Antiprotozoal and Antibacterial Activity of Ravenelin, a Xanthone Isolated from the Endophytic Fungus Exserohilum rostratum
Abstract
:1. Introduction
2. Results
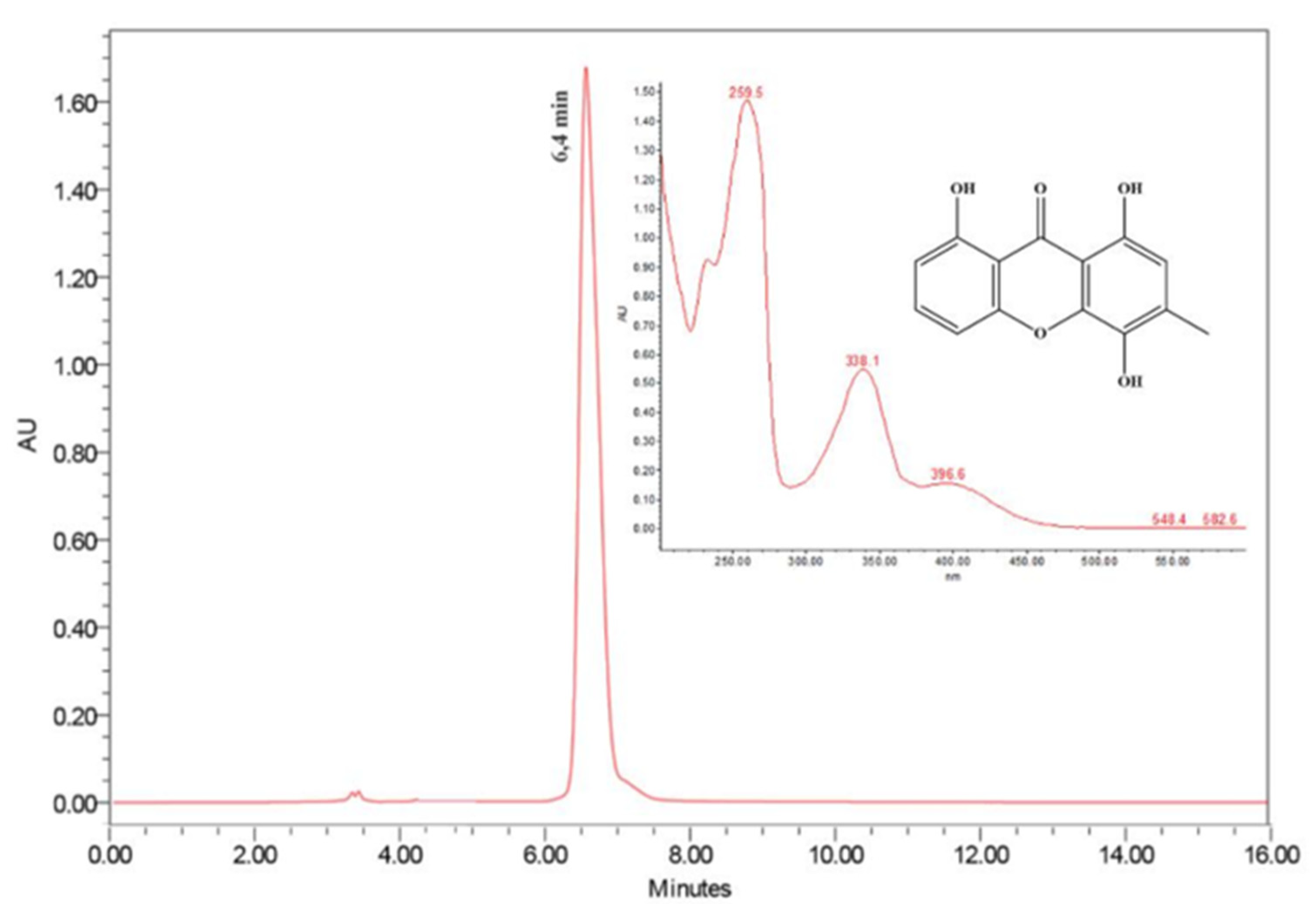
2.1. Isolation and Characterization of Ravenelin
2.2. Antibacterial Activity of Ravenelin
2.3. Antiplasmodial and Anti-Trypanosoma Effect of Ravenelin
2.4. Cytotoxicity and Selectivity Index of Ravenelin
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. E. rostratum Isolation
4.3. Culture of E. Rostratum in Rice and Compound Isolation
4.4. NMR and MS Analysis
4.5. Parasites
4.6. Animals
4.7. Cell Culture
4.8. Antimicrobial Assay
4.9. Antiplasmodial Assay
4.10. Antitrypanosomal Activity Assay
4.11. Cytotoxicity Test and Selectivity Index
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Camele, I.; Elshafie, H.S.; Caputo, L.; Sakr, S.H.; De Feo, V. Bacillus mojavensis: Biofilm formation and biochemical investigation of its bioactive metabolites. J. Biol. Res. 2019, 92, 8296. [Google Scholar]
- Gaynes, R. The Discovery of Penicillin—New Insights after More Than 75 Years of Clinical Use. Emerg. Infect. Dis. 2017, 23, 849–853. [Google Scholar]
- Newman, J.D.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar]
- Massand, M.; Jose, P.A.; Menghani, E.; Jebakumar, S.R.D. Continuing hunt for endophytic actinomycetes as a source of novel biologically active metabolites. World J. Microbiol. Biotechnol. 2015, 12, 1863–1875. [Google Scholar]
- Gutierrez, R.M.P.; Gonzalez, A.M.N.; Ramirez, A.M. Compounds Derived from Endophytes: A Review of Phytochemistry and Pharmacology. Curr. Med. Chem. 2012, 19, 2992–3030. [Google Scholar]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar]
- Capela, R.; Moreira, R.; Lopes, F. An Overview of Drug Resistance in Protozoal Diseases. Int. J. Mol. Sci. 2019, 20, 5748. [Google Scholar]
- Wang, C.; Tang, S.; Cao, S. Antimicrobial compounds from marine fungi. Phytochem. Rev. 2021, 20, 85–117. [Google Scholar]
- Sappapan, R.; Sommit, D.; Ngamrojanavanich, N.; Pengpreecha, S.; Wiyakrutta, S.; Sriubolmas, N.; Pudhom, K. 11-Hydroxymonocerin from the Plant Endophytic Fungus Exserohilum rostratum. J. Nat. Prod. 2008, 7, 1657–1659. [Google Scholar]
- Peres, V.; Nagem, T.J.; Oliveira, F.F. Tetraoxygenated naturally occurring xanthones. Phytochemistry 2000, 55, 683–710. [Google Scholar]
- Song, X.Q.; Zhang, X.; Han, Q.J.; Li, X.B.; Li, G.; Li, R.J.; Jiao, Y.; Zhou, J.C.; Lou, H.X. Xanthone derivatives from Aspergillus sydowii, an endophytic fungus from the liverwort Scapania ciliat S. Lac and their immunosuppressive activities. Phytochem. Lett. 2013, 6, 318–321. [Google Scholar]
- Mandal, S.; Das, P.C.; Joshi, P.C. Naturally occurring xanthones from terrestrial flora. J. Indian Chem. Soc. 1992, 69, 611–636. [Google Scholar]
- Zhang, S.J.; Ding, Z.S.; Jiang, F.S.; Ge, Q.F.; Guo, D.W.; Lid, H.B.; Hu, W.X. Synthesis, anticancer evaluation and docking study of vadimezan derivatives with carboxyl substitution. MedChemComm 2014, 5, 512–520. [Google Scholar]
- Pytka, K.; Żmudzka, E.; Lustyk, K.; Rapacz, A.; Olczyk, A.; Gałuszka, A.; Waszkielewicz, A.; Marona, H.; Sapa, J.; Barbara, F. The antidepressant- and anxiolytic-like activities of new xanthone derivative with piperazine moiety in behavioral tests in mice. Indian J. Pharmacol. 2016, 48, 286–291. [Google Scholar]
- Ghosal, S.; Chaudhuri, R.K. Chemical constituents of gentianaceae XVI: Antitubercular activity of xanthones of Canscora decussata Schult. J. Pharm. Sci. 1975, 64, 888–889. [Google Scholar]
- Araújo, J.; Fernandes, C.; Pinto, M.; Tiritan, M.E. Chiral Derivatives of Xanthones with Antimicrobial Activity. Molecules 2019, 24, 314. [Google Scholar]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar]
- Kang, H.H.; Zhang, H.B.; Zhong, M.J.; Ma, L.Y.; Liu, D.S.; Liu, W.Z.; Ren, H. Potential Antiviral Xanthones from a Coastal Saline Soil Fungus Aspergillus iizukae. Mar. Drugs 2018, 16, 449. [Google Scholar]
- Zhao, Y.; Liu, J.P.; Lu, D.; Li, P.Y.; Zhang, L.X. A new antioxidant xanthone from the pericarp of Garcinia mangostana Linn. Nat. Prod. Res. 2010, 24, 1664–1670. [Google Scholar]
- Feng, Z.; Lu, X.; Gan, L.; Zhang, Q.; Lin, L. Xanthones, A Promising Anti-Inflammatory Scaffold: Structure, Activity, and Drug Likeness Analysis. Molecules 2020, 25, 598. [Google Scholar]
- Mbwambo, Z.H.; Kapingu, M.C.; Moshi, M.J.; Machumi, F.; Apers, S.; Cos, P.; Ferreira, D.; Marais, J.P.; Vanden Berghe, D.; Maes, L.; et al. Antiparasitic activity of some xanthones and biflavonoids from the root bark of Garcinia livingstonei. J. Nat. Prod. 2006, 69, 369–372. [Google Scholar]
- Cechinel Filho, V. Chemical composition and biological potential of plants from the genus Bauhinia. Phytother. Res. 2009, 23, 1347–1354. [Google Scholar]
- Pinheiro, E.A.A.; Borges, F.C.; Pina, J.R.S.; Ferreira, L.R.S.; Cordeiro, J.S.; Carvalho, J.M.; Feitosa, A.O.; Campos, F.R.; Barison, A.; Souza, A.D.L.; et al. Annularins I and J: New metabolites isolated from endophytic fungus Exserohilum rostratum. J. Braz. Chem. Soc. 2016, 27, 1432–1436. [Google Scholar]
- Raistrick, H.; Robinson, R.; White, D.E. Studies in the biochemistry of micro-organisms: Ravenelin (3-methyl-1:4:8-trihydroxyxanthone), a new metabolic product of Helminthosporium Ravenelii Curtis and of H. turcicum Passerini. Biochem. J. 1936, 30, 1303–1314. [Google Scholar]
- Westerman, P.W.; Gunasekera, S.P.; Uvais, M.; Sultanbawa, S.; Kazlauskas, R. Carbon-13 N.M.R. study of naturally occurring xanthones. Org. Magn. Reson. 1977, 9, 631–636. [Google Scholar]
- Auranwiwat, C.; Trisuwan, K.; Saiai, A.; Pyne, S.G.; Ritthiwigrom, T. Antibacterial tetraoxygenated xanthones from the immature fruits of Garcinia cowa. Fitoterapias 2014, 98, 179–183. [Google Scholar]
- Al-Massarani, S.M.; El-Gamal, A.A.; Al-Musayeib, N.M.; Mothana, R.A.; Basudan, O.A.; Al-Rehaily, A.J.; Farag, M.; Assaf, M.H.; El Tahir, K.H.; Maes, L. Phytochemical, antimicrobial and antiprotozoal evaluation of Garcinia mangostana pericarp and α-mangostin, its major xanthone derivative. Molecules 2013, 18, 10599–10608. [Google Scholar]
- Padhi, S.; Masi, M.; Cimmino, A.; Tuzi, A.; Jena, A.; Tayung, K.; Evidente, A. Funiculosone, a substituted dihydroxanthene-1,9-dione with of its analogues proced by na endolichenic fungus Talaromyces funiculosus and their antimicrobial activity. Phytochemistry 2019, 157, 175–183. [Google Scholar]
- Isaka, M.; Palasarn, S.; Kocharin, K.; Saenboonrueng, J. A Cytotoxic Xanthone Dimer from the Entomopathogenic Fungus Aschersonia sp. BCC 8401. J. Nat. Prod. 2005, 68, 945–946. [Google Scholar]
- Isaka, M.; Jaturapat, A.; Rukseree, K.; Danwisetkanjana, K.; Tanticharoen, M.; Thebtaranonth, Y. Phomoxanthones A and B, novel xanthone dimers from the endophytic fungus Phomopsis species. J. Nat. Prod. 2001, 64, 1015–1018. [Google Scholar]
- Dua, V.K.; Verma, G.; Dash, A.P. In Vitro Antiprotozoal Activity of Some Xanthones Isolated from the Roots of Andrographis paniculata. Phytother. Res. 2009, 23, 126–128. [Google Scholar]
- Weniger, B.; Robledo, S.; Arango, G.J.; Deharo, E.; Aragón, R.; Muñoz, V.; Callapa, J.; Lobstein, A.; Anton, R. Antiprotozoal activities of Colombian plants. J. Ethnopharmacol. 2001, 78, 193–200. [Google Scholar]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar]
- Pereira da Silva, L.H.; Nussenzweig, V. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para camundongos brancos. Folia Clin. Biol. 1953, 20, 191–208. [Google Scholar]
- Castellani, O.; Ribeiro, L.V.; Fernandes, J.F. Differentiation of Trypanosoma cruzi in culture. J. Protozool. 1967, 14, 447–451. [Google Scholar]
- Teles, A.M.; Silva, J.V.S.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.S.; Mendes-Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evid. Based Complementary Altern. Med. 2021, 2021, 6663255. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 8th ed.; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2017. [Google Scholar]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar]
- Vossen, M.G.; Pferschy, S.; Chiba, P.; Noedl, H. The SYBR Green I malaria drug sensitivity assay: Performance in low parasitemia samples. Am. J. Trop. Med. Hyg. 2010, 82, 398–401. [Google Scholar]
- Teles, A.M.; Silva, J.V.S.; Fernandes, J.M.P.; Calabrese, K.S.; Abreu-Silva, A.L.; Marinho, S.C.; Mouchrek, A.N.; Mouchrek-Filho, V.E.; Almeida-Souza, F. Aniba rosaeodora (Var. amazonica Ducke) Essential Oil: Chemical Composition, Antibacterial, Antioxidant and Antitrypanosomal Activity. Antibiotics 2021, 10, 24. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- Almeida-Souza, F.; Silva, V.D.; Silva, G.X.; Taniwaki, N.N.; Hardoim, D.J.; Buarque, C.D.; Abreu-Silva, A.L.; Calabrese, K.S. 1,4-Disubstituted-1,2,3-Triazole Compounds Induce Ultrastructural Alterations in Leishmania amazonensis Promastigote: An in Vitro Antileishmanial and in Silico Pharmacokinetic Study. Int. J. Mol. Sci. 2020, 21, 6839. [Google Scholar]

| Compound | MIC (µM) | ||||
|---|---|---|---|---|---|
| Gram (+) Bacteria | Gram (−) Bacteria | ||||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | S. typhimurium | |
| Ravenelin | 7.5 | 484 | >1000 | >1000 | >1000 |
| Amoxicillin | 1.3 | 21.4 | 21.4 | 21.4 | 21.4 |
| Terramycin | 16.3 | 16.3 | 16.3 | 16.3 | 16.3 |
| Compounds | P. falciparum | T. cruzi | |
|---|---|---|---|
| IC50 (µM) | Epimastigote | Intracellular Amastigote | |
| IC50 (µM) | IC50 (µM) | ||
| Ravenelin | 3.4 ± 0.4 | 5 ± 1 | 9 ± 2 |
| Artesunate | 0.0085 ± 0.0008 | NA | NA |
| Benznidazole | NA | 22 ± 1 | 2 ± 1 |
| Compound | HepG2 | SI a | Peritoneal Macrophage | SI b |
|---|---|---|---|---|
| CC50 (µM) | CC50 (µM) | |||
| Ravenelin | >50 | >15 | 185 ± 1 | 21 |
| Artesunate | 110 ± 30 | 13,000 | NA | NA |
| Benznidazole | NA | ND | 666 ± 1 | 333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pina, J.R.S.; Silva-Silva, J.V.; Carvalho, J.M.; Bitencourt, H.R.; Watanabe, L.A.; Fernandes, J.M.P.; Souza, G.E.d.; Aguiar, A.C.C.; Guido, R.V.C.; Almeida-Souza, F.; et al. Antiprotozoal and Antibacterial Activity of Ravenelin, a Xanthone Isolated from the Endophytic Fungus Exserohilum rostratum. Molecules 2021, 26, 3339. https://doi.org/10.3390/molecules26113339
Pina JRS, Silva-Silva JV, Carvalho JM, Bitencourt HR, Watanabe LA, Fernandes JMP, Souza GEd, Aguiar ACC, Guido RVC, Almeida-Souza F, et al. Antiprotozoal and Antibacterial Activity of Ravenelin, a Xanthone Isolated from the Endophytic Fungus Exserohilum rostratum. Molecules. 2021; 26(11):3339. https://doi.org/10.3390/molecules26113339
Chicago/Turabian StylePina, Jeferson Rodrigo Souza, João Victor Silva-Silva, Josiwander Miranda Carvalho, Heriberto Rodrigues Bitencourt, Luciano Almeida Watanabe, Juan Matheus Pereira Fernandes, Guilherme Eduardo de Souza, Anna Caroline Campos Aguiar, Rafael Victorio Carvalho Guido, Fernando Almeida-Souza, and et al. 2021. "Antiprotozoal and Antibacterial Activity of Ravenelin, a Xanthone Isolated from the Endophytic Fungus Exserohilum rostratum" Molecules 26, no. 11: 3339. https://doi.org/10.3390/molecules26113339
APA StylePina, J. R. S., Silva-Silva, J. V., Carvalho, J. M., Bitencourt, H. R., Watanabe, L. A., Fernandes, J. M. P., Souza, G. E. d., Aguiar, A. C. C., Guido, R. V. C., Almeida-Souza, F., Calabrese, K. d. S., Marinho, P. S. B., & Marinho, A. M. d. R. (2021). Antiprotozoal and Antibacterial Activity of Ravenelin, a Xanthone Isolated from the Endophytic Fungus Exserohilum rostratum. Molecules, 26(11), 3339. https://doi.org/10.3390/molecules26113339










