Abstract
During the last few decades, pyridazine derivatives have emerged as privileged structures in heterocyclic chemistry, both because of their excellent chemistry and because of their potential applications in medicinal chemistry and optoelectronics. This review is focused on the recent advances in [3 + n] cycloaddition reactions in the pyridazine series as well as their medicinal chemistry and optoelectronic applications over the last ten years. The stereochemistry and regiochemistry of the cycloaddition reactions are discussed. Applications in optoelectronics (in particular, as fluorescent materials and sensors) and medicinal chemistry (in particular, antimicrobials and anticancer) are also reviewed.
1. Introduction
[3 + n] cycloaddition reactions represent one of the most important and efficient tools in the synthesis of heterocyclic rings, consisting of the addition of a multiple bond dipolarophile to a three-atom component system (TACS). Because of their complexity, [3 + 2] cycloaddition reactions have been extensively studied in the last 60 years [1,2,3,4,5,6,7,8,9,10,11,12,13,14], with interesting and heated discussions being involved in order to explain them.
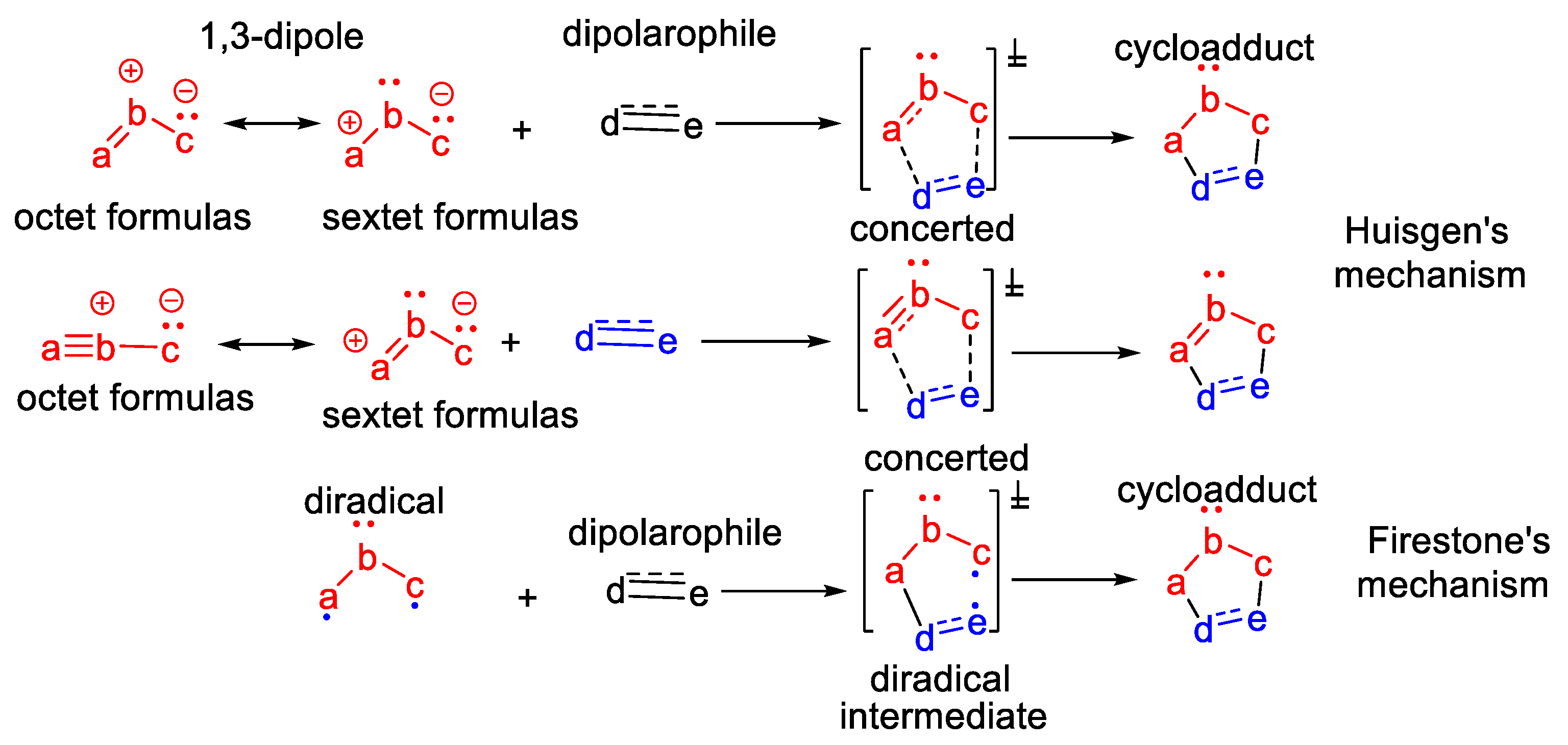
The [3 + 2] dipolar cycloaddition reactions were discovered by Rolf Huisgen in the early 1960s, more than 60 years ago [1,2]. The Huisgen [3 + 2] dipolar cycloaddition reactions represent an addition of a 1,3-dipole to a dipolarophile, leading to a five-membered heterocyclic ring [1,2,3,4,5,6,7], via a concerted mechanism, as presented in Scheme 1. The 1,3-dipole is a molecule with three atoms (abc), with a sextet of electrons (consequently, with a positive charge) to a marginal atom, and to the other marginal atom, a pair of non-bonding electrons (consequently, with a negative charge). The sextet formulas of the 1,3-dipole are stabilized by the non-bonding electrons of the central atom, adopting another canonical structure—the octet zwitterionic form. The dipolarophile is represented by an activated alkene or alkyne.
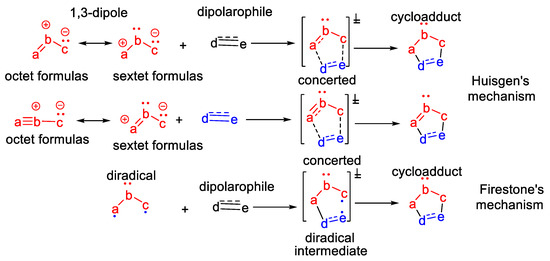
Scheme 1.
The [3 + 2] cycloadditions approach for obtaining five-membered heterocycles: Huisgen dipolar mechanism and Firestone diradical mechanism.
At the end of the 1960s, Firestone [9,10] proposed an alternative explanation for [3 + 2] cycloaddition reactions, via a radical mechanism (Scheme 1). In his approach, Firestone proposed a two-step mechanism, via the formation of a diradical intermediate.
Over the last decade, Domingo’s team [11,12,13,14] have revealed new, interesting considerations concerning the mechanistic inside of [3 + 2] cycloaddition reactions, based on molecular electron density theory (MEDT). Using their research, they classify the TACS in pseudo-diradical, pseudoradical, zwitterionic and carbenoid. Using MEDT theory, [3 + 2] cycloaddition reactions have been classified by Domingo into the corresponding pseudo-diradical, pseudoradical, zwitterionic and carbenoid reactions [11,12,13,14].
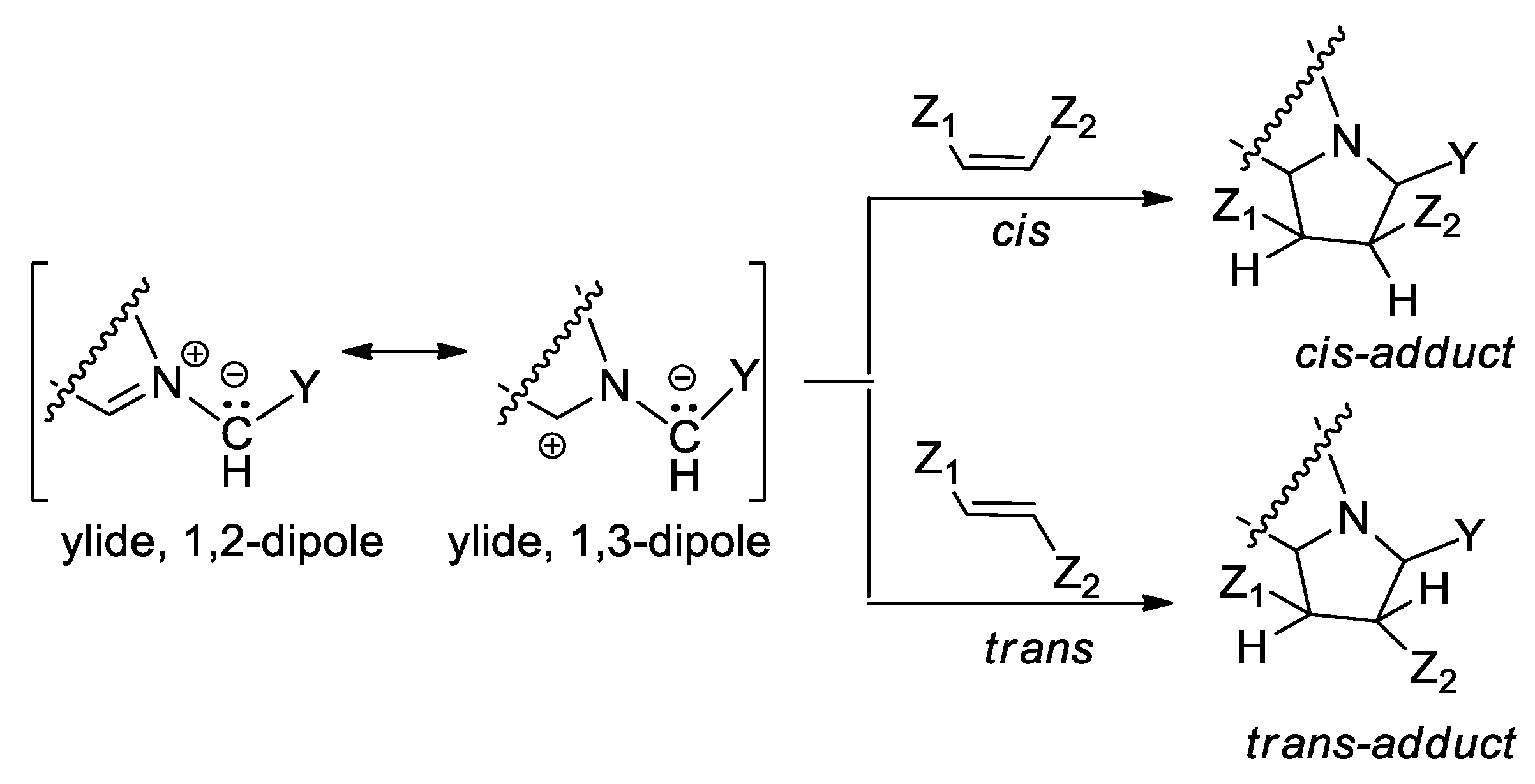
An interesting case of [3 + 2] cycloaddition reactions is represented by the cycloaddition of cycloimmonium ylides to various symmetrically and non-symmetrically substituted dipolarophiles, Scheme 2. The [3 + 2] cycloaddition reactions of cycloimmonium ylides to symmetrically substituted Z (or cis) and E (or trans) olefins involve interesting problems of stereochemistry, these reactions being highly stereospecific [8,15,16].
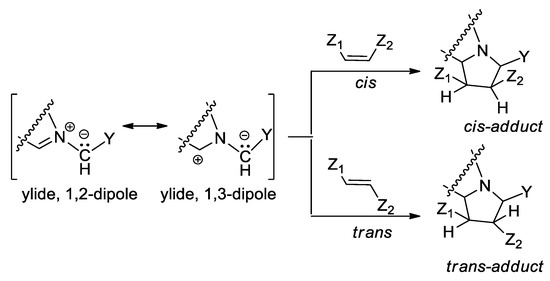
Scheme 2.
Stereochemistry of addition of cycloimmonium ylides to symmetrically substituted cis and trans olefins.
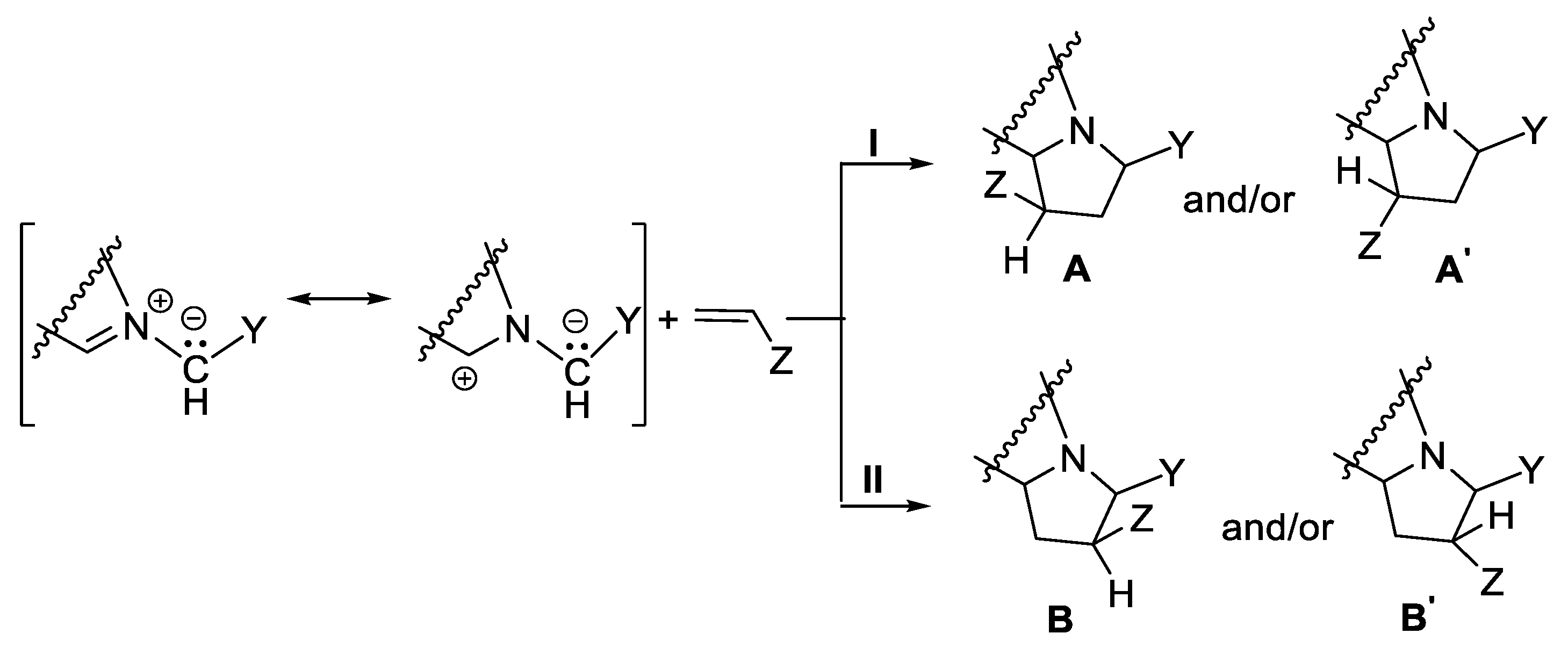
The [3 + 2] cycloaddition reactions of cycloimmonium ylides to non-symmetrically substituted dipolarophiles (with double or triple bond) involve interesting combined stereo- and regiochemistry aspects [17,18,19,20,21]. In the regiochemistry case, because of the double sense of the addition of ylide to dipolarophile, two reactions pathways (I or II) are possible, with the formation of two pairs of regioisomers (A, A’ and B, B’, Scheme 3), according to orbital, steric and electronic factors.
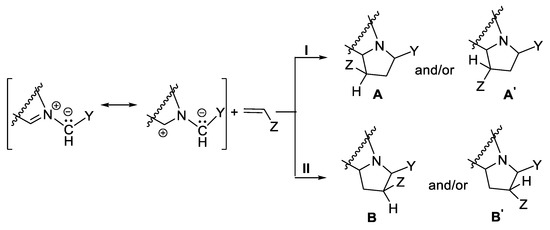
Scheme 3.
Regiochemistry of addition of cycloimmonium ylides to non-symmetrically substituted dipolarophiles, with the formation of endo/exo A and A’, and B and B’ pairs of stereoisomers.
On the other hand, [3 + 2] cycloaddition reactions represent an accessible and facile tool to obtain pyridazine derivatives of potential interest in medicinal chemistry (as antibacterials, antifungals, antituberculars, antivirals, anticancer agents, anti-inflammatories, antihypertensives, diuretics, etc.) [22,23] and in optoelectronics (as highly fluorescent materials, sensors and biosensors, lasers, etc.) [24].
From a previous review conducted ten years ago [23], it is clear that the focus has been on the most important achievements in the chemistry and applications of 1,2-diazine (pyridazine and phthalazine). In the present review, we focused on the recent advances in [3 + n] cycloaddition reactions in the pyridazine series as well as their medicinal chemistry and optoelectronic applications in the last ten years.
2. Results and Discussions
2.1. [3 + 2] Cycloaddition Reactions in the Pyridazinium and Phthalazinium Ylides Series
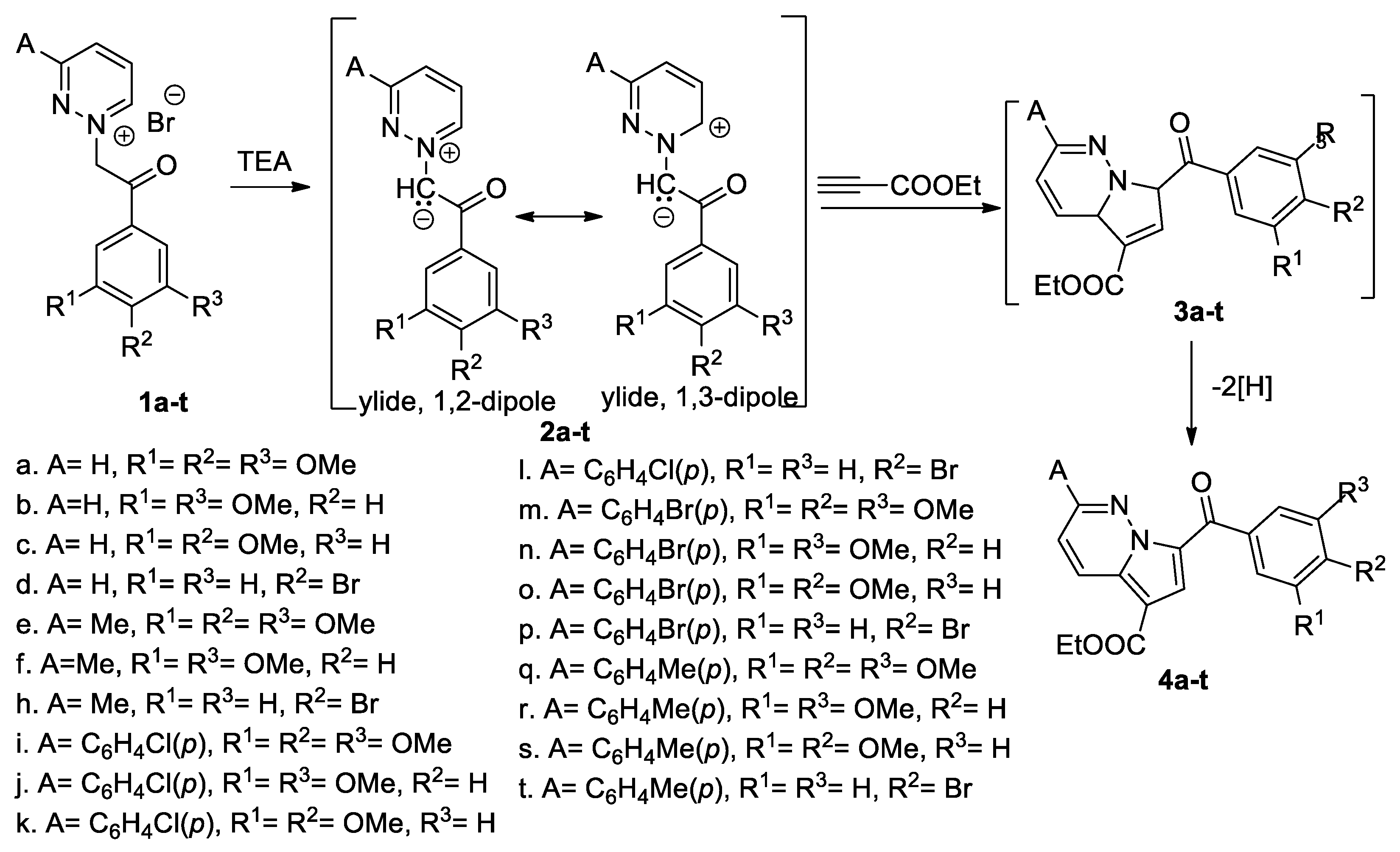
Popovici et al. [25] synthesized different pyrrolo [1,2-b]pyridazine derivatives (4a–t), using the [3 + 2] cycloaddition reactions of different pyridazinium ylides (2a–t; generated in situ from the corresponding pyridazinium salts (1a–t), in the presence of triethylamine (TEA) as the base), to ethyl propiolate, as shown in Scheme 4.
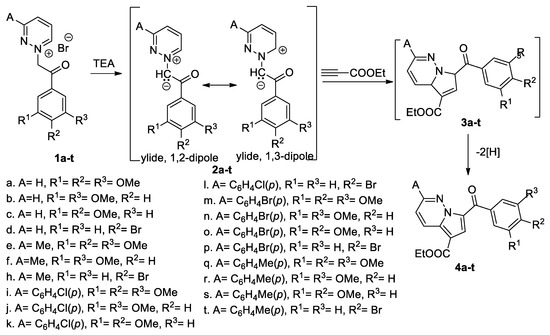
Scheme 4.
The [3 + 2] cycloaddition of pyrrolo[1,2-b]pyridazine derivatives 4a–t.
In the first instance, the corresponding intermediate dihydropyrrolo[1,2-b]pyridazines 3a–t were obtained and underwent an oxidative dehydrogenation under atmospheric conditions, leading to the final pyrrolo[1,2-b]pyridazine derivatives 4a–t, in moderate yields (40–52%). The [3 + 2] cycloadditions occur completely regioselectively, with a single type of regioisomer (4) being obtained. Some of the obtained pyrrolo[2,1-b]pyridazines were tested in vitro against a panel of 60 human tumor cell lines at a single dose up to five doses (according to National Cancer Institute protocol). The adducts 4a, 4b, 4e, and 4f showed a very good growth inhibition effect on almost all 60 cell lines, the best results being registered on HL-60 (TB) leukemia cells, COLO205 colon cancer cells, MDA-MB-435 melanoma cells, OVCAR-3 ovarian cancer cells and A498 renal cancer cells. The most active compound was 4f with GI50 values <100 nM on thirteen cell lines including colon, ovarian, renal, prostate, brain and breast cancer, melanoma and leukemia. Docking experiments have been performed for the biologically active cycloadducts (4a, 4b, 4e, 4f) and showed good compatibility with the colchicine binding site of tubulin. The authors claim that cycloadduct 4f could serve as a useful lead in anticancer drug design.
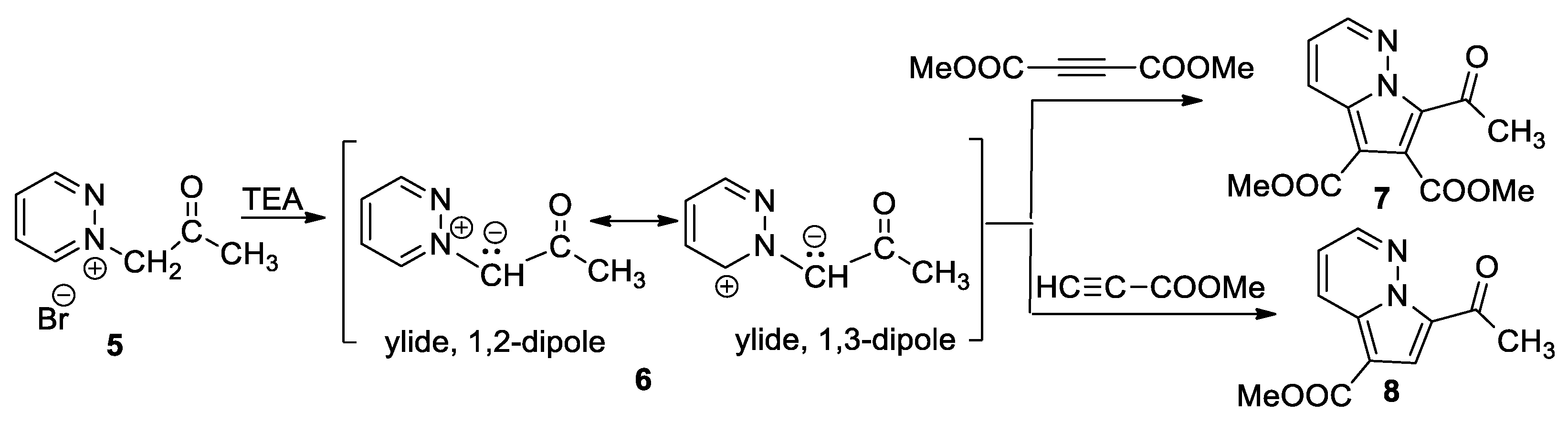
In continuation of their concern for new fluorescent pyrrolodiazine derivatives, Moldoveanu et al. [26] performed an interesting study concerning the cycloaddition reactions of pyridazinium ylides 6 (generated in situ from the corresponding pyridazinium salts 5 in the presence of TEA) in activated symmetrically and non-symmetrically substituted dipolarophiles with a triple bond (dimethyl acetylenedicarboxylate–DMAD and methyl propiolate), as shown in Scheme 5.
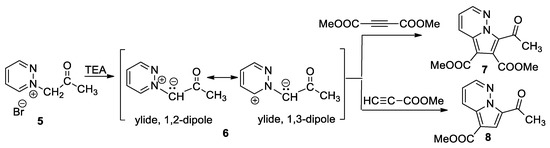
Scheme 5.
The [3 + 2] cycloaddition of pyridazinium ylides with DMAD and methyl propiolate.
A reaction with DMAD leads to the azabicycle 7, while [3 + 2] cycloaddition of ylide with methyl propiolate occurs completely regioselectively, with a single regioisomer (8) being obtained. The reactions were performed both under conventional thermal heating (TH) as well as under unconventional heating (microwave (MW) irradiation). The authors claim that under MW irradiation, the yields are higher, at around 10–15%. The authors prove that the cycloadducts 7 and 8 are intense blue emitters and have a quantum yield around 25%.
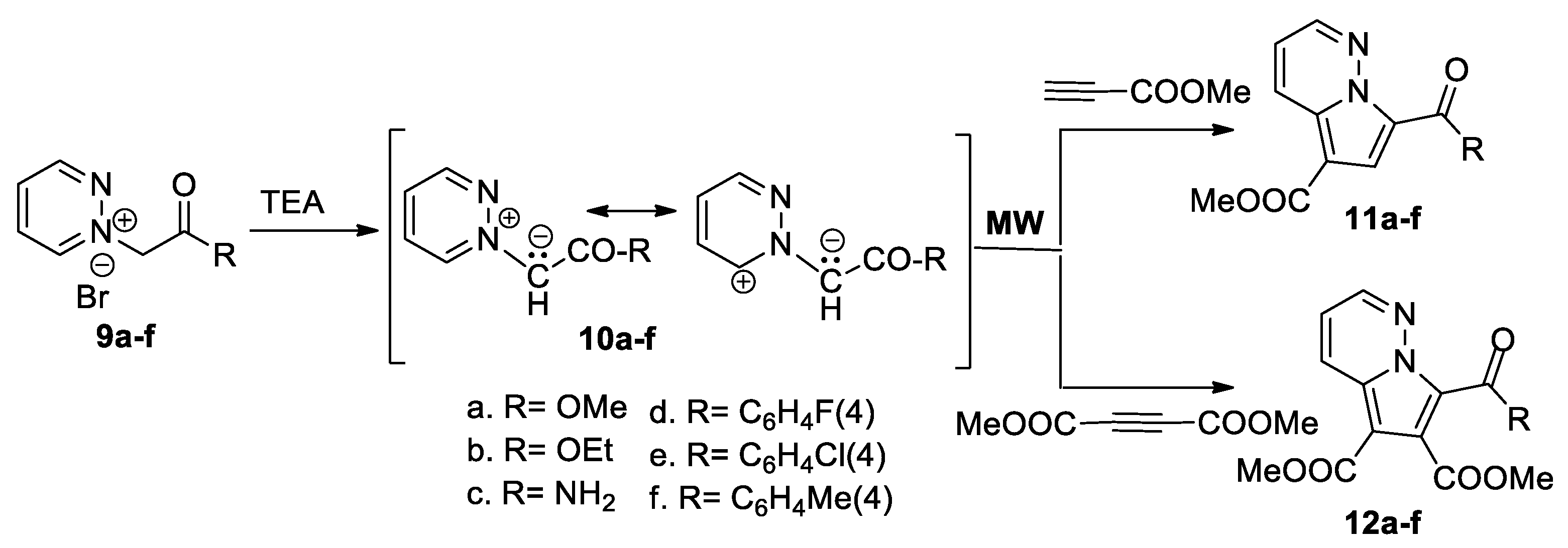
In continuation of their work in the field of cycloaddition reactions, Zbancioc et al. [27,28] reported an interesting and detailed study concerning the thermal versus specific effects of MW in the [3 + 2] cycloaddition reactions. In this respect, they studied the [3 + 2] cycloaddition reactions of pyridazinium ylides 10a–f (generated in situ from the corresponding pyridazinium salts 9a–f in the presence of TEA) to methyl propiolate and DMAD, as shown in Scheme 6.
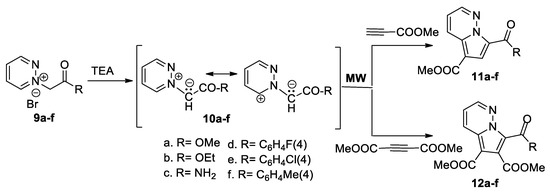
Scheme 6.
The [3 + 2] cycloaddition of pyridazinium ylides 10a–f with DMAD and methyl propiolate.
The cycloaddition reactions of pyridazinium ylides 10a–f to methyl propiolate occur completely regioselectively, with a single type of regioisomer being obtained, namely pyrrolopyridazine 11a–f. The reaction with DMAD leads to the pyrrolopyridazines 12a–f. The reactions were studied under MW irradiation, in order to elucidate the intrinsic mechanism of MW effects. On the basis of their study, the authors conclude that there are no specific MW effects in the studied [3 + 2] cycloaddition reactions, the observed effects being purely thermal. Time-dependent density functional theory computations and steady-state electronic spectroscopy measurements were performed for the pyrrolopyridazine 11a and 11e, revealing that these compounds possess an intense blue fluorescence [28].
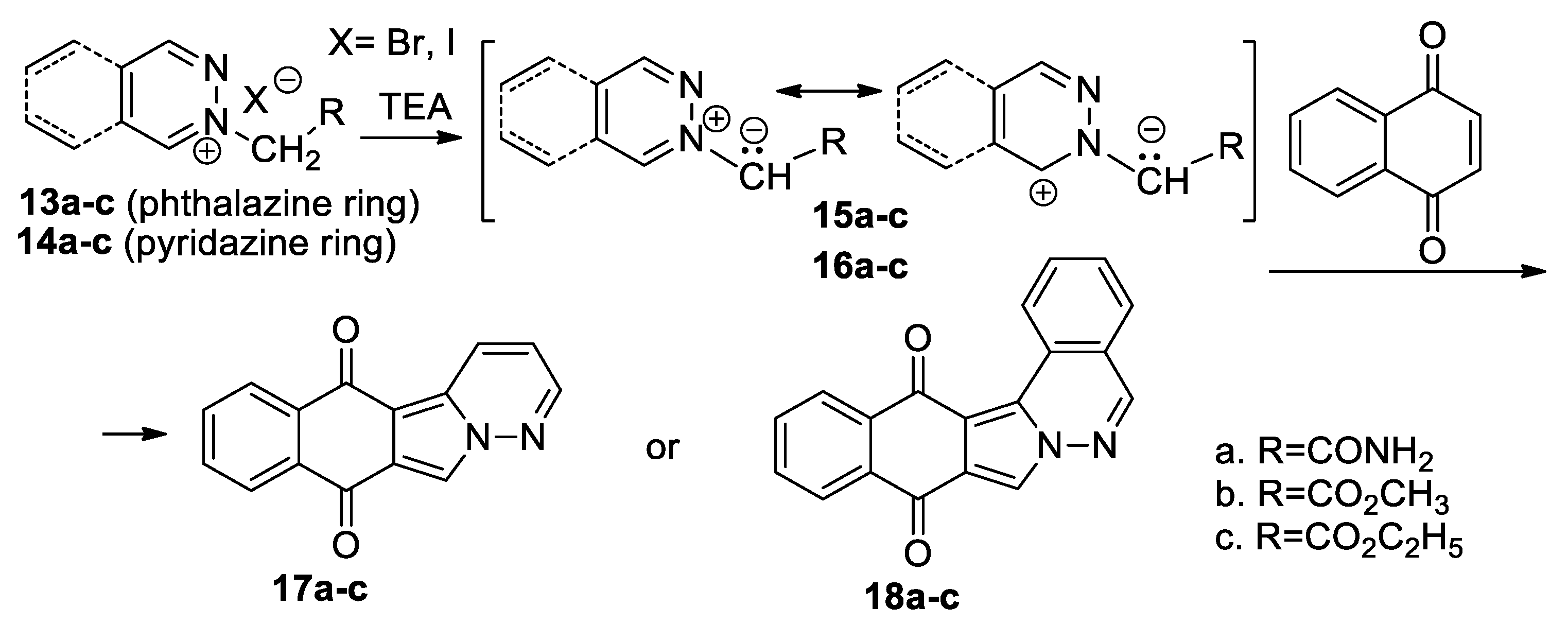
Mantu et al. [29,30,31] performed a straightforward and efficient study concerning the [3 + 2] cycloaddition reactions of pyridazinium 15a–c and phthalazinium 16a–c ylides (generated in situ from the corresponding pyridazinium 13a–c and phthalazinium 14a–c salts in the presence of TEA), to 1,4-naphthoquinone, as symmetrically activated cyclic alkene (Scheme 7).
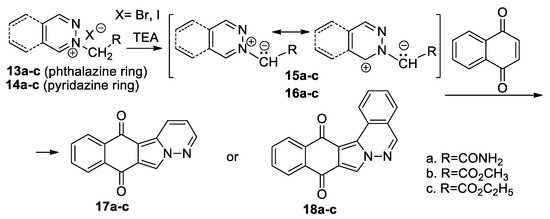
Scheme 7.
The [3 + 2] cycloaddition of pyridazinium and phthalazinium ylides to 1,4-naphthoquinone.
The reaction leads either to polycyclic dihydrobenzo[f]pyridazino[6,1-a] isoindole 17a–c or to dihydrobenzo[5,6]isoindolo[1,2-a]phthalazine 18a–c. The reactions were performed under conventional TH, as well as under MW or ultrasound (US) irradiation. The obtained data show that MW and US irradiation produce a remarkable acceleration for reactions (from hours to minutes), the consumed energy decreases considerably and the yields are higher. The US irradiation was found to be the most effective method, in terms of yields and reaction time, for obtaining polycyclic 1,2-diazines. The in vitro anticancer assay [30] against an NCI 60 human tumor cell line panel reveals that the polycyclic compounds 17a–c and 18a–c have anticancer activity. The obtained data led the authors to claim that polycyclic compounds 17a–c and 18a–c will act as an anticancer vector through multiple mechanisms of action, the intercalation with the DNA prevailing in competition with the other mechanisms.
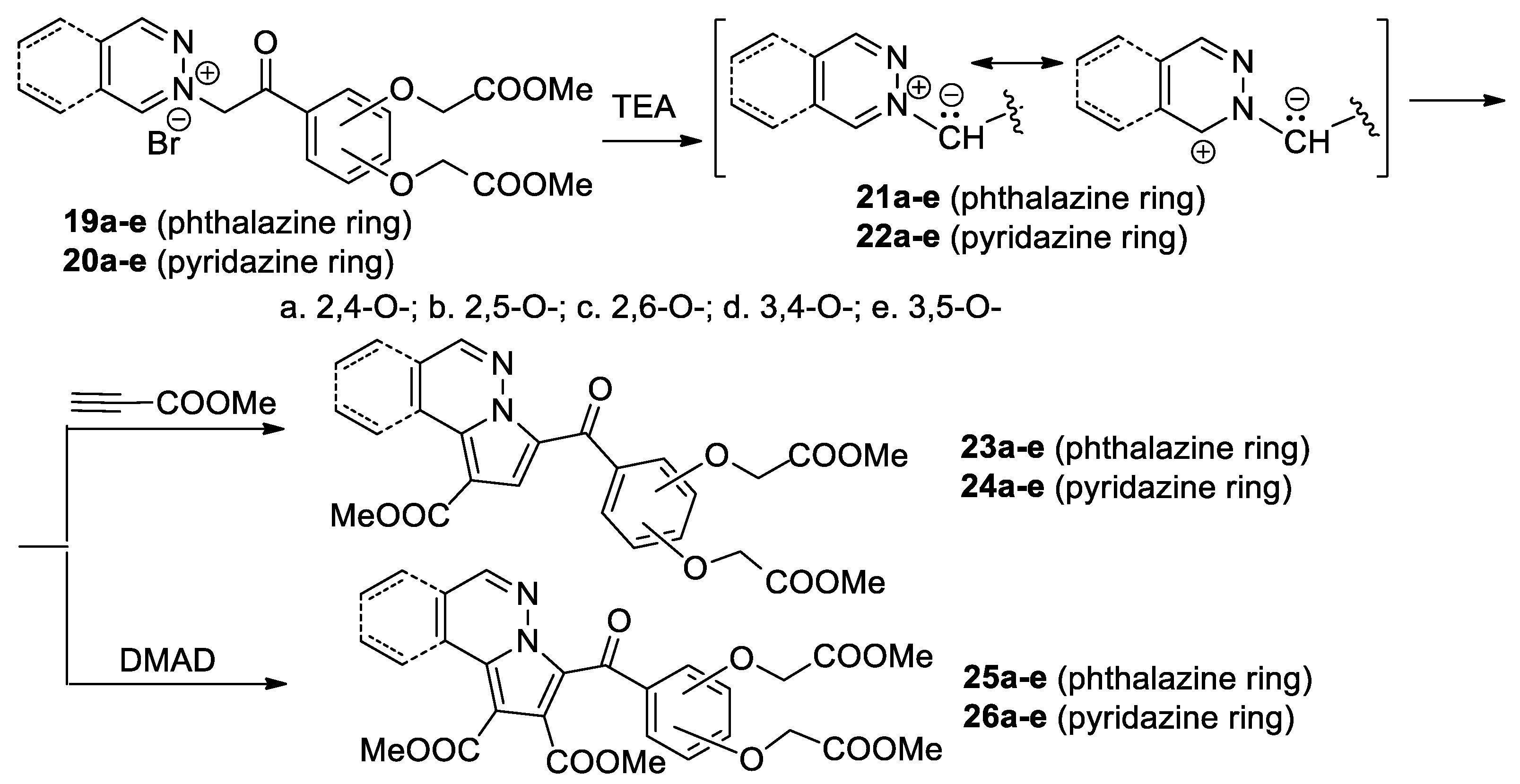
Zbancioc et al. [32,33,34] performed a thorough study concerning the [3 + 2] cycloaddition reactions of pyridazinium 21a–e and phthalazinium 22a–e ylides with the dihydroxyacetophenone skeleton (generated in situ from the corresponding pyridazinium 19a–e and phthalazinium 20a–c salts in the presence of TEA), to methyl propiolate and DMAD, as shown in Scheme 8.
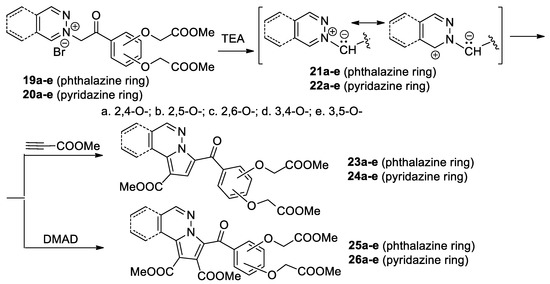
Scheme 8.
The [3 + 2] cycloaddition of pyridazinium and phthalazinium ylides with dihydroxyacetophenone skeleton.
The [3 + 2] cycloaddition reaction of the ylides 21a–e and 22a–e with methyl propiolate occur completely regioselectively, with a single type of regioisomer being obtained—the fused pyrrolo-diazines with the dihydroxyacetophenone skeleton 23a–e and 24a–e. In the case of the cycloaddition reaction of ylides 21a–e and 22a–e with the symmetrically substituted alkynes DMAD, the aromatized fused pyrrolo-diazines with the dihydroxyacetophenone skeleton 25a–e and 26a–e are obtained. The reactions were performed under conventional TH, as well as under MW or US irradiation. Under MW and US irradiation, the yields are higher, the reaction time decreases substantially (from hours to minutes), the consumed energy decreases considerably, the amount of used solvent also decreases, and the reaction conditions are milder. As a result, these reactions could be considered environmentally friendly. Overall, the use of US proved to be more efficient than MW or TH. The in vitro anticancer assay proves that some of the obtained compounds have a significant antitumor activity and a moderate antifungal activity, while the antibacterial activity is negligible [34].
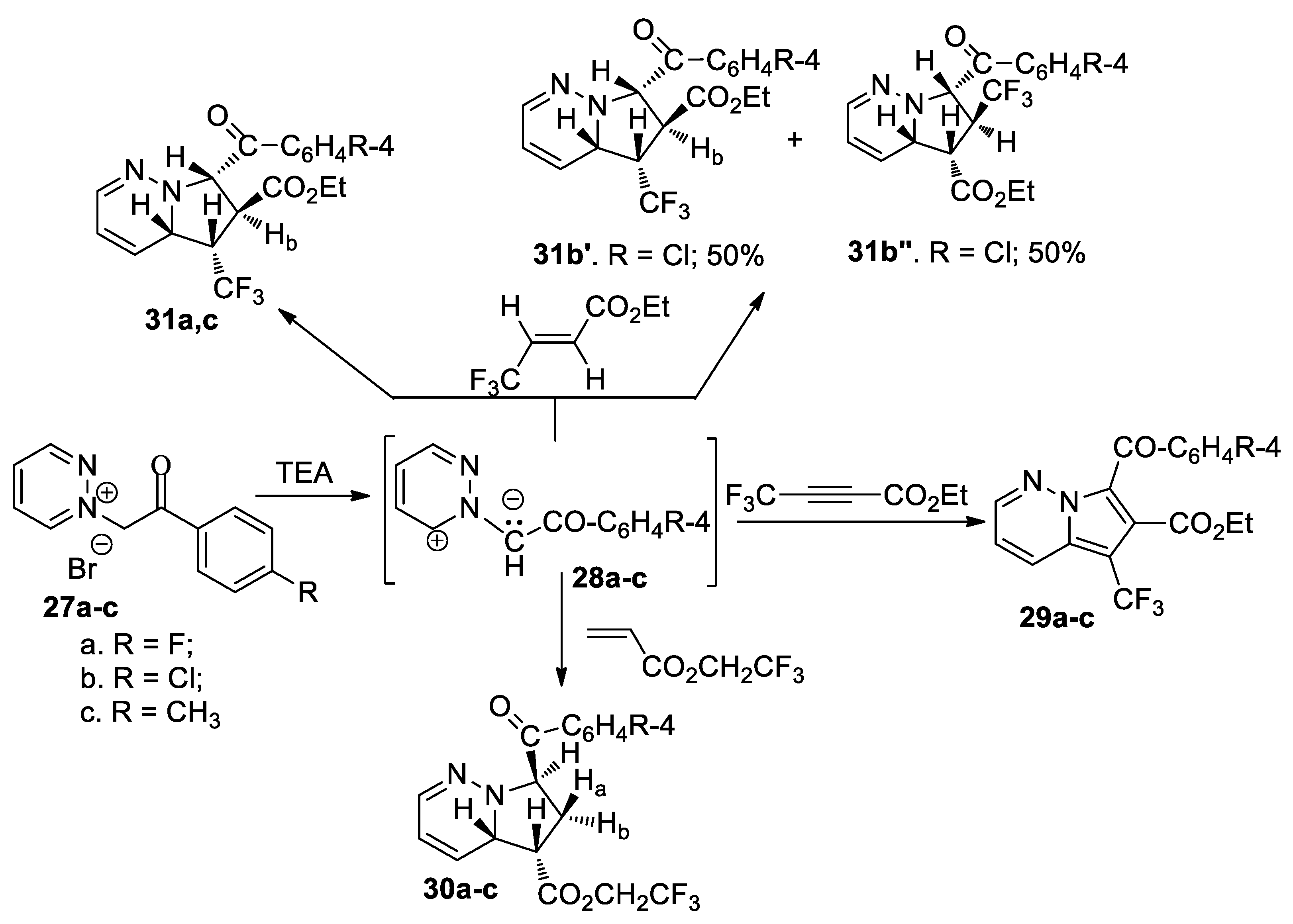
Tucaliuc et al. [35] performed an interesting study concerning the [3 + 2] cycloaddition reactions of pyridazinium ylides 28a–c with various activated non-symmetrically substituted dipolarophiles (alkenes and alkynes) containing fluorine moiety, as shown in Scheme 9.
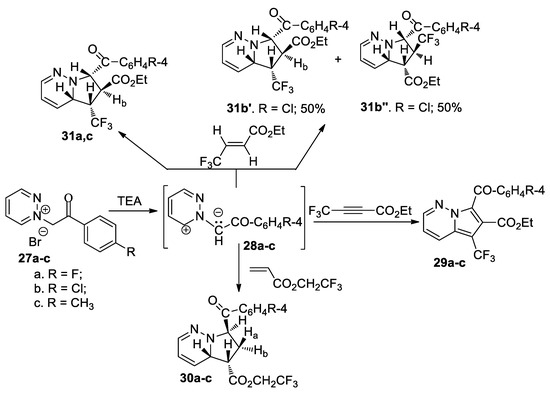
Scheme 9.
The [3 + 2] cycloaddition of pyridazinium ylides 28a–c to various fluorine dipolarophiles with double and triple bond.
The [3 + 2] cycloaddition reaction of ylides 28a–c to 2,2,2-trifluoroethyl acrylate occur completely regioselectively, involving a single type of regioisomer being obtained, with a tetrahydropyrrolopyridazine structure 30a–c. The cycloaddition reaction of ylides 28a–c to ethyl 4,4,4-trifluorobutinoate leads to the aromatized pyrrolopyridazine derivatives 29a–c, again with the reaction being completely regioselective.
When the dipolarophile was ethyl 4,4,4-trifluorocrotonate (E-isomer), the reactions involved additional stereo- and regiochemical problems. The cycloaddition reaction of ylides 28a and 28c occurs completely stereo- and regioselectively, with only isomers 31a and 31c being obtained. In the case of ylide 28b (R=Cl), the cycloaddition reaction occurs regioselectively, with two regisomers 31b′ and 31b’’ being obtained, in a molar ratio of 1:1.
These reactions were performed under conventional TH and under MW or US irradiation (both in liquid phase and phase-transfer catalysis). The use of MW irradiation proved to be more efficient (with the reaction time decreasing substantially, higher yields, smaller amount of used solvent), with these reactions being considered environmentally friendly. The in vitro antimicrobial activity of the obtained compounds indicated that the introduction of a trifluoromethyl moiety on the pyridazine skeleton is beneficial for antimicrobial activity. Practically all compounds have a spectacular antimicrobial activity against Gram positive germs, and very good activity against Gram negative germs. The antifungal activity of compounds was negligible.
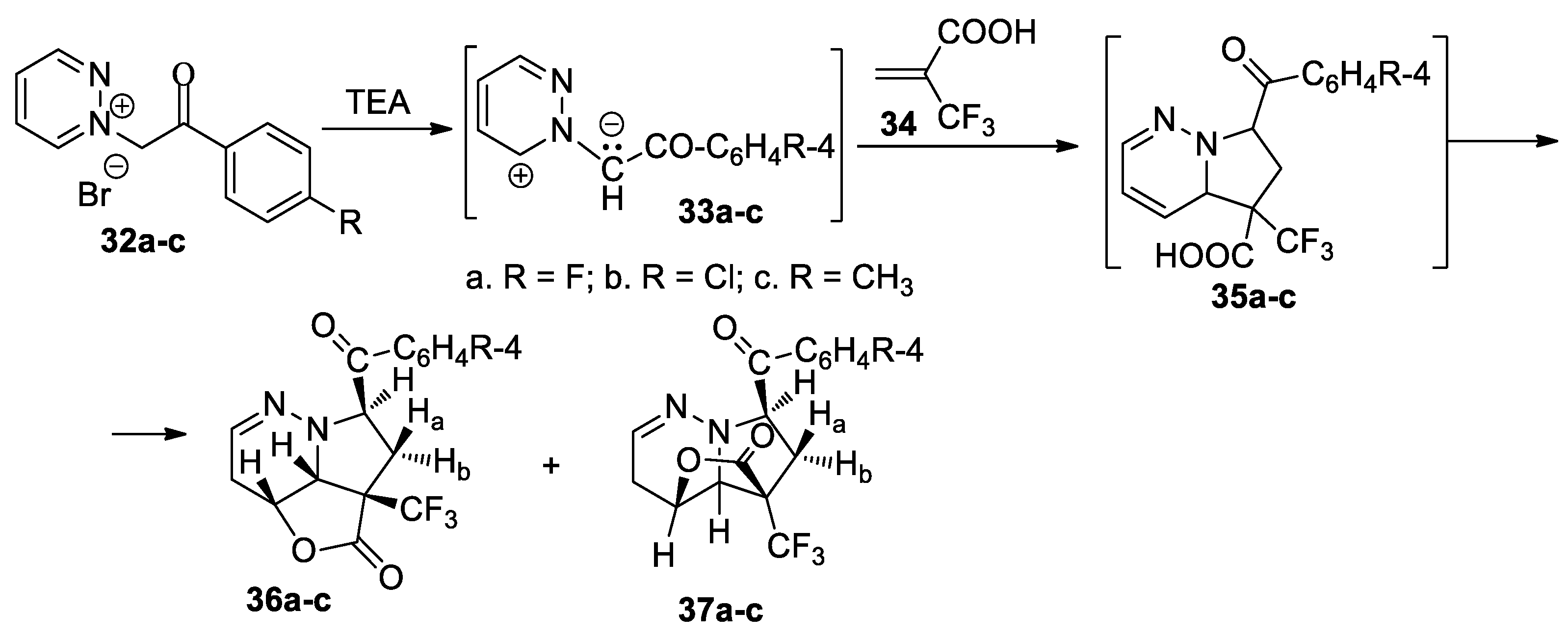
Butnariu et al. [36] performed a straightforward, efficient and selective study for obtaining hybrid trifluoromethyl-substituted γ-lactones with nitrogen heterocyclic skeletons 36a–c and 37a–c, as shown in Scheme 10.
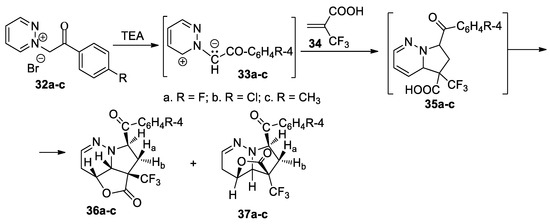
Scheme 10.
The [3 + 2] cycloaddition of pyridazinium ylides 33a–c to 2-(trifluoromethyl)acrylic acid.
The [3 + 2] cycloaddition reaction of ylides 33a–c to 2-(trifluoromethyl)acrylic acid 34 occurs unexpectedly; instead of the cycloadducts 35a–c, a new class of organic compounds were obtained: fused γ-lactones with nitrogen heterocyclic skeletons—36a–c and 37a–c. As for regiochemistry, the cycloaddition reactions occur regioselectively, with the formation of one pair of stereoisomers (type A and A’, Scheme 3), namely 36a–c (major) and 37a–c. A feasible reaction mechanism is presented for γ-lactone formation via a cascade reaction: an initial [3 + 2] cycloaddition leads to intermediary cycloadducts 35a–c, which underwent a concomitant protonation of the nitrogen atom and nucleophilic attack of the carboxylate oxygen at the adjacent endocyclic carbocation.
2.2. [3 + n] Cycloaddition Reactions in the Nitrile Imine series
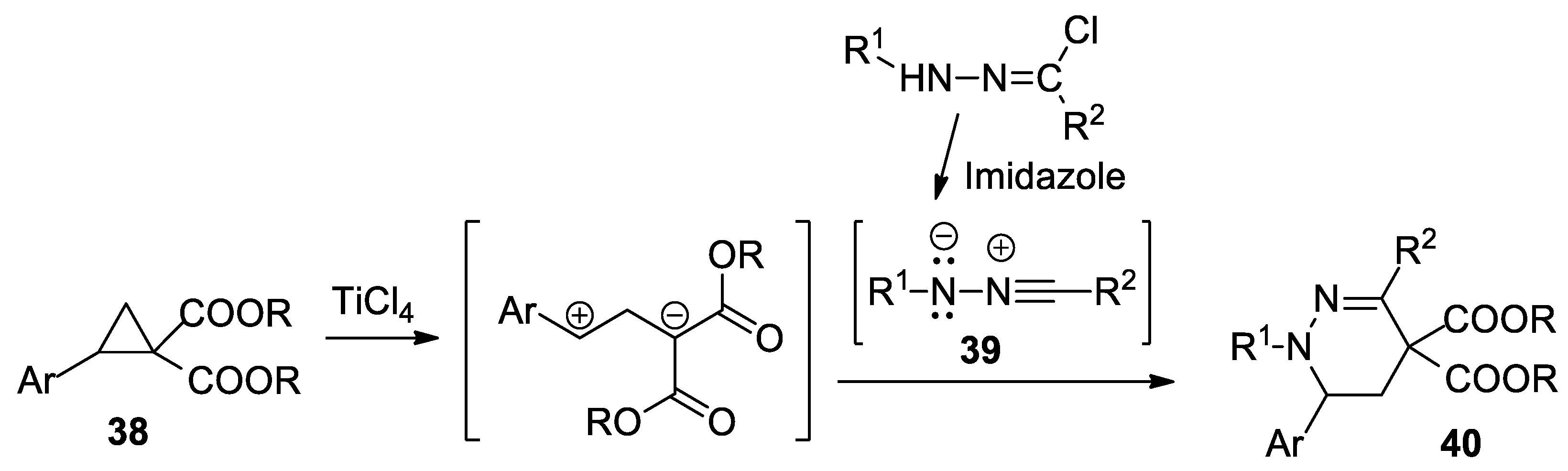
Grave et al. [37], using [3 + 3] cycloaddition reactions of various nitrile imines 39 to donor–acceptor cyclopropanes 28, report a straightforward and efficient synthesis of tetrahydropyridazine derivatives 40 (23 products), as shown in Scheme 11.
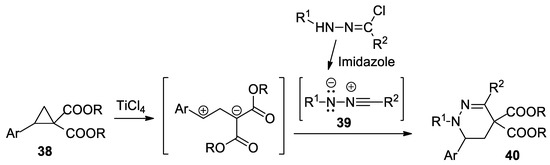
Scheme 11.
The [3 + 3] cycloaddition reactions of nitrile imines to donor–acceptor cyclopropanes.
The donor–acceptor cyclopropanes (Ar are various aromatic radicals: phenyl, 2-/3-/4-substituted-phenyl) were activated by a catalytic amount of TiCl4. The nitrile imines (R1 and R2 are different radicals—phenyl, substituted phenyl, naphthyl, benzoyl, thienyl) were generated in situ from hydrazonyl chlorides by treatment with imidazole.
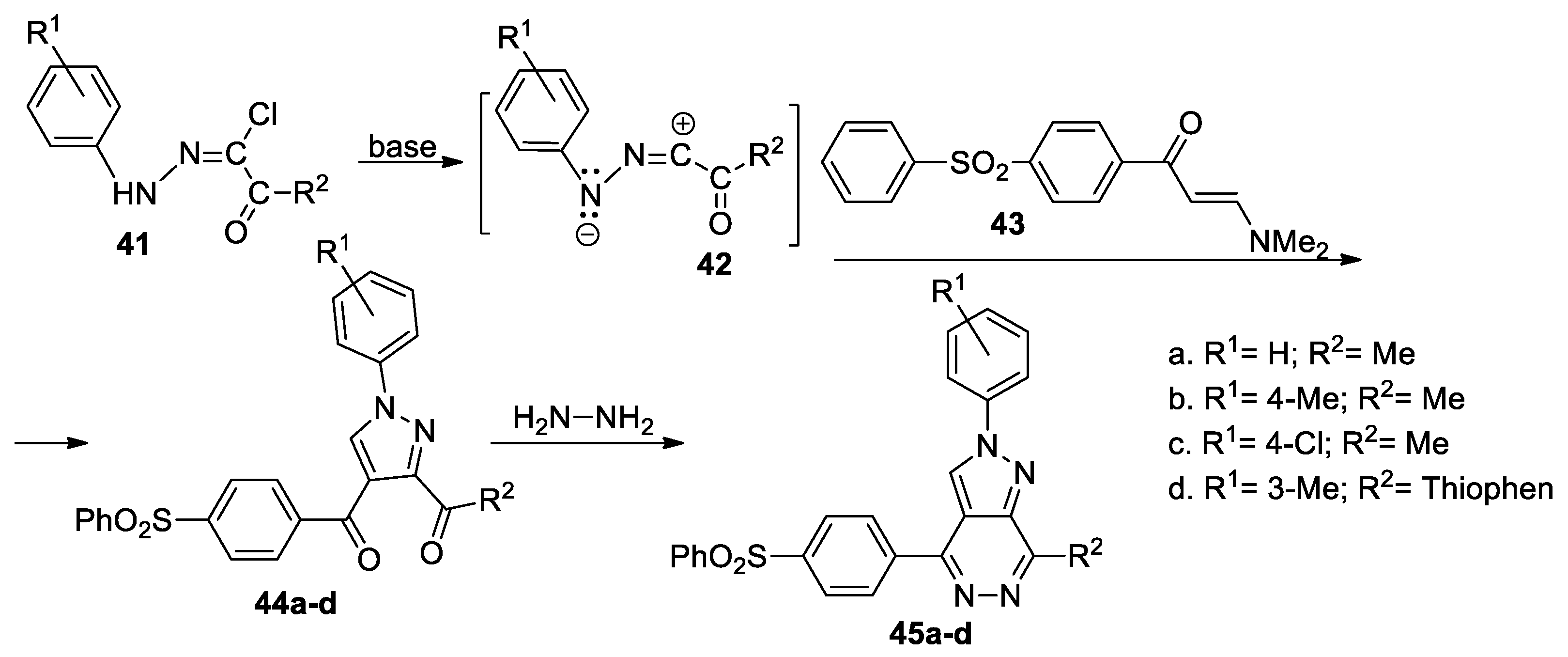
Mady et al. [38] report a green, facile and efficient synthetic approach for pyrazolo[3,4-d]pyridazines linked to sulfone moiety 45a–d, as shown in Scheme 12. In order to obtain the desired compounds 45a–d, initially, they obtain the pyrazole derivatives 44a–d, using the cycloaddition reaction of the enaminones 43 with the nitrile imines 42 (generated in situ by the action of the base on the hydrazonoyl chlorides 41), followed by a cyclocondensation with hydrazine.
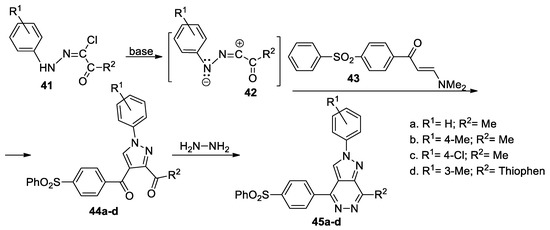
Scheme 12.
Two-step synthesis of pyrazolo[3,4-d]pyridazines 45a–d.
The reactions were performed using both CT heating and MW irradiation, with the reactions under MW being more efficient in terms of yields and time. The pyrazolo[3,4-d]pyridazine derivatives 45a–d were tested for their antimicrobial activity, the compounds having a very good antibacterial activity but not antifungal.
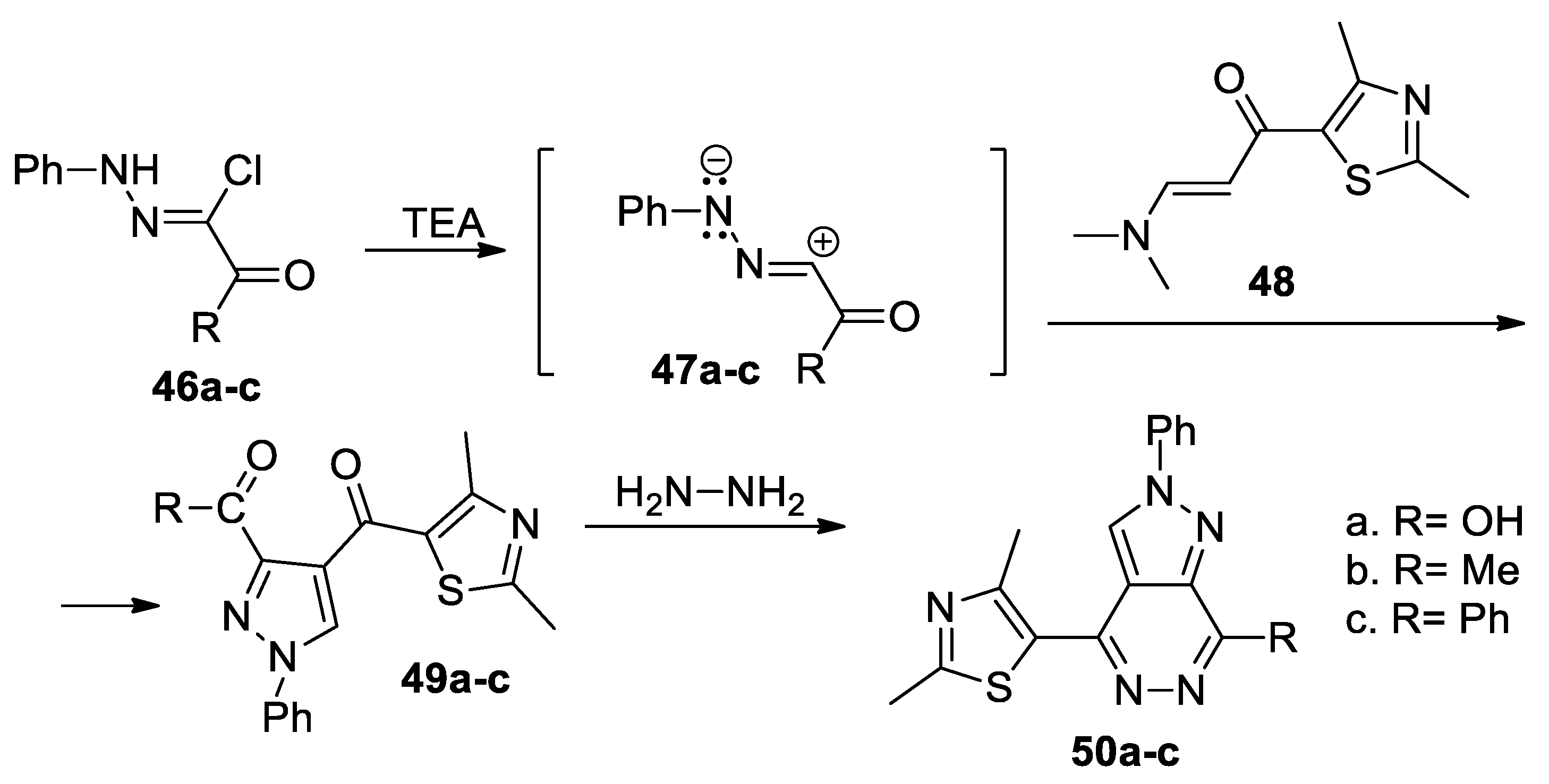
Zaki et al. [39] synthesized a series of pyrazolo[3,4-d]pyridazines 50a–c, as shown in Scheme 13. In order to obtain the desired compounds 50a–c, initially they obtain the pyrazole derivatives 49a–c using the cycloaddition reaction of the nitrile imines 47a–c (generated in situ by the action of the base TEA on the hydrazonoyl chlorides 46a–c) with thiazole derivative 48, followed by a cyclocondensation with hydrazine.
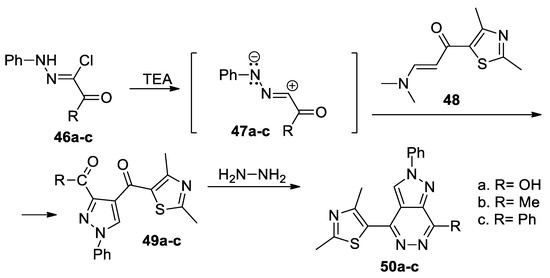
Scheme 13.
Two-step synthesis of pyrazolo[3,4-d]pyridazines 50a–c.
The pyrazolo[3,4-d]pyridazine derivatives 50a–c were tested for their antimicrobial activity, the compounds having a good antibacterial activity (both on Gram positive and Gram negative bacteria) but not antifungal.
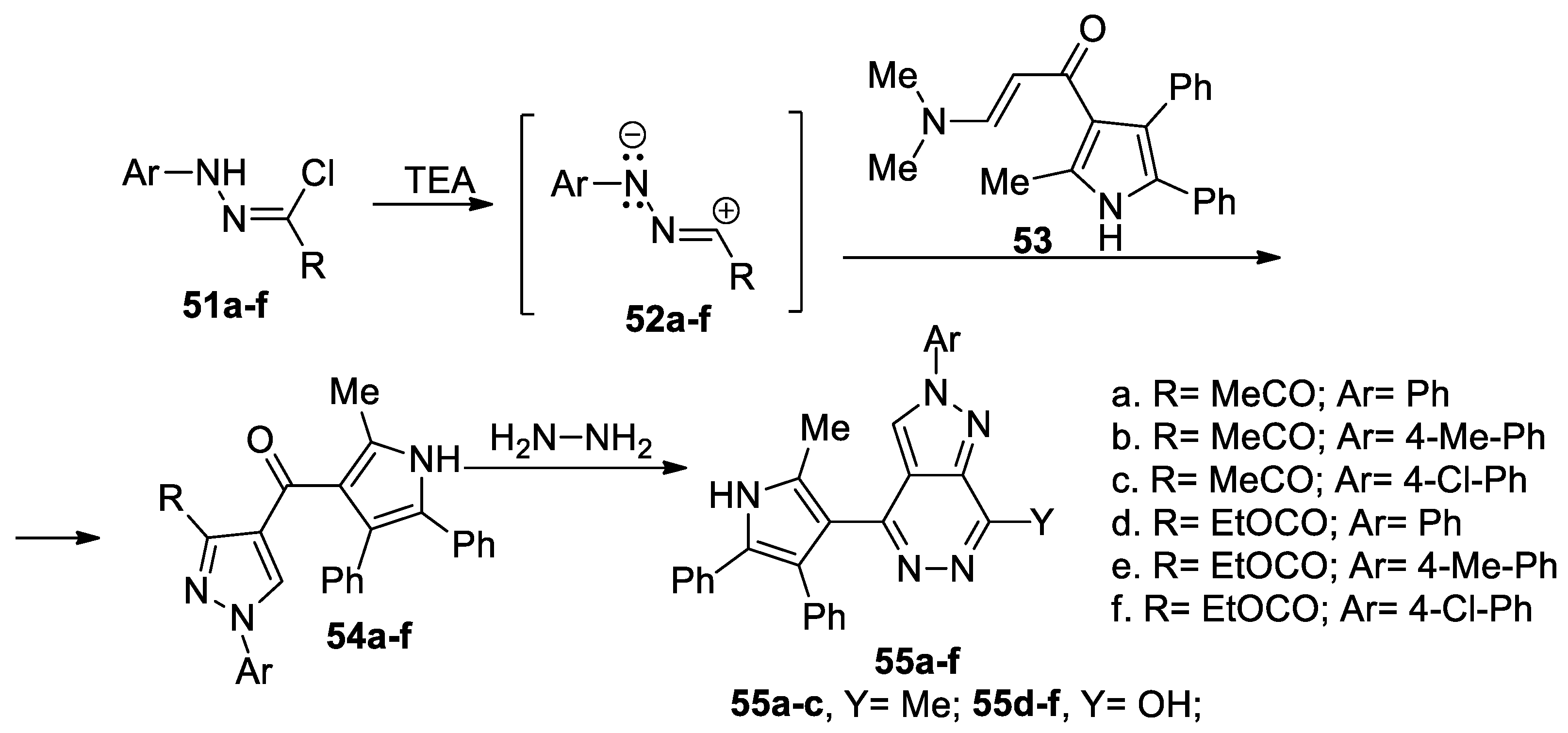
Eldebss et al. [40] synthesized a series of pyrazolo-pyridazines 55a–f, as shown in Scheme 14. In order to obtain the desired compounds 55a–f, initially they obtain the pyrazole derivatives 54a–f, using the cycloaddition reaction of the nitrile imines 52a–f (generated in situ by the action of the base TEA on the hydrazonoyl chlorides 51a–f) with enaminone-pyrrolo derivative 53, followed by a cyclocondensation with hydrazine. The cycloaddition reaction occur regioselective, in good yields.
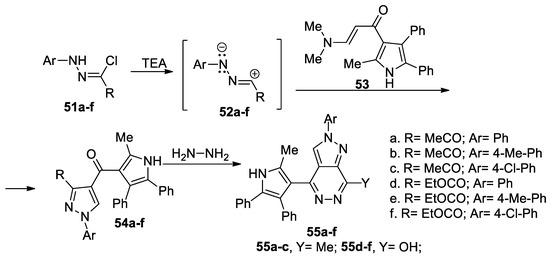
Scheme 14.
Two-step synthesis of pyrazolo-pyridazines 55a–f.
The pyrazolo-pyridazine derivatives 55a–f were tested for their protein kinase inhibitory activities against 25 kinases (belonging to four kinase groups), with the obtained results revealing that the compounds are selective and very good inhibitors against VEGFR-2, EGFR and CHK1, with IC50 values in the sub-micromolar range.
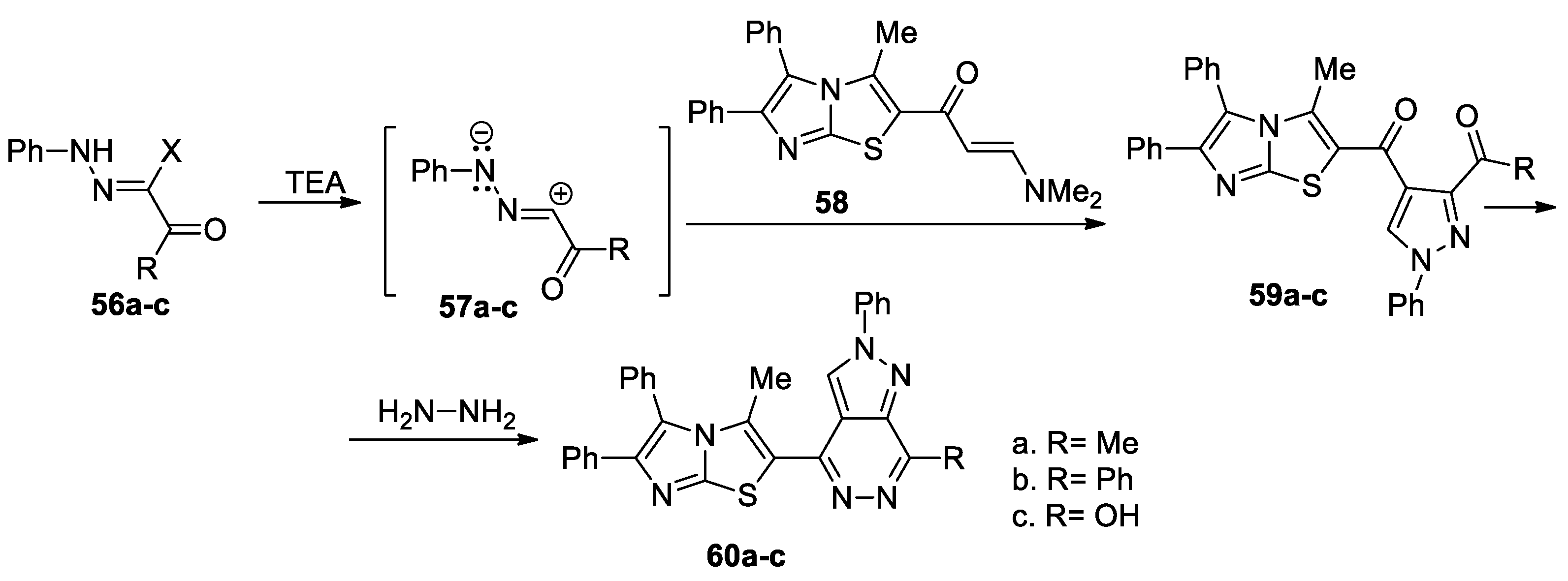
Gomha et al. [41] report the synthesis of a large variety of pyrazolo-azaheterocycles of potential interest in medicinal chemistry, as shown in Scheme 15. In order to obtain the desired compounds 60a–c, initially they obtain the pyrazole derivatives 59a–c, using a regioselective cycloaddition of nitrile imines 57a–c (generated in situ by the action of the base on the hydrazonoyl chlorides 56a–c) with enaminone 58, followed by a cyclocondensation with hydrazine hydrate when the corresponding pyrazolo[3,4-d]pyridazines 60a–c are obtained.
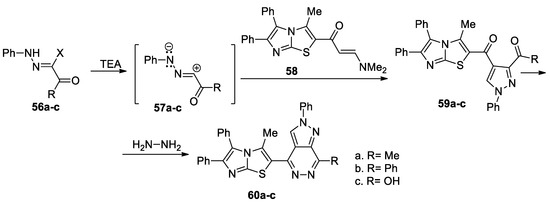
Scheme 15.
Two-step synthesis of pyrazolo[3,4-d]pyridazines 60a–c.
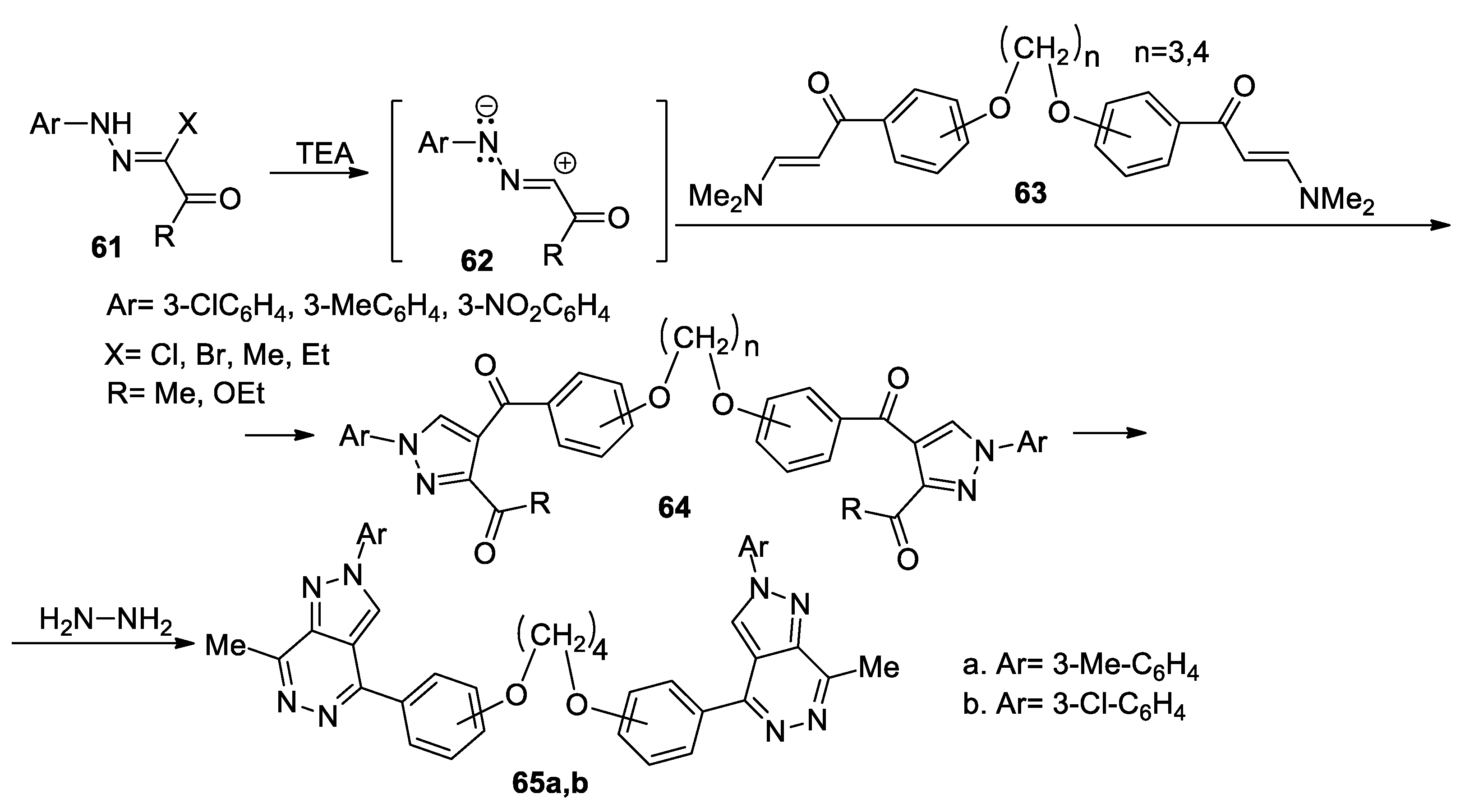
Using a similar strategy, Elwahy et al. [42] report an interesting study concerning synthesis of bis-pyrazolo[3,4-d]pyridazines 65a,b, as shown in Scheme 16. In order to obtain the desired compounds 65a,b, initially they obtain the bis-pyrazole derivatives 64, using the cycloaddition reactions of nitrile imines 62 (generated in situ by the action of the base on the hydrazonoyl chlorides 61) with bis(enaminones) 63, followed by a cyclocondensation with hydrazine hydrate when the corresponding bis-pyrazolo[3,4-d]pyridazines 65a,b are obtained.
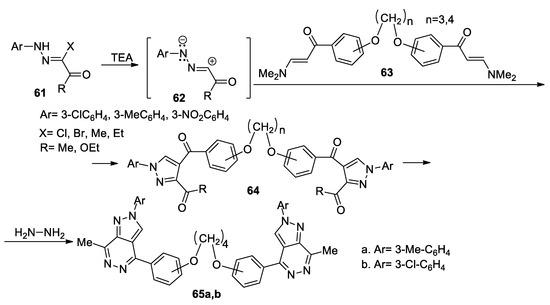
Scheme 16.
Two-step synthesis of bis-pyrazolo[3,4-d]pyridazines 65a,b.
These reactions were performed under conventional TH and MW irradiation. The author claims that MW irradiation is beneficial for the reaction pathway, the reaction conditions are milder, the yields are higher and the reaction time decreases.
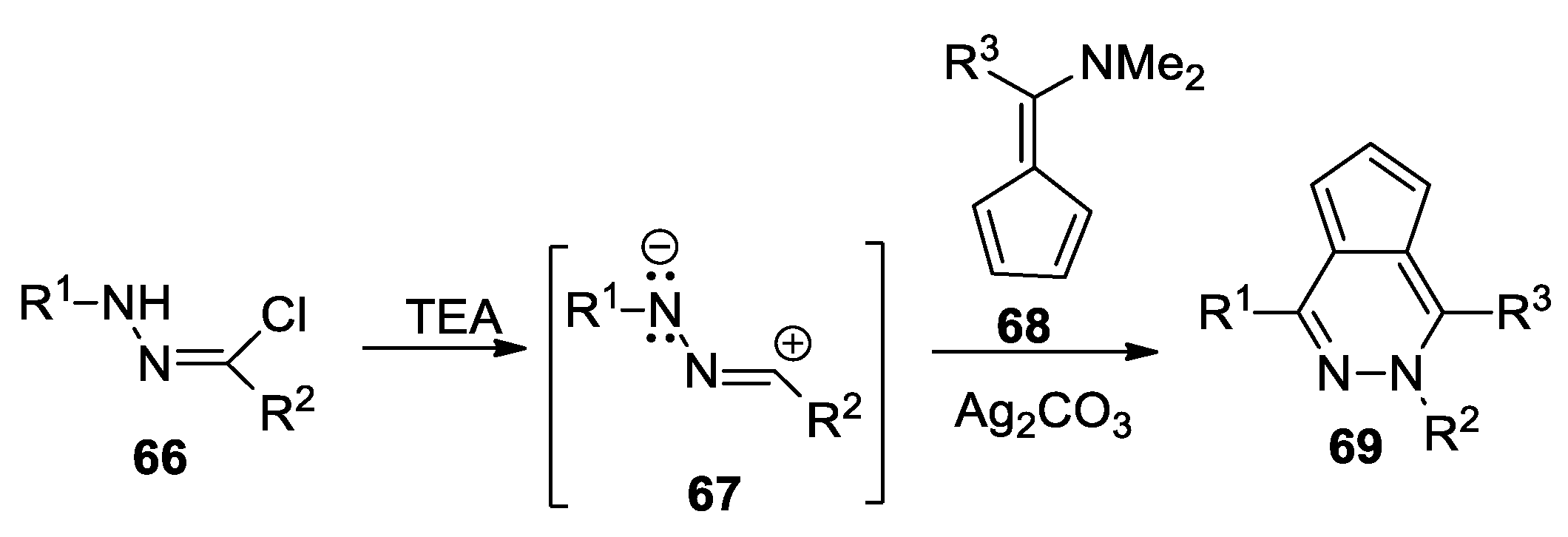
Abranyi-Balogh et al. [43] report a convenient and direct one-pot synthesis of cyclopenta[d]pyridazines, as shown in Scheme 17. In order to obtain the desired compound 69, they use the cycloaddition reactions of nitrile imine 67 (generated in situ from the hydrazonoyl chlorides 66; R1 = methyl, iso-propyl, tert-butyl, cyclohexyl, 3-OMe-phenyl; R2 = 4-OMe-phenyl) with fulvene 68 (R3 = H, methyl), using Ag2CO3 as the catalyst.
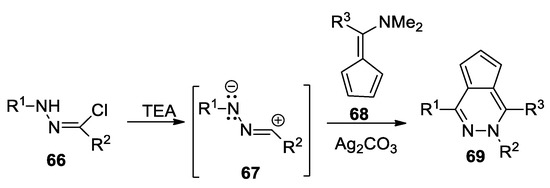
Scheme 17.
Synthesis of cyclopenta[d]pyridazines 69 by cycloaddition reactions.
These reactions were performed under mild conditions and the yields were good to high.
2.3. Pyridazine Derivatives Obtained by Cycloaddition and/or by Click Reactions of Azomethine Ylides or Imines
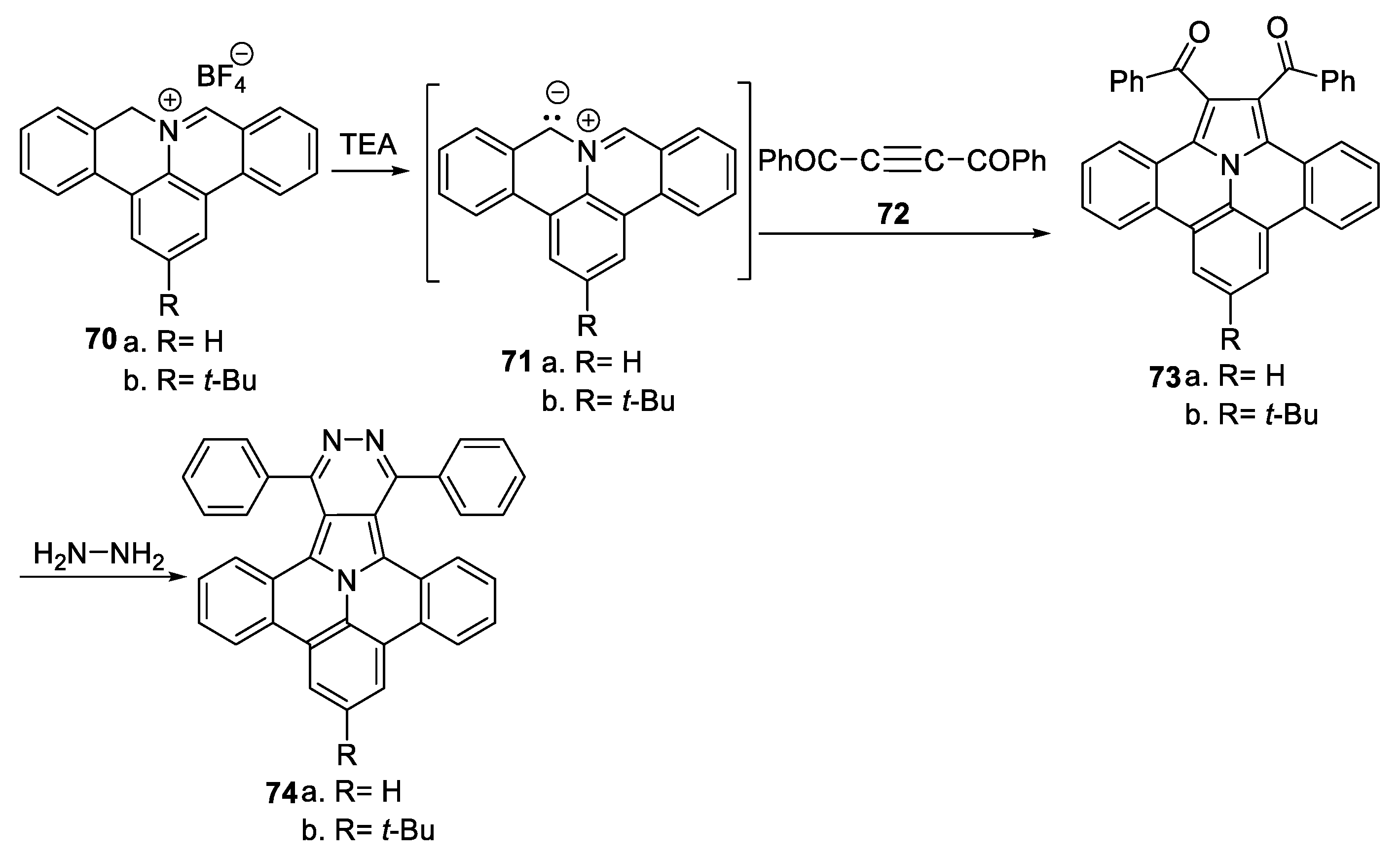
Richter et al. [44,45] performed an in-depth study concerning the optoelectronic properties of and obtaining some polycyclic aromatic hydrocarbons with pyrrolopyridazine core 74a,b, as shown in Scheme 18.
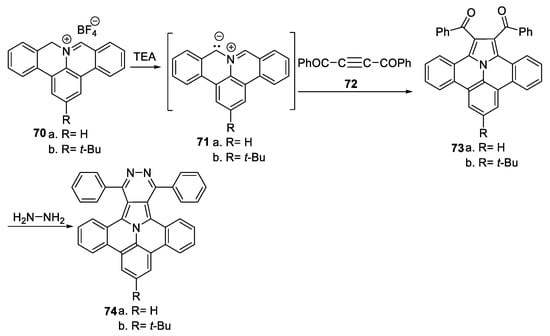
Scheme 18.
Two-step synthesis of polycyclic aromatic compounds with pyrrolopyridazine core 74a,b.
The synthesis was performed in two steps: an initial cycloaddition reaction of azomethine ylides 71a,b (generated from the salts 70a,b and TEA) to symmetrically substituted dipolarophiles with a triple bond (namely, 1,4-diphenylbut-2-yne-1,4-dione 72) leads to the intermediary ullazine 73a,b; this reaction is followed by a cyclocondensation of 73a,b with hydrazine, when the desired products 74a,b are obtained. In the obtained polycyclic aromatic hydrocarbons with pyrrolopyridazine core 74, interesting intramolecular push–pull phenomena between the ullazine part (as donor) and the pyridazine core (as acceptor part) were observed, which makes these compounds suitable candidates for organic field effect transistor (OFET) applications or chemical/bio-sensing. Similar results were obtained in this group (Berger) for other azaheterocycles [45].
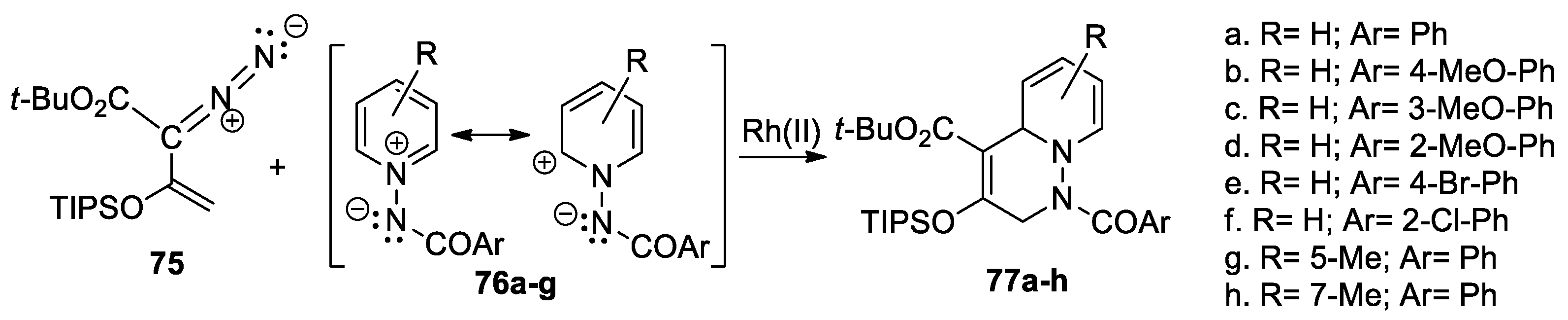
Xu et al. [46,47] synthesized a series of substituted 1,2,3,6-tetrahydropyridazine derivatives 77a–h, using the dirhodium-catalyzed [3 + 3] cycloaddition reactions of different N-acylimino-pyridinium ylides 76a–g, to enol diazoacetates 75 [TIPS = triisopropylsilyl], as shown in Scheme 19.
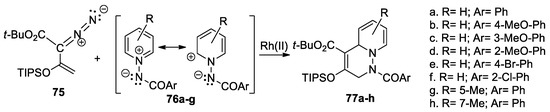
Scheme 19.
Synthesis of tetrahydropyridazine derivatives 80a–h by cycloaddition reactions.
The reactions occur regioselectively, with high yields, and excellent enantio-selectivities, controlled by the reaction conditions and catalysts. The sequence of reactions is triggered by Rh(II)-catalyzed dinitrogen extrusion, followed by vinylogous addition with N-acyliminopyridinium ylides.
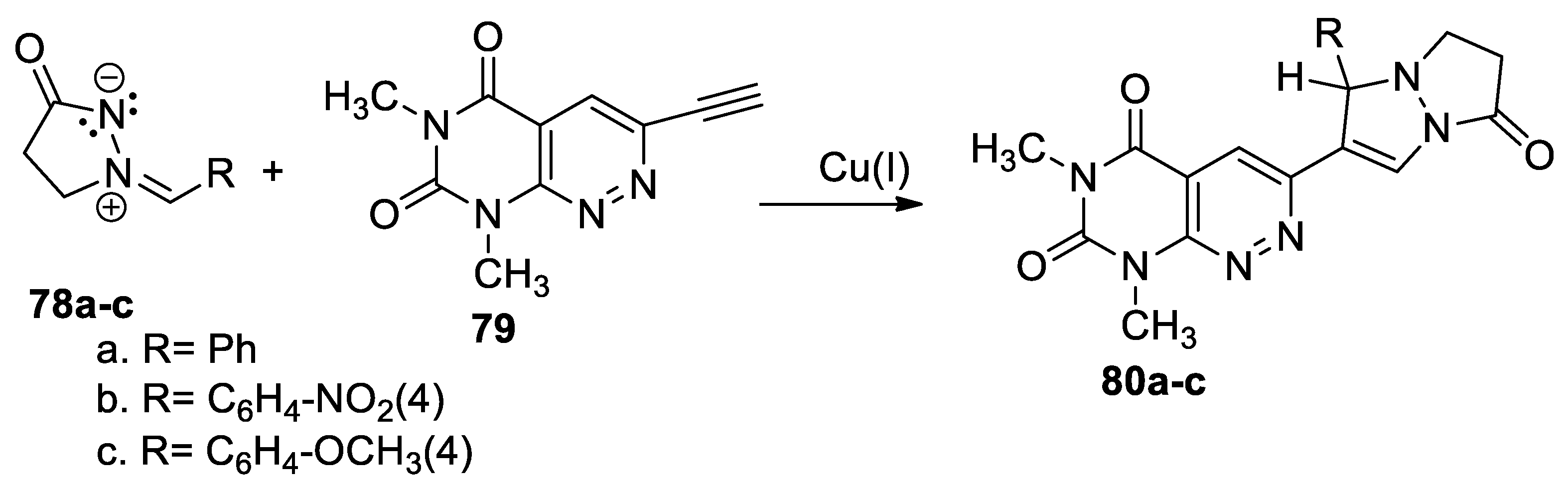
Using the cycloaddition reactions of azometine imines to π-deficient alkynyl hetarenes (used as dipolarophiles), Nelina-Nemtseva et al. [48] obtained various classes of fused azaheterocycles, these including pyrimido[4,5-c]pyridazine derivatives 80a–c, as shown in Scheme 20.
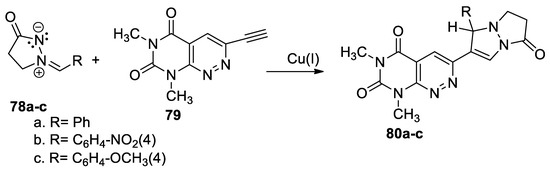
Scheme 20.
Copper-catalyzed [3 + 2] cycloaddition reactions for synthesis of 80a–c.
In order to obtain the fused pyridazine derivatives 80a–c, the authors used the copper-catalyzed [3 + 2] cycloaddition reactions. In this respect, 2-arylidene-5-oxopyrazolidin-2-ium-1-ides 78a–c were coupled with 3-ethynyl-6,8-dimethylpyrimido[4,5-c]pyridazine-5,7(6H,8H)-dione 79 (as dipolarophile), using Cu(I) as the catalyst. The reactions occur in moderate to excellent yields.
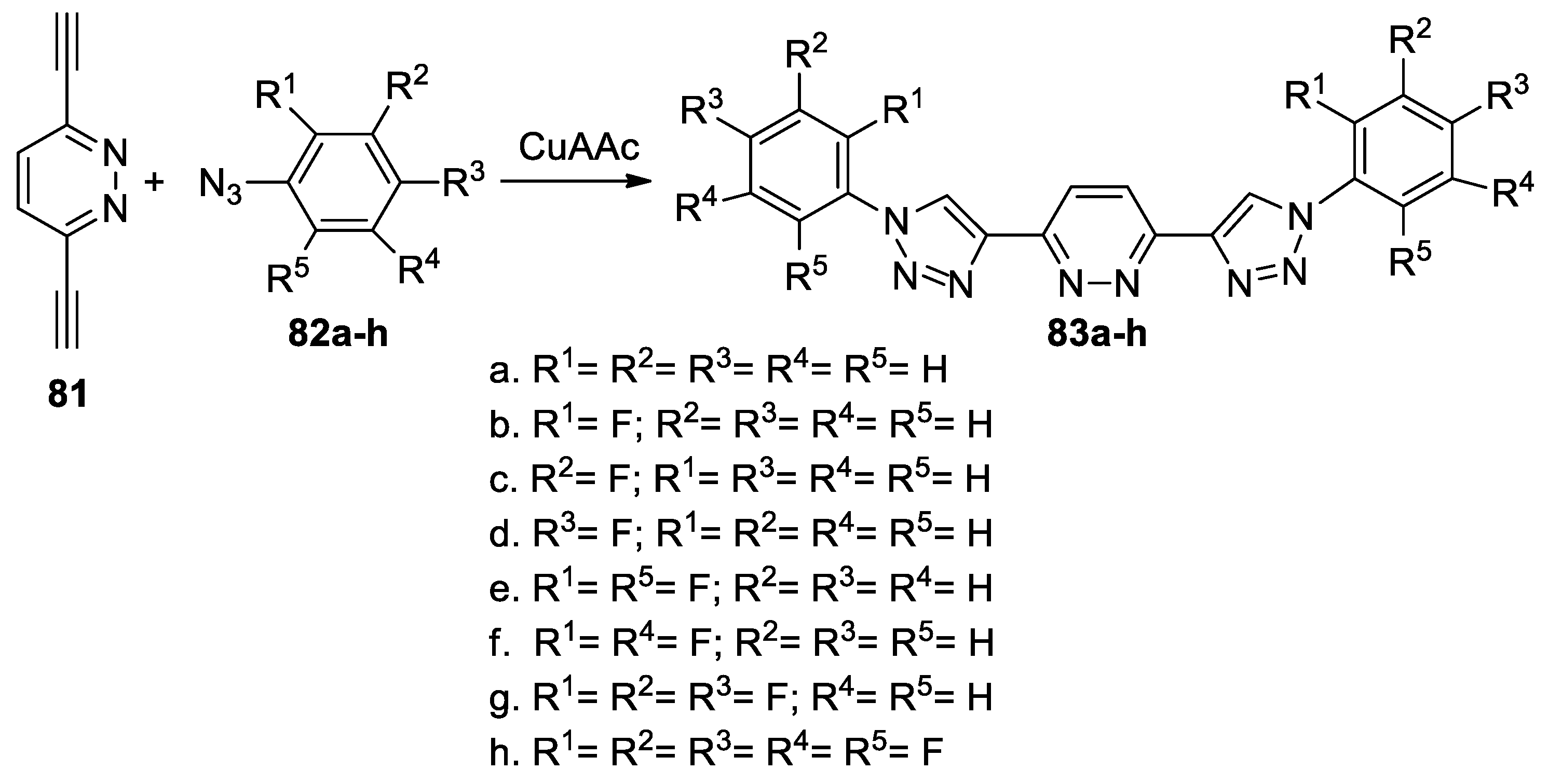
Birkenfelder et al. [49] synthesized a series of 3,6-bis(4-triazolyl)pyridazines by using a facile copper-catalyzed [3 + 2] cycloaddition reaction, as shown in Scheme 21.
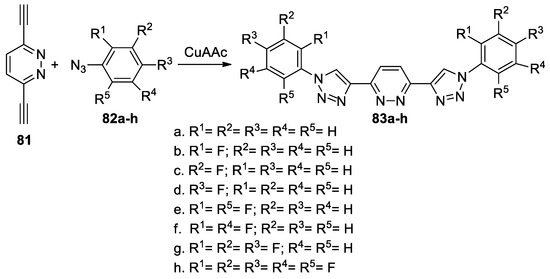
Scheme 21.
Copper-catalyzed [3 + 2] cycloaddition reactions for synthesis of 83a–h.
In this respect, 3,6-ethynylpyridazine 81 (used as dipolarophile with a triple bond) was treated with the corresponding azides 82a–h (CuAAC was used as a catalyst for click reactions), leading to the desired 3,6-bis(4-triazolyl)pyridazines 83a–h. Electrochemistry and optical spectroscopy of the obtained 3,6-bis(4-triazolyl)pyridazines 83a–h suggest that these compounds have an n-type organic semiconductor behavior, which make them useful as electron-transporting/hole-blocking materials in optoelectronics.
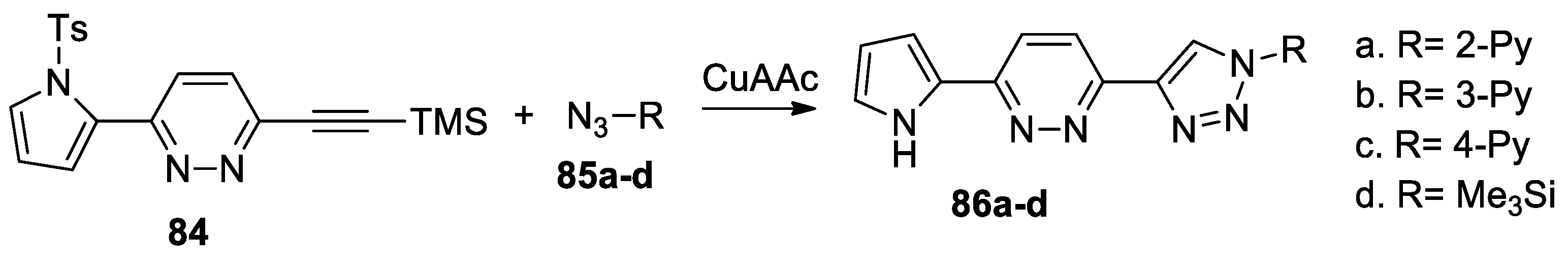
Yang et al. [50] synthesized a series of pyrrolo-pyridazyl-triazolyl derivatives 86a–d, by using a facile copper-catalyzed azide-alkyne cycloaddition reaction, as shown in Scheme 22.
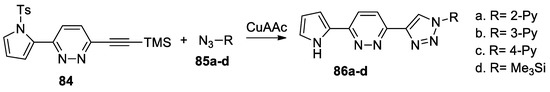
Scheme 22.
Azide-alkyne cycloaddition reactions for synthesis of pyridazine derivatives 86a–d.
In this respect, pyrrolo-pyridazine derivative 84 (used as dipolarophile with triple bond) was treated with the corresponding azides 85a–d (using CuAAC as catalyst for click reactions), leading to the desired pyrrolo-pyridazyl-triazolyl derivatives 86a–d. The authors suggest that these compounds could have applications in optoelectronics as π-conjugated D1-A1-D2-A2 materials.
2.4. Miscellaneous [3 + n] Cycloaddition Reactions
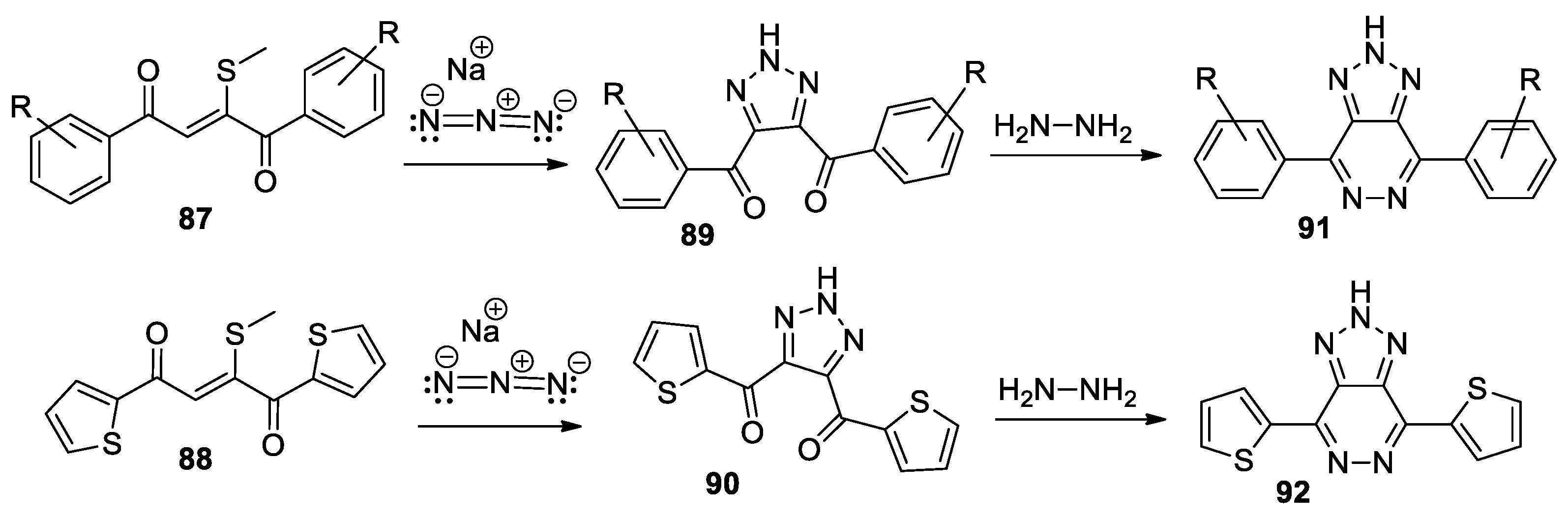
Swarup et al. [51] synthesized a series of 1,2,3-triazolo-pyridazine derivatives 91 and 92, using a straightforward and efficient procedure. In this respect, initially they obtain the 4,5-disubstituted-1,2,3-triazoles 89 and 90, through the cycloaddition reactions of R-benzo- or 2-methylthio- 1,4-ene-dione (compounds 87 and 88) and sodium azide (Scheme 23), followed by a cyclocondensation with hydrazine.
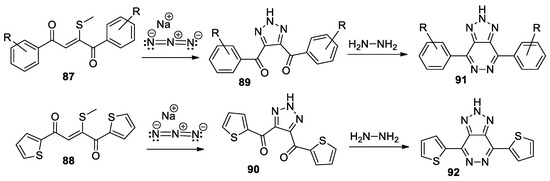
Scheme 23.
Two-step synthesis of 1,2,3-triazolo-pyridazine 91 and 92.
The triazolo-pyridazine derivatives, especially 92, could be used as key starting materials for obtaining solar cells.
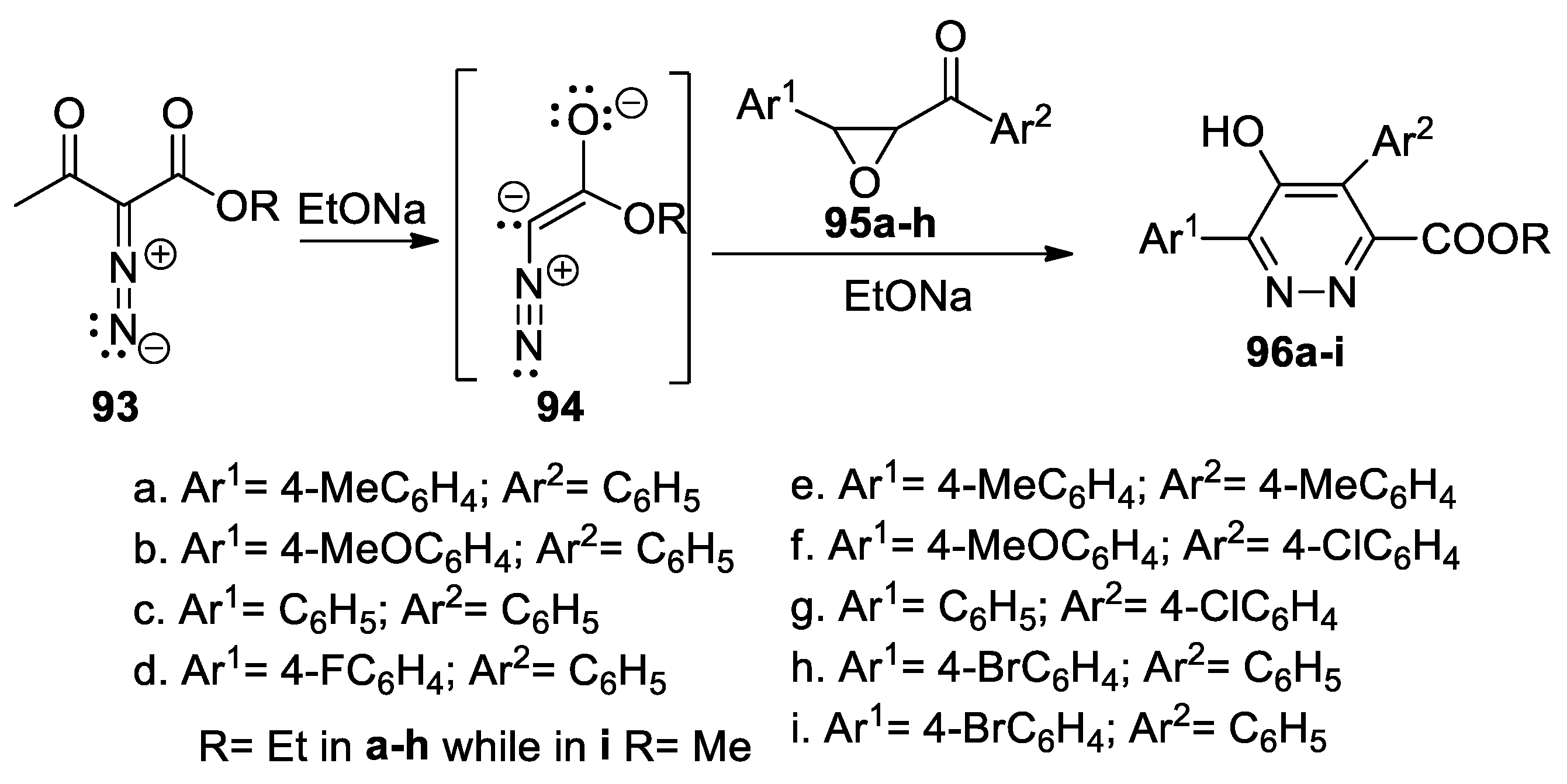
Nair et al. [52] synthesized a series of highly functionalized pyridazine esters 96a–i by cycloaddition reactions, as shown in Scheme 24. As 1,3-dipole, they used an α-diazoester anion 94 (generated in situ from the α-diazo-β-ketoester 93 in the presence of a base, EtONa), which undergoes a [3 + 3] annulation with chalcone epoxides 95a–h.
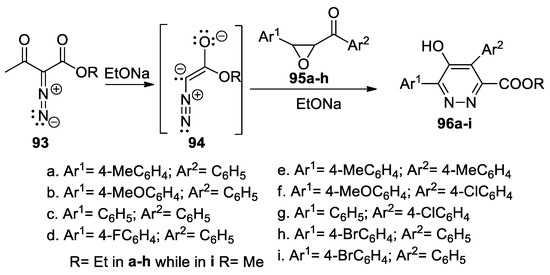
Scheme 24.
Synthesis of pyridazine esters 96a–i by [3 + 3] annulation reactions.
The reactions occur in moderate yields but completely regioselectively, in mild conditions, and with a wide variety of functional groups.
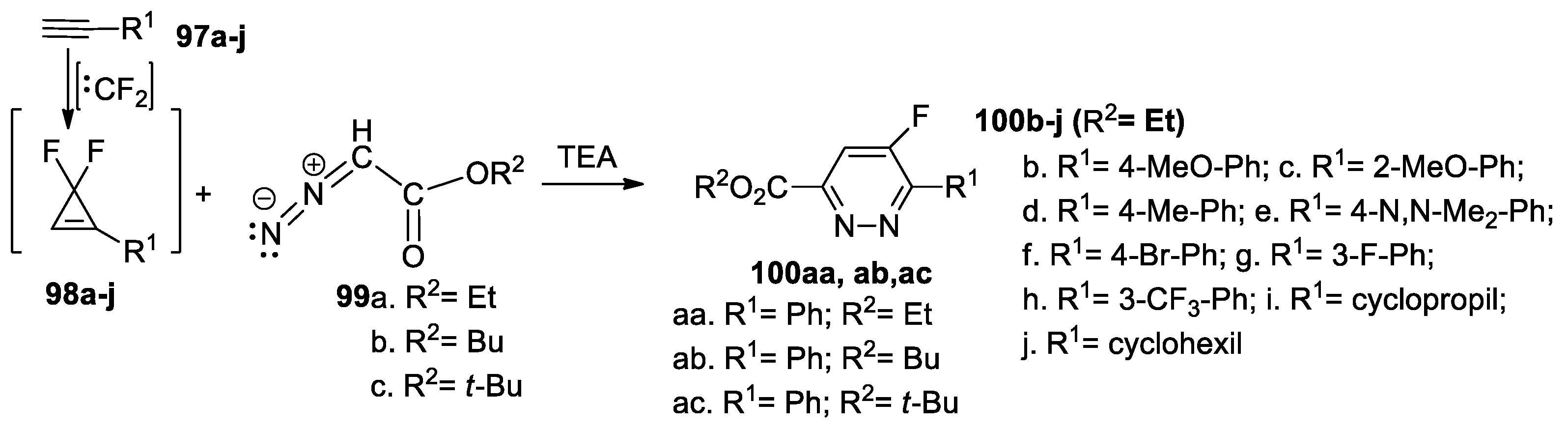
Tran et al. [53] synthesized different fluoro-pyridazine derivatives 100a–j, using the [3 + 2] cycloadditions of different R2-diazoacetate derivatives 99a–c, in the presence of TEA, to different 1-R1-2,2-difluorocyclopropene 98a–j (generated in situ from the corresponding acetylenic derivatives 97a–j and difluorocarbene), as shown in Scheme 25.
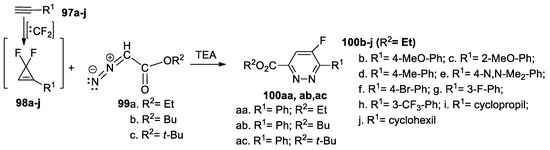
Scheme 25.
Synthesis of fluoro-pyridazine derivatives 100a–j by [3 + 2] cycloaddition reactions.
Overall, the reaction pathway is direct and efficient, with the desired 5-fluoro-pyridazines 100a–j being obtained in modest to good yields (30−86%).
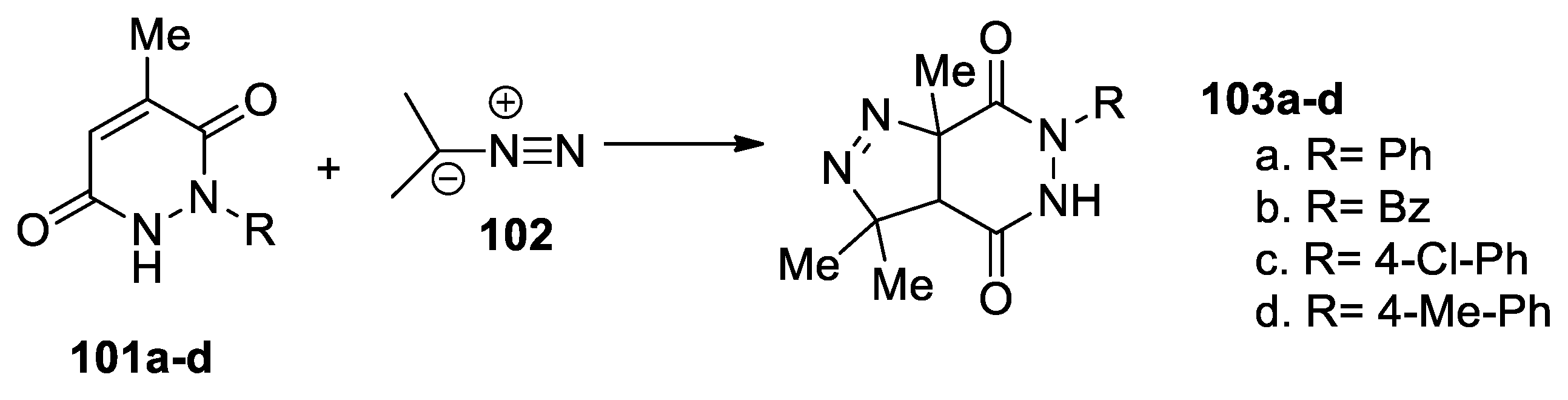
Ben Hamadi et al. [54] report an efficient and straightforward synthesis of saturated pyrazolo-pyridazinone via cycloaddition reactions, as shown in Scheme 26. In this respect, they use a [3 + 2] cycloaddition reaction of diazopropane 102 to pyridazinones 101a–d, when the corresponding saturated pyrazolo[3,4-d]pyridazinones 103a–d are obtained.
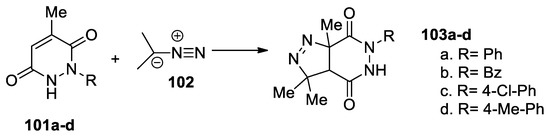
Scheme 26.
Synthesis of pyrazolo[3,4-d]pyridazinones 103a–d by [3 + 2] cycloaddition reactions.
These reactions were performed under conventional TH and US irradiation, concluding that the use of US irradiation has substantial advantages, with yields being higher, the reaction time decreasing, and the toxicity decreasing by using nontoxic solvents.
3. Concluding Remarks
In conclusion, it is clear that the [3 + n] cycloaddition reactions in the pyridazine series remain a versatile and useful tool to obtain new compounds of potential interest in medicinal chemistry and optoelectronics. The theoretical and experimental aspects of stereochemistry and regiochemistry involved in the [3 + n] cycloadditions are complex and attractive, and continue to rouse the interest of scientific community.
Author Contributions
Design and conception were carried out by V.M. and I.I.M. All authors contributed to writing, reviewing and approving the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Romanian Ministry of Education and Research, CNCS-UEFISCDI, project number PN-III-P4-ID-PCE-2020-0371, within PNCDI III.
Acknowledgments
Acknowledgment is given to infrastructure support from the Operational Program Competitiveness 2014–2020, Axis 1, under POC/448/1/1 Research infrastructure projects for public R&D institutions/Sections F 2018, through the Research Center with Integrated Techniques for Atmospheric Aerosol Investigation in Romania (RECENT AIR) project, under grant agreement MySMIS no. 127324.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huisgen, R.; Grashey, R.; Gotthardt, H.; Schmidt, R. 1,3-Dipolar additions of sydnones to Alkynes. A new route into the pyrazole series. Angew. Chem. Int. Ed. Engl. 1962, 1, 48–49. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Padwa, A. 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Houk, K.N., Yamaguchi, K., Eds.; John Wiley & Sons: New York, NY, USA, 1984; Chapters 1–3; pp. 1–450. ISBN1 047108364X. ISBN2 9780471083641. [Google Scholar]
- Zugravescu, I.; Petrovanu, M. N-Ylid-Chemistry; Mc Graw Hill: London, UK, 1976; ISBN 0-07-073080-6. [Google Scholar]
- Methoden der Organischen Chemie (Houben-Weyl). Organische Stickstoff-Verbindungen mit einer C,N-Doppelbindungen; Thieme Stuttgart: New York, NY, USA, 1991; pp. 100–1200. [Google Scholar]
- Epiotis, N.D. Theory of Organic Reactions; Springer: Berlin, Germany, 1978; ISBN 978-3-642-66827-2. [Google Scholar]
- Breugst, M.; Reissig, H.U. The Huisgen reaction: Milestones of the 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Tsuge, O.; Kanemasa, S.; Takenaka, S. Stereochemical study on 1,3-dipolar cycloaddition reactions of heteroaromatic N-ylides with symmetrically substituted cis and trans olefins. Bull. Chem. Soc. Jpn. 1985, 58, 3137–3157. [Google Scholar] [CrossRef]
- Firestone, R.A. On the mechanism of 1,3-dipolar cycloadditions. J. Org. Chem. 1968, 33, 2285–2290. [Google Scholar] [CrossRef]
- Firestone, R.A. The low energy of concert in many symmetry-allowed cycloadditions supports a stepwise-diradical mechanism. Int. J. Chem. Kinet. 2013, 45, 415–428. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the mysteries of the [3 + 2] cycloaddition reactions. Eur. J. Org. Chem. 2019, 267–282. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A molecular electron density theory study of the reactivity of azomethine imine in [3 + 2] cycloaddition reactions. Molecules 2017, 22, 750. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. The molecular electron density theory: A modern view of molecular reactivity in organic chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the high reactivity of the azomethineylides in [3 + 2]cycloaddition reactions. Lett. Org. Chem. 2010, 7, 432–439. [Google Scholar] [CrossRef]
- Dima, S.; Mangalagiu, I.I.; Caprosu, M.; Constantinescu, M.; Humelnicu, I.; Petrovanu, M. Stereochemistry of the cycloaddition reaction of 1-methylphthalazinium ylides to maleic and fumaric esters. J. Serb. Chem. Soc. 1997, 62, 1167–1174. [Google Scholar]
- Caprosu, M.; Mangalagiu, I.I.; Sirbu-Maftei, D.; Olariu, I.; Petrovanu, M. Studies on pyridazinium ylides. II. Stereochemistry of 3+2 dipolar cycloadditions of E-Z olefins. An. Stiint. Univ. Al. I. Cuza Iasi 1997, 5, 95–102. [Google Scholar]
- Mangalagiu, I.I.; Druta, I.; Constantinescu, M.; Humelnicu, I.; Petrovanu, M. Pyridazinium ylides. Regiochemistry. Tetrahedron 1996, 52, 8853–8862. [Google Scholar] [CrossRef]
- Mangalagiu, I.I.; Petrovanu, M. Pyridazinium ylides. Regiochemistry of addition. Acta Chim. Scand. 1997, 51, 927–931. [Google Scholar] [CrossRef][Green Version]
- Dima, S.; Mangalagiu, I.I.; Caprosu, M.; Constantinescu, M.; Humelnicu, I.; Petrovanu, M. The regiochemistry of the cycloaddition of 1-methylphthalazinium ylides to non-symmetrically substituted olefins. J. Serb. Chim. Soc. 1997, 62, 105–111. [Google Scholar]
- Caprosu, M.; Olariu, I.; Mangalagiu, I.I.; Constantinescu, M.; Petrovanu, M. The regiochemistry of the cycloaddition of 4-R-phenacylpyridazinium ylides to nonsymmetrical substituted olefins. Eur. J. Org. Chem. 1999, 12, 3501–3504. [Google Scholar] [CrossRef]
- Mangalagiu, I.I.; Mangalagiu, G.; Drochioiu, G.; Deleanu, C.; Petrovanu, M. 4-Methyl pyrimidinium ylides. Part 7: 3+2 dipolar cycloadditions to non-symmetrical substituted alkenes and alkynes. Tetrahedron 2003, 59, 111–114. [Google Scholar] [CrossRef]
- Amariucai-Mantu, D.; Mangalagiu, V.; Danac, R.; Mangalagiu, I.I. Microwave assisted reactions of azaheterocycles for medicinal chemistry applications. Molecules 2020, 25, 716. [Google Scholar] [CrossRef]
- Mangalagiu, I.I. Recent achievements in the chemistry of 1,2-diazines. Curr. Org. Chem. 2011, 15, 730–752. [Google Scholar] [CrossRef]
- Zbancioc, G.; Mangalagiu, I.I. Microwave-assisted synthesis of highly fluorescent pyrrolopyridazine derivatives. Synlett 2006, 5, 804–806. [Google Scholar] [CrossRef]
- Popovici, L.; Amarandi, R.M.; Mangalagiu, I.I.; Mangalagiu, V.; Danac, R. Synthesis, molecular modelling and anticancer evaluation of new pyrrolo[1,2-b]pyridazine and pyrrolo[2,1-a]phthalazine derivatives. J. Enz. Inhib. Med. Chem. 2019, 34, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, C.; Amariucai-Mantu, D.; Mangalagiu, V.; Antoci, V.; Maftei, D.; Mangalagiu, I.I.; Zbancioc, G. Microwave assisted reactions of fluorescent pyrrolodiazine building blocks. Molecules 2019, 24, 3760. [Google Scholar] [CrossRef] [PubMed]
- Zbancioc, G.; Moldoveanu, C.; Zbancioc, A.M.; Humelnicu, I.; Mangalagiu, I.I. New inside concerning microwave mechanism in cycloaddition reactions: Thermal heating versus specific effects of microwave. Rev. Roum. Chim. 2016, 61, 441–444. [Google Scholar]
- Maftei, D.; Zbancioc, G.; Humelnicu, I.; Mangalagiu, I.I. Conformational effects on the lowest excited states of benzoyl-pyrrolopyridazine: Insights from PCM time-dependent DFT. J. Phys. Chem. A 2013, 117, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Mantu, D.; Maftei, D.; Iurea, D.; Ursu, C.; Bejan, V. Synthesis, structure, and in vitro anticancer activity of new polycyclic 1,2-diazines. Med. Chem. Res. 2014, 23, 2909–2915. [Google Scholar] [CrossRef]
- Antoci, V.; Mantu, D.; Cozna, D.G.; Ursu, C.; Mangalagiu, I.I. Hybrid anticancer 1,2-diazine derivatives with multiple mechanism of action. Part 3. Med. Hypothesis 2014, 82, 11–15. [Google Scholar] [CrossRef]
- Bejan, V.; Mantu, D.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of isoindolo-1,2-diazine: A comparative study. Ultrason. Sonochem. 2012, 19, 999–1002. [Google Scholar] [CrossRef]
- Zbancioc, G.; Moldoveanu, C.; Zbancioc, A.M.; Mangalagiu, I.I. Microwave assisted synthesis of new pyrrolopyridazine derivatives with acetophenone skeleton. Part, V. Curr. Microw. Chem. 2014, 1, 41–46. [Google Scholar] [CrossRef]
- Zbancioc, G.; Zbancioc, A.M.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of dihydroxyacetophenone derivatives with or without 1,2-diazine skeleton. Ultrason. Sonochem. 2014, 21, 802–811. [Google Scholar] [CrossRef]
- Zbancioc, A.M.; Miron, A.; Tuchilus, C.; Rotinberg, P.; Mihai, C.T.; Mangalagiu, I.I.; Zbancioc, G. Synthesis and in vitro analysis of novel dihydroxyacetophenone derivatives with antimicrobial and antitumor activities. Med. Chem. 2014, 10, 476–483. [Google Scholar] [CrossRef]
- Tucaliuc, R.; Cotea, V.; Niculaua, M.; Tuchilus, C.; Mantu, D.; Mangalagiu, I.I. New pyridazine–fluorine derivatives: Synthesis, chemistry and biological activity. Part II. Eur. J. Med. Chem. 2013, 67, 367–372. [Google Scholar] [CrossRef]
- Butnariu, R.; Cotea, V.; Moldoveanu, C.; Zbancioc, G.; Deleanu, C.; Jones, P.; Mangalagiu, I.I. An efficient and selective way to hybrid trifluoromethyl-substituted γ-lactones or fused nitrogen derivatives via cascade reactions. Tet. Lett. 2011, 52, 6439–6442. [Google Scholar] [CrossRef]
- Garve, L.K.B.; Petzold, M.; Jones, P.G.; Werz, D.B. [3 + 3]-Cycloaddition of donor-acceptor cyclopropanes with nitrile imines generated in situ: Access to tetrahydropyridazines. Organic Letters 2016, 18, 564–567. [Google Scholar] [CrossRef]
- Mady, M.F.; Saleh, T.S.; El-Kateb, A.A.; Abd El-Rahman, N.M.; Abd El-Moez, S.I. Microwave-assisted synthesis of novel pyrazole and pyrazolo[3,4-d]pyridazine derivatives incorporating diaryl sulfone moiety as potential antimicrobial agents. Res. Chem. Intermed. 2016, 42, 753–769. [Google Scholar] [CrossRef]
- Zaki, Y.H.; Sayed, A.R.; Elroby, S.A. Regioselectivity of 1,3-dipolar cycloadditions and antimicrobial activity of isoxazoline, pyrrolo[3,4-d]isoxazole-4,6-diones, pyrazolo[3,4-d]pyridazines and pyrazolo[1,5-a]pyrimidines. Chem. Cent. J. 2016, 10, 17. [Google Scholar] [CrossRef][Green Version]
- Eldebss, T.M.A.; Gomha, S.M.; Abdulla, M.M.; Arafa, R.K. Novel pyrrole derivatives as selective CHK1 inhibitors: Design, regioselective synthesis and molecular modeling. MedChemComm 2015, 6, 852–859. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-Aziz, H.A. Enaminones as building blocks in heterocyclic preparations: Synthesis of novel pyrazoles, pyrazolo-[3,4-d]pyridazines, pyrazolo[1,5-a]pyrimidines, pyrido[2,3-d]pyrimidines linked to imidazo[2,1-b]thiazole system. Heterocycles 2012, 85, 2291–2303. [Google Scholar] [CrossRef]
- Elwahy, A.H.M.; Darweesh, A.F.; Shaaban, M.R. Microwave-assisted synthesis of bis(enaminoketones): Versatile precursors for novel bis(pyrazoles) via regioselective 1,3-dipolar cycloaddition with nitrileimines. J. Het. Chem. 2012, 49, 1120–1125. [Google Scholar] [CrossRef]
- Abranyi-Balogh, P. 1,3-Dipoles: Nitrile imines, nitrile oxides and nitrile sulfides. Synlett 2012, 23, 640–641. [Google Scholar] [CrossRef]
- Richter, M.; Fu, Y.; Dmitrieva, E.; Weigand, J.J.; Popov, A.; Berger, R.; Liu, J.; Feng, X. A polycyclic aromatic hydrocarbons containing a pyrrolopyridazine core. ChemPlusChem 2019, 84, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Hahn, S.; Dmitrieva, E.; Rominger, F.; Popov, A.; Bunz, U.H.; Feng, X.; Berger, R. Helical ullazine-quinoxaline-based polycyclic aromatic hydrocarbons. Chem. Eur. J. 2019, 25, 1345–1352. [Google Scholar] [CrossRef]
- Xu, X.; Doyle, M.P. The [3 + 3]-cycloaddition alternative for heterocycle syntheses: Catalytically generated metalloenolcarbenes as dipolar adducts. Acc. Chem. Res. 2014, 47, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zavalij, P.Y.; Doyle, M.P. Highly enantioselective dearomatizing formal [3 + 3] cycloaddition reactions of N-acyliminopyridinium ylides with electrophilic enol carbene intermediates. Angew. Chem. Int. Ed. 2013, 52, 12664–12668. [Google Scholar] [CrossRef] [PubMed]
- Nelina-Nemtseva, J.I.; Gulevskaya, A.V.; Suslonov, V.V.; Misharev, A.D. 1,3-Dipolar cycloaddition of azomethine imines to ethynylhetarenes: A synthetic route to 2,3-dihydropyrazolo[1,2-a]pyrazol-1(5H)-one based heterobiaryls. Tetrahedron 2018, 74, 1101–1109. [Google Scholar] [CrossRef]
- Birkenfelder, I.; Gurke, J.; Grubert, L.; Hecht, S.; Schmidt, B.M. Click chemistry derived pyridazines: Electron-deficient building blocks with defined conformation and packing structure. Chem. Asian, J. 2017, 12, 3156–3161. [Google Scholar] [CrossRef]
- Yang, W.; Fu, L.; Wu, J.; Song, C. Synthesis of pyrrol-pyridazyl-triazolyl-pyridines via Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction. Synthetic Comm. 2016, 46, 1118–1123. [Google Scholar] [CrossRef]
- Swarup, H.A.; Kempegowda Mantelingu, K.; Rangappa, K.S. Effective and transition-metal-free construction of disubstituted, trisubstituted 1,2,3-NH-triazoles and triazolopyridazine via intermolecular 1,3-dipolar cycloaddition reaction. ChemistrySelect 2018, 3, 703–708. [Google Scholar] [CrossRef]
- Nair, D.; Pavashe, P.; Katiyar, S.; Namboothiri, I.N.N. Regioselective synthesis of pyrazole and pyridazine esters from chalcones and α-diazo-β-ketoesters. Tetrahedron Lett. 2016, 57, 3146–3149. [Google Scholar] [CrossRef]
- Tran, G.; Gomez Pardo, D.; Tsuchiya, T.; Hilebrand, S.; Vors, J.-P.; Cossy, J. Modular, concise, and efficient synthesis of highly functionalized 5-fluoropyridazines by a [2+1]/[3 + 2]-cycloaddition sequence. Org. Lett. 2015, 17, 3414–3417. [Google Scholar] [CrossRef]
- Ben Hamadi, N.; Msaddek, M. A facile and efficient ultrasound-assisted stereospecific synthesis of novel bicyclo-cyclopropanes. CR Chim. 2012, 15, 409–413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).