Y2O3 Nanoparticles and X-ray Radiation-Induced Effects in Melanoma Cells
Abstract
1. Introduction
2. Results
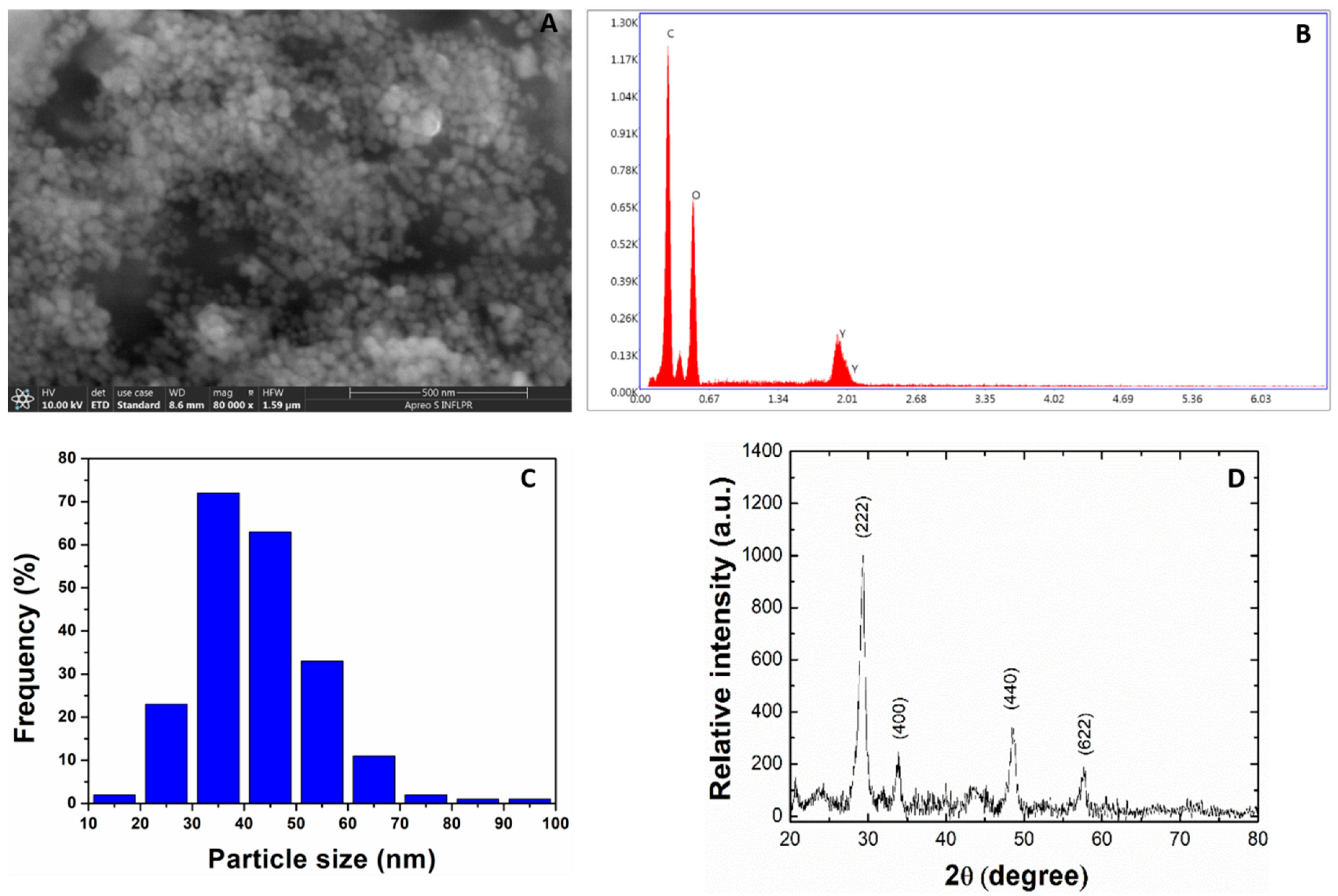
2.1. Size and Morphological Analysis of Y2O3 NPs
2.2. Treatment with Y2O3 Generates ROS in Concentration-Dependent Manner
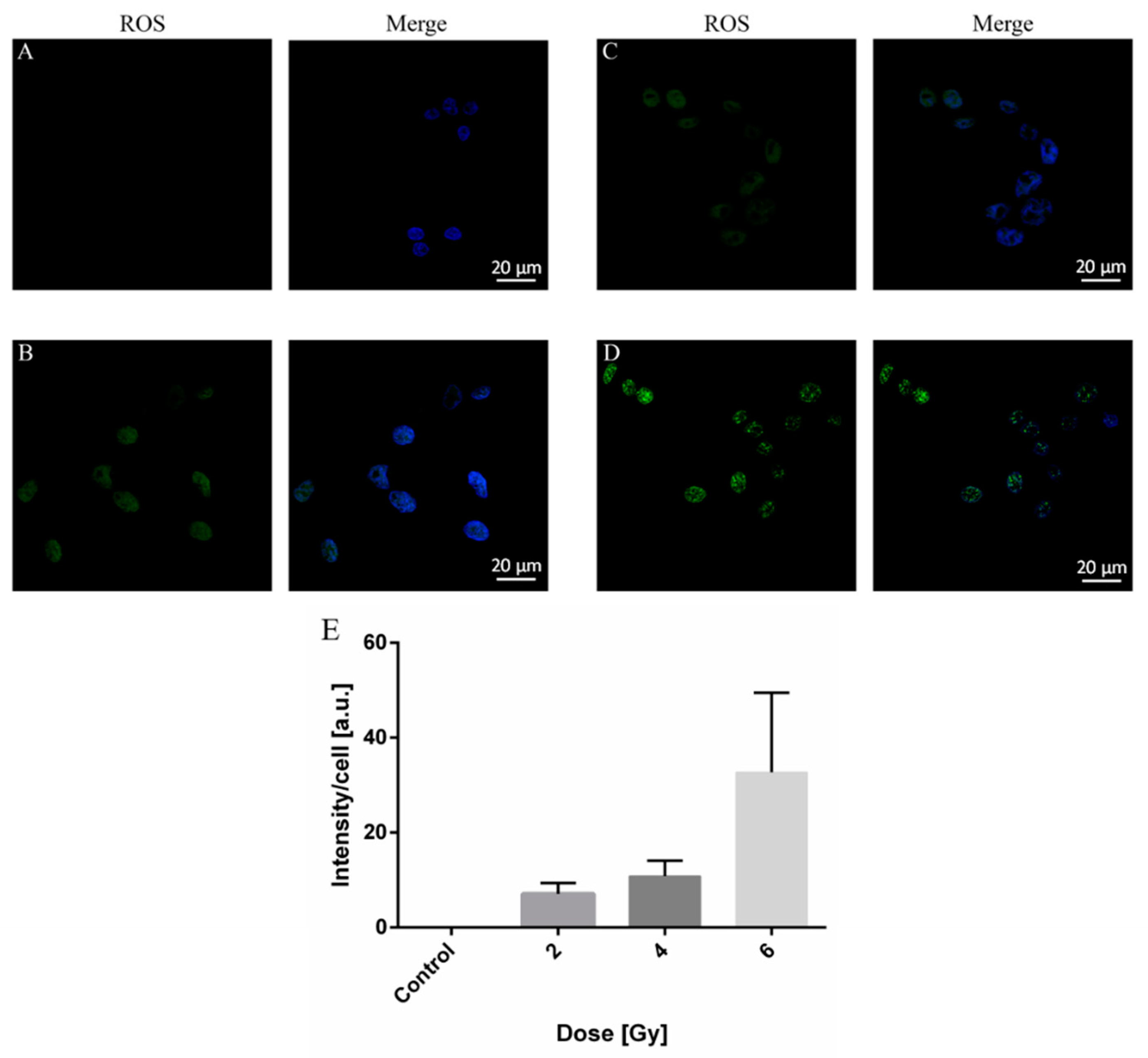
2.3. ROS Production in A375 Cancer Cells by X-ray Irradiation
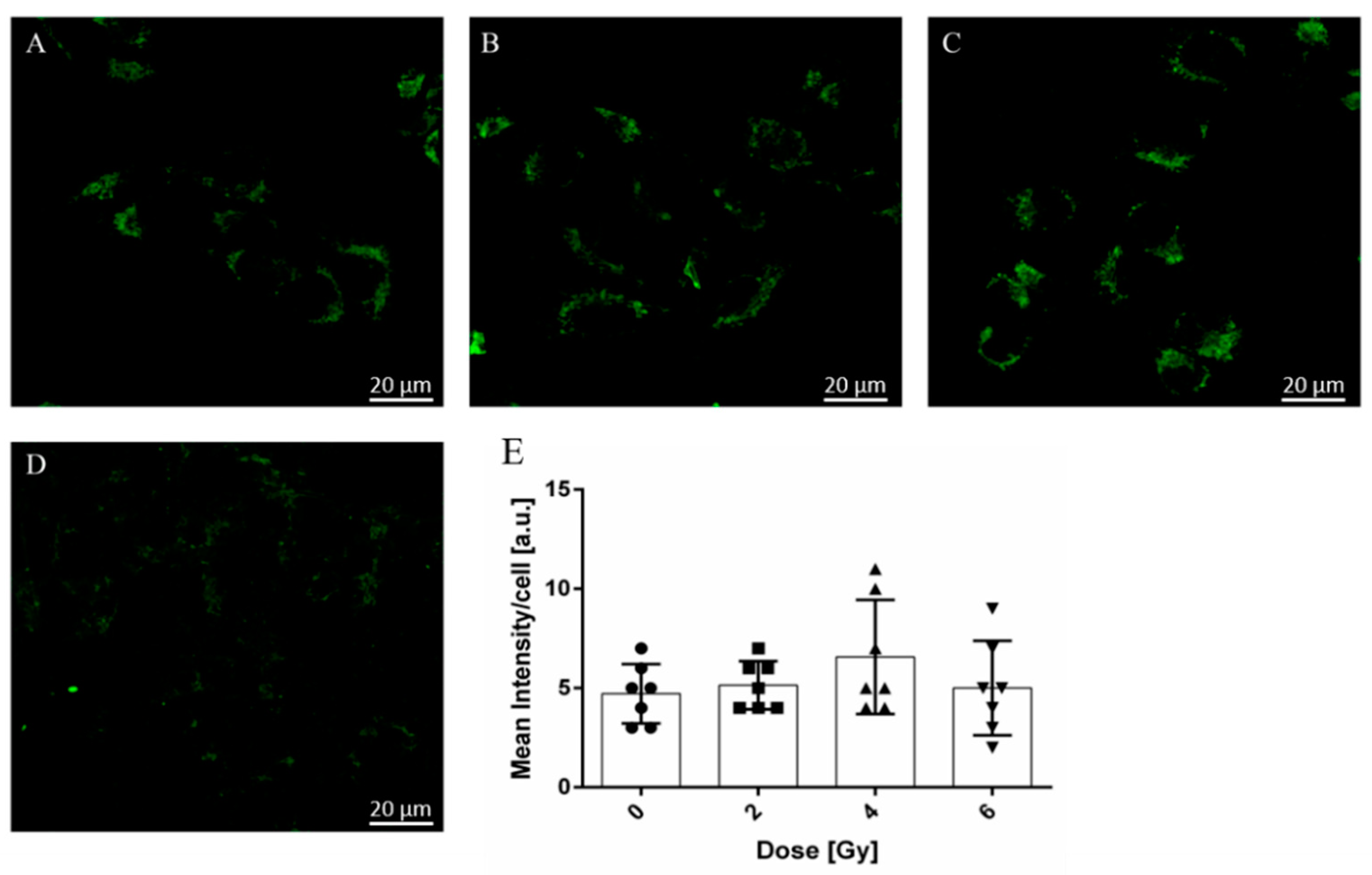
2.4. Radiation-Induced Modifications of A375 Cancer Cell Mitochondria Activity
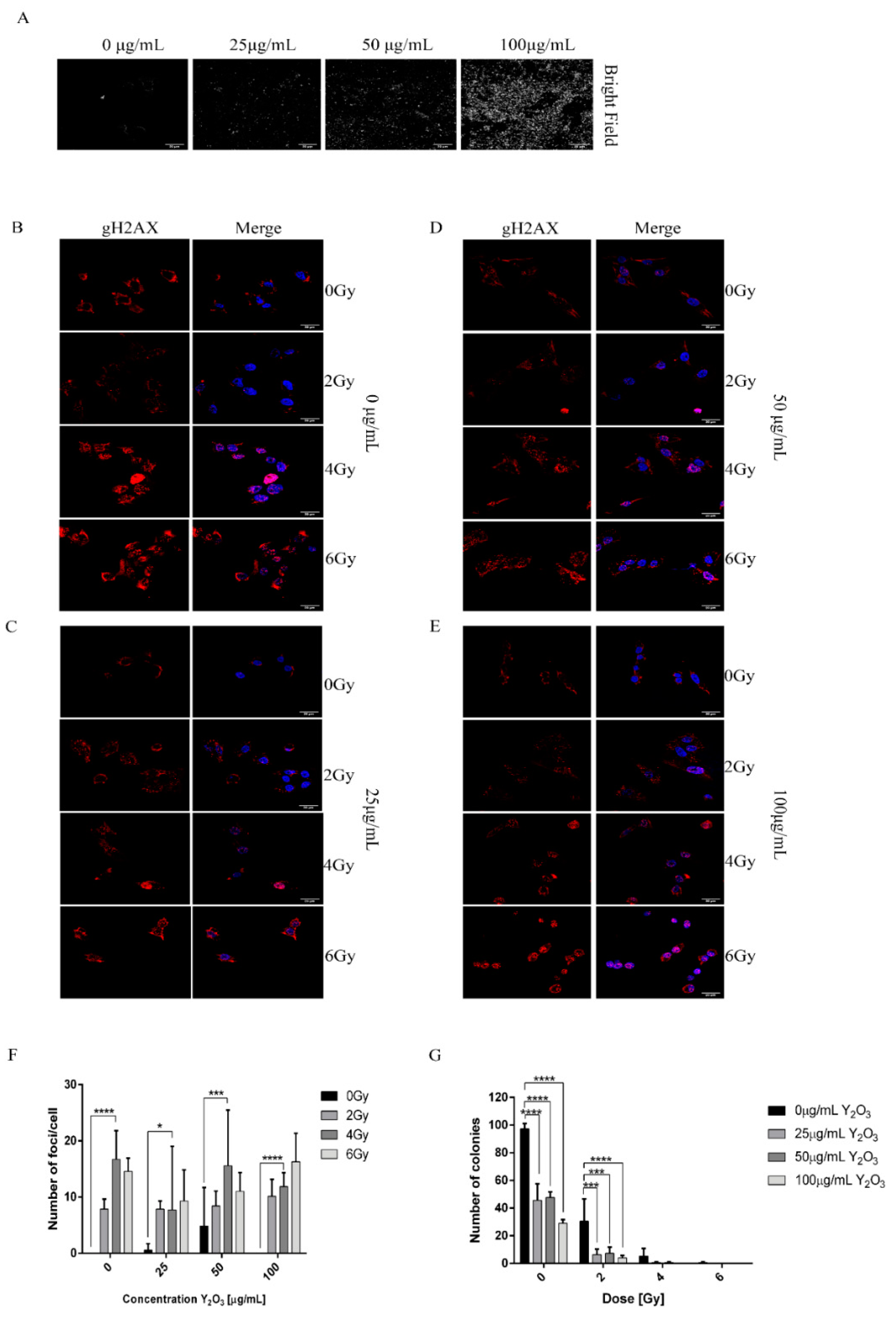
2.5. Combined Effect of NPs and Radiation Induced DNA Damage in A375 Cancer Cells
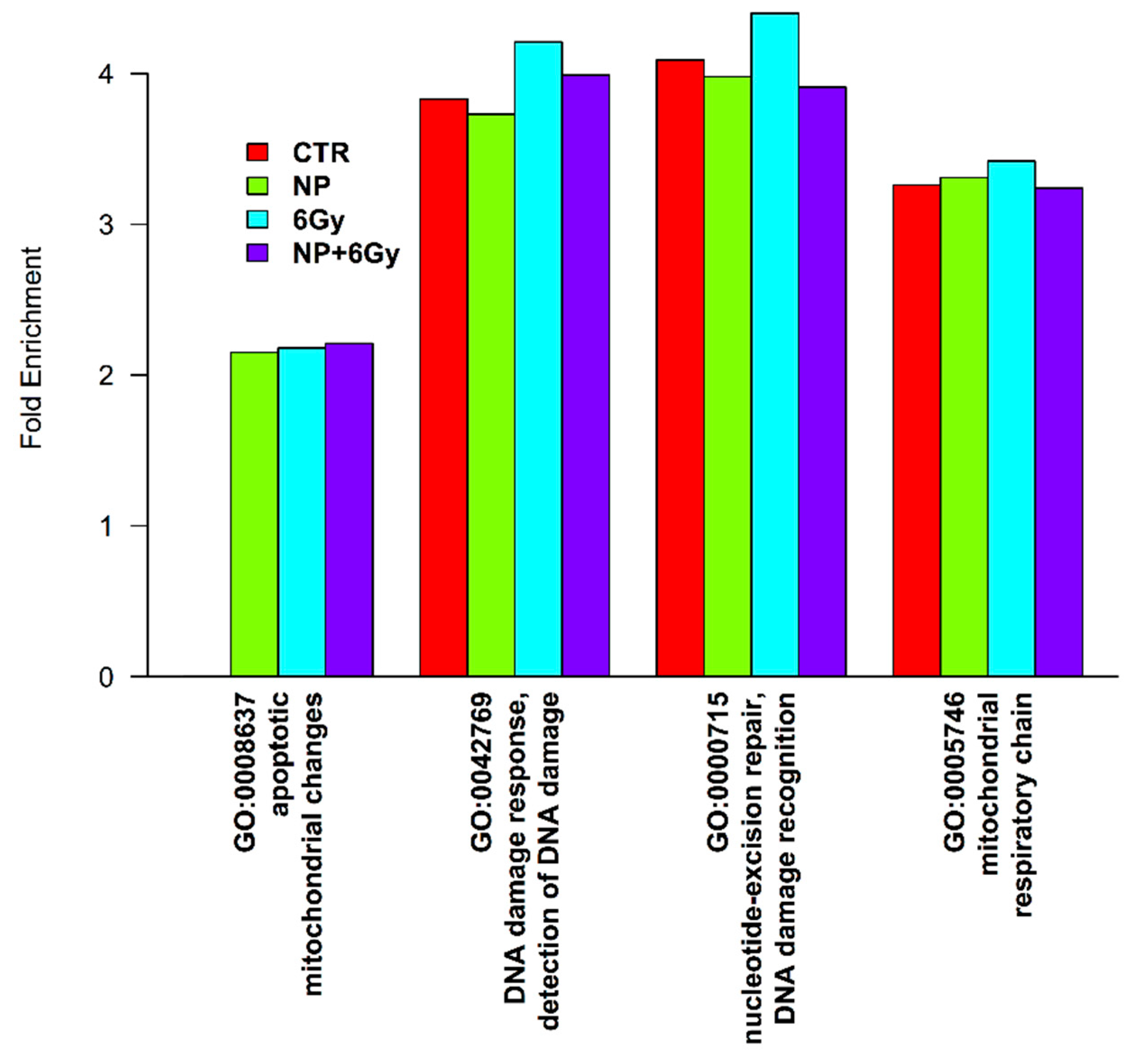
2.6. Proteomic Profile of A375 Cancer Cells Exposed to Y2O3 NPs X-ray
3. Discussion
4. Materials and Methods
4.1. XRD and SEM
4.2. X-ray Irradiation Set-Up
4.3. Cell Culture
4.4. Y2O3 NPs Treatment
4.5. Intracellular ROS Assay
4.6. DNA Double-Strand Breaks Assay
4.7. Clonogenic Assay
4.8. Mass Spectrometry Sample Preparation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setua, S.; Menon, D.; Asok, A.; Nair, S.; Koyakutty, M. Folate receptor targeted, rare-earth oxide nanocrystals for bi-modal fluorescence and magnetic imaging of cancer cells. Biomaterials 2010, 31, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Teo, R.; Termini, J.; Gray, H. Lanthanides: Applications in Cancer Diagnosis and Therapy. J. Med. Chem. 2016, 59, 6012–6024. [Google Scholar] [CrossRef] [PubMed]
- Cavouras, D.; Kandarakis, I.; Panayiotakis, G.S.; Evangelou, E.K.; Nomicos, C.D. An evaluation of the Y2O3:Eu3+ scintillator for application in medical xray detectors and image receptors. Med Phys. 1996, 23, 1965–1975. [Google Scholar] [CrossRef]
- Skandani, A.; Pham, T.; Luhrs, C.; El-Genk, M.; Al-Haik, M. Effects of composition and transparency on photo and radioluminescence of Y2O3:Eu complexes. Radiat. Eff. Defects Solids 2011, 166, 501–512. [Google Scholar] [CrossRef]
- Cavouras, D.; Kandarakis, I.; Maris, T.; Panayiotakis, G.S.; Nomicos, C.D. Assessment of the gain transfer function of phosphors for application in medical imaging radiation detectors. Eur. J. Radiol. 2000, 35, 70–77. [Google Scholar] [CrossRef]
- Shivaramu, N.; Lakshminarasappa, B.; Nagabhushana, K.; Singh, F.; Swart, H. Synthesis, thermoluminescence and defect centres in Eu3+ doped Y2O3 nanophosphor for gamma dosimetry applications. Mater. Res. Express 2017, 4, 115033. [Google Scholar] [CrossRef]
- Santos, S.; Rodrigues, O.; Campos, L. Bio-prototyping of europium-yttria based rods for radiation dosimetry. Mater. Chem. Phys. 2017, 199, 557–566. [Google Scholar] [CrossRef]
- Souris, J.; Cheng, S.; Pelizzari, C.; Chen, N.; La Riviere, P.; Chen, C.; Lo, L. Radioluminescence characterization of in situ X-ray nanodosimeters: Potential real-time monitors and modulators of external beam radiation therapy. Appl. Phys. Lett. 2014, 105, 203110. [Google Scholar] [CrossRef]
- Kertzscher, G.; Beddar, S. Inorganic scintillation detectors for 192Ir brachytherapy. Phys. Med. Biol. 2019, 64, 225018. [Google Scholar] [CrossRef]
- Chuang, Y.; Chu, C.; Cheng, S.; Liao, L.; Chu, T.; Chen, N.; Paldino, A.; Hsia, Y.; Chen, C.; Lo, L. Annealing-modulated nanoscintillators for nonconventional X-ray activation of comprehensive photodynamic effects in deep cancer theranostics. Theranostics 2020, 10, 6758–6773. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.; Chan, W.; Yarmush, M. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Andelman, T.; Gordonov, S.; Busto, G.; Moghe, P.; Riman, R. Synthesis and cytotoxicity of Y2O3 nanoparticles of various morphologies. Nanoscale Res. Lett. 2010, 5, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, Y.; Ma, Y.; Liu, Z.; Cao, L.; Wang, D.; Liu, S.; Xu, W.; Wang, W. Size-dependent cytotoxicity of yttrium oxide nanoparticles on primary osteoblasts in vitro. J. Nanoparticle Res. 2016, 18, 18. [Google Scholar] [CrossRef]
- Gao, C.; Jin, Y.; Jia, G.; Suo, X.; Liu, H.; Liu, D.; Yang, X.; Ge, K.; Liang, X.; Wang, S.; et al. Y2O3 nanoparticles caused bone tissue damage by breaking the intracellular phosphate balance in bone marrow stromal cells. Acs Nano 2019, 13, 313–323. [Google Scholar] [CrossRef]
- Schubert, D.; Dargusch, R.; Raitano, J.; Chan, S. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006, 342, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Baeeri, M.; Rahimifard, M.; Navaei-Nigjeh, M.; Mohammadirad, A.; Pourkhalili, N.; Hassani, S.; Kamali, M.; Abdollahi, M. Antiapoptotic effects of cerium oxide and yttrium oxide nanoparticles in isolated rat pancreatic islets. Hum. Exp. Toxicol. 2013, 32, 544–553. [Google Scholar] [CrossRef]
- Bowling, A.; Beal, M. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 1995, 56, 1151–1171. [Google Scholar] [CrossRef]
- Mitra, R.; Merwin, M.; Han, Z.; Conley, S.; Al-Ubaidi, M.; Naash, M. Yttrium oxide nanoparticles prevent photoreceptor death in a light-damage model of retinal degeneration. Free Radic. Biol. Med. 2014, 75, 140–148. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Pandurangan, M.; Veerappan, M.; Kim, D.; Sreekanth, T.; Shim, J. Green synthesis, characterization and anticancer activity of yttrium oxide nanoparticles. Mater. Lett. 2018, 216, 58–62. [Google Scholar] [CrossRef]
- Hendrickx, A.; Cozzio, A.; Plasswilm, L.; Panje, C. Radiotherapy for lentigo maligna and lentigo maligna melanoma—A systematic review. Radiat. Oncol. 2020, 15. [Google Scholar] [CrossRef]
- Strojan, P. Role of radiotherapy in melanoma management. Radiol. Oncol. 2010, 44, 1–12. [Google Scholar] [CrossRef]
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Buontempo, F.; Orsini, E.; Zironi, I.; Stolen, L.; Cappellini, A.; Rapino, S.; Tartari, A.; Mostacci, D.; Cucchi, G.; Martelli, A.; et al. Enhancing radiosensitivity of melanoma cells through very high dose rate pulses released by a plasma focus device. PLoS ONE 2018, 13, e0199312. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Garcia, D.; Juarez-Moreno, K.; Calderon-Osuna, I.; Navarro, P.; Hirata, G. Nanotoxicological study of downconversion Y2O3:Eu(3+) luminescent nanoparticles functionalized with folic acid for cancer cells bioimaging. J. Biomed. Mater. Res. Part B 2020, 108, 2396–2406. [Google Scholar] [CrossRef]
- Zako, T.; Hyodo, H.; Tsuji, K.; Tokuzen, K.; Kishimoto, H.; Ito, M.; Kaneko, K.; Maeda, M.; Soga, K. Development of near infrared-fluorescent nanophosphors and applications for cancer diagnosis and therapy. J. Nanomater. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Okumura, Y.; Soga, K.; Fukuda, R.; Tsuji, T. Bioimaging of M1 cells using ceramic nanophosphors: Synthesis and toxicity assay of Y2O3 nanoparticles. J. Phys. Conf. Ser. 2009, 191. [Google Scholar] [CrossRef]
- Chávez-García, D.; Sengar, P.; Juarez-Moreno, K.; Flores, D.L.; Calderón, I.; Barrera, J.; Hirata, G.A. Luminescence properties and cell uptake analysis of Y2O3:Eu, Bi nanophosphors for bio-imaging applications. J. Mater. Res. Technol. 2021, 10, 797–807. [Google Scholar] [CrossRef]
- Criddle, D.; Gillies, S.; Baumgartner-Wilson, H.; Jaffar, M.; Chinje, E.; Passmore, S.; Chvanov, M.; Barrow, S.; Gerasimenko, O.; Tepikin, A.; et al. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J. Biol. Chem. 2006, 281, 40485–40492. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Sherman, B.; Lempicki, R. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Huang, D.; Sherman, B.; Lempicki, R. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Stracker, T.H.; Roig, I.; Knobel, P.A.; Marjanovic, M. The ATM signaling network in development and disease. Front. Genet. 2013, 4, 37. [Google Scholar] [CrossRef]
- Zhou, B.; Bartek, J. Targeting the checkpoint kinases: Chemosensitization versus chemoprotection. Nat. Rev. Cancer 2004, 4, 216–225. [Google Scholar] [CrossRef]
- Reinhardt, H.; Schumacher, B. The p53 network: Cellular and systemic DNA damage responses in aging and cancer. Trends Genet. 2012, 28, 128–136. [Google Scholar] [CrossRef]
- Mirzayans, R.; Andrais, B.; Scott, A.; Murray, D. New insights into p53 signaling and cancer cell response to DNA damage: Implications for cancer therapy. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Pflaum, J.; Schlosser, S.; Müller, a.M. p53 family and cellular stress responses in cancer. Front. Oncol. 2014, 4, 285. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, A.; Komarova, E. The role of p53 in determining sensitivity to radiotherapy. Nat. Rev. Cancer 2003, 3, 117–129. [Google Scholar] [CrossRef]
- Weinberg, W.; Denning, M. p21(waf1) control of epithelial cell cycle and cell fate. Crit. Rev. Oral Biol. Med. 2002, 13, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Smits, V.A.; Klompmaker, R.; Vallenius, T.; Rijksen, G.; Mäkelä, T.P.; Medema, R.H. p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J. Biol. Chem. 2000, 275, 30638–30643. [Google Scholar] [CrossRef]
- Frank, A.M. A Ranking-Based Scoring Function for Peptide− Spectrum Matches. J. Proteome Res. 2009, 8, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Taylor, M.; Boulton, S. Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Lieber, M.; Kornberg, R.; Raetz, C.; Rothman, J.; Thorner, J. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010, 79, 181–211. [Google Scholar] [CrossRef]
- Chernikova, S.; Game, J.; Brown, J. Inhibiting homologous recombination for cancer therapy. Cancer Biol. Ther. 2012, 13, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.; Paget, J.; Khan, A.; Harrington, K. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409. [Google Scholar] [CrossRef] [PubMed]
- Sambade, M.; Peters, E.; Thomas, N.; Kaufmann, W.; Kimple, R.; Shields, J. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother. Oncol. 2011, 98, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Mousavi, M.; Nedaei, H.; Khoei, S.; Eynali, S.; Khoshgard, K.; Robatjazi, M.; Rad, R. Enhancement of radiosensitivity of melanoma cells by pegylated gold nanoparticles under irradiation of megavoltage electrons. Int. J. Radiat. Biol. 2017, 93, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Chompoosor, A.; Saha, K.; Ghosh, P.; Macarthy, D.; Miranda, O.; Zhu, Z.; Arcaro, K.; Rotello, V. The Role of Surface Functionality on Acute Cytotoxicity, ROS Generation and DNA Damage by Cationic Gold Nanoparticles. Small 2010, 6, 2246–2249. [Google Scholar] [CrossRef]
- Wason, M.; Colon, J.; Das, S.; Seal, S.; Turkson, J.; Zhao, J.; Baker, C. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomed. -Nanotechnol. Biol. Med. 2013, 9, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Patra, H.; Banerjee, S.; Chaudhuri, U.; Lahiri, P.; Dasgupta, A. Cell selective response to gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 111–119. [Google Scholar] [CrossRef]
- Ivosev, V.; Sanchez, G.; Stefancikova, L.; Haidar, D.; Vargas, C.; Yang, X.; Bazzi, R.; Porcel, E.; Roux, S.; Lacombe, S. Uptake and excretion dynamics of gold nanoparticles in cancer cells and fibroblasts. Nanotechnology 2020, 31, 135102. [Google Scholar] [CrossRef]
- Pashazadeh, A.; Boese, A.; Friebe, M. Radiation therapy techniques in the treatment of skin cancer: An overview of the current status and outlook. J. Dermatol. Treat. 2019, 30, 831–839. [Google Scholar] [CrossRef]
- Suski, J.M.; Magdalena, L.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. In Mitochondrial Bioenergetics; Humana Press: New York, NY, USA, 2012; pp. 357–381. [Google Scholar]
- Cairns, R.; Harris, I.; Mak, T. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Ben-Neriah, Y.; Karin, M. Inflammation meets cancer, with NF-kappa B as the matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa-B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Bodapati, S.; Murray, E.; Rice, K.; Winston, N.; Shokuhfar, T.; Zhao, Y.; Blough, E. Cytotoxicity and genotoxicity caused by yttrium oxide nanoparticles in HEK293 cells. Int. J. Nanomed. 2014, 9, 1379–1391. [Google Scholar] [CrossRef]
- Ghita, M.; McMahon, S.; Taggart, L.; Butterworth, K.; Schettino, G.; Prise, K. A mechanistic study of gold nanoparticle radiosensitisation using targeted microbeam irradiation. Sci. Rep. 2017, 7, 44752. [Google Scholar] [CrossRef]
- Zabierowski, S.; Herlyn, M. Melanoma stem cells: The dark seed of melanoma. J. Clin. Oncol. 2008, 26, 2890–2894. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Wilhite, T.; Balboni, T.; Alexander, B.; Spektor, A.; Ott, P.; Ng, A.; Hodi, F.; Schoenfeld, J. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015, 4, 44752. [Google Scholar] [CrossRef] [PubMed]
- Angheluta, L. 3D reconstruction of archaeological artefacts from 2-D X-ray images. Optoelectron. Adv. Mater. Rapid Commun. 2018, 12, 705–712. [Google Scholar]
- Aglan, A.H.; Mabrouk, M.; Aly, R.M.; Beherei, H.H.; Ahmed, H.H. Harnessing the antioxidant property of cerium and yttrium oxide nanoparticles to enhance mesenchymal stem cell proliferation. Asian J. Pharm. Clin. Res. 2018, 11, 436–442. [Google Scholar] [CrossRef]
- Franken, N.; Rodermond, H.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Chiritoiu, M.; Chiritoiu, G.; Munteanu, C.; Pastrama, F.; Ivessa, N.; Petrescu, S. EDEM1 drives misfolded protein degradation via erad and exploits er-phagy as back-up mechanism when ERAD is impaired. Int. J. Mol. Sci. 2020, 21, 3468. [Google Scholar] [CrossRef] [PubMed]

| Accession | Description | Unique Peptides | PSM-CTRL | PSM-NP | PSM-6Gy | PSM-NP+6Gy |
|---|---|---|---|---|---|---|
| O14757 | Serine/threonine-protein kinase Chk1 OS =Homo sapiens (Human) OX = 9606 GN = CHEK1 PE = 1 SV = 2 − [CHK1_HUMAN] | 1 | 4 | 3 | 5 | 1 |
| P38936 | Cyclin-dependent kinase inhibitor 1 OS = Homo sapiens (Human) OX = 9606 GN=CDKN1A PE = 1 SV = 3 − [CDN1A_HUMAN] | 3 | 1 | 6 | NA | 5 |
| P11802 | Cyclin-dependent kinase 4 OS = Homo sapiens (Human) OX = 9606 GN = CDK4 PE = 1 SV = 2 − [CDK4_HUMAN] | 2 | 1 | 3 | 1 | 5 |
| Q00534 | Cyclin-dependent kinase 6 OS = Homo sapiens (Human) OX = 9606 GN = CDK6 PE = 1 SV = 1 − [CDK6_HUMAN] | 6 | 13 | 9 | 9 | 21 |
| P13010 | X-ray repair cross-complementing protein 5 OS = Homo sapiens (Human) OX = 9606 GN = XRCC5 PE = 1 SV = 3 − [XRCC5_HUMAN] | 41 | 78 | 94 | 73 | 86 |
| P12956 | X-ray repair cross-complementing protein 6 OS = Homo sapiens (Human) OX = 9606 GN = XRCC6 PE = 1 SV = 2 − [XRCC6_HUMAN] | 51 | 130 | 139 | 139 | 155 |
| P49959 | Double-strand break repair protein MRE11 OS = Homo sapiens (Human) OX = 9606 GN = MRE11 PE = 1 SV = 3 − [MRE11_HUMAN] | 9 | 9 | 16 | 10 | 16 |
| Q92878 | DNA repair protein RAD50 OS = Homo sapiens (Human) OX = 9606 GN = RAD50 PE = 1 SV = 1 − [RAD50_HUMAN] | 6 | 9 | 11 | 7 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porosnicu, I.; Butnaru, C.M.; Tiseanu, I.; Stancu, E.; Munteanu, C.V.A.; Bita, B.I.; Duliu, O.G.; Sima, F. Y2O3 Nanoparticles and X-ray Radiation-Induced Effects in Melanoma Cells. Molecules 2021, 26, 3403. https://doi.org/10.3390/molecules26113403
Porosnicu I, Butnaru CM, Tiseanu I, Stancu E, Munteanu CVA, Bita BI, Duliu OG, Sima F. Y2O3 Nanoparticles and X-ray Radiation-Induced Effects in Melanoma Cells. Molecules. 2021; 26(11):3403. https://doi.org/10.3390/molecules26113403
Chicago/Turabian StylePorosnicu, Ioana, Cristian M. Butnaru, Ion Tiseanu, Elena Stancu, Cristian V. A. Munteanu, Bogdan I. Bita, Octavian G. Duliu, and Felix Sima. 2021. "Y2O3 Nanoparticles and X-ray Radiation-Induced Effects in Melanoma Cells" Molecules 26, no. 11: 3403. https://doi.org/10.3390/molecules26113403
APA StylePorosnicu, I., Butnaru, C. M., Tiseanu, I., Stancu, E., Munteanu, C. V. A., Bita, B. I., Duliu, O. G., & Sima, F. (2021). Y2O3 Nanoparticles and X-ray Radiation-Induced Effects in Melanoma Cells. Molecules, 26(11), 3403. https://doi.org/10.3390/molecules26113403









