, Ala200, Ser204, Ser207, Phe108, Trp286, Asn312 | Val114, Val117, Phe193, Tyr199, Phe290 |
| 7c | −9.67 | 2 | Tyr308, Tyr316 | Asp113, Ser204, Ser207, Phe108, Asn312 | Val114, Phe193, Phe290 |
| 7h | −6.88 | - | - | Trp109, Thr110, Asp113, Ala200, Ser204, Phe108, Trp286, Asn312 | Val114, Val117, Phe290 |
| Alprenolol | −13.19 | 4 | Asp113, Asn312, Tyr316 | Met82, Thr110, Tyr118, Ser203, Ser207, Trp286, Phe289, Ala200, Tyr308 | Trp109, Val114, Phe193, Tyr199, Phe290 |
| No. | MW | Number of HBA a | Number of HBD b | Log Po/w (iLOGP) c | Log S d | TPSA e | BBB Permeant f | Lipinski, Ghose, Veber, Egan, and Muegge Violations | Bioavailability SCORE | Drug-Likeness Model Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 295.34 | 4 | 0 | 2.83 | Moderately soluble | 60.92 | Yes | 0 | 0.55 | 0.61 |
| 5b | 309.36 | 4 | 0 | 3.11 | Moderately soluble | 60.92 | Yes | 0 | 0.55 | 0.90 |
| 5c | 323.35 | 5 | 0 | 0 | Moderately soluble | 70.15 | No | 0 | 0.55 | 0.33 |
| 5d | 357.36 | 5 | 0 | 0 | Poorly soluble | 70.15 | Yes | 0 | 0.55 | 0.33 |
| 6a | 323.39 | 4 | 0 | 3.44 | Moderately soluble | 60.92 | Yes | 0 | 0.55 | 0.61 |
| 6b | 337.42 | 4 | 0 | 3.60 | Poorly soluble | 60.92 | Yes | 0 | 0.55 | 0.97 |
| 6c | 363.45 | 4 | 0 | 3.80 | Poorly soluble | 60.92 | Yes | 0 | 0.55 | 0.64 |
| 6d | 309.36 | 4 | 0 | 3.07 | Moderately soluble | 60.92 | Yes | 0 | 0.55 | 0.57 |
| 6e | 323.39 | 4 | 0 | 3.31 | Moderately soluble | 60.92 | Yes | 0 | 0.55 | 0.63 |
| 6f | 337.42 | 4 | 0 | 3.56 | Moderately soluble | 60.92 | Yes | 0 | 0.55 | 0.55 |
| 6g | 351.44 | 4 | 0 | 3.68 | Poorly soluble | 60.92 | Yes | 0 | 0.55 | 1.01 |
| 6h | 339.39 | 5 | 0 | 3.28 | Moderately soluble | 70.15 | Yes | 0 | 0.55 | 0.22 |
| 6i | 353.41 | 5 | 0 | 3.48 | Poorly soluble | 70.15 | Yes | 0 | 0.55 | 0.51 |
| 6j | 367.44 | 5 | 0 | 3.73 | Poorly soluble | 70.15 | Yes | 0 | 0.55 | 0.57 |
| 6k | 381.47 | 5 | 0 | 3.99 | Poorly soluble | 70.15 | Yes | 0 | 0.55 | 0.52 |
| 6l | 381.47 | 5 | 0 | 4.00 | Poorly soluble | 70.15 | Yes | 0 | 0.55 | 0.54 |
| 6m | 401.46 | 5 | 0 | 3.76 | Poorly soluble | 70.15 | Yes | 0 | 0.55 | 0.33 |
| 6n | 391.42 | 6 | 0 | 3.62 | Poorly soluble | 83.29 | Yes | 0 | 0.55 | 0.26 |
| 7a | 377.48 | 4 | 0 | 3.95 | Poorly soluble | 60.92 | Yes | 0 | 0.55 | 0.64 |
| 7b | 361.39 | 5 | 0 | 3.49 | Poorly soluble | 74.06 | Yes | 0 | 0.55 | 0.53 |
| 7c | 323.29 | 4 | 0 | 3.24 | Moderately soluble | 60.92 | Yes | 0 | 0.55 | 0.57 |
| 7d | 337.42 | 4 | 0 | 3.43 | Poorly soluble | 60.92 | Yes | 0 | 0.55 | 0.51 |
| 7e | 381.47 | 5 | 0 | 3.84 | Poorly soluble | 70.15 | Yes | 0 | 0.55 | 0.54 |
| 7f | 395.49 | 5 | 0 | 4.05 | Poorly soluble | 70.15 | Yes | 0 | 0.55 | 0.66 |
| 7g | 415.48 | 5 | 0 | 3.93 | Poorly soluble | 70.15 | Yes | 0 | 0.55 | 0.33 |
| 7h | 405.45 | 6 | 0 | 3.77 | Poorly soluble | 83.29 | No | 0 | 0.55 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Kartsev, V.; Hakobyan, E.K.; Petrou, A.; Paronikyan, R.G.; Nazaryan, I.M.; Akopyan, H.H.; Hovakimyan, A.A. Synthesis and Neurotropic Activity of New Heterocyclic Systems: Pyridofuro[3,2-d]pyrrolo[1,2-a]pyrimidines, Pyridofuro[3,2-d]pyrido[1,2-a]pyrimidines and Pyridofuro[3′,2′:4,5]pyrimido[1,2-a]azepines. Molecules 2021, 26, 3320. https://doi.org/10.3390/molecules26113320
Sirakanyan SN, Spinelli D, Geronikaki A, Kartsev V, Hakobyan EK, Petrou A, Paronikyan RG, Nazaryan IM, Akopyan HH, Hovakimyan AA. Synthesis and Neurotropic Activity of New Heterocyclic Systems: Pyridofuro[3,2-d]pyrrolo[1,2-a]pyrimidines, Pyridofuro[3,2-d]pyrido[1,2-a]pyrimidines and Pyridofuro[3′,2′:4,5]pyrimido[1,2-a]azepines. Molecules. 2021; 26(11):3320. https://doi.org/10.3390/molecules26113320
Chicago/Turabian StyleSirakanyan, Samvel N., Domenico Spinelli, Athina Geronikaki, Victor Kartsev, Elmira K. Hakobyan, Anthi Petrou, Ruzanna G. Paronikyan, Ivetta M. Nazaryan, Hasmik H. Akopyan, and Anush A. Hovakimyan. 2021. "Synthesis and Neurotropic Activity of New Heterocyclic Systems: Pyridofuro[3,2-d]pyrrolo[1,2-a]pyrimidines, Pyridofuro[3,2-d]pyrido[1,2-a]pyrimidines and Pyridofuro[3′,2′:4,5]pyrimido[1,2-a]azepines" Molecules 26, no. 11: 3320. https://doi.org/10.3390/molecules26113320
APA StyleSirakanyan, S. N., Spinelli, D., Geronikaki, A., Kartsev, V., Hakobyan, E. K., Petrou, A., Paronikyan, R. G., Nazaryan, I. M., Akopyan, H. H., & Hovakimyan, A. A. (2021). Synthesis and Neurotropic Activity of New Heterocyclic Systems: Pyridofuro[3,2-d]pyrrolo[1,2-a]pyrimidines, Pyridofuro[3,2-d]pyrido[1,2-a]pyrimidines and Pyridofuro[3′,2′:4,5]pyrimido[1,2-a]azepines. Molecules, 26(11), 3320. https://doi.org/10.3390/molecules26113320







