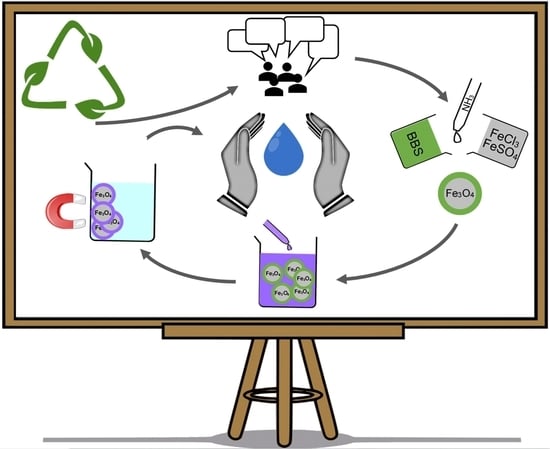
The “Lab4treat” Outreach Experience: Preparation of Sustainable Magnetic Nanomaterials for Remediation of Model Wastewater
Abstract
1. Introduction
2. Materials and Methods
3. Results: The Experiment
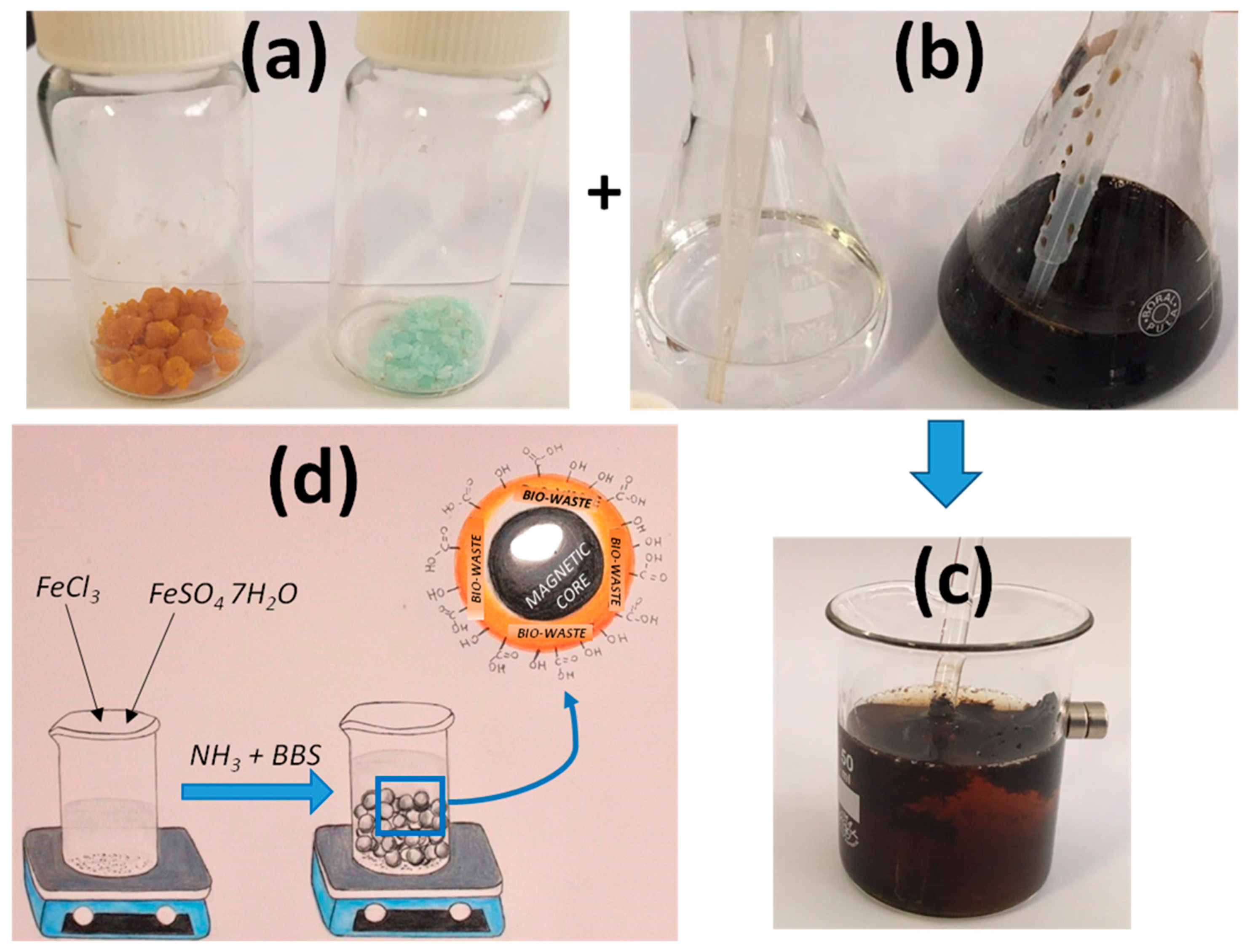
3.1. Magnetic Material Preparation
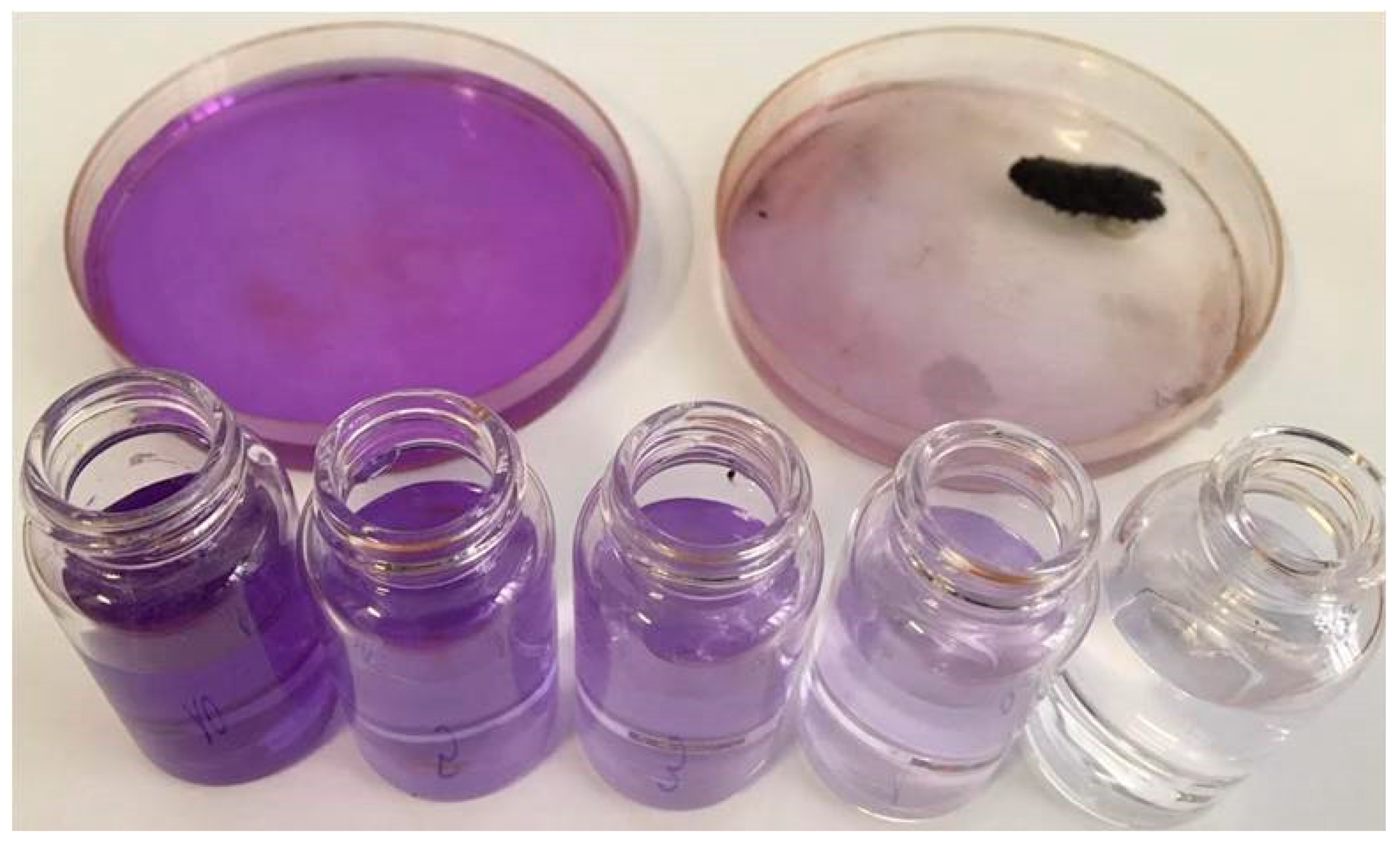
3.2. Water Depollution Experiment
4. Discussion: Chemical and Physical Phenomena Occurring during the Experiment: Analytical, Inorganic, Physical Chemistry for Dummies
4.1. Collection of Organic Waste, Aerobic and Anaerobic Digestion, Production of Gas and Compost. BBS Extraction and Composition
4.2. Coprecipitation Synthesis for the Preparation of the Magnetic Material
4.3. Role of Humic Acid in the Preparation of Magnetic Material: Interaction of BBS Carboxylate Groups with Material Iron Cations. Protection Against Material Oxidation. Exposition of Reactive Moieties for Substrate Capture
4.4. Introduction to Magnetism and Use in Environmental Applications
Industrial Applications of Magnetic Systems: Magnetic Separation
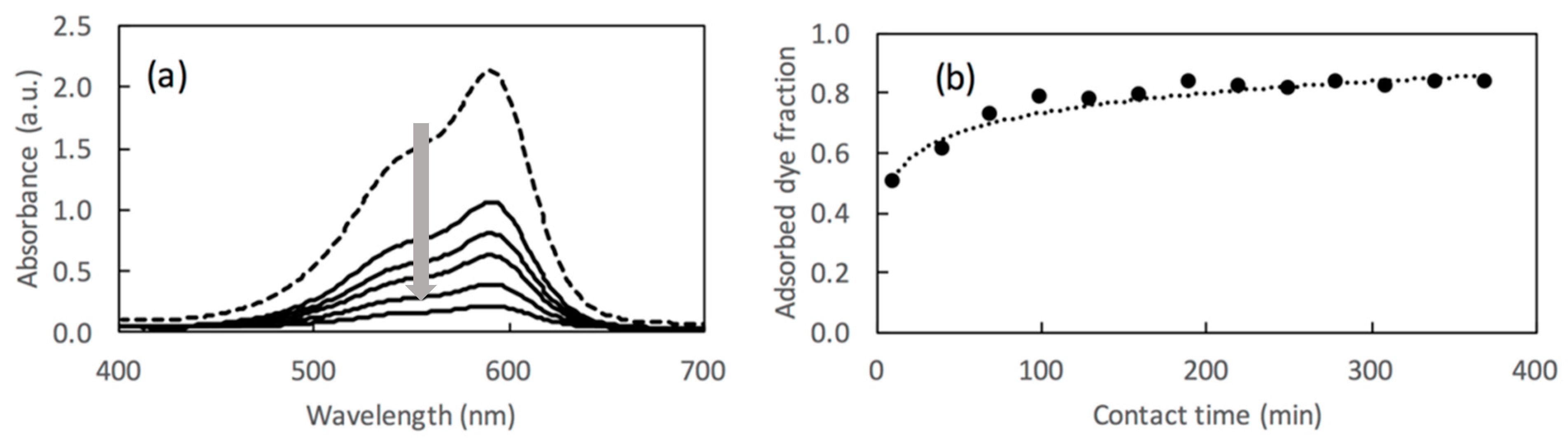
4.5. Evaluation of Adsorption Capacities. Lambert–Beer law and Evaluation of Adsorbed Amounts
4.6. Perspective towards the Photocatalytic Abatement of the Captured Pollutants (BBS as Photosensitizers in Photocatalytic Processes)
5. Conclusions
- (1)
- acquiring manual skills by means of simple synthetic procedures, in particular evaluating the reactivity of chemicals after mixing reactants to obtain new substances;
- (2)
- verifying the magnetic properties of the material synthesized by using a simple external commercially available magnet;
- (3)
- verifying the capability of interactions between different materials, in particular focusing on the capacity of the produced material for “capturing” dye molecules, thus cleaning the aqueous solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, S.B. Iron and Iron Oxide-Based Eco-nanomaterials for Catalysis and Water Remediation. In Handbook of Ecomaterials; Martínez, L., Kharissova, O., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Mayo, J.T.; Yavuz, C.; Yean, S.; Cong, L.; Shipley, H.; Yu, W.; Falkner, J.; Kan, A.; Tomson, M.; Colvin, V.L. The effect of nanocrystalline magnetite size on arsenic removal. Sci. Technol. Adv. Mater. 2007, 8, 71–75. [Google Scholar] [CrossRef]
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xu, Z.; Kuznicki, S.M. Mercury removal from flue gases by novel regenerable magnetic nanocomposite sorbents. Environ. Sci. Technol. 2009, 43, 3266–3271. [Google Scholar] [CrossRef]
- Girginova, P.I.; Daniel-da-Silva, A.L.; Lopes, C.B.; Figueira, P.; Otero, M.; Amaral, V.S.; Pereira, E.; Trindade, T. Silica coated magnetite particles for magnetic removal of Hg2+ from water. J. Colloid Interf. Sci. 2010, 345, 234–240. [Google Scholar] [CrossRef]
- Iram, M.; Guo, C.; Guan, Y.; Ishfaq, A.; Liu, H. Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. J. Hazard. Mater. 2010, 181, 1039–1050. [Google Scholar] [CrossRef]
- Wang, C.; Tao, S.; Wei, W.; Meng, C.; Liua, F.; Han, M. Multifunctional mesoporous material for detection, adsorption and removal of Hg2+ in aqueous solution. J. Mater. Chem. 2010, 20, 4635–4641. [Google Scholar] [CrossRef]
- Hu, J.; Shao, D.; Chen, C.; Sheng, G.; Li, J.; Wang, X.; Nagatsu, M. Plasma-induced grafting of cyclodextrin onto multiwall carbon nanotube/iron oxides for adsorbent application. J. Phys. Chem. B 2010, 114, 6779–6785. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Chen, J.; Chen, L.; Huai, J.; Gong, W.; Yuan, Z.; Wang, J.; Ma, J. Magnetic carbon nanotubes synthesis by Fenton’s reagent method and their potential application for removal of azo dye from aqueous solution. J. Colloid Interf. Sci. 2012, 378, 175–183. [Google Scholar] [CrossRef]
- He, H.-B.; Li, B.; Dong, J.-P.; Lei, Y.-Y.; Wang, T.-L.; Yu, Q.-W.; Feng, Y.-Q.; Sun, Y.-B. Mesostructured Nanomagnetic Polyhedral Oligomeric Silsesquioxanes (POSS) Incorporated with Dithiol Organic Anchors for Multiple Pollutants Capturing in Wastewater. ACS Appl. Mater. Interf. 2013, 5, 8058–8066. [Google Scholar] [CrossRef]
- Mao, J.; Jiang, W.; Gu, J.; Zhou, S.; Lu, Y.; Xie, T. Synthesis of P (St-DVB)/Fe3O4 microspheres and application for oil removal in aqueous environment. App. Surf. Sci. 2014, 317, 787–793. [Google Scholar] [CrossRef]
- Ricco, R.; Konstas, K.; Styles, M.J.; Richardson, J.J.; Babarao, R.; Suzuki, K.; Scopecec, P.; Falcaro, P. Lead(II) uptake by aluminium based magnetic framework composites (MFCs) in water. J. Mater. Chem. A 2015, 3, 19822–19831. [Google Scholar] [CrossRef]
- Zhang, C.; Ai, L.; Jiang, J. Solvothermal synthesis of MIL–53(Fe) hybrid magnetic composites for photoelectrochemical water oxidation and organic pollutant photodegradation under visible light. J. Mater. Chem. A 2015, 3, 3074–3081. [Google Scholar] [CrossRef]
- Khan, M.; Lo, I.M.C. Removal of ionizable aromatic pollutants from contaminated water using nano γ-Fe2O3 based magnetic cationic hydrogel: Sorptive performance, magnetic separation and reusability. J. Hazard Mater. 2017, 322, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Tao, F.; Pan, Q. Fast and Selective Removal of Oils from Water Surface via Highly Hydrophobic Core−Shell Fe2O3@C Nanoparticles under Magnetic Field. ACS Appl. Mater. Interf. 2010, 2, 3141–3146. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, H.; Hu, Z.; Cai, Y.; Shi, Y. Preparation of carbon coated Fe3O4 nanoparticles and their application for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2010, 1217, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, J.; Zhu, Z.; Cui, F.; Zhu, Y.-A.; Duan, X.; Wang, S. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 2018, 354, 507–516. [Google Scholar] [CrossRef]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. Research progress on iron oxide-based magnetic materials: Synthesis techniques and photocatalytic applications. Ceram. Int. 2016, 42, 9–34. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, T.; Hao, L.; Guo, Y.; Liu, W.; Guo, L.; Wang, C.; Wang, Z.; Wu, Q. Advances in magnetic porous organic frameworks for analysis and adsorption applications. Trends Anal. Chem. 2020, 132, 116048. [Google Scholar] [CrossRef]
- Sriplai, N.; Pinitsoontorn, S. Bacterial cellulose-based magnetic nanocomposites: A review. Carbohydr. Polym. 2021, 254, 117228. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Naz, I.; Lin, D.; Saroj, D.P.; Ali, M. Development and application of novel biomagnetic membrane capsules for the removal of the cationic dye malachite green in wastewater treatment. RSC Adv. 2019, 9, 3625–3646. [Google Scholar] [CrossRef]
- Ali, N.; Zaman, H.; Bilal, M.; Shah, A.H.A.; Shahzad Nazir, M.; Iqbal, H.M.N. Environmental perspectives of interfacially active and magnetically recoverable composite materials—A review. Sci. Total Environ. 2019, 670, 523–538. [Google Scholar] [CrossRef]
- Cui, S.; Wang, X.; Zhang, X.; Xia, W.; Tang, X.; Lin, B.; Wu, Q.; Zhang, X.; Shen, X. Preparation of magnetic MnFe2O4-Cellulose aerogel composite and its kinetics and thermodynamics of Cu(II) adsorption. Cellulose 2018, 25, 735–751. [Google Scholar] [CrossRef]
- Peralta, M.E.; Ocampo, S.; Funes, I.G.; Onaga, M.F.; Parolo, M.E.; Carlos, L. Nanomaterials with Tailored Magnetic Properties as Adsorbents of Organic Pollutants from Wastewaters. Inorganics 2020, 8, 24. [Google Scholar] [CrossRef]
- Han, L.-J.; Ge, F.-Y.; Sun, G.H.; Gao, X.-J.; Zheng, H.-G. Effective adsorption of Congo red by a MOF-based magnetic material. Dalton Trans. 2019, 48, 4650–4656. [Google Scholar] [CrossRef]
- Gupta, S.V.; Ahmaruzzaman, M. Development of efficient magnetic Fe2O3-activated Bakelite nanocomposite as an adsorbent for removal of Victoria blue dye from water. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Ardisson, J.D.; Lago, R.M. Preparation of magnetic mesoporous composites from glycerol and iron(III) salt. J. Chem. Technol. Biotechnol. 2020, 95, 1038–1045. [Google Scholar] [CrossRef]
- Cui, Y.; Atkinson, J.D. Glycerol-derived magnetic mesoporous Fe/C composites for Cr(VI) removal, prepared via acid-assisted one-pot pyrolysis. Chemosphere 2019, 228, 694–701. [Google Scholar] [CrossRef]
- Liu, S.; Yua, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 nanoparticles. Adv. Colloid Interf. Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Plohl, O.; Simonič, M.; Kolar, K.; Gyergyek, S.; Fras Zemljič, L. Magnetic nanostructures functionalized with a derived lysine coating applied to simultaneously remove heavy metal pollutants from environmental systems. Sci. Technol. Adv. Mater. 2021, 22, 55–71. [Google Scholar] [CrossRef]
- Matos Oliveira, R.V.; Alves Lima, J.R.; da Costa Cunha, G.; Cruz Romão, L.P. Use of eco-friendly magnetic materials for the removal of polycyclic aromatic hydrocarbons and metals from environmental water samples. J. Environ. Chem. Eng. 2020, 8, 104050. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Fu, H.; Ma, S.; Zhao, P.; Xu, S.; Zhan, S. Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: Structure dependence and mechanism. Chem. Eng. J. 2019, 360, 157–170. [Google Scholar] [CrossRef]
- He, H.-Y.; He, Z.; Shen, Q. Synthesis and Enhanced Photocatalysis of Magnetic Reduced Graphene Oxide-MnFe2O4 Nanohybrids. Micro Nanosyst. 2018, 10, 94–101. [Google Scholar] [CrossRef]
- Palma, D.; Bianco Prevot, A.; Brigante, M.; Fabbri, D.; Magnacca, G.; Richard, C.; Mailhot, G.; Nisticò, R. New Insights on the Photodegradation of Caffeine in the Presence of Bio-Based Substances-Magnetic Iron Oxide Hybrid Nanomaterials. Materials 2018, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kaneti, Y.V.; Wulan Septiani, N.L.; Dou, S.X.; Bando, Y.; Hossain, S.A.; Kim, J.; Yamauchi, Y. A Review on Iron Oxide-Based Nanoarchitectures for Biomedical, Energy Storage, and Environmental Applications. Small Methods 2019, 3, 1800512. [Google Scholar] [CrossRef]
- Manohar, A.; Krishnamoorthi, C.; Pavithra, C.; Thota, N. Magnetic Hyperthermia and Photocatalytic Properties of MnFe2O4 Nanoparticles Synthesized by Solvothermal Reflux Method. J. Supercond. Novel Magn. 2021, 34, 251–259. [Google Scholar] [CrossRef]
- Muñoz Medina, G.A.; Fernández van Raap, M.B.; Coral, D.F.; Muraca, D.; Sánchez, F.H. Synthesis of highly stable Fe/FeOx@citrate colloids with strong magnetic response by mechanochemistry and coprecipitation for biomedical and environmental applications. J. Magnet. Magnet. Mater. 2020, 508. [Google Scholar] [CrossRef]
- Ravanello Mariosi, F.; Venturini, J.; Cas Viegas, A.; Perez Bergmann, C. Lanthanum-doped spinel cobalt ferrite (CoFe2O4) nanoparticles for environmental applications. Ceram. Int. 2020, 46, 2772–2779. [Google Scholar] [CrossRef]
- Talbot, D.; Abramson, S.; Gri_ete, N.; Bée, A. pH-sensitive magnetic alginate/-Fe2O3 nanoparticles for adsorption/desorption of a cationic dye from water. J. Water Process Eng. 2018, 25, 301–308. [Google Scholar] [CrossRef]
- Mohammadi, A.; Daemi, H.; Barikani, M. Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int. J. Biol. Macromol. 2014, 69, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Safarik, I.; Lunackova, P.; Weyda, F.; Safarikova, M. Adsorption of water-soluble organic dyes on ferrofluidmodified sawdust. Holzforschung 2007, 61, 247–253. [Google Scholar] [CrossRef]
- Safarik, I.; Safarikova, M. Magnetic fluid modified peanut husks as an adsorbent for organic dyes removal. Phys. Proc. 2010, 9, 274–278. [Google Scholar] [CrossRef]
- Zuorro, A.; Di Battista, A.; Lavecchia, R. Magnetically modified coffee silverskin for the removal of xenobiotics from wastewater. Chem. Eng. Trans. 2013, 35, 1375–1380. [Google Scholar]
- Stan, M.; Lung, I.; Soran, M.; Opris, O.; Leostean, C.; Popa, A.; Copaciu, F.; Diana, M.; Kacso, I.; Silipas, T. Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 100, 65–73. [Google Scholar] [CrossRef]
- Jodeh, S.; Hamed, O.; Melhem, A.; Salghi, R.; Jodeh, D.; Azzaoui, K.; Benmassaoud, Y.; Murtada, K. Magnetic nanocellulose from olive industry solid waste for the effective removal of methylene blue from wastewater. Environ. Sci. Pollut. Res. 2018, 25, 22060–22074. [Google Scholar] [CrossRef] [PubMed]
- Magnacca, G.; Allera, A.; Montoneri, E.; Celi, L.; Benito, D.E.; Gagliardi, L.G.; Carlos, L. Novel magnetite nanoparticles coated with waste sourced bio- based substances as sustainable and renewable adsorbing materials. ACS Sustain. Chem. Eng. 2014, 2, 1518–1524. [Google Scholar] [CrossRef]
- Nisticò, R.; Cesano, F.; Franzoso, F.; Magnacca, G.; Scarano, D.; Funes, I.G.; Carlos, L.; Parolo, M.E. From biowaste to magnet-responsive materials for water remediation from polycyclic aromatic hydrocarbons. Chemosphere 2018, 202, 686–693. [Google Scholar] [CrossRef]
- Nisticò, R.; Celi, L.R.; Bianco Prevot, A.; Carlos, L.; Magnacca, G.; Zanzo, E.; Martin, M. Sustainable magnet-responsive nanomaterials for the removal of arsenic from contaminated water. J. Hazard. Mater. 2018, 342, 260–269. [Google Scholar] [CrossRef]
- Ioana, I. Clean technology from waste management. In Proceedings of the 4th WSEAS International Conference on Waste management, Water Pollution, Air Pollution, Indoor Climate (WWAI ‘10), Kantaoui, Sousse, Tunisia, 3–6 May 2010; Kallel, A., Hassairi, A., Bulucea, C.A., Mastorakis, N., Eds.; WSEAS Press: Athens, Greece, 2010. Plenary Lecture 2. p. 13, ISBN 978-960-474-190-8. [Google Scholar]
- European Commission. Preparatory Study on Food Waste Across EU 27; European Commission: Brussel, Belgium, 2011; ISBN 978-92-79-22138-5. [Google Scholar]
- Biochemenergy. Available online: https://ideas.repec.org/a/ids/ijgenv/v11y2011i2p170-196.html (accessed on 30 April 2021).
- Isolation, Characterization and Screening of Environmental Applications of Bio-Organic Substances Obtained from Urban Biomasses (EnvironBOS). Available online: https://cordis.europa.eu/project/id/269128 (accessed on 30 April 2021).
- Enhancing Water Quality by Developing Novel Materials for Organic Pollutant Removal in Tertiary Water Treatments. Available online: https://cordis.europa.eu/project/id/645551 (accessed on 30 April 2021).
- Palma, D.; Bianco Prevot, A.; Celi, L.; Martin, M.; Fabbri, D.; Magnacca, G.; Chierotti, M.R.; Nisticò, R. Isolation, characterization, and environmental application of bio-based materials as auxiliaries in photocatalytic processes. Catalysts 2018, 8, 197. [Google Scholar] [CrossRef]
- Nisticò, R.; Barrasso, M.; Carrillo Le Roux, G.A.; Seckler, M.M.; Sousa, W.; Malandrino, M.; Magnacca, G. Biopolymers from composted biowaste as stabilizers for the synthesis of spherical and homogeneously sized silver nanoparticles for textile applications on natural fibers. ChemPhysChem 2015, 16, 3902–3909. [Google Scholar] [CrossRef]
- Savarino, P.; Montoneri, E.; Biasizzo, M.; Quagliotto, P.; Viscardi, G.; Boffa, V. Upgrading biomass wastes in chemical technology. Humic acid-like matter isolated from compost as chemical auxiliary for textile dyeing. J. Chem. Technol. Biotechnol. 2007, 82, 939–948. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Arques, A.; Carlos, L.; Laurenti, E.; Magnacca, G.; Nisticò, R. Innovative sustainable materials for the photoinduced remediation of polluted water, Chapter 7. In Sustainable Water and Wastewater Processes; Galanakis, C.M., Agrafioti, E., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 203–238. ISBN 978-0-12-816170-8. [Google Scholar] [CrossRef]
- Franzoso, F.; Nisticò, R.; Cesano, F.; Corazzari, I.; Turci, F.; Scarano, D.; Bianco Prevot, A.; Magnacca, G.; Carlos, L.; Martire, D.O. Biowaste-derived substances as a tool for obtaining magnet-sensitive materials for environmental applications in wastewater treatments. Chem. Eng. J. 2017, 310, 307–316. [Google Scholar] [CrossRef]
- ACEA Pinerolese Industriale. Available online: http://ambiente.aceapinerolese.it/polo-ecologico (accessed on 30 April 2021).
- Montoneri, E.; Savarino, P.; Bottigliengo, S.; Boffa, V.; Bianco Prevot, A.; Fabbri, D.; Pramauro, E. Biomass wastes as renewable source of energy and chemicals for the industry with friendly environmental impact. Fresenius Environ. Bull. 2009, 18, 219–223. [Google Scholar]
- Nisticò, R. Magnetic materials and water treatments for a sustainable future. Res. Chem. Intermediates. 2017, 43, 6911–6949. [Google Scholar] [CrossRef]
- Nisticò, R.; Cesano, F.; Garello, F. Magnetic materials and systems: Domain structure visualization and other characterization techniques for the application in the materials science and biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef]
- Tabasso, S.; Ginepro, M.; Tomasso, L.; Montoneri, E.; Nisticò, R.; Francavilla, M. Integrated biochemical and chemical processing of municipal bio-waste to obtain bio based products for multiple uses. The case of soil remediation. J. Clean. Prod. 2020, 245, 119191. [Google Scholar] [CrossRef]
- Nisticò, R. A synthetic guide toward the tailored production of magnetic iron oxide nanoparticles. Boletin Sociedad Espanola Ceramica Vidrio 2021, 60, 29–40. [Google Scholar] [CrossRef]
- Sinha, A.; Ganguly, R.; Puri, I.K. Magnetic separation from superparamagnetic particles suspensions. J. Magnet. Magnet. Mater. 2009, 321, 2251–2256. [Google Scholar] [CrossRef]
- Leong, S.S.; Yeap, S.P.; Lim, J. Working principle and application of magnetic separation for biomedical diagnostic at high- and low-field gradients. Interf. Focus 2016, 6, 20160048. [Google Scholar] [CrossRef]
- Ge, W.; Encinas, A.; Araujo, E.; Song, S. Magnetic matrices used in high gradient magnetic separation (HGMS): A review. Results Phys. 2017, 7, 4278–4286. [Google Scholar] [CrossRef]
- Wang, F.; Tang, D.; Gao, L.; Dai, H.; Jiang, P.; Lu, M. Dynamic capture and accumulation of multiple types of magnetic particles based on fully coupled multiphysics model in multiwire for high-gradient magnetic separation. Adv. Powder Technol. 2020, 31, 1040–1050. [Google Scholar] [CrossRef]
- Menzel, K.; Lindner, J.; Nirschl, H. Removal of magnetite particles and lubricant contamination from viscous oil by High-Gradient Magnetic Separation technique. Sep. Purif. Technol. 2012, 92, 122–128. [Google Scholar] [CrossRef]
- Calza, P.; Avetta, P.; Rubulotta, G.; Sangermano, M.; Laurenti, E. TiO2-Soybean Peroxidase composite materials as a new photocatalytic system. Chem. Eng. J. 2014, 239, 87–92. [Google Scholar] [CrossRef]
- Avetta, P.; Bella, F.; Bianco Prevot, A.; Laurenti, E.; Montoneri, E.; Arques, A.; Carlos, L. Waste clearing waste: Photodegradation of monochlorophenols in the presence of waste derived organic catalysts. ACS Sustain. Chem. Eng. 2013, 1, 1545–1550. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Avetta, P.; Fabbri, D.; Laurenti, E.; Marchis, T.; Perrone, D.G.; Montoneri, E.; Boffa, V. Waste-derived bioorganic substances for light-induced generation of reactive oxygenated species. ChemSusChem 2011, 4, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tummino, M.L.; Testa, M.L.; Malandrino, M.; Gamberini, R.; Bianco Prevot, A.; Magnacca, G.; Laurenti, E. Green waste-derived substances immobilized on SBA-15 silica: Surface properties, adsorbing and photosensitizing activities towards organic and inorganic substrates. Nanomaterials 2019, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Calza, P.; Di Sarro, J.; Magnacca, G.; Bianco Prevot, A.; Laurenti, E. Use of low-cost magnetic materials containing waste derivatives for the (photo)-Fenton removal of organic pollutants. Materials 2019, 12, 3942. [Google Scholar] [CrossRef]
- Testa, M.L.; Tummino, M.L.; Agostini, S.; Avetta, P.; Deganello, F.; Montoneri, E.; Magnacca, G.; Bianco Prevot, A. Synthesis, characterization and environmental application of silica grafted photoactive substances isolated from urban biowaste. RSC Adv. 2015, 5, 47920–47927. [Google Scholar] [CrossRef]
- Aparicio, F.; Escalada, J.P.; De Gerónimo, E.; Aparicio, V.C.; García Einschlag, F.S.; Magnacca, G.; Carlos, L.; Mártire, D.O. Carbamazepine degradation mediated by light in the presence of humic substances-coated magnetite nanoparticles. Nanomaterials 2019, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R.; Bianco Prevot, A.; Magnacca, G.; Canone, L.; Garcia-Ballesteros, S.; Arques, A. Sustainable magnetic materials (from chitosan and municipal biowaste) for the removal of Diclofenac from water. Nanomaterials 2019, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Polliotto, V.; Pomilla, F.R.; Maurino, V.; Marcì, G.; Bianco Prevot, A.; Nisticò, R.; Magnacca, G.; Paganini, M.C.; Ponce Robles, L.; Perez, L.; et al. Different approaches for the solar photocatalytic removal of micro-contaminants from aqueous environment: Titania vs. hybrid magnetic iron oxides. Catal. Today 2019, 328, 164–171. [Google Scholar] [CrossRef]
- Gomis, J.; Carlos, L.; Bianco Prevot, A.; Teixeira, A.C.S.C.; Mora, M.; Amat, A.M.; Vicente, R.; Arques, A. Bio-based substances from urban waste as auxiliaries for solar photo-Fenton treatment under mild conditions: Optimization of operational variables. Catal. Today 2015, 240, 39–45. [Google Scholar] [CrossRef]
| Ref. | First Author, Year | Type of Article | Materials | Action Mechanism | Targets | Notes |
|---|---|---|---|---|---|---|
| Materials for adsorption | ||||||
| [21] | Wang 2020 | Review | Magnetic porous organic frameworks | Adsorption | Emerging contaminants, metals, polycyclic aromatic hydrocarbons, other organic pollutants | |
| [22] | Sriplai 2021 | Review | Magnetic bacterial cellulose | Adsorption | Heavy metal ions | Reuse of C-sources for bacterial metabolism |
| [23] | Imran Ali 2019 | Research article | Biomagnetic membrane capsules | Adsorption | Malachite green | Encapsulation in polyvinyl alcohol/alginate |
| [24] | Nisar Ali 2019 | Review | Fe-based magnetic compounds with high wettability properties | Adsorption and separation | Oil–water separation, dye-based pollutants, heavy metals | Overview of magnetic material synthesis, interfacial materials |
| [25] | Cui 2018 | Research article | MnFe2O4-cellulose aerogel | Adsorption | Cu2+ | |
| [26] | Peralta 2020 | Review | Nanoabsorbents with magnetic core | Adsorption | Organic pollutants | |
| [27] | Han 2019 | Research article | MOF-based magnetic materials | Adsorption | Congo red | |
| [28] | Gupta 2021 | Research article | Fe2O3-activated bakelite nanocomposites | Adsorption | Victoria blue dye | |
| [29] | Medeiros 2019 | Research article | Mesoporous composites glycerol-based with magnetic core | Adsorption | Organic contaminants (methylene blue, indigo carmine) | Reuse of glycerol by pyrolysis |
| [30] | Cui 2019 | Research article | Fe/C | Adsorption | Cr(VI) | Pyrolysis of glycerol base precursors, |
| [31] | Liu 2020 | Review | Fe3O4-based composites | Adsorption | Dye removal, oily wastewater treatment | Overview of Fe3O4 synthesis, pyrolysis and surface functionalization |
| [32] | Plohl 2021 | Research article | Magnetic nanostructures with lysine | Adsorption | Heavy metals | |
| [33] | Matos Oliveira 2020 | Research article | CoFe2O4 and natural organic matter | Adsorption | Polycyclic aromatic hydrocarbons, metals | |
| Materials with multiple actions | ||||||
| [34] | Yi 2020 | Review | Magnetic biochar | Adsorption, catalytic activation of H2O2 and persulfate | Adsorption of heavy metals, nuclear and organic pollutants, inorganic ions | Study of papers in the period 2011–2019 |
| [1] | Singh 2019 | Review | Zero valent iron, several materials | Precipitation, reduction, oxidation | ||
| Materials for catalysis/photocatalysis | ||||||
| [35] | Fu 2019 | Research article | Biochar + MnFe2O4 | Peroxymonosulfate activation | Organic pollutants | |
| [36] | He 2018 | Research article | Reduced graphene oxide-MnFe2O4 | Fenton-like, sunlight excitation | Malachite green | |
| [37] | Palma 2018 | Research article | Magnetic Bio-based substances nanoparticles | (Photo)Fenton-like processes | Caffeine | |
| [38] | Tanaka 2019 | Review | Iron-oxide-based nanoarchitectures, hybridization with inorganic and C-based materials | Catalysis | Air pollution, catalytic oxidation of CO | |
| [39] | Manohar 2021 | Research article | MnFe2O4 | Photocatalysis | Rhodamine B | |
| Promising materials to test | ||||||
| [40] | Muños Medina 2020 | Research article | Fe/FeOx/citrate | Adsorption | Good potential for environmental applications | High stability |
| [41] | Ravanello Mariosi 2020 | Research article | La-doped spinel CoFe2O4 | Adsorption | Good potential for environmental applications | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tummino, M.L.; Nisticò, R.; Franzoso, F.; Bianco Prevot, A.; Calza, P.; Laurenti, E.; Paganini, M.C.; Scalarone, D.; Magnacca, G. The “Lab4treat” Outreach Experience: Preparation of Sustainable Magnetic Nanomaterials for Remediation of Model Wastewater. Molecules 2021, 26, 3361. https://doi.org/10.3390/molecules26113361
Tummino ML, Nisticò R, Franzoso F, Bianco Prevot A, Calza P, Laurenti E, Paganini MC, Scalarone D, Magnacca G. The “Lab4treat” Outreach Experience: Preparation of Sustainable Magnetic Nanomaterials for Remediation of Model Wastewater. Molecules. 2021; 26(11):3361. https://doi.org/10.3390/molecules26113361
Chicago/Turabian StyleTummino, Maria Laura, Roberto Nisticò, Flavia Franzoso, Alessandra Bianco Prevot, Paola Calza, Enzo Laurenti, Maria Cristina Paganini, Dominique Scalarone, and Giuliana Magnacca. 2021. "The “Lab4treat” Outreach Experience: Preparation of Sustainable Magnetic Nanomaterials for Remediation of Model Wastewater" Molecules 26, no. 11: 3361. https://doi.org/10.3390/molecules26113361
APA StyleTummino, M. L., Nisticò, R., Franzoso, F., Bianco Prevot, A., Calza, P., Laurenti, E., Paganini, M. C., Scalarone, D., & Magnacca, G. (2021). The “Lab4treat” Outreach Experience: Preparation of Sustainable Magnetic Nanomaterials for Remediation of Model Wastewater. Molecules, 26(11), 3361. https://doi.org/10.3390/molecules26113361