Modulation of Serotonin Receptors in Neurodevelopmental Disorders: Focus on 5-HT7 Receptor
Abstract
:1. Introduction
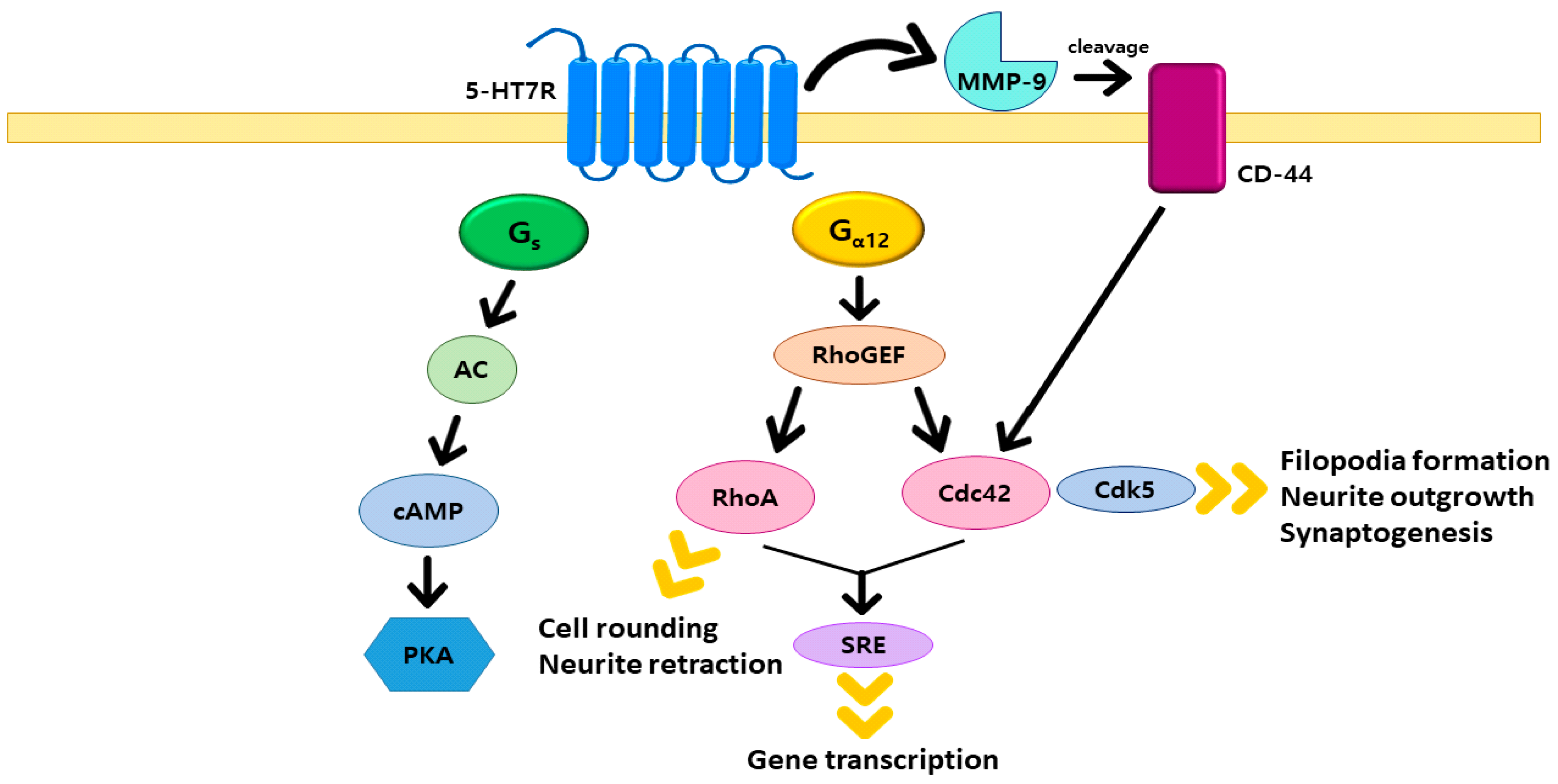
2. 5-HT7R/G12 Signaling Pathway
3. Autism Spectrum Disorder
4. Fragile X Syndrome (FXS)
5. Rett Syndrome
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morris-Rosendahl, D.J.; Crocq, M.-A. Neurodevelopmental disorders—The history and future of a diagnostic concept. Dialog-Clin. Neurosci. 2020, 22, 65–72. [Google Scholar] [CrossRef]
- Niemi, M.; Martin, H.C.; Rice, D.L.; Gallone, G.; Gordon, S.; Kelemen, M.; McAloney, K.; McRae, J.; Radford, E.J.; Yu, S.; et al. Common genetic variants contribute to risk of rare severe neurodevelopmental disorders. Nat. Cell Biol. 2018, 562, 268–271. [Google Scholar] [CrossRef] [Green Version]
- Tărlungeanu, D.C.; Novarino, G. Genomics in neurodevelopmental disorders: An avenue to personalized medicine. Exp. Mol. Med. 2018, 50, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristino, A.S.; Williams, S.M.; Hawi, Z.; An, J.Y.; Bellgrove, M.A.; Schwartz, C.E.; Costa, L.D.F.; Claudianos, C. Neurodevelopmental and Neuropsychiatric Disorders Represent an Interconnected Molecular System. Mol. Psychiatry 2014, 19, 294–301. [Google Scholar] [CrossRef]
- Avni, E.; Ben-Itzchak, E.; Zachor, D.A. The Presence of Comorbid ADHD and Anxiety Symptoms in Autism Spectrum Disorder: Clinical Presentation and Predictors. Front. Psychiatry 2018, 9, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-P. Synaptic Formation, Neural Circuits and Neurodevelopmental Disorders Controlled by Signaling, Translation, and Epigenetic Regulation. Dev. Neurobiol. 2019, 79, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Ismail, F.Y.; Shapiro, B.K. What Are Neurodevelopmental Disorders? Curr. Opin. Neurol. 2019, 32, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Häusser, M.; Spruston, N.; Stuart, G.J. Diversity and Dynamics of Dendritic Signaling. Science 2000, 290, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.; Alshammari, F.; Hughes, E.; Khanbabaei, M.; Rho, J.M. Dendritic overgrowth and elevated ERK signaling during neonatal development in a mouse model of autism. PLoS ONE 2017, 12, e0179409. [Google Scholar] [CrossRef]
- Jiang, M.; Ash, R.T.; Baker, S.A.; Suter, B.; Ferguson, A.; Park, J.; Rudy, J.; Torsky, S.P.; Chao, H.T.; Zoghbi, H.Y.; et al. Dendritic aroborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. J. Neurosci. 2013, 33, 19518–19533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgeron, T. A synaptic trek to autism. Curr. Opin. Neurobiol. 2009, 19, 231–234. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V. Dendrite and spine modifications in autism and related neurodevelopmental disorders in patients and animal models. Dev. Neurobiol. 2017, 77, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Stigler, K.A.; Diener, J.T.; Kohn, A.E.; Li, L.; Erickson, C.A.; Posey, D.J.; McDougle, C.J. Aripiprazole in Pervasive Developmental Disorder Not Otherwise Specified and Asperger’s Disorder: A 14-Week, Prospective, Open-Label Study. J. Child Adolesc. Psychopharmacol. 2009, 19, 265–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, R.N.; Owen, R.; Kamen, L.; Manos, G.; McQuade, R.D.; Carson, W.H.; Aman, M.G. A Placebo-Controlled, Fixed-Dose Study of Aripiprazole in Children and Adolescents with Irritability Associated with Autistic Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 1110–1119. [Google Scholar] [CrossRef]
- Blankenship, K.; Erickson, C.A.; Stigler, K.A.; Posey, D.J.; McDougle, C.J. Aripiprazole for irritability associated with autistic disorder in children and adolescents aged 6–17 years. Pediatr. Health 2010, 4, 375–381. [Google Scholar] [CrossRef]
- Knight, J.A.; Smith, C.; Toohey, N.; Klein, M.T.; Teitler, M. Pharmacological Analysis of the Novel, Rapid, and Potent Inactivation of the Human 5-Hydroxytryptamine7 Receptor by Risperidone, 9-OH-Risperidone, and Other Inactivating Antagonists. Mol. Pharmacol. 2009, 75, 374–380. [Google Scholar] [CrossRef]
- Smith, C.; Toohey, N.; Knight, J.A.; Klein, M.T.; Teitler, M. Risperidone-Induced Inactivation and Clozapine-Induced Reactivation of Rat Cortical Astrocyte 5-Hydroxytryptamine7 Receptors: Evidence for In Situ G Protein-Coupled Receptor Homodimer Protomer Cross-Talk. Mol. Pharmacol. 2011, 79, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Loebel, A.; Brams, M.; Goldman, R.S.; Silva, R.; Hernandez, D.; Deng, L.; Mankoski, R.; Findling, R.L. Lurasidone for the Treatment of Irritability Associated with Autistic Disorder. J. Autism Dev. Disord. 2016, 46, 1153–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homberg, J.R.; Kolk, S.M.; Schubert, D. Editorial perspective of the Research Topic “Deciphering serotonin’s role in neurodevelopment”. Front. Cell. Neurosci. 2013, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Leopoldo, M.; Lacivita, E.; Berardi, F.; Perrone, R.; Hedlund, P.B. Serotonin 5-HT7 receptor agents: Structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol. Ther. 2011, 129, 120–148. [Google Scholar] [CrossRef] [Green Version]
- Krobert, K.; Bach, T.; Syversveen, T.; Kvingedal, A.; Levy, F. The cloned human 5-HT 7 receptor splice variants: A comparative characterization of their pharmacology, function and distribution. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001, 363, 620–632. [Google Scholar] [CrossRef]
- Kvachnina, E.; Liu, G.; Dityatev, A.; Renner, U.; Dumuis, A.; Richter, D.W.; Dityateva, G.; Schachner, M.; Voyno-Yasenetskaya, T.A.; Ponimaskin, E.G. 5-HT7 Receptor Is Coupled to Gα Subunits of Heterotrimeric G12-Protein to Regulate Gene Transcription and Neuronal Morphology. J. Neurosci. 2005, 25, 7821–7830. [Google Scholar] [CrossRef] [Green Version]
- Fukuhara, S.; Chikumi, H.; Gutkind, J.S. RGS-containing RhoGEFs: The missing link between transforming G proteins and Rho? Oncogene 2001, 20, 1661–1668. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Van Aelst, L.; Cline, H.T. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nat. Neurosci. 2000, 3, 217–225. [Google Scholar] [CrossRef]
- Ruchhoeft, M.L.; Ohnuma, S.-I.; McNeill, L.; Holt, C.E.; Harris, W.A. The Neuronal Architecture of Xenopus Retinal Ganglion Cells Is Sculpted by Rho-Family GTPases In Vivo. J. Neurosci. 1999, 19, 8454–8463. [Google Scholar] [CrossRef] [Green Version]
- Kobe, F.; Guseva, D.; Jensen, T.P.; Wirth, A.; Renner, U.; Hess, D.; Müller, M.; Medrihan, L.; Zhang, W.; Zhang, M.; et al. 5-HT7R/G12 Signaling Regulates Neuronal Morphology and Function in an Age-Dependent Manner. J. Neurosci. 2012, 32, 2915–2930. [Google Scholar] [CrossRef]
- Speranza, L.; Labus, J.; Volpicelli, F.; Guseva, D.; LaCivita, E.; Leopoldo, M.; Bellenchi, G.C.; Di Porzio, U.; Bijata, M.; Perrone-Capano, C.; et al. Serotonin 5-HT7 receptor increases the density of dendritic spines and facilitates synaptogenesis in forebrain neurons. J. Neurochem. 2017, 141, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Bijata, M.; Labus, J.; Guseva, D.; Stawarski, M.; Butzlaff, M.; Dzwonek, J.; Schneeberg, J.; Böhm, K.; Michaluk, P.; Rusakov, D.A.; et al. Synaptic Remodeling Depends on Signaling between Serotonin Receptors and the Extracellular Matrix. Cell Rep. 2017, 19, 1767–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.-F.; Chen, Y.-J.; Chu, M.-C.; Hsu, Y.-T.; Lu, T.-Y.; Chen, I.-T.; Chen, P.S.; Lin, H.-C. Deep Brain Stimulation Modified Autism-Like Deficits via the Serotonin System in a Valproic Acid-Induced Rat Model. Int. J. Mol. Sci. 2018, 19, 2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses, A. Serotonin, neural markers, and memory. Front. Pharmacol. 2015, 6, 143. [Google Scholar] [CrossRef]
- Neul, J.L.; Fang, P.; Barrish, J.; Lane, J.; Caeg, E.B.; Smith, E.O.; Zoghbi, H.; Percy, A.; Glaze, D.G. Specific Mutations in Methyl-CpG-Binding Protein 2 Confer Different Severity in Rett Syndrome. Neurology 2008, 70, 1313–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulikovskaja, L.; Sarajlija, A.; Savic-Pavicevic, D.; Dobricic, V.; Klein, C.; Westenberger, A. WDR45 mutations may cause a MECP2 mutation-negative Rett syndrome phenotype. Neurol. Genet. 2018, 4, e227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogliati, F.; Giorgini, V.; Masciadri, M.; Bonati, M.T.; Marchi, M.; Cracco, I.; Gentilini, D.; Peron, A.; Savini, M.N.; Spaccini, L.; et al. Pathogenic Variants in STXBP1 and in Genes for GABAa Receptor Subunities Cause Atypical Rett/Rett-like Phenotypes. Int. J. Mol. Sci. 2019, 20, 3621. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, B.; Valenti, D.; Chiodi, V.; Ferrante, A.; de Bari, L.; Fiorentini, C.; Domenici, M.R.; Ricceri, L.; Vacca, R.A.; Fabbri, A.; et al. Modulation of Rho GTPases rescues brain mitochondrial dysfunction, cognitive deficits and aberrant synaptic plasticity in female mice modeling Rett syndrome. Eur. Neuropsychopharmacol. 2015, 25, 889–901. [Google Scholar] [CrossRef]
- Ricceri, L.; De Filippis, B.; Laviola, G. Rett syndrome treatment in mouse models: Searching for effective targets and strategies. Neuropharmacology 2013, 68, 106–115. [Google Scholar] [CrossRef]
- De Filippis, B.; Nativio, P.; Fabbri, A.; Ricceri, L.; Adriani, W.; Lacivita, E.; Leopoldo, M.; Passarelli, F.; Fuso, A.; Laviola, G. Pharmacological Stimulation of the Brain Serotonin Receptor 7 as a Novel Therapeutic Approach for Rett Syndrome. Neuropsychopharmacology 2014, 39, 2506–2518. [Google Scholar] [CrossRef]
- De Filippis, B.; Chiodi, V.; Adriani, W.; Lacivita, E.; Mallozzi, C.; Leopoldo, M.; Domenici, M.R.; Fuso, A.; Laviola, G. Long-lasting beneficial effects of central serotonin receptor 7 stimulation in female mice modeling Rett syndrome. Front. Behav. Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Valenti, D.; De Bari, L.; Vigli, D.; Lacivita, E.; Leopoldo, M.; Laviola, G.; Vacca, R.A.; De Filippis, B. Stimulation of the brain serotonin receptor 7 rescues mitochondrial dysfunction in female mice from two models of Rett syndrome. Neuropharmacology 2017, 121, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Vigli, D.; Rusconi, L.; Valenti, D.; La Montanara, P.; Cosentino, L.; Lacivita, E.; Leopoldo, M.; Amendola, E.; Gross, C.; Landsberger, N.; et al. Rescue of prepulse inhibition deficit and brain mitochondrial dysfunction by pharmacological stimulation of the central serotonin receptor 7 in a mouse model of CDKL5 Deficiency Disorder. Neuropharmacology 2019, 144, 104–114. [Google Scholar] [CrossRef]
- Huber, K.M.; Gallagher, S.M.; Warren, S.T.; Bear, M.F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA 2002, 99, 7746–7750. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.T.; Spatuzza, M.; D’Antoni, S.; Bonaccorso, C.M.; Trovato, C.; Musumeci, S.A.; Leopoldo, M.; Lacivita, E.; Catania, M.V.; Ciranna, L. Activation of 5-HT7 Serotonin Receptors Reverses Metabotropic Glutamate Receptor-Mediated Synaptic Plasticity in Wild-Type and Fmr1 Knockout Mice, a Model of Fragile X Syndrome. Biol. Psychiatry 2012, 72, 924–933. [Google Scholar] [CrossRef]
- Costa, L.T.; Sardone, L.M.; LaCivita, E.; Leopoldo, M.; Ciranna, L. Novel agonists for serotonin 5-HT7 receptors reverse metabotropic glutamate receptor-mediated long-term depression in the hippocampus of wild-type and Fmr1 KO mice, a model of Fragile X Syndrome. Front. Behav. Neurosci. 2015, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Riobo, N.A.; Manning, D.R. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol. Sci. 2005, 26, 146–154. [Google Scholar] [CrossRef]
- Strathmann, M.P.; Simon, M.I. Gα12 and Gα13 Subunits Define a Fourth Class of G Protein α Subunits. Proc. Natl. Acad. Sci. USA 1991, 88, 5582–5586. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Singer, W.D.; Sternweis, P.C.; Sprang, S.R. Structure of the p115RhoGEF rgRGS domain–Gα13/i1 chimera complex suggests convergent evolution of a GTPase activator. Nat. Struct. Mol. Biol. 2005, 12, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Murga, C.; Zohar, M.; Igishi, T.; Gutkind, J.S. A Novel PDZ Domain Containing Guanine Nucleotide Ex-change Factor Links Heterotrimeric G Proteins to Rho. J. Biol. Chem. 1999, 274, 5868–5879. [Google Scholar] [CrossRef] [Green Version]
- Boureux, A.; Vignal, E.; Faure, S.; Fort, P. Evolution of the Rho Family of Ras-Like GTPases in Eukaryotes. Mol. Biol. Evol. 2007, 24, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zipkin, I.D.; Kindt, R.M.; Kenyon, C.J. Role of a New Rho Family Member in Cell Migration and Axon Guidance in C. elegans. Cell 1997, 90, 883–894. [Google Scholar] [CrossRef] [Green Version]
- Sit, S.-T.; Manser, E. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 2011, 124, 679–683. [Google Scholar] [CrossRef] [Green Version]
- Fromm, C.; Coso, O.A.; Montaner, S.; Xu, N.; Gutkind, J.S. The Small GTP-Binding Protein Rho Links G Protein-Coupled Receptors and Gα12 to the Serum Response Element and to Cellular Transformation. Proc. Natl. Acad. Sci. USA 1997, 94, 10098–10103. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Yuan, H.; Xie, W.; Simon, M.I.; Wu, D. Specific Involvement of G Proteins in Regulation of Serum Response Factor-mediated Gene Transcription by Different Receptors. J. Biol. Chem. 1998, 273, 27118–27123. [Google Scholar] [CrossRef] [Green Version]
- Herlenius, E.; Lagercrantz, H. Neurotransmitters and neuromodulators during early human development. Early Hum. Dev. 2001, 65, 21–37. [Google Scholar] [CrossRef]
- Yoon, S.H.; Choi, J.; Lee, W.J.; Do, J.T. Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder. J. Clin. Med. 2020, 9, 966. [Google Scholar] [CrossRef] [Green Version]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [Green Version]
- Eissa, N.; Al-Houqani, M.; Sadeq, A.; Ojha, S.K.; Sasse, A.; Sadek, B. Current Enlightenment about Etiology and Phar-macological Treatment of Autism Spectrum Disorder. Front. Neurosci. 2018, 12, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schain, R.J.; Freedman, D.X. Studies on 5-Bydroxyindole Metabolism in Autistic and Other Mentally Retarded Children. J. Pediatrics 1961, 58, 315–320. [Google Scholar] [CrossRef]
- Cook, E.; Leventhal, B.L. The serotonin system in autism. Curr. Opin. Pediatrics 1996, 8, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Oblak, A.; Gibbs, T.; Blatt, G.J. Reduced Serotonin Receptor Subtypes in a Limbic and a Neocortical Region in Autism. Autism Res. 2013, 6, 571–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenburg, C.; Blatt, G.J. Differential Serotonin Transporter (5-HTT) and 5-HT2 Receptor Density in Limbic and Ne-ocortical Areas of Adults and Children with Autism Spectrum Disorders: Implications for Selective Serotonin Reuptake Inhibitor Efficacy. J. Neurochem. 2019, 151, 642–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassig, J.P.; Vachirasomtoon, K.; Hartzell, K.; Leventhal, M.; Courchesne, E.; Lord, C.; Leventhal, B.L.; Cook, E.H. Physical mapping of the serotonin 5-HT7 receptor gene (HTR7) to chromosome 10 and pseudogene (HTR7P) to chromosome 12, and testing of linkage disequilibrium betweenHTR7 and autistic disorder. Am. J. Med Genet. 1999, 88, 472–475. [Google Scholar] [CrossRef]
- Ciranna, L.; Catania, M.V. 5-HT7 Receptors as Modulators of Neuronal Excitability, Synaptic Transmission and Plasticity: Physiological Role and Possible Implications in Autism Spectrum Disorders. Front. Cell. Neurosci. 2014, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-C.; Lin, H.-C.; Chan, Y.-H.; Gean, P.-W.; Yang, Y.K.; Chen, P.S. 5-HT1A-receptor agonist modified amygdala activity and amygdala-associated social behavior in a valproate-induced rat autism model. Int. J. Neuropsychopharmacol. 2013, 16, 2027–2039. [Google Scholar] [CrossRef] [Green Version]
- Canal, C.E.; Felsing, D.E.; Liu, Y.; Zhu, W.; Wood, J.T.; Perry, C.K.; Vemula, R.; Booth, R.G. An Orally Active Phenyl-aminotetralin-Chemotype Serotonin 5-HT7 and 5-HT1A Receptor Partial Agonist That Corrects Motor Stereotypy in Mouse Models. ACS Chem. Neurosci. 2015, 6, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A Comprehensive Resource for in Silico Drug Discovery and Exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef]
- Lacivita, E.; Niso, M.; Mastromarino, M.; Garcia Silva, A.; Resch, C.; Zeug, A.; Loza, M.I.; Castro, M.; Ponimaskin, E.; Leopoldo, M. Knowledge-Based Design of Long-Chain Arylpiperazine Derivatives Targeting Multiple Serotonin Re-ceptors as Potential Candidates for Treatment of Autism Spectrum Disorder. ACS Chem. Neurosci. 2021, 12, 1313–1327. [Google Scholar] [CrossRef]
- Kelleher, R.J.; Bear, M.F. The Autistic Neuron: Troubled Translation? Cell 2008, 135, 401–406. [Google Scholar] [CrossRef] [Green Version]
- DiCicco-Bloom, E.; Lord, C.; Zwaigenbaum, L.; Courchesne, E.; Dager, S.R.; Schmitz, C.; Schultz, R.T.; Crawley, J.; Young, L.J. The Developmental Neurobiology of Autism Spectrum Disorder. J. Neurosci. 2006, 26, 6897–6906. [Google Scholar] [CrossRef] [Green Version]
- Akins, M.R.; Berk-Rauch, H.E.; Fallon, J.R. Presynaptic translation: Stepping out of the postsynaptic shadow. Front. Neural Circuits 2009, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-C.; Frei, J.A.; Kilander, M.B.C.; Shen, W.; Blatt, G.J. A Subset of Autism-Associated Genes Regulate the Structural Stability of Neurons. Front. Cell. Neurosci. 2016, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Threadgill, R.; Bobb, K.; Ghosh, A. Regulation of Dendritic Growth and Remodeling by Rho, Rac, and Cdc. Neuron 1997, 19, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Duffney, L.J.; Zhong, P.; Wei, J.; Matas, E.; Cheng, J.; Qin, L.; Ma, K.; Dietz, D.; Kajiwara, Y.; Buxbaum, J.D.; et al. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep. 2015, 11, 1400–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, M.; Murtaza, N.; Scharrenberg, R.; White, S.H.; Johanns, O.; Walker, S.; Yuen, R.K.C.; Schwanke, B.; Bedürftig, B.; Henis, M.; et al. Altered TAOK2 activity causes autism-related neurodevelopmental and cognitive abnormalities through RhoA signaling. Mol. Psychiatry 2019, 24, 1329–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speranza, L.; Chambery, A.; Di Domenico, M.; Crispino, M.; Severino, V.; Volpicelli, F.; Leopoldo, M.; Bellenchi, G.; Di Porzio, U.; Perrone-Capano, C. The serotonin receptor 7 promotes neurite outgrowth via ERK and Cdk5 signaling pathways. Neuropharmacology 2013, 67, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Giuliano, T.; Volpicelli, F.; De Stefano, M.E.; Lombardi, L.; Chambery, A.; Lacivita, E.; Leopoldo, M.; Bellenchi, G.C.; di Porzio, U.; et al. Activation of 5-HT7 receptor stimulates neurite elongation through mTOR, Cdc42 and actin filaments dynamics. Front. Behav. Neurosci. 2015, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdala, A.P.L.; Dutschmann, M.; Bissonnette, J.M.; Paton, J.F.R. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2010, 107, 18208–18213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, J.L.; Casey, A.B.; Saraf, T.S.; Mukherjee, M.; Booth, R.G.; Canal, C.E. (S)-5-(2′-Fluorophenyl)- N, N-Dimethyl-1,2,3,4-Tetrahydronaphthalen-2-Amine, a Serotonin Receptor Modulator, Possesses Anticonvulsant, Proso-cial, and Anxiolytic-like Properties in an Fmr1 Knockout Mouse Model of Fragile X Syndrome and Autism Spectrum Disorder. ACS Pharmacol. Transl. Sci. 2020, 3, 509–523. [Google Scholar]
- Maurin, T.; Zongaro, S.; Bardoni, B. Fragile X Syndrome: From molecular pathology to therapy. Neurosci. Biobehav. Rev. 2014, 46, 242–255. [Google Scholar] [CrossRef]
- Osterweil, E.K.; Krueger, D.D.; Reinhold, K.; Bear, M.F. Hypersensitivity to MGluR5 and ERK1/2 Leads to Excessive Protein Synthesis in the Hippocampus of a Mouse Model of Fragile X Syndrome. J. Neurosci. 2010, 30, 15616–15627. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.-S.; Hoang, E.T.; Viar, K.E.; Stornetta, R.L.; Scott, M.M.; Zhu, J.J. Pharmacological rescue of Ras signaling, GluA1-dependent synaptic plasticity, and learning deficits in a fragile X model. Genes Dev. 2014, 28, 273–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.; Sardone, L.M.; Bonaccorso, C.M.; D’Antoni, S.; Spatuzza, M.; Gulisano, W.; Tropea, M.R.; Puzzo, D.; Leopoldo, M.; Lacivita, E.; et al. Activation of Serotonin 5-HT7 Receptors Modulates Hippocampal Synaptic Plas-ticity by Stimulation of Adenylate Cyclases and Rescues Learning and Behavior in a Mouse Model of Fragile X Syndrome. Front. Mol. Neurosci. 2018, 11, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry-Kravis, E.; Huttenlocher, P.R. Cyclic AMP metabolism in fragile X syndrome. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1992, 31, 22–26. [Google Scholar] [CrossRef]
- Kelley, D.J.; Davidson, R.J.; Elliott, J.L.; Lahvis, G.P.; Yin, J.C.P.; Bhattacharyya, A. The Cyclic AMP Cascade Is Altered in the Fragile X Nervous System. PLoS ONE 2007, 2, e931. [Google Scholar] [CrossRef]
- Wold, E.A.; Wild, C.T.; Cunningham, K.A.; Zhou, J. Targeting the 5-HT2C Receptor in Biological Context and the Current State of 5-HT2C Receptor Ligand Development. Curr. Top. Med. Chem. 2019, 19, 1381–1398. [Google Scholar] [CrossRef]
- Albert-Gascó, H.; Ros-Bernal, F.; Castillo-Gómez, E.; Olucha-Bordonau, F.E. MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes. Int. J. Mol. Sci. 2020, 21, 4471. [Google Scholar] [CrossRef] [PubMed]
- Gazestani, V.H.; Pramparo, T.; Nalabolu, S.; Kellman, B.P.; Murray, S.; Lopez, L.; Pierce, K.; Courchesne, E.; Lewis, N.E. A Perturbed Gene Network Containing PI3K–AKT, RAS–ERK and WNT–β-Catenin Pathways in Leukocytes Is Linked to ASD Genetics and Symptom Severity. Nat. Neurosci. 2019, 22, 1624–1634. [Google Scholar] [CrossRef]
- Werry, T.D.; Gregory, K.J.; Sexton, P.M.; Christopoulos, A. Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. J. Neurochem. 2005, 93, 1603–1615. [Google Scholar] [CrossRef]
- Lacivita, E.; Niso, M.; Stama, M.L.; Arzuaga, A.; Altamura, C.; Costa, L.; Desaphy, J.-F.; Ragozzino, M.E.; Ciranna, L.; Leopoldo, M. Privileged scaffold-based design to identify a novel drug-like 5-HT7 receptor-preferring agonist to target Fragile X syndrome. Eur. J. Med. Chem. 2020, 199, 112395. [Google Scholar] [CrossRef]
- Fu, C.; Armstrong, D.; Marsh, E.; Lieberman, D.; Motil, K.; Witt, R.; Standridge, S.; Nues, P.; Lane, J.; Dinkel, T.; et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr. Open 2020, 4, e000717. [Google Scholar] [CrossRef]
- Renieri, A.; Meloni, I.; Longo, I.; Ariani, F.; Mari, F.; Pescucci, C.; Cambi, F. Rett syndrome: The complex nature of a monogenic disease. J. Mol. Med. 2003, 81, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.L.; Levenson, J.M.; Vilaythong, A.P.; Richman, R.; Armstrong, D.L.; Noebels, J.L.; Sweatt, J.D.; Zoghbi, H.Y. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 2004, 13, 2679–2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, E.S.; Hunnicutt, B.J.; Knopp, S.J.; Williams, J.T.; Bissonnette, J.M. A selective 5-HT1a receptor agonist improves respiration in a mouse model of Rett syndrome. J. Appl. Physiol. 2013, 115, 1626–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdala, A.P.; Bissonnette, J.M.; Newman-Tancredi, A. Pinpointing brainstem mechanisms responsible for autonomic dysfunction in Rett syndrome: Therapeutic perspectives for 5-HT1A agonists. Front. Physiol. 2014, 5, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdala, A.P.; Lioy, D.T.; Garg, S.K.; Knopp, S.J.; Paton, J.F.R.; Bissonnette, J.M. Effect of Sarizotan, a 5-HT1aand D2-Like Receptor Agonist, on Respiration in Three Mouse Models of Rett Syndrome. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1031–1039. [Google Scholar] [CrossRef] [Green Version]
- Landry, E.S.; Lapointe, N.P.; Rouillard, C.; Lévesque, D.; Hedlund, P.B.; Guertin, P.A. Contribution of spinal 5-HT1Aand 5-HT7receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur. J. Neurosci. 2006, 24, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Vogelgesang, S.; Niebert, S.; Renner, U.; Möbius, W.; Hülsmann, S.; Manzke, T.; Niebert, M. Analysis of the Serotonergic System in a Mouse Model of Rett Syndrome Reveals Unusual Upregulation of Serotonin Receptor 5b. Front. Mol. Neurosci. 2017, 10, 61. [Google Scholar] [CrossRef]
- Vogelgesang, S.; Niebert, M.; Bischoff, A.M.; Hülsmann, S.; Manzke, T. Persistent Expression of Serotonin Receptor 5b Alters Breathing Behavior in Male MeCP2 Knockout Mice. Front. Mol. Neurosci. 2018, 11, 28. [Google Scholar] [CrossRef]
- Stiedl, O.; Pappa, E.; Konradsson-Geuken, Å.; Ögren, S.O. The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front. Pharmacol. 2015, 6, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumenko, V.S.; Popova, N.K.; Lacivita, E.; Leopoldo, M.; Ponimaskin, E.G. Interplay between Serotonin 5-HT1Aand 5-HT7Receptors in Depressive Disorders. CNS Neurosci. Ther. 2014, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.R.; Mukhin, Y.V.; Gettys, T.W.; Garnovskaya, M.N. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br. J. Pharmacol. 1999, 127, 1751–1764. [Google Scholar] [CrossRef] [Green Version]
- Guseva, D.; Wirth, A.; Ponimaskin, E. Cellular mechanisms of the 5-HT7receptor-mediated signaling. Front. Behav. Neurosci. 2014, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Meunier, C.; Cancela, J.-M.; Fossier, P. Lack of GSK3β activation and modulation of synaptic plasticity by dopamine in 5-HT1A-receptor KO mice. Neuropharmacology 2017, 113, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Mogha, A.; Guariglia, S.R.; Debata, P.R.; Wen, G.Y.; Banerjee, P. Serotonin 1A receptor-mediated signaling through ERK and PKCα is essential for normal synaptogenesis in neonatal mouse hippocampus. Transl. Psychiatry 2012, 2, e66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druse, M.; Tajuddin, N.F.; Gillespie, R.A.; Le, P. Signaling pathways involved with serotonin1A agonist-mediated neuroprotection against ethanol-induced apoptosis of fetal rhombencephalic neurons. Dev. Brain Res. 2005, 159, 18–28. [Google Scholar] [CrossRef]
- Purkayastha, S.; Ford, J.; Kanjilal, B.; Diallo, S.; Del Rosario Inigo, J.; Neuwirth, L.; El Idrissi, A.; Ahmed, Z.; Wieraszko, A.; Azmitia, E.C.; et al. Clozapine functions through the prefrontal cortex serotonin 1A receptor to heighten neuronal activity via calmodulin kinase II-NMDA receptor interactions. J. Neurochem. 2011, 120, 396–407. [Google Scholar] [CrossRef]
- Albert, P.R.; Sajedi, N.; Lemonde, S.; Ghahremani, M.H. Constitutive G(I2)-Dependent Activation of Adenylyl Cyclase Type II by the 5-HT1A Receptor. Inhibition by Anxiolytic Partial Agonists. J. Biol. Chem. 1999, 274, 35469–35474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuyama, T.; Inagaki, S.; Takagi, H. Distribution of type II adenylyl cyclase mRNA in the rat brain. Mol. Brain Res. 1993, 19, 165–170. [Google Scholar] [CrossRef]
- Renner, U.; Zeug, A.; Woehler, A.; Niebert, M.; Dityatev, A.; Dityateva, G.; Gorinski, N.; Guseva, D.; Abdel-Galil, D.; Fröhlich, M.; et al. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012, 125, 2486–2499. [Google Scholar] [CrossRef] [Green Version]
| Names | Structures | Targets | Effects |
|---|---|---|---|
| 8-OH DPAT |  | 5-HT1AR/5-HT7R agonist | decreased mGluR-mediated LTD, prevented internalization of AMPA receptors [43]; normalization of respiratory function [77] |
| (+)-5-FPT |  | 5-HT1AR/5-HT2CR/5-HT7R agonist | reduction of stereotypic behavior, social activity increase [65]; reduced the number of audiogenic seizures [78] |
| aripiprazole |  | partial 5-HT1AR/ HT2AR/5-HT2CR/5-HT7R/D1R/D2R/D3R/D4R/D5R agonist; 5-HT1BR/5-HT1DR/5-HT2AR/5-HT2CR/5-HT3AR/HT6R/5-HT7R/D1R/D2R/D3R/ D4R/D5R/some alpha adrenergic and histamine receptors antagonist | irritability amelioration [14,15,66] |
| risperidone |  | 5-HT1A/5-HT1DR/5-HT2AR/5-HT2CR/5-HT7R/D1R/D2R/some alpha adrenergenic and histamine receptors antagonist | irritability and aggressive behavior amelioration [17,18,66] |
| lurasidone |  | 5-HT1AR/5-HT2AR/5-HT7/D2R/ some alpha adrenergic receptors antagonist; 5-HT1AR partial agonist | irritability and aggressive behavior amelioration [19,66] |
| 1 |  | 5-HT1AR/5-HT7R agonist | metabolically stable and have suitable CNS druglike properties [67] |
| 2 |  | 5-HT1AR/5-HT7R agonist | metabolically stable and have suitable CNS druglike properties [67] |
| 3 |  | 5-HT1AR/5-HT7R agonist; 5-HT2BR antagonist | metabolically stable and have suitable CNS druglike properties [67] |
| LP-211 |  | 5-HT7R agonist; affinity to 5-HT1AR/D2R | neurite growth promotion (increased the number of dendritic spines and synaptic connections) [54,68,69]; decreased mGluR-mediated LTD, prevented internalization of AMPA receptors [43,44]; reduction of stereotypic behavior, improvement of recognition memory [38,39]; anxiety alleviation, exploratory behavior and learning ability improvement [38,39]; normalization of mitochondrial ETC function [40,41] |
| Names | Structures | Targets | Effects |
|---|---|---|---|
| BA-10 |  | 5-HT7R agonist | decreased mGluR-mediated LTD [44] |
| Forskolin |  | adenylate cyclase stimulation | decreased mGluR-mediated LTD [82] |
| PACAP | a protein encoded by ADCYAP1 gene | adenylate cyclase stimulation | decreased mGluR-mediated LTD [82] |
| 4 |  | 5-HT7R agonist | reduction of stereotypic behavior [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Avramets, D.; Jeon, B.; Choo, H. Modulation of Serotonin Receptors in Neurodevelopmental Disorders: Focus on 5-HT7 Receptor. Molecules 2021, 26, 3348. https://doi.org/10.3390/molecules26113348
Lee J, Avramets D, Jeon B, Choo H. Modulation of Serotonin Receptors in Neurodevelopmental Disorders: Focus on 5-HT7 Receptor. Molecules. 2021; 26(11):3348. https://doi.org/10.3390/molecules26113348
Chicago/Turabian StyleLee, Jieon, Diana Avramets, Byungsun Jeon, and Hyunah Choo. 2021. "Modulation of Serotonin Receptors in Neurodevelopmental Disorders: Focus on 5-HT7 Receptor" Molecules 26, no. 11: 3348. https://doi.org/10.3390/molecules26113348
APA StyleLee, J., Avramets, D., Jeon, B., & Choo, H. (2021). Modulation of Serotonin Receptors in Neurodevelopmental Disorders: Focus on 5-HT7 Receptor. Molecules, 26(11), 3348. https://doi.org/10.3390/molecules26113348








