The Effect of High Fat Diet on Cerebrovascular Health and Pathology: A Species Comparative Review
Abstract
1. Introduction
1.1. Environmental Factors in Translating HFD Research
1.2. Metabolic Factors in Translating HFD Research
2. Effects of HFD on the Cerebrovasculature
2.1. Cerebral Arterial Stiffening and Inflammation
2.2. Blood Brain Barrier
2.3. Microvascular Rarefaction and Other Microvascular Changes
2.4. Systemic Factors
2.5. Maternal HFD Effects on Offspring
3. Synthesis: Species Considerations for Assessing the Effects of HFD in Preclinical Models
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greenwood, C.E.; Winocur, G. Learning and memory impairment in rats fed a high saturated fat diet. Behav. Neural Biol. 1990, 53, 74–87. [Google Scholar] [CrossRef]
- Greenwood, C.E.; Winocur, G. Cognitive Impairment in Rats Fed High-Fat Diets: A Specific Effect of Saturated Fatty-Acid Intake. Behav. Neurosci. 1996, 110, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.R.; Haley-Zitlin, V.; Rosenberger, D.S.; Granholm, A.-C. Damaging effects of a high-fat diet to the brain and cognition: A review of proposed mechanisms. Nutr. Neurosci. 2014, 17, 241–251. [Google Scholar] [CrossRef]
- Winocur, G.; Greenwood, C.E. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging 2005, 26, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Zhang, Y.; Zheng, W.; Davidson, T.L. The Effects of a High-Energy Diet on Hippocampal Function and Blood-Brain Barrier Integrity in the Rat. J. Alzheimer’s Dis. 2010, 21, 207–219. [Google Scholar] [CrossRef]
- Kalmijn, S. Fatty acid intake and the risk of dementia and cognitive decline: A review of clinical and epidemiological studies. J. Nutr. Health Aging 2000, 4, 202–207. [Google Scholar] [PubMed]
- Luchsinger, J.A.; Tang, M.-X.; Shea, S.; Mayeux, R. Caloric Intake and the Risk of Alzheimer Disease. Arch. Neurol. 2002, 59, 1258–1263. [Google Scholar] [CrossRef]
- Johnson, L.A.; Torres, E.R.; Boutros, S.W.; Patel, E.; Akinyeke, T.; Alkayed, N.J.; Raber, J. Apolipoprotein E4 mediates insulin resistance-associated cerebrovascular dysfunction and the post-prandial response. Br. J. Pharmacol. 2019, 39, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Arbones-Mainar, J.M.A.; Johnson, L.; Torres-Perez, E.E.; Garcia, A.; Díaz, S.P.; Raber, J.; Maeda, N. Metabolic shifts toward fatty-acid usage and increased thermogenesis are associated with impaired adipogenesis in mice expressing human APOE4. Int. J. Obes. 2016, 40, 1574–1581. [Google Scholar] [CrossRef]
- Rusu, M.E.; Mocan, A.; Ferreira, I.C.F.R.; Popa, D.-S. Health Benefits of Nut Consumption in Middle-Aged and Elderly Population. Antioxidants 2019, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef]
- Showalter, M.R.; Nonnecke, E.B.; Linderholm, A.L.; Cajka, T.; Sa, M.R.; Lönnerdal, B.; Kenyon, N.J.; Fiehn, O. Obesogenic diets alter metabolism in mice. PLoS ONE 2018, 13, e0190632. [Google Scholar] [CrossRef]
- Rusu, M.E.; Georgiu, C.; Pop, A.; Mocan, A.; Kiss, B.; Vostinaru, O.; Fizesan, I.; Stefan, M.-G.; Gheldiu, A.-M.; Mates, L.; et al. Antioxidant Effects of Walnut (Juglans regia L.) Kernel and Walnut Septum Extract in a D-Galactose-Induced Aging Model and in Naturally Aged Rats. Antioxidants 2020, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metab. 2018, 28, 415–431.e4. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; Mazlan, N.; Whybrow, S. Carbohydrates, Appetite and Feeding Behavior in Humans. J. Nutr. 2001, 131, 2775S–2781S. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Yasoshima, A.; Doi, K.; Nakayama, H.; Uetsuka, K. Involvement of Sex, Strain and Age Factors in High Fat Diet-Induced Obesity in C57BL/6J and BALB/cA Mice. Exp. Anim. 2007, 56, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, S.D.; Lecanda, M.; Solis, F.; Ezquer, M.; Gutiérrez, J.; Brandan, E.; Leiva, A.; Sobrevia, L.; Conget, P. Mice Long-Term High-Fat Diet Feeding Recapitulates Human Cardiovascular Alterations: An Animal Model to Study the Early Phases of Diabetic Cardiomyopathy. PLoS ONE 2013, 8, e60931. [Google Scholar] [CrossRef]
- Zimmerman, B.; Kundu, P.; Liu, Z.; Urbanski, H.F.; Kroenke, C.D.; Kohama, S.G.; Bethea, C.L.; Raber, J. Longitudinal Effects of Immediate and Delayed Estradiol on Cognitive Performance in a Spatial Maze and Hippocampal Volume in Menopausal Macaques Under an Obesogenic Diet. Front. Neurol. 2020, 11, 539. [Google Scholar] [CrossRef]
- Havel, P.J.; Kievit, P.; Comuzzie, A.G.; Bremer, A.A. Use and Importance of Nonhuman Primates in Metabolic Disease Research: Current State of the Field. ILAR J. 2017, 58, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Borghjid, S.; Feinman, R.D. Response of C57Bl/6 mice to a carbohydrate-free diet. Nutr. Metab. 2012, 9, 69. [Google Scholar] [CrossRef]
- Karam, J.G.; Nessim, F.; McFarlane, S.I.; Feinman, R.D. Carbohydrate restriction and cardiovascular risk. Curr. Cardiovasc. Risk Rep. 2008, 2, 88–94. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Hu, T.; Reynolds, K.; Yao, L.; Bunol, C.; Liu, Y.; Chen, C.-S.; Klag, M.J.; Whelton, P.K.; He, J. Effects of Low-Carbohydrate and Low-Fat Diets. Ann. Intern. Med. 2014, 161, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Feinman, R.D. Carbohydrate restriction improves the features of Metabolic Syndrome. Metabolic Syndrome may be defined by the response to carbohydrate restriction. Nutr. Metab. 2005, 2, 31. [Google Scholar] [CrossRef]
- Volek, J.S.; Sharman, M.J.; Forsythe, C.E. Modification of Lipoproteins by Very Low-Carbohydrate Diets. J. Nutr. 2005, 135, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of Dietary, Circulating, and Supplement Fatty Acids with Coronary Risk. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Wilde, D.W.; Massey, K.D.; Walker, G.K.; Vollmer, A.; Grekin, R.J. High-Fat Diet Elevates Blood Pressure and Cerebrovascular Muscle Ca 2+ Current. Hypertension 2000, 35, 832–837. [Google Scholar] [CrossRef]
- Al-Regaiey, K.A. The effects of calorie restriction on aging: A brief review. Eur Rev. Med. Pharmacol Sci 2016, 20, 2468–2473. [Google Scholar] [PubMed]
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, R.; de Cabo, R.; Anderson, R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017, 8, 14063. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, C.; Lu, M.; Dong, Q.; Wang, Z.; Wang, Z.; Xiong, W.; Zhang, N.; Zhou, J.; Liu, Q.; et al. Calorie restriction is the most reasonable anti-ageing intervention: A meta-analysis of survival curves. Sci. Rep. 2018, 8, 5779. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu. Rev. Nutr. 2020, 40, 105–133. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Forster, M.J. Caloric restriction and the aging process: A critique. Free Radic. Biol. Med. 2014, 73, 366–382. [Google Scholar] [CrossRef] [PubMed]
- McMaster, P.D. Do Species Lacking A Gall Bladder Possess Its Functional Equivalent? J. Exp. Med. 1922, 35, 127–140. [Google Scholar] [CrossRef][Green Version]
- Turumin, J.; Shanturov, V.; Turumina, H. The role of the gallbladder in humans. Rev. Gastroenterol. México 2013, 78, 177–187. [Google Scholar] [CrossRef]
- Housset, C.; Chrétien, Y.; Debray, D.; Chignard, N. Functions of the Gallbladder. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2016; pp. 1549–1577. [Google Scholar]
- Bonora, E. The metabolic syndrome and cardiovascular disease. Ann. Med. 2006, 38, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Nyenhuis, D. Understanding and Treating Vascular Cognitive Impairment. Contin. Lifelong Learn. Neurol. 2013, 19, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Yamamoto, Y. Emerging Evidence for Pathogenesis of Sporadic Cerebral Small Vessel Disease. Stroke 2016, 47, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, Y.; Lu, Z.; Leak, R.K.; Zhang, F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res. Rev. 2017, 34, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Duncombe, J.; Kitamura, A.; Hase, Y.; Ihara, M.; Kalaria, R.N.; Horsburgh, K. Chronic cerebral hypoperfusion: A key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 2451–2468. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.C.; Dai, W.; Grossman, M.; Detre, J.A. Arterial Spin Labeling Blood Flow MRI: Its Role in the Early Characterization of Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 20, 871–880. [Google Scholar] [CrossRef]
- Chao, L.L.; Buckley, S.T.; Kornak, J.; Schuff, N.; Madison, C.; Yaffe, K.; Miller, B.L.; Kramer, J.H.; Weiner, M.W. ASL Perfusion MRI Predicts Cognitive Decline and Conversion from MCI to Dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Edrissi, H.; Schock, S.C.; Cadonic, R.; Hakim, A.M.; Thompson, C.S. Cilostazol reduces blood brain barrier dysfunction, white matter lesion formation and motor deficits following chronic cerebral hypoperfusion. Brain Res. 2016, 1646, 494–503. [Google Scholar] [CrossRef]
- Shibata, M.; Ohtani, R.; Ihara, M.; Tomimoto, H. White Matter Lesions and Glial Activation in a Novel Mouse Model of Chronic Cerebral Hypoperfusion. Stroke 2004, 35, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Enmi, J.-I.; Kitamura, A.; Yamamoto, Y.; Saito, S.; Takahashi, Y.; Iguchi, S.; Tsuji, M.; Yamahara, K.; Nagatsuka, K.; et al. A Novel Mouse Model of Subcortical Infarcts with Dementia. J. Neurosci. 2015, 35, 3915–3928. [Google Scholar] [CrossRef] [PubMed]
- Washida, K.; Hattori, Y.; Ihara, M. Animal Models of Chronic Cerebral Hypoperfusion: From Mouse to Primate. Int. J. Mol. Sci. 2019, 20, 6176. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, E.; Safciuc, F.; Sima, A.V. A hyperlipidemic diet induces structural changes in cerebral blood vessels. Curr. Neurovascular Res. 2011, 8, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Obadia, N.; Lessa, M.A.; Daliry, A.; Silvares, R.R.; Gomes, F.; Tibiriçá, E.; Estato, V. Cerebral microvascular dysfunction in metabolic syndrome is exacerbated by ischemia—Reperfusion injury. BMC Neurosci. 2017, 18, 67. [Google Scholar] [CrossRef]
- Hong, J.E.; Stubbins, R.; Smith, R.R.E.; Harvey, A.; Núñez, N.P. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J. 2009, 8, 11. [Google Scholar] [CrossRef]
- Stubbins, R.E.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2011, 51, 861–870. [Google Scholar] [CrossRef]
- Yang, Y.D.L.S., Jr.; Keating, K.D.; Allison, D.B.; Nagy, T.R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 2014, 22, 2147–2155. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Schemmel, R.; Mickelsen, O.; Gill, J.L. Dietary Obesity in Rats: Body Weight and Body Fat Accretion in Seven Strains of Rats. J. Nutr. 1970, 100, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Dunn-Meynell, A.A.; Balkan, B.; Keesey, R.E. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. Integr. Comp. Physiol. 1997, 273, R725–R730. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Casellas, A.; Proenza, A.M.; Lladó, I.; Gianotti, M. Sex-dependent differences in rat hepatic lipid accumulation and insulin sensitivity in response to diet-induced obesity. Biochem. Cell Biol. 2012, 90, 164–172. [Google Scholar] [CrossRef]
- Garg, N.; Thakur, S.; Mcmahan, C.A.; Adamo, M.L. High fat diet induced insulin resistance and glucose intolerance are gender-specific in IGF-1R heterozygous mice. Biochem. Biophys. Res. Commun. 2011, 413, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Hevener, A.; Reichart, D.; Janez, A.; Olefsky, J. Female Rats Do Not Exhibit Free Fatty Acid-Induced Insulin Resistance. Diabetes 2002, 51, 1907–1912. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Medrikova, D.; Jilkova, Z.M.; Bardova, K.; Janovska, P.; Rossmeisl, M.; Kopecky, J. Sex differences during the course of diet-induced obesity in mice: Adipose tissue expandability and glycemic control. Int. J. Obes. 2012, 36, 262–272. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Waldén, T.B.; Carlsson, P.-O.; Jansson, L.; Phillipson, M. Female Mice are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6, 60–75. [Google Scholar] [CrossRef]
- Høeg, L.D.; Sjøberg, K.A.; Jeppesen, J.; Jensen, T.E.; Frøsig, C.; Birk, J.B.; Bisiani, B.; Hiscock, N.; Pilegaard, H.; Wojtaszewski, J.F.; et al. Lipid-Induced Insulin Resistance Affects Women Less Than Men and Is Not Accompanied by Inflammation or Impaired Proximal Insulin Signaling. Diabetes 2010, 60, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.M.; on behalf of the Scottish Diabetes Research Network Epidemiology Group; Walker, J.J.; Colhoun, H.M.; Leese, G.P.; Lindsay, R.S.; McKnight, J.A.; Morris, A.P.; Pearson, D.W.; Petrie, J.R.; et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetology 2011, 54, 3003–3006. [Google Scholar] [CrossRef]
- Ter Horst, K.W.; Gilijamse, P.W.; de Weijer, B.A.; Kilicarslan, M.; Ackermans, M.T.; Nederveen, A.J.; Nieuwdorp, M.; Romijn, J.A.; Serlie, M.J. Sexual Dimorphism in Hepatic, Adipose Tissue, and Peripheral Tissue Insulin Sensitivity in Obese Humans. Front. Endocrinol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Rosner, B.; Monson, R.R.; Speizer, F.E.; Hennekens, C.H. A Prospective Study of Obesity and Risk of Coronary Heart Disease in Women. N. Engl. J. Med. 1990, 322, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Tarumi, T.; Khan, M.A.; Liu, J.; Tseng, B.M.; Parker, R.; Riley, J.; Tinajero, C.; Zhang, R. Cerebral Hemodynamics in Normal Aging: Central Artery Stiffness, Wave Reflection, and Pressure Pulsatility. Br. J. Pharmacol. 2014, 34, 971–978. [Google Scholar] [CrossRef]
- Zimmerman, B.; Rypma, B.; Gratton, G.; Fabiani, M. Age-related changes in cerebrovascular health and their effects on neural function and cognition: A comprehensive review. Psychophysiology 2021, 13796. [Google Scholar] [CrossRef]
- Fabiani, M.; Low, K.A.; Tan, C.-H.; Zimmerman, B.; Fletcher, M.A.; Schneider-Garces, N.; Maclin, E.L.; Chiarelli, A.M.; Sutton, B.P.; Gratton, G. Taking the pulse of aging: Mapping pulse pressure and elasticity in cerebral arteries with optical methods. Psychophysiology 2014, 51, 1072–1088. [Google Scholar] [CrossRef]
- Tan, C.H.; Low, K.A.; Kong, T.; Fletcher, M.A.; Zimmerman, B.; Maclin, E.L.; Chiarelli, A.M.; Gratton, G.; Fabiani, M. Mapping cerebral pulse pressure and arterial compliance over the adult lifespan with optical imaging. PLoS ONE 2017, 12, e0171305. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Fletcher, M.A.; Tan, C.H.; Low, K.A.; Maclin, E.L.; Zimmerman, B.; Kong, T.; Gorsuch, A.; Gratton, G.; Fabiani, M. Individual differences in regional cortical volumes across the life span are associated with regional optical measures of arterial elasticity. NeuroImage 2017, 162, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Low, K.A.; Chiarelli, A.M.; Fletcher, M.A.; Navarra, R.; Burzynska, A.Z.; Kong, T.S.; Zimmerman, B.; Maclin, E.L.; Sutton, B.P.; et al. Optical measures of cerebral arterial stiffness are associated with white matter signal abnormalities and cognitive performance in normal aging. Neurobiol. Aging 2019, 84, 200–207. [Google Scholar] [CrossRef]
- Henson, G.D.; Walker, A.E.; Reihl, K.D.; Donato, A.J.; Lesniewski, L.A. Dichotomous mechanisms of aortic stiffening in high-fat diet fed young and old B6D2F1 mice. Physiol. Rep. 2014, 2, e00268. [Google Scholar] [CrossRef]
- Winder, N.R.; Reeve, E.H.; Walker, A.E. Large artery stiffness and brain health: Insights from animal models. Am. J. Physiol. Circ. Physiol. 2021, 320, H424–H431. [Google Scholar] [CrossRef]
- Atkinson, J. Animal Models of Arterial Stiffness. Pathophysiol. Eval. Manag. Valv. Heart Dis. 2006, 44, 96–116. [Google Scholar] [CrossRef]
- Walker, A.E.; Henson, G.D.; Reihl, K.D.; Morgan, R.G.; Dobson, P.S.; Nielson, E.I.; Ling, J.; Mecham, R.P.; Li, D.Y.; Lesniewski, L.A.; et al. Greater impairments in cerebral artery compared with skeletal muscle feed artery endothelial function in a mouse model of increased large artery stiffness. J. Physiol. 2015, 593, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, R.H.; Beeman, S.C.; Broekelmann, T.J.; Liu, D.; Tsang, K.M.; Kovacs, A.; Ye, L.; Danback, J.R.; Watson, A.; Wardlaw, A.; et al. Minoxidil improves vascular compliance, restores cerebral blood flow, and alters extracellular matrix gene expression in a model of chronic vascular stiffness. Am. J. Physiol. Circ. Physiol. 2018, 315, H18–H32. [Google Scholar] [CrossRef] [PubMed]
- Onetti, Y.; Meirelles, T.; Dantas, A.P.; Schröder, K.; Vila, E.; Egea, G.; Jiménez-Altayó, F. NADPH oxidase 4 attenuates cerebral artery changes during the progression of Marfan syndrome. Am. J. Physiol. Circ. Physiol. 2016, 310, H1081–H1090. [Google Scholar] [CrossRef] [PubMed]
- De Montgolfier, O.; Pinçon, A.; Pouliot, P.; Gillis, M.-A.; Bishop, J.; Sled, J.G.; Villeneuve, L.; Ferland, G.; Lévy, B.I.; Lesage, F.; et al. High Systolic Blood Pressure Induces Cerebral Microvascular Endothelial Dysfunction, Neurovascular Unit Damage, and Cognitive Decline in Mice. Hypertension 2019, 73, 217–228. [Google Scholar] [CrossRef]
- Gentile, M.T.; Poulet, R.; Di Pardo, A.; Cifelli, G.; Maffei, A.; Vecchione, C.; Passarelli, F.; Landolfi, A.; Carullo, P.; Lembo, G. β-Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol. Aging 2009, 30, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sadekova, N.; Vallerand, D.; Guevara, E.; Lesage, F.; Girouard, H. Carotid Calcification in Mice: A New Model to Study the Effects of Arterial Stiffness on the Brain. J. Am. Heart Assoc. 2013, 2, e000224. [Google Scholar] [CrossRef] [PubMed]
- Muhire, G.; Iulita, M.F.; Vallerand, D.; Youwakim, J.; Gratuze, M.; Petry, F.R.; Planel, E.; Ferland, G.; Girouard, H. Arterial Stiffness Due to Carotid Calcification Disrupts Cerebral Blood Flow Regulation and Leads to Cognitive Deficits. J. Am. Heart Assoc. 2019, 8, e011630. [Google Scholar] [CrossRef]
- Zuloaga, K.L.; Zhang, W.; Yeiser, L.A.; Stewart, B.; Kukino, A.; Nie, X.; Roese, N.E.; Grafe, M.R.; Pike, M.M.; Raber, J.; et al. Neurobehavioral and Imaging Correlates of Hippocampal Atrophy in a Mouse Model of Vascular Cognitive Impairment. Transl. Stroke Res. 2015, 6, 390–398. [Google Scholar] [CrossRef]
- Zuloaga, K.L.; Johnson, A.L.; Roese, E.N.; Marzulla, T.; Zhang, W.; Nie, X.; Alkayed, F.N.; Hong, C.; Grafe, M.R.; Pike, M.M.; et al. High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. Br. J. Pharmacol. 2015, 36, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Yaffe, K.; Biller, J.; Bratzke, L.C.; Faraci, F.M.; Gorelick, P.B.; Gulati, M.; Kamel, H.; Knopman, D.S.; Launer, L.J.; et al. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension 2016, 68, e67–e94. [Google Scholar] [CrossRef]
- Jennings, J.R.; Muldoon, M.F.; Allen, B.; Ginty, A.T.; Gianaros, P.J. Cerebrovascular function in hypertension: Does high blood pressure make you old? Psychophysiology 2020, 13654. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, G.; van der Laan, S.W.; Haitjema, S.; Asl, H.F.; Siemelink, M.A.; Bezemer, T.; van Setten, J.; Dichgans, M.; Malik, R.; Worrall, B.B.; et al. Human Validation of Genes Associated with a Murine Atherosclerotic Phenotype. Arter. Thromb. Vasc. Biol. 2016, 36, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Von Scheidt, M.; Zhao, Y.; Kurt, Z.; Pan, C.; Zeng, L.; Yang, X.; Schunkert, H.; Lusis, A.J. Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metab. 2017, 25, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Bergen, W.G.; Mersmann, H.J. Comparative Aspects of Lipid Metabolism: Impact on Contemporary Research and Use of Animal Models. J. Nutr. 2005, 135, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, H.; Glagov, S. Comparison of Abdominal and Thoracic Aortic Medial Structure in Mammals. Circ. Res. 1969, 25, 677–686. [Google Scholar] [CrossRef]
- Aroor, A.R.; Jia, G.; Sowers, J.R. Cellular mechanisms underlying obesity-induced arterial stiffness. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R387–R398. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, 217. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, C.C.; Leitão, R.A.; Alves, L.A.O.; Coelho-Santos, V.; Guerrant, R.L.; Ribeiro, C.F.; Malva, J.O.; Silva, A.P.; Oriá, R.B. Effect of Hypoproteic and High-Fat Diets on Hippocampal Blood-Brain Barrier Permeability and Oxidative Stress. Front. Nutr. 2019, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Kanoski, S.E. Blood-brain barrier disruption: Mechanistic links between Western diet consumption and dementia. Front. Aging Neurosci. 2014, 6, 88. [Google Scholar] [CrossRef]
- Schachter, J.; Martel, J.; Lin, C.-S.; Chang, C.-J.; Wu, T.-R.; Lu, C.-C.; Ko, Y.-F.; Lai, H.-C.; Ojcius, D.M.; Young, J.D. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav. Immun. 2018, 69, 1–8. [Google Scholar] [CrossRef]
- Ngkelo, A.; Meja, K.; Yeadon, M.; Adcock, I.A.; Kirkham, P. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Gi-alpha dependent PI-3kinase signalling. J. Inflamm. 2012, 9, 1. [Google Scholar] [CrossRef] [PubMed]
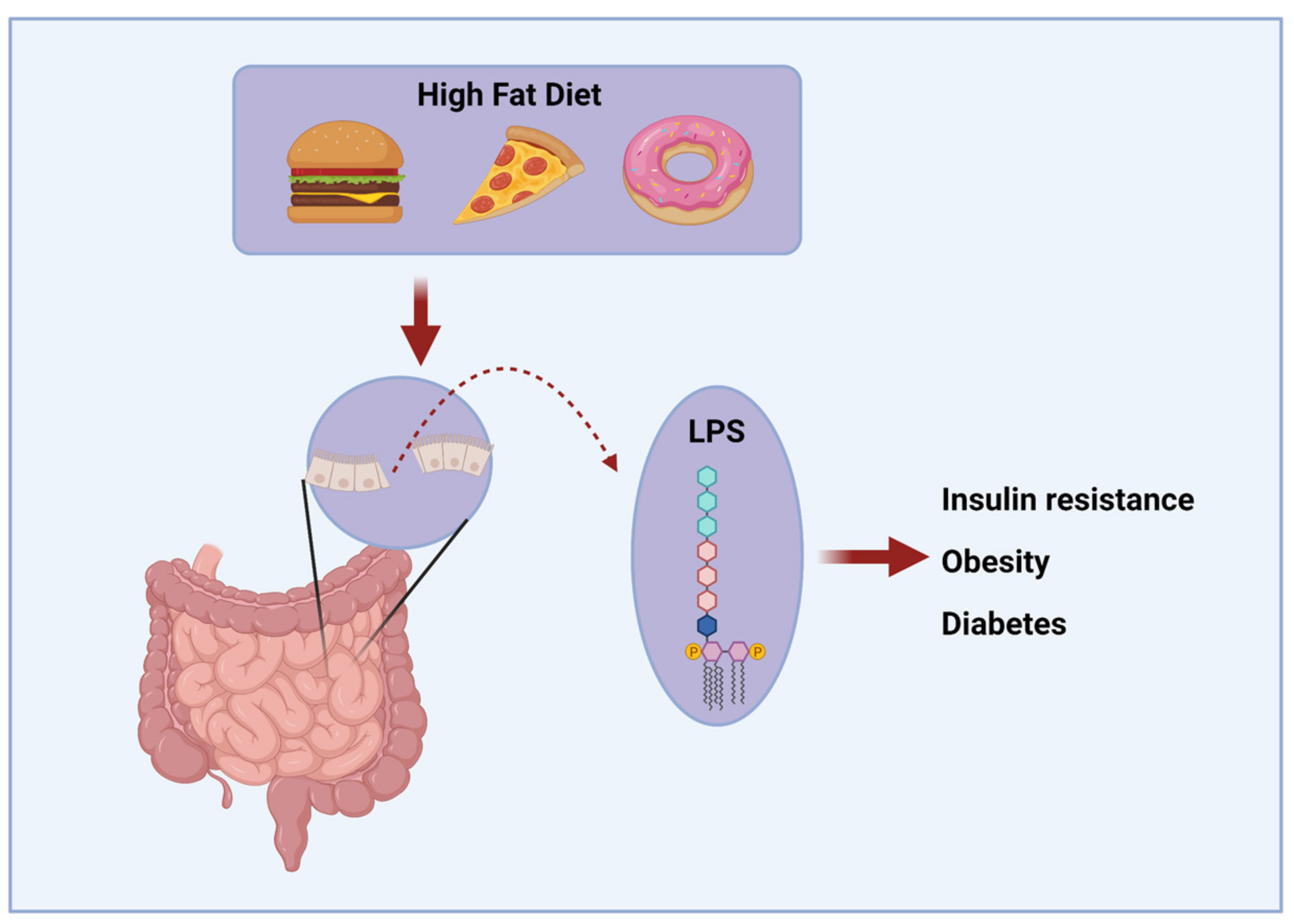
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Van Rooy, M.-J.; Pretorius, E. Metabolic syndrome, platelet activation and the development of transient ischemic attack or thromboembolic stroke. Thromb. Res. 2015, 135, 434–442. [Google Scholar] [CrossRef]
- Omran, F.; Christian, M. Inflammatory Signaling and Brown Fat Activity. Front. Endocrinol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Freeman, L.R.E.; Granholm, A.-C. Vascular Changes in Rat Hippocampus following a High Saturated Fat and Cholesterol Diet. Br. J. Pharmacol. 2011, 32, 643–653. [Google Scholar] [CrossRef]
- Kim, D.W.; Glendining, K.A.; Grattan, D.R.; Jasoni, C.L. Maternal Obesity in the Mouse Compromises the Blood-Brain Barrier in the Arcuate Nucleus of Offspring. Endocrinology 2016, 157, 2229–2242. [Google Scholar] [CrossRef]
- Davidson, T.L.; Monnot, A.; Neal, A.U.; Martin, A.A.; Horton, J.J.; Zheng, W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood–brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol. Behav. 2012, 107, 26–33. [Google Scholar] [CrossRef]
- Chang, H.-C.; Tai, Y.-T.; Cherng, Y.-G.; Lin, J.-W.; Liu, S.-H.; Chen, T.-L.; Chen, R.-M. Resveratrol Attenuates High-Fat Diet-Induced Disruption of the Blood–Brain Barrier and Protects Brain Neurons from Apoptotic Insults. J. Agric. Food Chem. 2014, 62, 3466–3475. [Google Scholar] [CrossRef] [PubMed]
- Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Szalai, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Obesity in Aging Exacerbates Blood-Brain Barrier Disruption, Neuroinflammation, and Oxidative Stress in the Mouse Hippocampus: Effects on Expression of Genes Involved in Beta-Amyloid Generation and Alzheimer’s Disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 1212–1226. [Google Scholar] [CrossRef]
- Ghribi, O.; Golovko, M.; Larsen, B.; Schrag, M.; Murphy, E.J. Deposition of iron and ?-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol-enriched diets. J. Neurochem. 2006, 99, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Farrall, A.J.; Wardlaw, J.M. Blood–brain barrier: Ageing and microvascular disease systematic review and meta-analysis. Neurobiol. Aging 2009, 30, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Owen, J.B.; Erickson, M.A. Insulin in the brain: There and back again. Pharmacol. Ther. 2012, 136, 82–93. [Google Scholar] [CrossRef]
- Heni, M.; Schöpfer, P.; Peter, A.; Sartorius, T.; Fritsche, A.E.; Synofzik, M.B.; Häring, H.-U.; Maetzler, W.; Hennige, A.M. Evidence for altered transport of insulin across the blood–brain barrier in insulin-resistant humans. Acta Diabetol. 2013, 51, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Tschritter, O.; Preissl, H.; Hennige, A.M.; Stumvoll, M.; Porubska, K.; Frost, R.; Marx, H.; Klösel, B.; Lutzenberger, W.; Birbaumer, N.; et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: A magnetoencephalographic study. Proc. Natl. Acad. Sci. USA 2006, 103, 12103–12108. [Google Scholar] [CrossRef]
- Rhea, E.M.; Salameh, T.S.; Logsdon, A.F.; Hanson, A.J.; Erickson, M.A.; Banks, W.A. Blood-Brain Barriers in Obesity. AAPS J. 2017, 19, 921–930. [Google Scholar] [CrossRef]
- He, Y.; Han, D.; Shi, M.; Sun, Z.; Xie, J.; Zhu, J.; Liu, Y. Effects of High-Fat Diet on the Morphological Characteristics of Cerebral Microvasculature without Hyperlipidemia in Wistar Rat. Int. J. Clin. Exp. Pathol 2016, 9, 11752–11759. [Google Scholar]
- Bernier, M.; Wahl, D.; Ali, A.; Allard, J.; Faulkner, S.; Wnorowski, A.; Sanghvi, M.; Moaddel, R.; Alfaras, I.; Mattison, J.A.; et al. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging 2016, 8, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Eudave, D.M.; BeLow, M.N.; Flandreau, E.I. Effects of high fat or high sucrose diet on behavioral-response to social defeat stress in mice. Neurobiol. Stress 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Keleher, M.R.; Zaidi, R.; Patel, K.; Ahmed, A.; Bettler, C.; Pavlatos, C.; Shah, S.; Cheverud, J.M. The effect of dietary fat on behavior in mice. J. Diabetes Metab. Disord. 2018, 17, 297–307. [Google Scholar] [CrossRef]
- Deal, A.W.; Seshie, O.; Lenzo, A.; Cooper, N.; Ozimek, N.; Woods, L.C.S. High-fat diet negatively impacts both metabolic and behavioral health in outbred heterogeneous stock rats. Physiol. Genom. 2020, 52, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Akana, S.F.; Bhatnagar, S.; Dallman, M.F.; Wong, D.; Mucke, L. Hypothalamic-Pituitary-Adrenal Dysfunction in Apoe Mice: Possible Role in Behavioral and Metabolic Alterations. J. Neurosci. 2000, 20, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Malnick, S.D.H.; Knobler, H. The Medical Complications of Obesity. QJM 2006, 99, 565–579. [Google Scholar] [CrossRef]
- Johnson, L.A.; Zuloaga, K.L.; Kugelman, T.L.; Mader, K.S.; Morré, J.T.; Zuloaga, D.G.; Weber, S.; Marzulla, T.; Mulford, A.; Button, D.; et al. Amelioration of Metabolic Syndrome-Associated Cognitive Impairments in Mice via a Reduction in Dietary Fat Content or Infusion of Non-Diabetic Plasma. eBioMedicine 2016, 3, 26–42. [Google Scholar] [CrossRef]
- Osmond, J.M.; Mintz, J.D.; Stepp, D.W. Preventing increased blood pressure in the obese Zucker rat improves severity of stroke. Am. J. Physiol. Circ. Physiol. 2010, 299, H55–H61. [Google Scholar] [CrossRef]
- Osmond, J.M.; Mintz, J.D.; Dalton, B.; Stepp, D.W. Obesity Increases Blood Pressure, Cerebral Vascular Remodeling, and Severity of Stroke in the Zucker Rat. Hypertension 2009, 53, 381–386. [Google Scholar] [CrossRef]
- Chantler, P.D.; Shrader, C.D.; Tabone, L.E.; D’Audiffret, A.C.; Huseynova, K.; Brooks, S.D.; Branyan, K.W.; Grogg, K.A.; Frisbee, J.C. Cerebral Cortical Microvascular Rarefaction in Metabolic Syndrome is Dependent on Insulin Resistance and Loss of Nitric Oxide Bioavailability. Microcirculation 2015, 22, 435–445. [Google Scholar] [CrossRef]
- Frisbee, J.C. Hypertension-Independent Microvascular Rarefaction in the Obese Zucker Rat Model of the Metabolic Syndrome. Microcirculation 2005, 12, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Animal Models of Atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. Effect of a Single High-Fat Meal on Endothelial Function in Healthy Subjects. Am. J. Cardiol. 1997, 79, 350–354. [Google Scholar] [CrossRef]
- Cuevas, A.M.; Guasch, V.; Castillo, O.; Irribarra, V.; Mizon, C.; Martin, A.S.; Strobel, P.; Perez, D.; Germain, A.M.; Leighton, F. A high-fat diet induces and red wine counteracts endothelial dysfunction in human volunteers. Lipids 2000, 35, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Gryglewski, R.J.; Palmer, R.M.J.; Moncada, S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nat. Cell Biol. 1986, 320, 454–456. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Mietus-Snyder, M.; Malloy, M.J. Endothelial dysfunction occurs in children with two genetic hyperlipidemias: Improvement with antioxidant vitamin therapy. J. Pediatr. 1998, 133, 35–40. [Google Scholar] [CrossRef]
- Chambers, J.C.; McGregor, A.; Jean-Marie, J.; Obeid, O.A.; Kooner, J.S. Demonstration of Rapid Onset Vascular Endothelial Dysfunction After Hyperhomocysteinemia. Circulation 1999, 99, 1156–1160. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef]
- Xiao, L.; Sonne, S.B.; Feng, Q.; Chen, N.; Xia, Z.; Li, X.; Fang, Z.; Zhang, D.; Fjære, E.; Midtbø, L.K.; et al. High-fat feeding rather than obesity drives taxonomical and functional changes in the gut microbiota in mice. Microbiome 2017, 5, 43. [Google Scholar] [CrossRef]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nat. Cell Biol. 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Bultman, S.J. Bacterial butyrate prevents atherosclerosis. Nat. Microbiol. 2018, 3, 1332–1333. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut Microbiota Metabolism of Anthocyanin Promotes Reverse Cholesterol Transport in Mice Via Repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xiao, X.; Hu, M.; Zhang, X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018, 214, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef]
- Krych, L.; Hansen, C.H.F.; Hansen, A.K.; Berg, F.V.D.; Nielsen, D.S. Quantitatively Different, yet Qualitatively Alike: A Meta-Analysis of the Mouse Core Gut Microbiome with a View towards the Human Gut Microbiome. PLoS ONE 2013, 8, e62578. [Google Scholar] [CrossRef]
- De Matheus, A.S.M.; Tannus, L.R.M.; Cobas, R.A.; Palma, C.C.S.; Negrato, C.A.; Gomes, M.d.B. Impact of Diabetes on Cardiovascular Disease: An Update. Available online: https://www.hindawi.com/journals/ijhy/2013/653789/ (accessed on 22 February 2021).
- Islam, M.d.S.; Wilson, R.D. Experimentally Induced Rodent Models of Type 2 Diabetes. In Animal Models in Diabetes Research; Joost, H.-G., Al-Hasani, H., Schürmann, A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 161–174. ISBN 978-1-62703-068-7. [Google Scholar]
- Chang-Chen, K.J.; Mullur, R.; Bernal-Mizrachi, E. β-cell failure as a complication of diabetes. Rev. Endocr. Metab. Disord. 2008, 9, 329–343. [Google Scholar] [CrossRef]
- Parilla, J.H.; Willard, J.R.; Barrow, B.M.; Zraika, S. A Mouse Model of Beta-Cell Dysfunction as Seen in Human Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Chai, W.; Chen, M.-H.; Liu, Z.; Shi, W. Hyperglycemia in apolipoprotein E-deficient mouse strains with different atherosclerosis susceptibility. Cardiovasc. Diabetol. 2011, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Mathews, C.E.; Xue, S.; Posgai, A.; Lightfoot, Y.L.; Li, X.; Lin, A.; Wasserfall, C.; Haller, M.J.; Schatz, D.; Atkinson, M.A. Acute Versus Progressive Onset of Diabetes in NOD Mice: Potential Implications for Therapeutic Interventions in Type 1 Diabetes. Diabetes 2015, 64, 3885–3890. [Google Scholar] [CrossRef]
- Lakka, H.-M.; Lakka, T.A.; Tuomilehto, J.; Sivenius, J.; Salonen, J.T. Hyperinsulinemia and the Risk of Cardiovascular Death and Acute Coronary and Cerebrovascular Events in Men. Arch. Intern. Med. 2000, 160, 1160–1168. [Google Scholar] [CrossRef]
- Yin, W.; Carballo-Jane, E.; McLaren, D.G.; Mendoza, V.H.; Gagen, K.; Geoghagen, N.S.; McNamara, L.A.; Gorski, J.N.; Eiermann, G.J.; Petrov, A.; et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J. Lipid Res. 2012, 53, 51–65. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Hagi, A.; Inoue, Y. The Relationship between Plasma High Density Lipoprotein Cholesterol Levels and Cholesteryl Ester Transfer Protein Activity in Six Species of Healthy Experimental Animals. Biol. Pharm. Bull. 2001, 24, 579–581. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Levin, B.E.; Dunn-Meynell, A.A. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am. J. Physiol. Integr. Comp. Physiol. 2002, 282, R46–R54. [Google Scholar] [CrossRef]
- Levin, B.E. Arcuate NPY neurons and energy homeostasis in diet-induced obese and resistant rats. Am. J. Physiol. Content 1999, 276, R382–R387. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J.P. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef]
- Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F. Early nutrition impact on the insulin-like growth factor axis and later health consequences. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 285–292. [Google Scholar] [CrossRef]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. ACTA (BBA) Mol. Basis Dis. 2014, 1842, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Lindsley, S.R.; Comstock, S.M.; Takahashi, D.L.; Evans, A.E.; He, G.-W.; Thornburg, K.L.; Grove, K.L. Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. Int. J. Obes. 2012, 37, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. Fetal origins of cardiovascular disease. Ann. Med. 1999, 31, 3–6. [Google Scholar] [CrossRef]
- Barker, D.J. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 2002, 13, 364–368. [Google Scholar] [CrossRef]
- Barker, D.; Godfrey, K.; Gluckman, P.; Harding, J.; Owens, J.; Robinson, J. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Symonds, M.; Mostyn, A.; Pearce, S.; Budge, H.; Stephenson, T.; Symonds, M. Endocrine and nutritional regulation of fetal adipose tissue development. J. Endocrinol. 2003, 179, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Budge, H.; Mostyn, A.; Pearce, S.; Webb, R.; Symonds, M. Fetal and neonatal adipose maturation: A primary site of cytokine and cytokine-receptor action. Biochem. Soc. Trans. 2001, 29, 80–85. [Google Scholar] [CrossRef]
- Kim, J.K.; Gavrilova, O.; Chen, Y.; Reitman, M.L.; Shulman, G.I. Mechanism of Insulin Resistance in A-ZIP/F-1 Fatless Mice. J. Biol. Chem. 2000, 275, 8456–8460. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Barnes, R.I.; Garg, A. Genetic basis of congenital generalized lipodystrophy. Int. J. Obes. 2003, 28, 336–339. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Pajvani, U.B.; Scherer, P.E. Apoptosis through Targeted Activation of Caspase8 (“ATTAC-mice”): Novel Mouse Models of Inducible and Reversible Tissue Ablation. Cell Cycle 2005, 4, 1141–1145. [Google Scholar] [CrossRef]
- Chehab, F.F. Minireview: Obesity and LipOdystrophy—Where Do the Circles Intersect? Endocrinology 2008, 149, 925–934. [Google Scholar] [CrossRef]
- McCurdy, C.; Bishop, J.M.; Williams, S.M.; Grayson, B.E.; Smith, M.S.; Friedman, J.E.; Grove, K.L. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Investig. 2009, 119, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; Cagampang, F.R.; Mukhtar, D.; Anthony, F.W.; Ohri, S.K.; Hanson, M. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br. J. Nutr. 2009, 102, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Parente, L.B.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Deleterious effects of high-fat diet on perinatal and postweaning periods in adult rat offspring. Clin. Nutr. 2008, 27, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.Y.; Dekou, V.; Douglas, G.; Jensen, R.; Hanson, M.; Poston, L.; Taylor, P.D. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R127–R133. [Google Scholar] [CrossRef]
- Vuguin, P.M.; Hartil, K.; Kruse, M.; Kaur, H.; Lin, C.-L.V.; Fiallo, A.; Glenn, A.S.; Patel, A.; Williams, L.; Seki, Y.; et al. Shared Effects of Genetic and Intrauterine and Perinatal Environment on the Development of Metabolic Syndrome. PLoS ONE 2013, 8, e63021. [Google Scholar] [CrossRef]
- Armitage, J.A.; Lakasing, L.; Taylor, P.D.; Balachandran, A.A.; Jensen, R.I.; Dekou, V.; Ashton, N.; Nyengaard, J.R.; Poston, L. Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J. Physiol. 2005, 565, 171–184. [Google Scholar] [CrossRef]
- Khan, I.Y.; Taylor, P.D.; Dekou, V.; Seed, P.T.; Lakasing, L.; Graham, D.; Dominiczak, A.F.; Hanson, M.A.; Poston, L. Gender-Linked Hypertension in Offspring of Lard-Fed Pregnant Rats. Hypertension 2003, 41, 168–175. [Google Scholar] [CrossRef]
- Mitra, A.; Alvers, K.M.; Crump, E.M.; Rowland, N.E. Effect of high-fat diet during gestation, lactation, or postweaning on physiological and behavioral indexes in borderline hypertensive rats. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R20–R28. [Google Scholar] [CrossRef]
- Taylor, P.D.; McConnell, J.; Khan, I.Y.; Holemans, K.; Lawrence, K.M.; Asare-Anane, H.; Persaud, S.J.; Jones, P.M.; Petrie, L.; Hanson, M.; et al. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R134–R139. [Google Scholar] [CrossRef] [PubMed]
- Clancy, B.; Finlay, B.L.; Darlington, R.B.; Anand, K. Extrapolating brain development from experimental species to humans. NeuroToxicology 2007, 28, 931–937. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmerman, B.; Kundu, P.; Rooney, W.D.; Raber, J. The Effect of High Fat Diet on Cerebrovascular Health and Pathology: A Species Comparative Review. Molecules 2021, 26, 3406. https://doi.org/10.3390/molecules26113406
Zimmerman B, Kundu P, Rooney WD, Raber J. The Effect of High Fat Diet on Cerebrovascular Health and Pathology: A Species Comparative Review. Molecules. 2021; 26(11):3406. https://doi.org/10.3390/molecules26113406
Chicago/Turabian StyleZimmerman, Benjamin, Payel Kundu, William D. Rooney, and Jacob Raber. 2021. "The Effect of High Fat Diet on Cerebrovascular Health and Pathology: A Species Comparative Review" Molecules 26, no. 11: 3406. https://doi.org/10.3390/molecules26113406
APA StyleZimmerman, B., Kundu, P., Rooney, W. D., & Raber, J. (2021). The Effect of High Fat Diet on Cerebrovascular Health and Pathology: A Species Comparative Review. Molecules, 26(11), 3406. https://doi.org/10.3390/molecules26113406





